High Pressure Processing under Mild Conditions for Bacterial Mitigation and Shelf Life Extension of European Sea Bass Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Sources and Proximate Analysis
2.2. Application of High Pressure on Fish Fillets
2.3. Microbiological Analysis
- TVCs were enumerated using spread plate methodology on tryptic glucose yeast agar (Biolife, IT) after aerobic incubation at 25 °C for 72 h;
- Pseudomonas spp. were enumerated using spread plate methodology on Cetrimide agar (CFC, Merck, Darmstadt, Germany) after aerobic incubation at 25 °C for 48 h;
- Brochothrix thermosphacta was enumerated using spread plate methodology on Streptomycin Thallous Acetate agar (STAA, Oxoid, Lenexa, KS, United States) after aerobic incubation at 25 °C for 48 h;
- LABs were enumerated using pour plate methodology in Mann, Rogosa, and Sharpe agar (MRS, Merck, Darmstadt, Germany) after incubation under a facultatively anaerobic condition at 25 °C for 96 h;
- H2S-producing bacteria were enumerated using pour plate methodology in LYNGBY Iron Agar (Condalab, Madrid, Spain) after incubation under a facultatively anaerobic condition at 25 °C for 72 h;
- Enterobacteriaceae spp. were enumerated using pour plate methodology in Violet Red Bile Glucose agar (VRBG, Merck, Darmstadt, Germany) after incubation under a facultatively anaerobic condition at 37 °C for 24 h.
2.4. Physicochemical Analyses
2.5. Sensory Evaluation
2.6. Data and Statistical Analyses
3. Results and Discussion
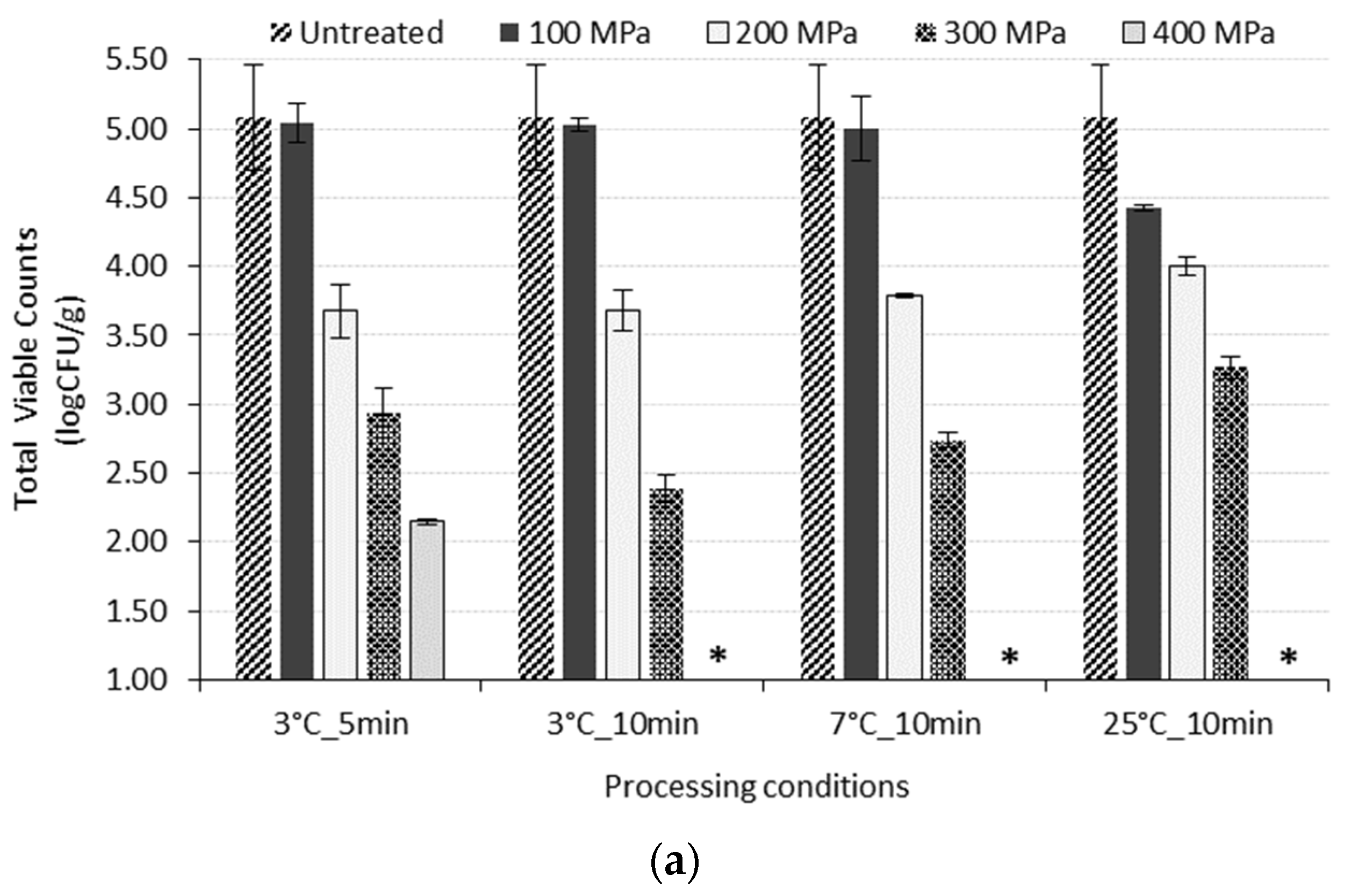
3.1. Effect of HP Treatment on the Reduction of Initial Microflora
3.2. Effect of HP on the Bacterial Growth and the Shelf Life of Sea Bass Fillets
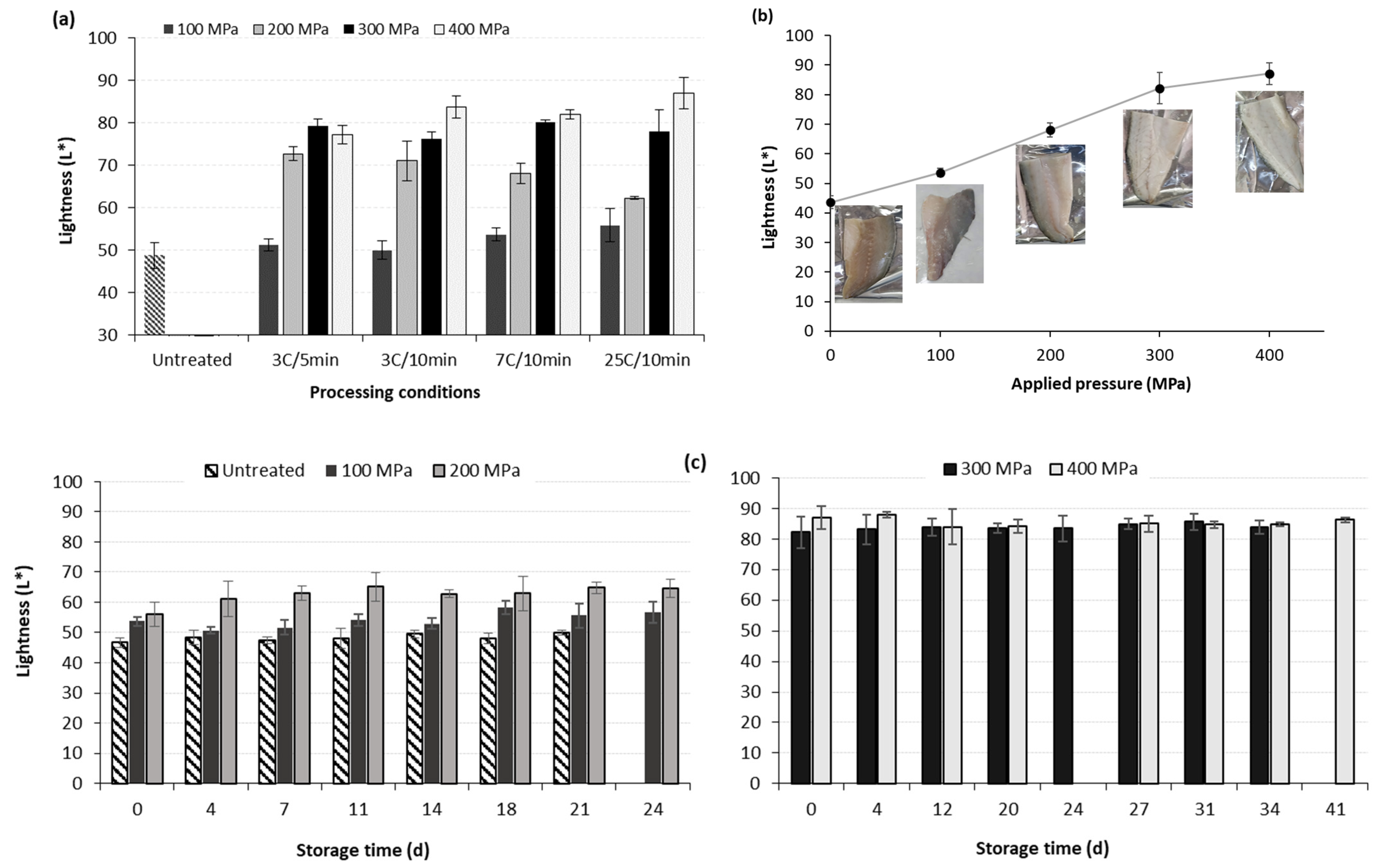
3.3. Effect of HP on the Physicochemical Characteristics of Sea Bass Fillets
3.4. Effect of HP on the Sensorial Characteristics of Fish Fillets
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Tsironi, T.; Houhoula, D.; Taoukis, P. Hurdle technology for fish preservation. Aquac. Fish. 2020, 5, 65–71. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Physical emerging technologies. Foods 2021, 10, 1–20. [Google Scholar]
- Adeyeye, S.A.O. Smoking of fish: A critical review. J. Culin. Sci. Technol. 2019, 17, 559–575. [Google Scholar] [CrossRef]
- Tsironi, T.; Taoukis, P.S. Modeling microbial spoilage and quality of gilthead seabream fillets: Combined effect of osmotic pretreatment, modified atmosphere packaging and nisin on shelf life. J. Food Sci. 2010, 75, 243–251. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Laub-Ekgreen, M.H.; Martinez-Lopez, B.; Frosch, S.; Jessen, F. The influence of processing conditions on the weight change of single herring (Clupea herengus) fillets during marinating. Food Res. Int. 2018, 108, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Tsevdou, M.; Gogou, E.; Taoukis, P. High hydrostatic pressure processing of foods. In Green Food Processing Techniques: Preservation, Transformation and Extraction; Vorobiev, E., Chemat, F., Eds.; Academic Press: London, UK, 2019; pp. 87–137. [Google Scholar]
- Tonello, C. Case Studies on High Pressure Processing of Foods. In Nonthermal Processing Technologies for Food; Zhang, H.Q., Barbosa-Cánovas, G.V., Balasubramaniam, V.M., Dunne, C.P., Farkas, D.F., Yuan, J.T.C., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 36–50. [Google Scholar]
- Roobab, U.; Fidalgo, L.G.; Arshad, R.N.; Khan, A.W.; Zeng, X.-A.; Bhat, Z.F.; Bekhit, A.E.-D.A.; Batool, Z.; Aadil, R.M. High pressure processing of fish and shellfish products: Safety, quality, and research prospects. Compr. Rev. Food Sci. Food Saf. 2021, 21, 3297–3325. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhu, C.; Zhang, D.; Liu, H. Effects of high pressure processing on aquatic products with an emphasis on sensory evaluation. Int. J. Food Sci. Technol. 2022, 57, 6980–6996. [Google Scholar] [CrossRef]
- Arnaud, C.; de Lamballerie, M.; Pottier, L. Effect of high pressure processing on the preservation of frozen and re-thawed sliced cod (Gadus morhua) and salmon (Salmo salar) fillets. High Press Res. 2018, 38, 62–79. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, Y.; Wang, Q.; Cheng, K.-W.; Chen, F. Application of high pressure processing to improve digestibility, reduce allergenicity, and avoid protein oxidation in cod (Gadus morhua). Food Chem. 2019, 298, 125087. [Google Scholar] [CrossRef] [PubMed]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. High pressure processing (HPP) for decreasing weight loss of fresh albacore (Thunnus alalunga) steaks. Food Bioprocess Technol. 2019, 12, 2074–2084. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. Evolution of quality parameters of high pressure processing (HPP) pretreated albacore (Thunnus alalunga) during long-term frozen storage. Innov. Food Sci. Emerg. Technol. 2020, 62, 102334. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. High pressure pretreatment in albacore (Thunnus alalunga) for reducing freeze-driven weight losses with minimal quality changes. J. Sci. Food Agric. 2020, 101, 2704–2711. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The effect of different high pressure conditions on the quality and shelf life of cold smoked fish. Innov. Food Sci. Emerg. Technol. 2011, 12, 104–110. [Google Scholar] [CrossRef]
- Teixeira, B.; Fidalgo, L.; Mendes, R.; Costa, G.; Cordeiro, C.; Marques, A.; Saraiva, J.A.; Nunes, M.L. Effect of high pressure processing in the quality of sea bass (Dicentrarchus labrax) fillets: Pressurization rate, pressure level and holding time. Innov. Food Sci. Emerg. Technol. 2014, 22, 31–39. [Google Scholar] [CrossRef]
- Tsironi, T.; Anjos, L.; Pinto, P.I.S.; Dimopoulos, G.; Santos, S.; Santa, C.; Manadas, B.; Canario, A.; Taoukis, P.; Power, D. High pressure processing of European sea bass (Dicentrarchus labrax) fillets and tools for flesh quality and shelf life monitoring. J. Food Eng. 2019, 262, 83–91. [Google Scholar] [CrossRef]
- Cropotova, J.; Mozuraityte, R.; Standal, I.B.; Ojha, S.; Rustad, T.; Tiwari, B. Influence of high pressure processing on quality attributes of haddock and mackerel minces during frozen storage, and fishcakes prepared thereof. Innov. Food Sci. Emerg. Technol. 2020, 59, 102236. [Google Scholar] [CrossRef]
- Roobab, U.; Afzal, R.; Ranjha, M.M.A.N.; Zeng, X.-A.; Ahmed, Z.; Aadil, R.M. High pressure-based hurdle interventions for raw and processed meat: A clean-label prospective. Int. J. Food Sci. 2022, 57, 816–826. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Dalgaard, P. Effects of high -pressure processing on Listeria monocytogenes, spoilage microflora and multiple compound quality indices in chilled cold-smoked salmon. J. Appl. Microbiol. 2004, 96, 398–408. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Zaman, S.U.; Smith, J.P. High pressure destruction kinetics of Escherichia coli (O157:H7) and Listeria monocytogenes (Scott A) in a fish slurry. J. Food Eng. 2008, 87, 99–106. [Google Scholar] [CrossRef]
- Ramirez-Suarez, J.C.; Morrissey, M.T. Effect of high pressure processing (HPP) on shelf life of albacore tuna (Thunnus alalunga) minced muscle. Innov. Food Sci. Emerg. Technol. 2006, 7, 19–27. [Google Scholar] [CrossRef]
- Panagou, E.; Tassou, C.C.; Manitsa, C.; Mallidis, C. Modelling the effect of high pressure on the inactivation kinetics of a pressure-resistant strain of Pediococcus damnosus in phosphate buffer and gilt-head seabream (Sparus aurata). J. Appl. Microbiol. 2007, 102, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Tsironi, T.; Maltezou, I.; Tsevdou, M.; Katsaros, G.; Taoukis, P. High Pressure Cold Pasteurization of Gilthead Seabream Fillets: Selection of Process Conditions and Validation of Shelf Life Extension. Food Bioprocess Technol. 2015, 8, 681–690. [Google Scholar] [CrossRef]
- terSteeg, P.F.; Hellemons, J.C.; Kok, A.E. Synergistic actions of nisin, sublethal ultrahigh pressure and reduced temperature on bacteria and yeast. Appl. Environ. Microbiol. 1999, 65, 4148–4154. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G. The effect of high hydrostatic pressure on the microbiological, chemical and sensory quality of fresh gilthead sea bream (Sparus aurata). Eur. Food Res. Technol. 2010, 230, 533–542. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F.; DeWitt, C.A.M. High pressure processing at ultra-low temperatures: Inactivation of foodborne bacterial pathogens and quality changes in frozen fish fillets. Innov. Food Sci. Emerg. Technol. 2021, 74, 102811. [Google Scholar] [CrossRef]
- Wirtz, C. Agriculture, forestry and fishery statistics. In Eurostat Products Statistical Books; Publications Office of the European Union: Luxembourg, Luxembourg, 2020. [Google Scholar]
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA). Species Profile: European Seabass; EUMOFA: Brussels, Belgium, 2022. [Google Scholar]
- Ntzimani, A.; Angelakopoulos, R.; Semenoglou, I.; Dermesonlouoglou, E.; Tsironi, T.; Moutou, K.; Taoukis, P. Slurry ice as an alternative cooling medium for fish harvesting and transportation—Study of the effect on seabass flesh quality and shelf life. Aquac. Fish. 2023, 8, 385–392. [Google Scholar] [CrossRef]
- Semenoglou, I.; Eliasson, L.; Uddstål, R.; Tsironi, T.; Taoukis, P.; Xanthakis, E. Supercritical CO2 extraction of oil from Arctic charr side streams from filleting processing. Innov. Food Sci. Emerg. Technol. 2021, 71, 10212. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Taoukis, P.S.; Drosinos, E.H.; Nychas, G.-J.E. Applicability of an Arrhenius Model for the Combined Effect of Temperature and CO2 Packaging on the Spoilage Microflora of Fish. Appl. Environ. Microbiol. 2000, 66, 3528–3534. [Google Scholar] [CrossRef]
- ISO 13300-1; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 1: Staff Responsibilities. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- ISO 13300-2; Sensory Analysis—General Guidance for the Staff of a Sensory Evaluation Laboratory—Part 2: Recruitment and Training of Panel Leaders. International Organization for Standardization (ISO): Geneva, Switzerland, 2006.
- ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- Baranyi, J.; Roberts, T.A. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef]
- Castrica, M.; Pavlovic, R.; Balzaretti, C.M.; Curone, G.; Brecchia, G.; Copelotti, E.; Panseri, S.; Pessina, D.; Arnoldi, C.; Chiesa, L.M. Effect of High Pressure Processing on Physico-Chemical, Microbiological and Sensory Traits in Fresh Fish Fillets (Salmo salar and Pleuronectes platessa). Foods 2021, 10, 1775. [Google Scholar] [CrossRef]
- Argyri, A.A.; Papadopoulou, O.S.; Sourri, P.; Chorianopoulos, N.; Tassou, C.C. Quality and Safety of Fresh Chicken Fillets after High Pressure Processing: Survival of Indigenous Brochothrix thermosphacta and Inoculated Listeria monocytogenes. Microorganisms 2019, 7, 520. [Google Scholar] [CrossRef]
- de Alba, M.; Pérez-Andrés, J.M.; Harrison, S.M.; Brunton, N.P.; Burgess, C.M.; Tiwari, B.K. High pressure processing on microbial inactivation, quality parameters and nutritional quality indices of mackerel fillets. Innov. Food Sci. Emerg. Technol. 2019, 55, 80–87. [Google Scholar] [CrossRef]
- Kumar, V.; Rao, P.S.; Purohit, S.R.; Kumar, Y. Effects of high pressure processing (HPP) and acid pre-treatment on quality attributes of hilsa (Tenualosa ilisha) fillets. LWT Food Sci. Technol. 2019, 111, 647–652. [Google Scholar] [CrossRef]
- Romulo, A. The impact of high pressure processing treatment on microbial inactivation of seafood—A review. Food Res. 2021, 5, 38–44. [Google Scholar] [CrossRef]
- Kung, H.-F.; Lin, C.-S.; Liu, S.-S.; Huang, C.-Y.; Chiu, K.; Lee, Y.-C.; Tsai, Y.-H. High pressure processing extend the shelf life of milkfish flesh during refrigerated storage. Food Control 2022, 134, 108768. [Google Scholar] [CrossRef]
- Alpas, H.; Kalchayanand, N.; Bozoglu, F.; Ray, B. Interactions of high hydrostatic pressure, pressurization temperature and pH on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. Int. J. Food Microb. 2000, 60, 33–42. [Google Scholar] [CrossRef]
- Moussa, M.; Perrier-Cornet, J.M.; Gervais, P. Damage in Escherichia coli cells treated with a combination of High Hydrostatic Pressure and subzero temperature. Appl. Environ. Microbiol. 2007, 73, 6508–6518. [Google Scholar] [CrossRef]
- Bulut, S. The effects of high pressure processing at low and subzero temperatures on inactivation of microorganisms in frozen and unfrozen beef mince inoculated with Escherichia coli strain ATCC 25922. Food Bioprocess Technol. 2014, 7, 3033–3044. [Google Scholar] [CrossRef]
- Bulut, S.; Chapleau, N.; de Lamballerie, M.; Le-Bail, A. High Pressure Processing of chicken meat: Change in total aerobic counts after pressure treatment and during chilled storage. Br. Microbiol. Res. J. 2014, 4, 540–549. [Google Scholar] [CrossRef]
- Chapleau, N.; Ritz, M.; Delépine, S.; Jugiau, F.; Federighi, M.; de Lamballerie, M. Influence of kinetic parameters of high pressure processing on bacterial inactivation in a buffer system. Int. J. Food Microbiol. 2006, 106, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Hovda, M.B. High pressure processing extend the shelf life of fresh salmon, cod and mackerel. Food Control 2016, 70, 242–248. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Stathopoulos, C.E.; Nguyen, M.H. Advances in High Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. [Google Scholar] [CrossRef]
- Cheftel, J.C. Review: High pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 1995, 1, 75–90. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Marshall, M.R. Effect of high pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). J. Food Sci. 2007, 72, C509–C515. [Google Scholar] [CrossRef]
- Chouhan, A.; Kaur, B.P.; Rao, P.S. Effect of high pressure processing and thermal treatment on quality of hilsa (Tenualosa ilisha) fillets during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 151–160. [Google Scholar] [CrossRef]
- Christensen, L.B.; Hovda, M.B.; Rode, T.M. Quality changes in high pressure processed cod, salmon and mackerel during storage. Food Control 2017, 72, 90–96. [Google Scholar] [CrossRef]
- Pazos, M.; Mendez, L.; Fidalgo, L.; Vásquez, M.; Torres, J.A.; Aubourg, S.P.; Saraiva, J.A. Effect of high pressure processing of Atlantic mackerel (Scomber scombrus) on biochemical changes during commercial frozen storage. Food Bioprocess Technol. 2015, 8, 2159–2170. [Google Scholar] [CrossRef]




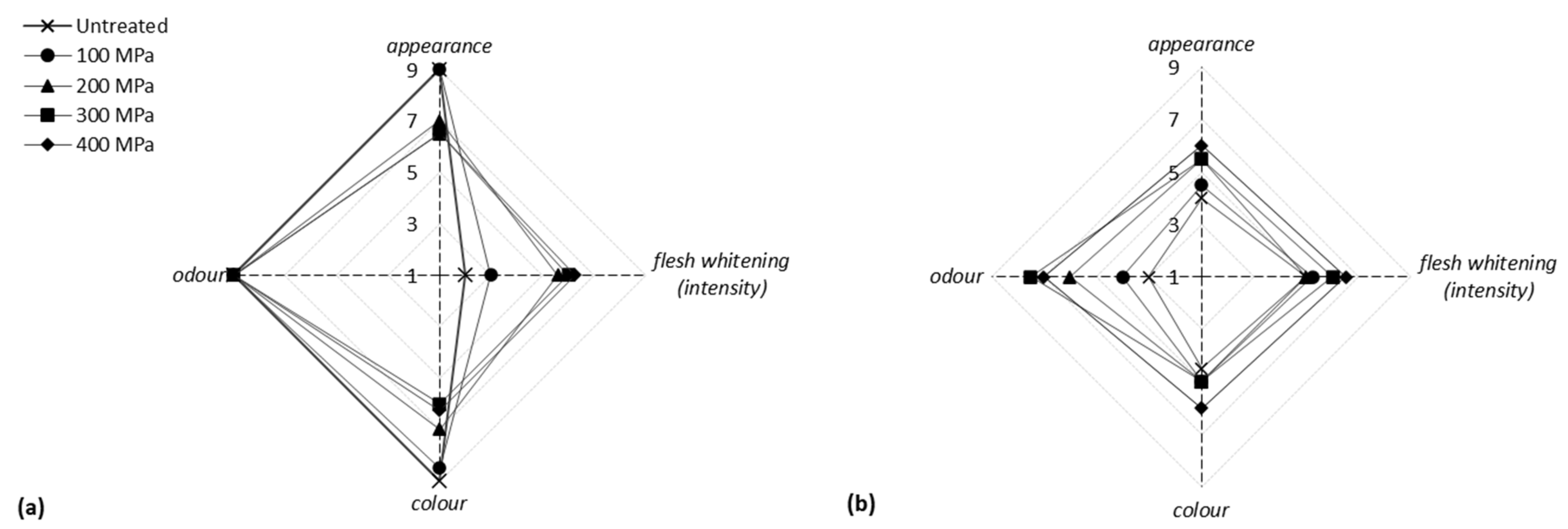

| Raw Fillet Characteristics | Cooked Fillet Characteristics |
|---|---|
| Total appearance (hedonic) Flesh “whitening” (intensity) Color (hedonic) Odor (hedonic) | Appearance (hedonic) Color (hedonic) Odor (hedonic) Taste (hedonic) Hardness (intensity) Hardness (hedonic) Adhesiveness * (intensity) Adhesiveness * (hedonic) Overall score |
| Fish Fillet Treatment | Growth Rate (d−1) | Lag Phase (d) | R-Square | Shelf Life * (d) |
|---|---|---|---|---|
| Untreated fillet | 0.432 d (±0.019) | 3.9 bc (±0.3) | 0.999 | 10 a (±0.1) |
| Untreated fillet (aerobically stored) | 0.301 abc (±0.031) | 0.1 a (±0.0) | 0.992 | 10 a (±0.8) |
| 100 MPa_3 °C_5 min | 0.459 3 (±0.036) | 3.3 2 (±0.5) | 0.997 | 11 1 (±0.0) |
| 200 MPa_3 °C_5 min | 0.439 3 (±0.052) | 3.3 2 (±0.8) | 0.993 | 13 12 (±0.1) |
| 300 MPa_3 °C_5 min | 0.401 3 (±0.035) | 2.7 2 (±0.8) | 0.994 | 15 23 (±0.1) |
| 400 MPa_3 °C_5 min | 0.218 2 (±0.021) | 9.8 3 (±0.2) | 0.990 | 32 6 (±2.3) |
| 100 MPa_3 °C_10 min | 0.153 AB,12 (±0.025) | 0.0 A,1 (±0.0) | 0.924 | 15 BC,34 (±2.3) |
| 200 MPa_3 °C_10 min | 0.212 BC,2 (±0.021) | 0.0 A,1 (±0.0) | 0.974 | 17 C,4 (±1.6) |
| 300 MPa_3 °C_10 min | 0.435 F,3 (±0.065) | 12 FG,4 (±1.2) | 0.952 | 23 E,5 (±0.3) |
| 400 MPa_3 °C_10 min | 0.144 AB,1 (±0.011) | 14 G,4 (±2.8) | 0.990 | 50 G,6 (±0.2) |
| 100 MPa_7 °C_10 min | 0.192 ABC (±0.012) | 1.7 AB (±0.6) | 0.996 | 11 A (±0.1) |
| 200 MPa_7 °C_10 min | 0.270 CD (±0.053) | 0.1 A (±0.0) | 0.960 | 14 B (±2.5) |
| 300 MPa_7 °C_10 min | 0.212 BC (±0.024) | 3.7 BC (±1.9) | 0.980 | 21 D (±0.2) |
| 400 MPa_7 °C_10 min | 0.114 A (±0.006) | 22 H (±2.3) | 0.992 | 67 H (±0.1) |
| 100 MPa_25 °C_10 min | 0.408 d,EF (±0.075) | 5.4 cd,CD (±0.9) | 0.980 | 11 b,A (±0.1) |
| 200 MPa_25 °C_10 min | 0.353 bcd,E (±0.087) | 9.9 f,EF (±0.5) | 0.928 | 20 e,D (±1.7) |
| 300 MPa_25 °C_10 min | 0.334 bcd,CD (±0.050) | 7.9 e,DE (±1.8) | 0.983 | 22 f,DE (±0.2) |
| 400 MPa_25 °C_10 min | 0.217 a,BC (±0.021) | 13 g,G (±2.4) | 0.977 | 37 h,F (±0.3) |
| aerobically stored | ||||
| 100 MPa_25 °C_10 min | 0.386 cd (±0.051) | 0.1 a (±0.0) | 0.983 | 10 ab (±1.1) |
| 200 MPa_25 °C_10 min | 0.283 ab (±0.019) | 2.8 b (±0.9) | 0.994 | 14 c (±0.2) |
| 300 MPa_25 °C_10 min | 0.376 bcd (±0.026) | 6.7 de (±0.8) | 0.995 | 18 d (±0.1) |
| 400 MPa_25 °C_10 min | 0.632 e (±0.083) | 16 h (±0.9) | 0.987 | 25 g (±0.1) |
| Fish Fillet Treatment | pH Value | Hardness (g) | Adhesiveness (g·s) |
|---|---|---|---|
| Untreated fillet | 6.53 a (±0.02) | 127 a (±9.94) | 20.2 e (±4.67) |
| Untreated fillet (aerobically stored) | 6.70 de (±0.12) | 151 a (±17.9) | 9.91 cd (±1.31) |
| 100 MPa_3 °C_5 min | 6.78 3 (±0.10) | 119 1 (±14.4) | 21.6 2 (±6.62) |
| 200 MPa_3 °C_5 min | 6.62 12 (±0.01) | 145 1 (±18.7) | 39.9 34 (±6.11) |
| 300 MPa_3 °C_5 min | 6.60 12 (±0.02) | 239 2 (±6.23) | 10.5 1 (±1.54) |
| 400 MPa_3 °C_5 min | 6.60 12 (±0.10) | 287 3 (±36.0) | 0.98 1 (±0.18) |
| 100 MPa_3 °C_10 min | 6.69 BCD,23 (±0.03) | 242 C,2 (±12.6) | 29.0 C,23 (±6.44) |
| 200 MPa_3 °C_10 min | 6.58 AB,1 (±0.02) | 249 C,2 (±17.8) | 46.1 D,4 (±14.2) |
| 300 MPa_3 °C_10 min | 6.57 AB,1 (±0.06) | 303 D,3 (±8.63) | 4.98 A,1 (±0.08) |
| 400 MPa_3 °C_10 min | 6.52 A,1 (±0.01) | 319 DE,3 (±12.9) | 6.47 AB,1 (±0.98) |
| 100 MPa_7 °C_10 min | 6.55 A (±0.12) | 139 A (±10.1) | 10.2 AB (±0.15) |
| 200 MPa_7 °C_10 min | 6.71 CD (±0.04) | 187 B (±2.46) | 17.5 B (±1.12) |
| 300 MPa_7 °C_10 min | 6.63 ABCD (±0.01) | 271 C (±39.4) | 3.92 A (±1.58) |
| 400 MPa_7 °C_10 min | 6.71 CD (±0.01) | 342 E (±12.3) | 8.41 AB (±1.87) |
| 100 MPa_25 °C_10 min | 6.63 bc,ABC (±0.05) | 161 a,A (±12.3) | 30.7 f,C (±2.75) |
| 200 MPa_25 °C_10 min | 6.60 ab,ABCD (±0.03) | 277 bc,C (±17.1) | 14.5 d,AB (±3.11) |
| 300 MPa_25 °C_10 min | 6.67 bcd,ABCD (±0.10) | 251 b,C (±29.8) | 13.9 d,AB (±2.58) |
| 400 MPa_25 °C_10 min | 6.73 e,D (±0.01) | 307 cd,D (±18.9) | 4.73 ab,A (±1.29) |
| (aerobically stored) | |||
| 100 MPa_25 °C_10 min | 6.66 bcd (±0.02) | 137 a (±17.4) | 7.88 bc (±1.35) |
| 200 MPa_25 °C_10 min | 6.74 e (±0.04) | 282 bc (±32.7) | 20.6 e (±5.05) |
| 300 MPa_25 °C_10 min | 6.67 bcd (±0.04) | 359 e (±20.0) | 4.05 ab (±1.51) |
| 400 MPa_25 °C_10 min | 6.72 de (±0.02) | 340 de (±25.7) | 2.52 a (±0.71) |
| Fish Fillet Treatment | Lipid Oxidation Rate * (d−1) | Lag Phase (d) | R-Square | Shelf Life ** (d) |
|---|---|---|---|---|
| Untreated fillet | 0.104 c (±0.011) | - | 0.957 | 9.67 a (±1.03) |
| 100 MPa_25 °C_10 min | 0.050 b (±0.005) | - | 0.950 | 20.3 b (±2.11) |
| 200 MPa_25 °C_10 min | 0.042 ab (±0.004) | - | 0.949 | 23.8 c (±2.26) |
| 300 MPa_25 °C_10 min | 0.048 b (±0.003) | 6.08 a (±1.76) | 0.989 | 27.0 d (±1.14) |
| 400 MPa_25 °C_10 min | 0.036 a (±0.002) | 5.85 a (±1.99) | 0.979 | 34.0 e (±1.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsevdou, M.; Dimopoulos, G.; Limnaios, A.; Semenoglou, I.; Tsironi, T.; Taoukis, P. High Pressure Processing under Mild Conditions for Bacterial Mitigation and Shelf Life Extension of European Sea Bass Fillets. Appl. Sci. 2023, 13, 3845. https://doi.org/10.3390/app13063845
Tsevdou M, Dimopoulos G, Limnaios A, Semenoglou I, Tsironi T, Taoukis P. High Pressure Processing under Mild Conditions for Bacterial Mitigation and Shelf Life Extension of European Sea Bass Fillets. Applied Sciences. 2023; 13(6):3845. https://doi.org/10.3390/app13063845
Chicago/Turabian StyleTsevdou, Maria, George Dimopoulos, Athanasios Limnaios, Ioanna Semenoglou, Theofania Tsironi, and Petros Taoukis. 2023. "High Pressure Processing under Mild Conditions for Bacterial Mitigation and Shelf Life Extension of European Sea Bass Fillets" Applied Sciences 13, no. 6: 3845. https://doi.org/10.3390/app13063845
APA StyleTsevdou, M., Dimopoulos, G., Limnaios, A., Semenoglou, I., Tsironi, T., & Taoukis, P. (2023). High Pressure Processing under Mild Conditions for Bacterial Mitigation and Shelf Life Extension of European Sea Bass Fillets. Applied Sciences, 13(6), 3845. https://doi.org/10.3390/app13063845







