Quantitative Tissue Elasticity Measurement of Human Cadaver Oesophagus by Using Vibrational Optical Coherence Elastography
Abstract
:1. Introduction
2. Materials and Methods
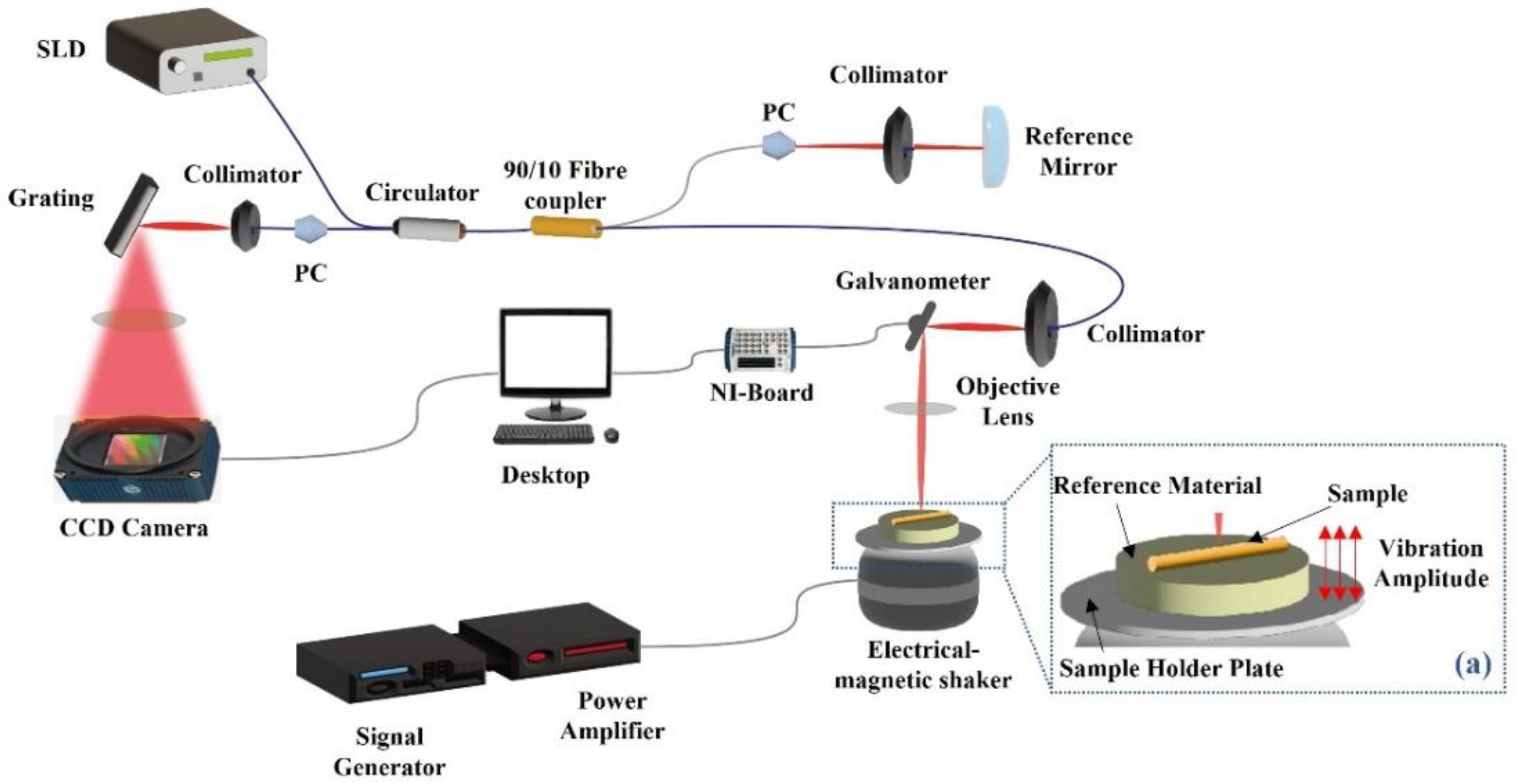
2.1. OCT/OCE System Configuration and Imaging Protocol
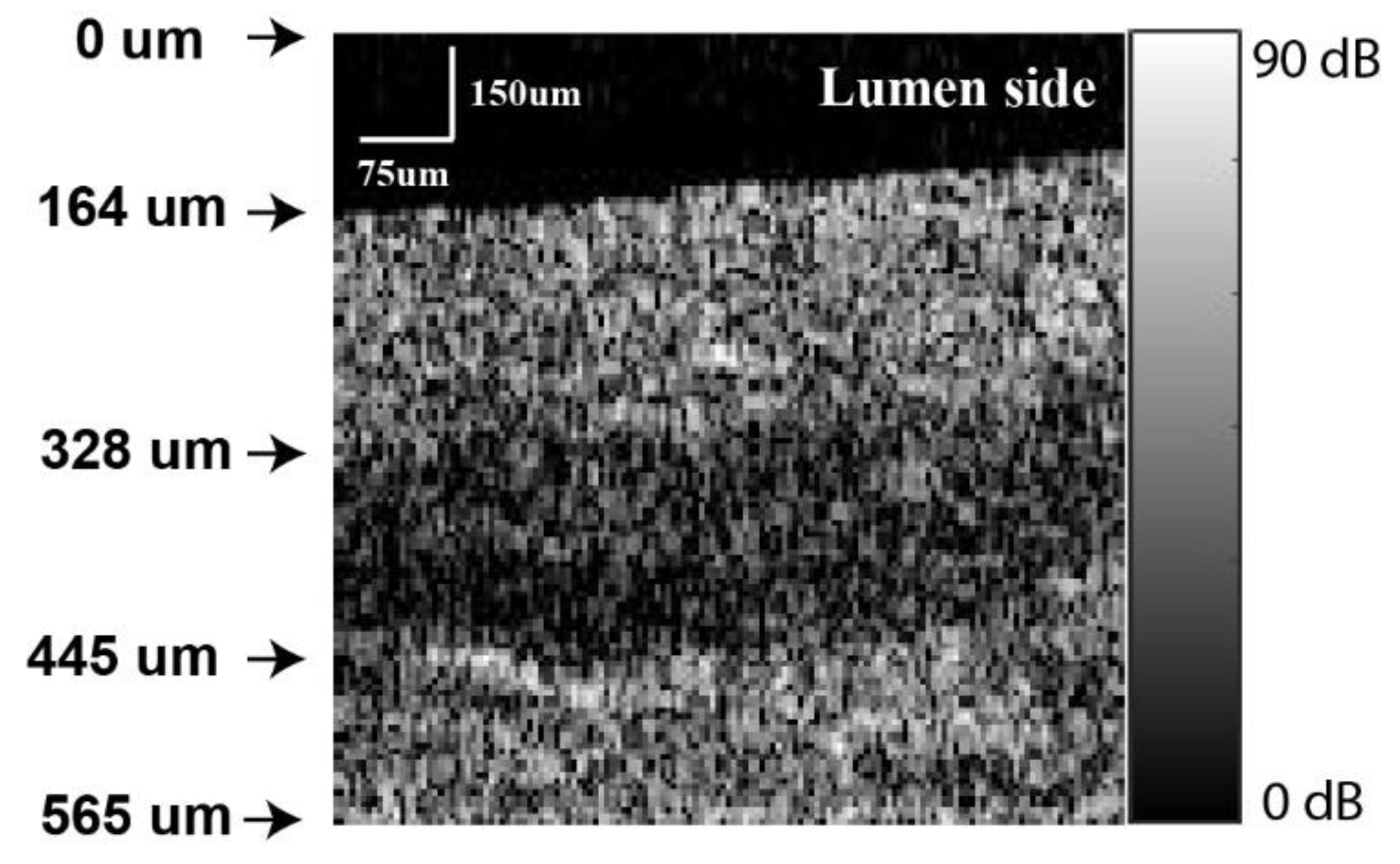
2.2. OCT Intensity and Tissue Layer Thickness Measurement
2.3. OCE Results Processing Method
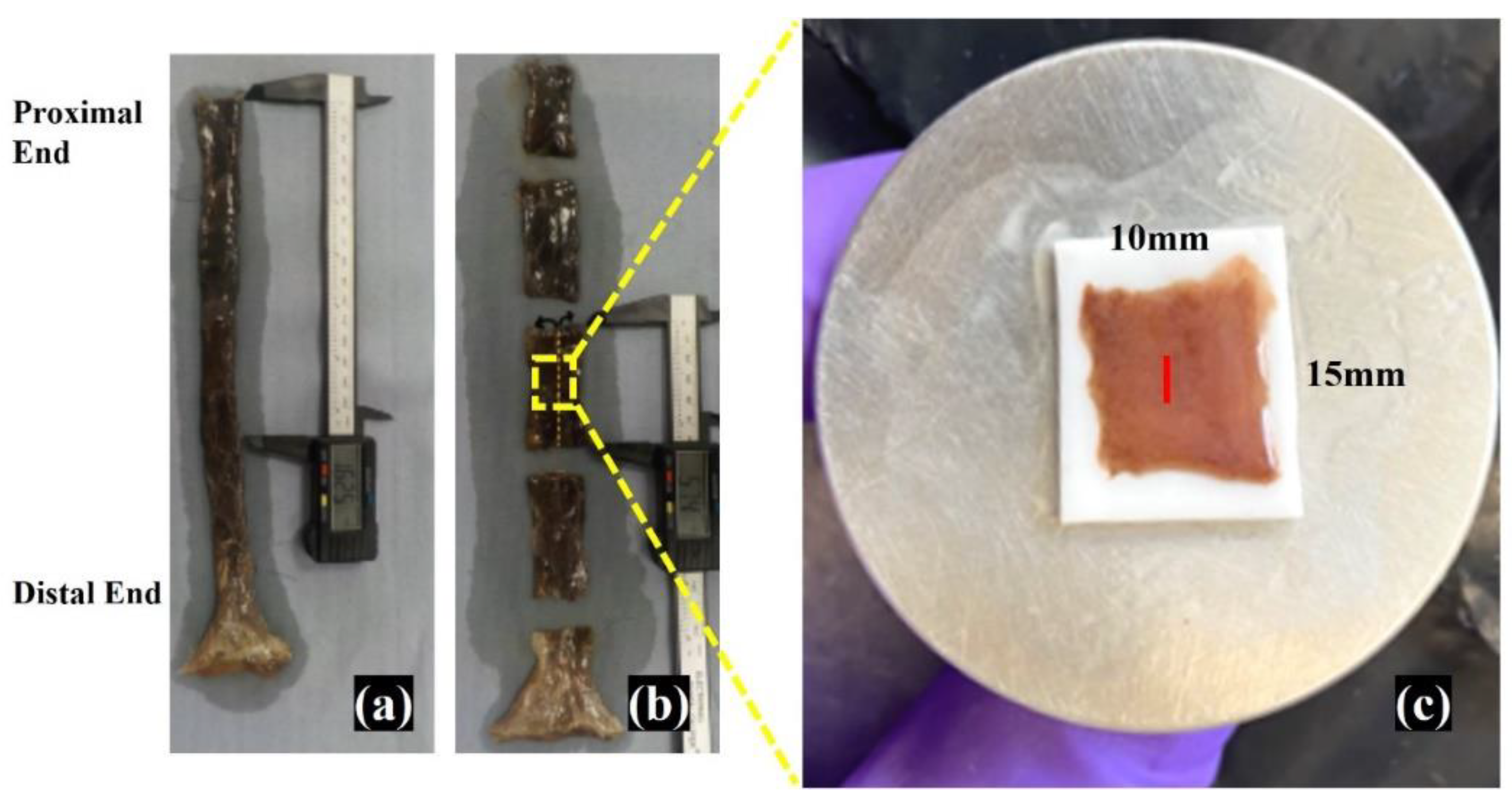
2.4. Oesophagus Wall Tissue Sample Handling
2.5. Histological Imaging
3. Results
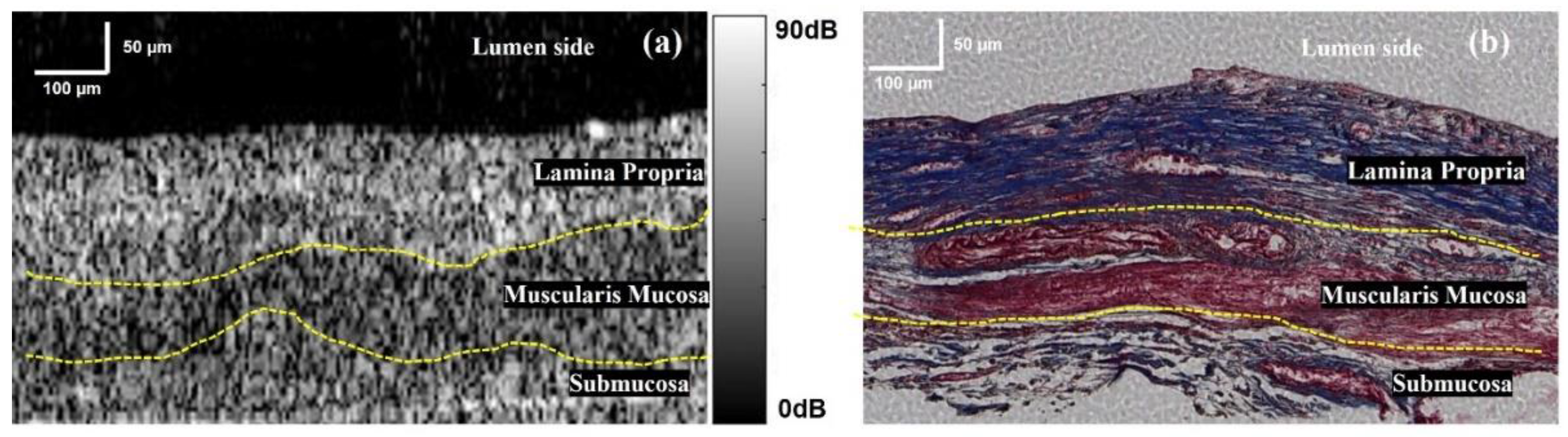
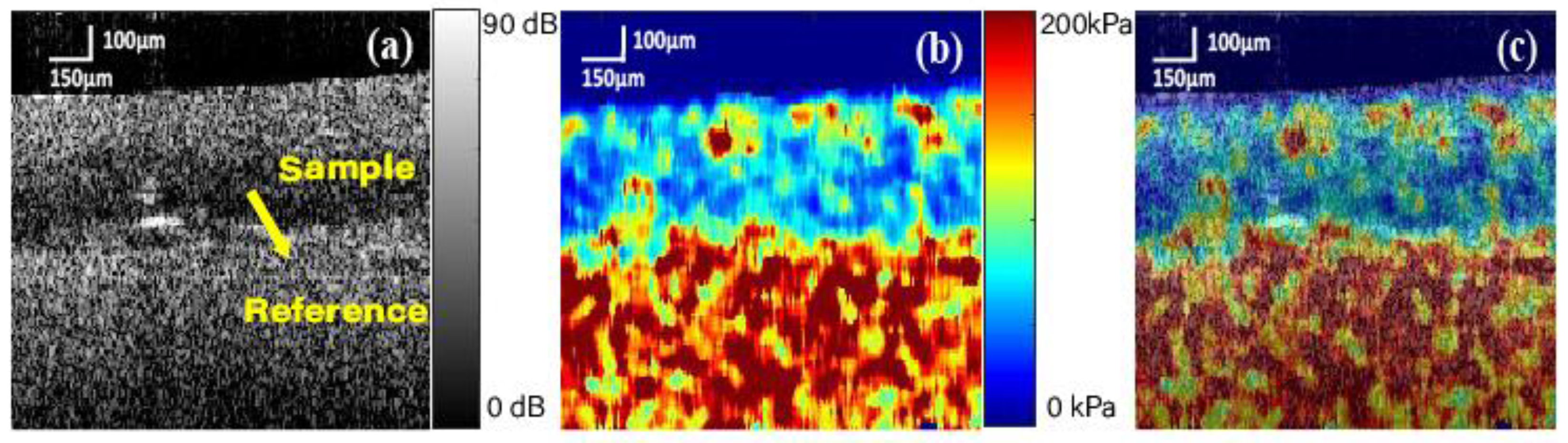
3.1. Cross-Section Observation of Oesophageal Tissue
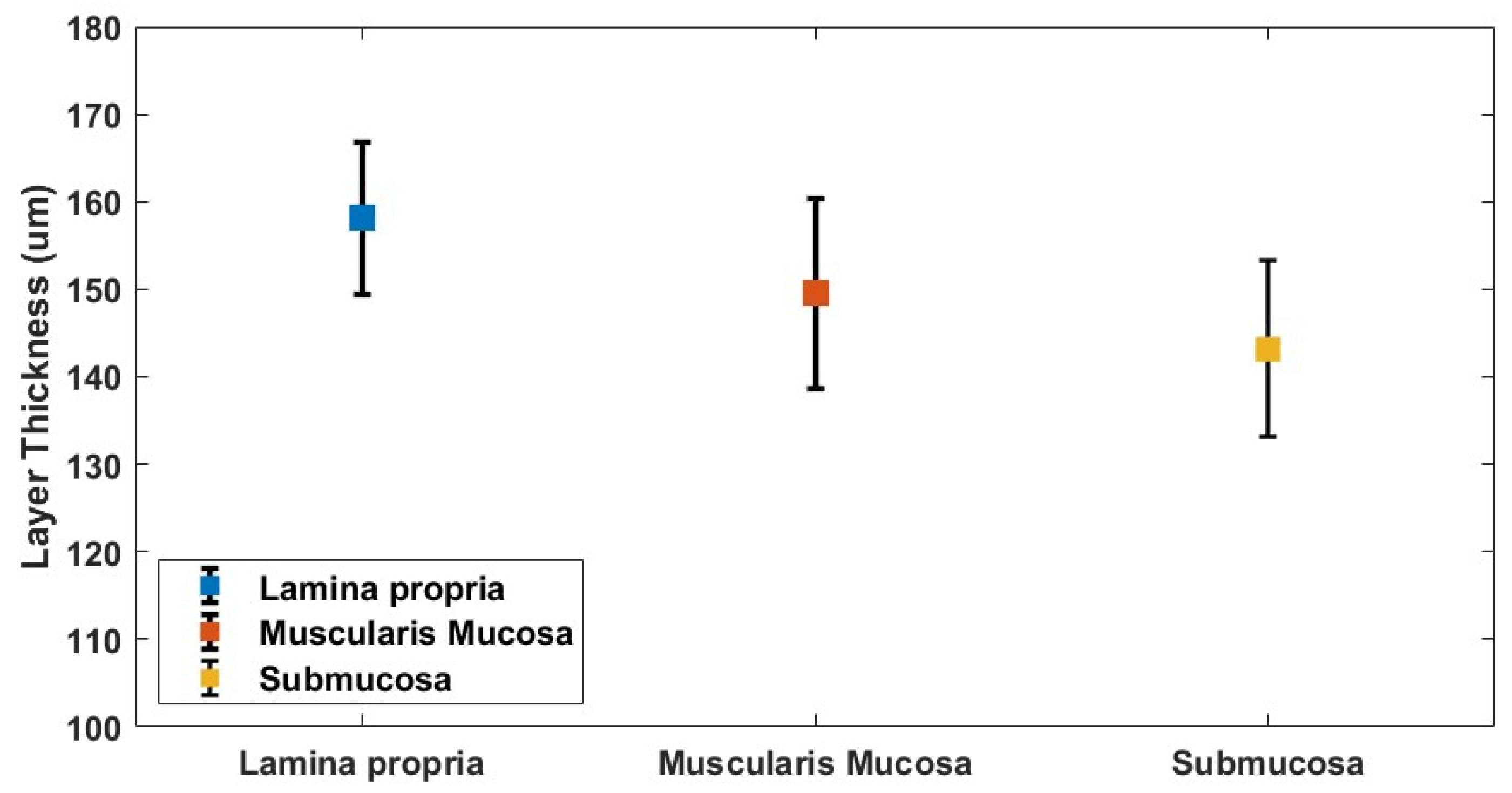
3.2. Oesophagus Layer Thickness Measurement
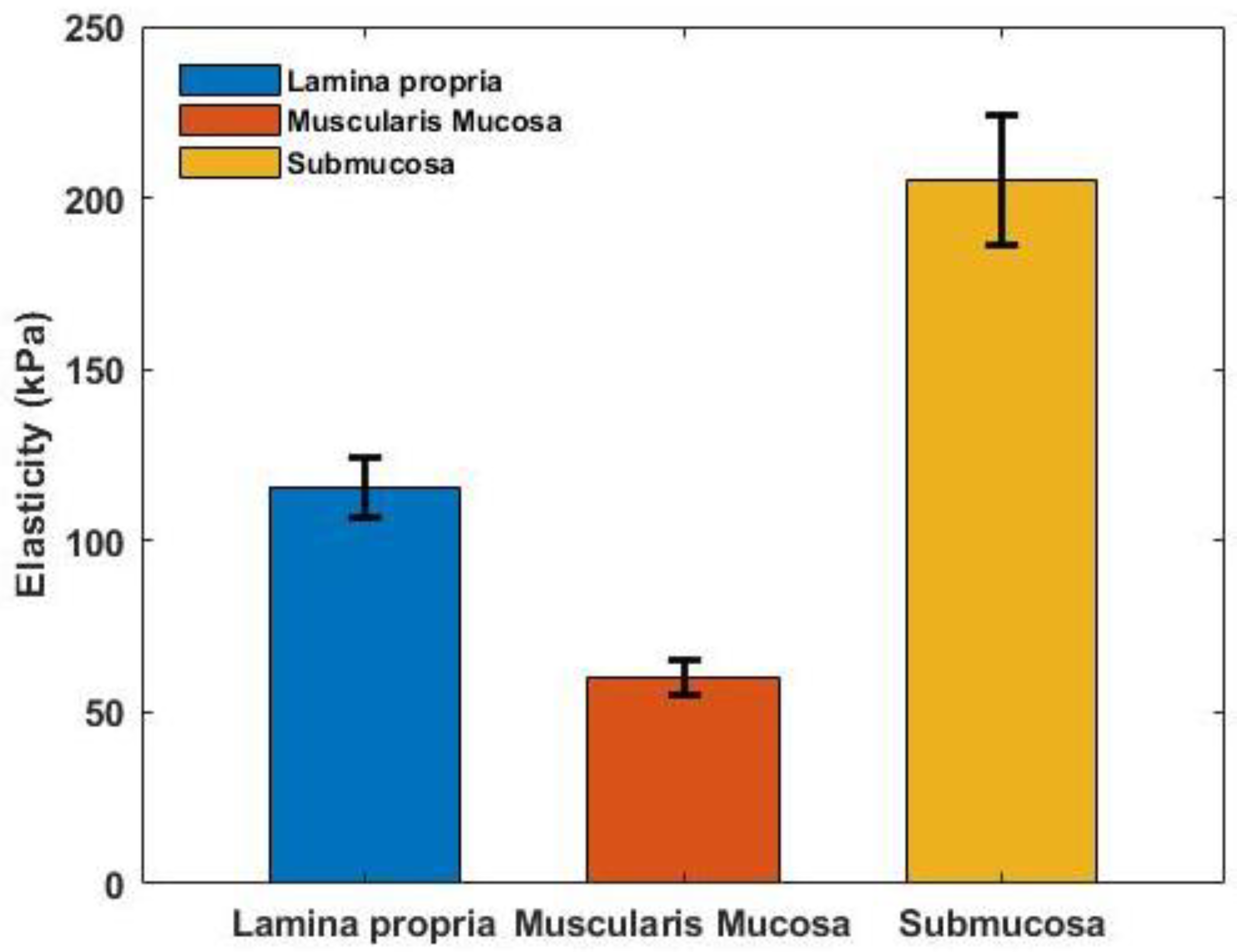
3.3. Vibrational OCE Results of Oesophageal Tissue Sample
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update Global Cancer Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Blot, W.J.; Devesa, S.S.; Fraumeni, J.F. Continuing Climb in Rates of Esophageal Adenocarcinoma: An Update. JAMA 1993, 270, 1320. [Google Scholar] [CrossRef] [PubMed]
- Blot, W.J.; Devesa, S.S.; Kneller, R.W.; Fraumeni, J.F. Rising Incidence of Adenocarcinoma of the Esophagus and nGastric Cardia. JAMA 1991, 265, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.; Chow, W.; Devesa, S. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Br. J. Cancer 2009, 101, 855–859. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598. [Google Scholar] [CrossRef] [PubMed]
- Meves, V.; Behrens, A.; Pohl, J. Diagnostics and Early Diagnosis of Esophageal Cancer. Visc. Med. 2015, 31, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.; Gilbert, D.; Kaye, P.; Penman, I.; Aithal, G.; Ragunath, K. High-resolution endoscopy and endoscopic ultrasound for evaluation of early neoplasia in Barrett’s esophagus. Surg. Endosc. 2010, 24, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Falk, G.W.; Catalano, M.F.; Sivak, M.V., Jr.; Rice, T.W.; Van Dam, J. Endosonography in the evaluation of patients with Barrett’s esophagus and high-grade dysplasia. Gastrointest. Endosc. 1994, 40, 207–212. [Google Scholar] [CrossRef]
- Bayramoglu, N.; Kannala, J.; Heikkila, J. Deep learning for magnification independent breast cancer histopathology image classification. In Proceedings of the 2016 23rd International Conference on Pattern Recognition (ICPR), Cancun, Mexico, 4–8 December 2016; pp. 2440–2445. [Google Scholar]
- Belsare, A.; Mushrif, M. Histopathological image analysis using image processing techniques: An overview. Signal Image Process. 2012, 3, 23. [Google Scholar] [CrossRef]
- Hanby, A. The pathology of breast cancer and the role of the histopathology laboratory. Clin. Oncol. 2005, 17, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Li, Q.; Melnichouk, O.; Huszti, E.; Martin, L.J.; Gunasekara, A.; Mawdsley, G.; Yaffe, M.J.; Minkin, S. Evidence That Breast Tissue Stiffness Is Associated with Risk of Breast Cancer. PLoS ONE 2014, 9, e100937. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, K.; Castaneda, B.; Zhang, M.; Nigwekar, P.; di Sant’Agnese, P.A.; Joseph, J.V.; Strang, J.; Rubens, D.J.; Parker, K.J. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomarkers 2008, 4, 213–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlins, P.H.; Wang, R. Theory, developments and applications of optical coherence tomography. J. Phys. D Appl. Phys. 2005, 38, 2519–2535. [Google Scholar] [CrossRef]
- Cheong, W.-F.; Prahl, S.A.; Welch, A.J. A review of the optical properties of biological tissues. IEEE J. Quantum Electron. 1990, 26, 2166–2185. [Google Scholar] [CrossRef] [Green Version]
- Tuchin, V.V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis; SPIE Press Book: Bellingham, WA, USA, 2007; p. 840. [Google Scholar]
- Zhou, F.; Wei, H.; Ye, X.; Hu, K.; Wu, G.; Yang, H.; He, Y.; Xie, S.; Guo, Z. Influence of nanoparticles accumulation on optical properties of human normal and cancerous liver tissue in vitro estimated by OCT. Phys. Med. Biol. 2015, 60, 1385. [Google Scholar] [CrossRef]
- Qin, N.; Liu, Y.; Huang, L.; Xin, Y.; Zhang, X.; Hu, X.; Li, Q. Research on optical properties of cardiovascular tissues based on OCT data. J. Innov. Opt. Health Sci. 2021, 14, 2140007. [Google Scholar] [CrossRef]
- Guan, G.; Li, C.; Ling, Y.; Yang, Y.; Vorstius, J.B.; Keatch, R.; Wang, R.; Huang, Z. Quantitative evaluation of degenerated tendon model using combined optical coherence elastography and acoustic radiation force method. J. Biomed. Opt. 2013, 18, 111417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, J.; Zhou, K.; Wang, R.; Li, C.; Huang, Z. Optimal frequency for vibrational optical coherence elastography (OCE) on tissue mechanical properties characterization. In Optical Elastography and Tissue Biomechanics VI; SPIE: Bellingham, WA, USA, 2019; Volume 10880, pp. 15–20. [Google Scholar]
- Zhang, Y.; Ling, Y.; Zhang, D.; Wang, M.; Purslow, C.; Yang, Y.; Li, C.; Huang, Z. Quantitative measurement of mechanical properties in wound healing processes in a corneal stroma model by using vibrational optical coherence elastography (OCE). Biomed. Opt. Express 2021, 12, 588–603. [Google Scholar] [CrossRef]
- Zvietcovich, F.; Yao, J.; Chu, Y.-J.; Meemon, P.; Rolland, J.P.; Parker, K.J. A comparative study of shear wave speed estimation techniques in optical coherence elastography applications. In Optical Elastography and Tissue Biomechanics III; SPIE: Bellingham, WA, USA, 2016; Volume 9710, pp. 99–109. [Google Scholar]
- Li, C.; Guan, G.; Ling, Y.; Hsu, Y.-T.; Song, S.; Huang, J.T.-J.; Lang, S.; Wang, R.K.; Huang, Z.; Nabi, G. Detection and characterisation of biopsy tissue using quantitative optical coherence elastography (OCE) in men with suspected prostate cancer. Cancer Lett. 2015, 357, 121–128. [Google Scholar] [CrossRef]
- Yuting, L.; Li, C.; Zhou, K.; Guan, G.; Appleton, P.L.; Lang, S.; McGloin, D.; Huang, Z.; Nabi, G. Microscale characterization of prostate biopsies tissues using optical coherence elastography and second harmonic generation imaging. Lab. Investig. 2018, 98, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brezinski, M.; Fujimoto, J. Optical coherence tomography: High-resolution imaging in nontransparent tissue. IEEE J. Sel. Top. Quantum Electron. 1999, 5, 1185–1192. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, J.; Li, C.; Huang, Z. Optimal stimulation frequency for vibrational optical coherence elastography. J. Biophotonics 2019, 13, e201960066. [Google Scholar] [CrossRef]
- Bille, J.F. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Suvik, A.; Effendy, A. The use of modified Masson’s trichrome staining in collagen evaluation in wound healing study. Mal. J. Vet. Res. 2012, 3, 39–47. [Google Scholar]
- Tateya, T.; Tateya, I.; Bless, D.M. Immuno-scanning electron microscopy of collagen types I and III in human vocal fold lamina propria. Ann. Otol. Rhinol. Laryngol. 2007, 116, 156–159. [Google Scholar] [CrossRef]
- Madruga de Melo, E.C.; Lemos, M.; Filho, J.A.X.; Sennes, L.U.; Saldiva, P.H.N.; Tsuji, D.H. Distribution of collagen in the lamina propria of the human vocal fold. Laryngoscope 2003, 113, 2187–2191. [Google Scholar] [CrossRef]
- Hahn, M.S.; Kobler, J.B.; Starcher, B.C.; Zeitels, S.M.; Langer, R. Quantitative and Comparative Studies of the Vocal Fold Extracellular Matrix I: Elastic Fibers and Hyaluronic Acid. Ann. Otol. Rhinol. Laryngol. 2006, 115, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.G.; Kumar, S.U.; Sowndarya, S.; Babu, P.S.; Rose, C. Isolation, characterization, and in vitro evaluation of bovine rumen submucosa films of collagen or chitosan-treated collagen. J. Biomater. Appl. 2015, 30, 780–792. [Google Scholar] [CrossRef]
- Kilarski, W.; Bigaj, J. The fine structure of striated muscle fibres of tunica muscularis of the intestine in some teleosts. Zeitschrift für Zellforschung und Mikroskopische Anatomie 1971, 113, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Kallmünzer, B.; Sorensen, B.; Neuhuber, W.L.; Wörl, J. Enteric co-innervation of striated muscle fibres in human oesophagus. Neurogastroenterol. Motil. 2008, 20, 597–610. [Google Scholar] [CrossRef]
- Durcan, C.; Hossain, M.; Chagnon, G.; Perić, D.; Bsiesy, L.; Karam, G.; Girard, E. Experimental investigations of the human oesophagus: Anisotropic properties of the embalmed muscular layer under large deformation. Biomech. Model. Mechanobiol. 2022, 21, 1169–1186. [Google Scholar] [CrossRef]
- Takeda, T.; Kassab, G.; Liu, J.; Puckett, J.L.; Mittal, R.R.; Mittal, R.K. A novel ultrasound technique to study the bio-mechanics of the human esophagus in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G785–G793. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Liao, N.; Zhao, J.; Gregersen, H. Shear modulus of elasticity of the esophagus. Ann. Biomed. Eng. 2004, 32, 1223–1230. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.; Liao, D.; Gregersen, H. Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J. Biomech. 2006, 39, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gregersen, H.; Kassab, G.S. A two-layered mechanical model of the rat esophagus. Experiment and theory. Biomed. Eng. Online 2004, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Sirotkina, M.A.; Shirmanova, M.V.; Bugrova, M.; Elagin, V.V.; Agrba, P.A.; Kirillin, M.Y.; Kamensky, V.A.; Zagaynova, E.V. Continuous optical coherence tomography monitoring of nanoparticles accumulation in biological tissues. J. Nanoparticle Res. 2011, 13, 283–291. [Google Scholar] [CrossRef]
- Kennedy, B.F.; Hillman, T.R.; McLaughlin, R.A.; Quirk, B.C.; Sampson, D.D. In vivo dynamic optical coherence elastography using a ring actuator. Opt. Express 2009, 17, 21762–21772. [Google Scholar] [CrossRef]
- Henninger, H.B.; Ellis, B.J.; Scott, S.A.; Weiss, J.A. Contributions of elastic fibers, collagen, and extracellular matrix to the multiaxial mechanics of ligament. J. Mech. Behav. Biomed. Mater. 2019, 99, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Karsdal, M.A. Elastin. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 197–201. [Google Scholar]
- Liles, D.T.; Lin, F. Silicone Elastomeric Particles in Skin Care Applications. In Polymeric Delivery of Therapeutics; American Chemical Society: Washington, DC, USA, 2010; Volume 1053, pp. 207–219. [Google Scholar] [CrossRef]
- Knabe, M.; Günter, E.; Ell, C.; Pech, O. Can EUS elastography improve lymph node staging in esophageal cancer? Surg. Endosc. 2012, 27, 1196–1202. [Google Scholar] [CrossRef]
- Sazuka, T.; Akai, T.; Uesato, M.; Horibe, D.; Kuboshima, M.; Kitabayashi, H.; Matsunaga, A.; Kagaya, A.; Muto, Y.; Takeshita, N.; et al. Assessment for diagnosis of lymph node metastasis in esophageal cancer using endoscopic ultrasound elastography. Esophagus 2016, 13, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Guan, G.; Cheng, X.; Huang, Z.; Wang, R.K. Quantitative elastography provided by surface acoustic waves measured by phase-sensitive optical coherence tomography. Opt. Lett. 2012, 37, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Li, C.; Feng, K.; Duncan, R.; Eisma, R.; Huang, Z.; Nabi, G. Effects of fixation and preservation on tissue elastic properties measured by quantitative optical coherence elastography (OCE). J. Biomech. 2016, 49, 1009–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cadaver No. | Days in Tank | Age at Death | Gender | Sample Number |
|---|---|---|---|---|
| 1 | 444 | 85 | Female | 6 |
| 2 | 298 | 88 | Female | 14 |
| 3 | 164 | 90 | Male | 2 |
| 4 | 166 | 89 | Female | 1 |
| 5 | 161 | 89 | Male | 4 |
| 6 | 155 | 95 | Male | 3 |
| 7 | 478 | 81 | Female | 21 |
| Lamina Propria | Muscularis Mucosa | Submucosa |
|---|---|---|
| dB | dB | dB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, J.; Boga, M.; Reid, L.; Li, C.; Huang, Z. Quantitative Tissue Elasticity Measurement of Human Cadaver Oesophagus by Using Vibrational Optical Coherence Elastography. Appl. Sci. 2023, 13, 3844. https://doi.org/10.3390/app13063844
Wang M, Li J, Boga M, Reid L, Li C, Huang Z. Quantitative Tissue Elasticity Measurement of Human Cadaver Oesophagus by Using Vibrational Optical Coherence Elastography. Applied Sciences. 2023; 13(6):3844. https://doi.org/10.3390/app13063844
Chicago/Turabian StyleWang, Mingkai, Jiaxuan Li, Mihrican Boga, Luke Reid, Chunhui Li, and Zhihong Huang. 2023. "Quantitative Tissue Elasticity Measurement of Human Cadaver Oesophagus by Using Vibrational Optical Coherence Elastography" Applied Sciences 13, no. 6: 3844. https://doi.org/10.3390/app13063844
APA StyleWang, M., Li, J., Boga, M., Reid, L., Li, C., & Huang, Z. (2023). Quantitative Tissue Elasticity Measurement of Human Cadaver Oesophagus by Using Vibrational Optical Coherence Elastography. Applied Sciences, 13(6), 3844. https://doi.org/10.3390/app13063844






