Influence of Ingestion of Lactulose on γ-Lactones Emanating from Human Skin Surface
Abstract
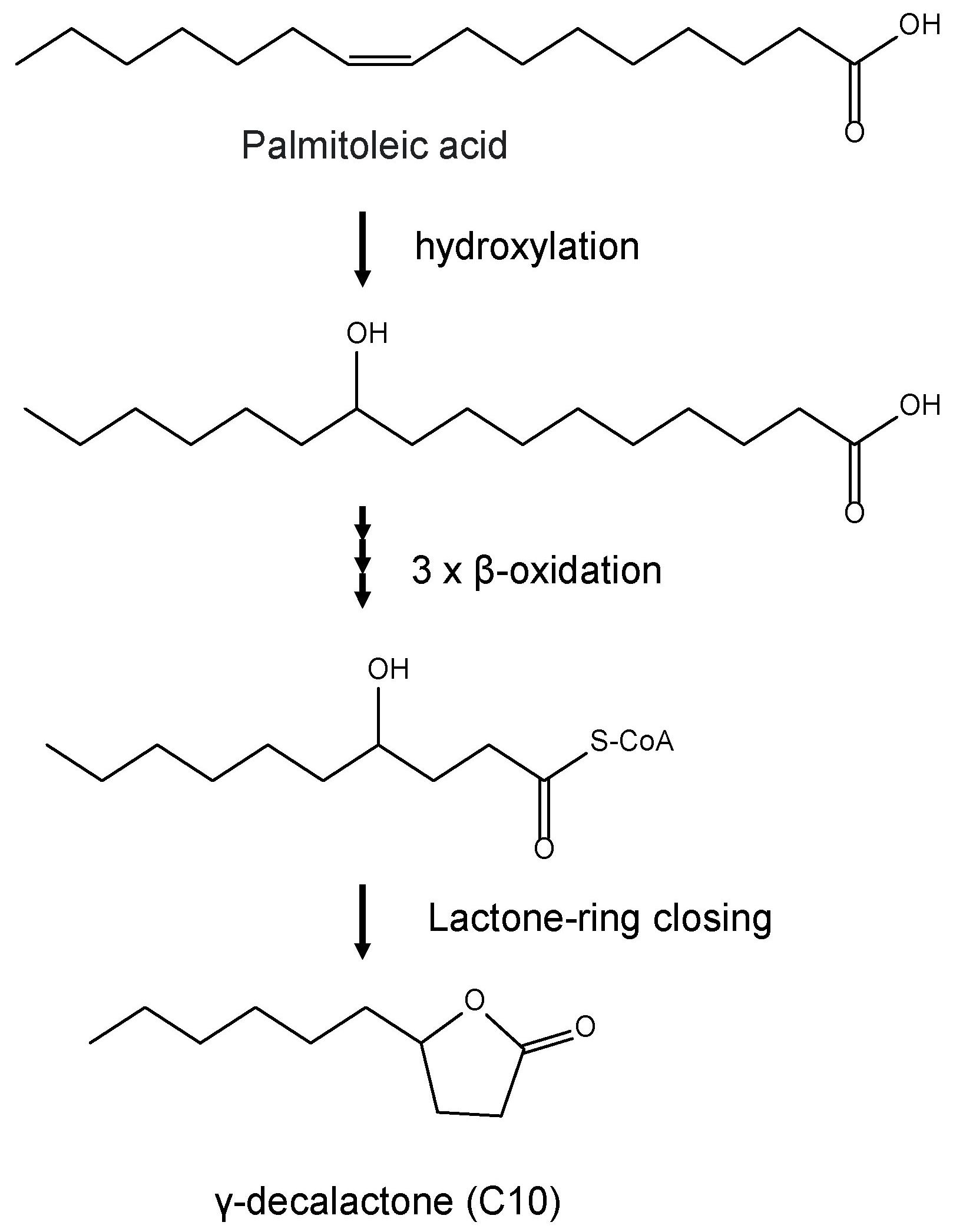
1. Introduction
2. Methods
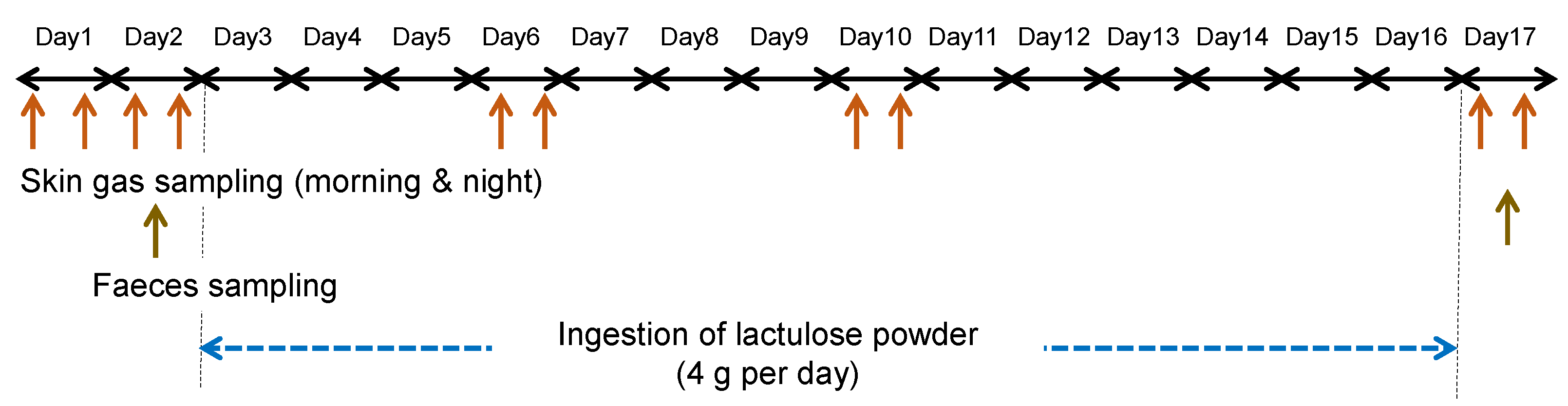
2.1. Participant Tests
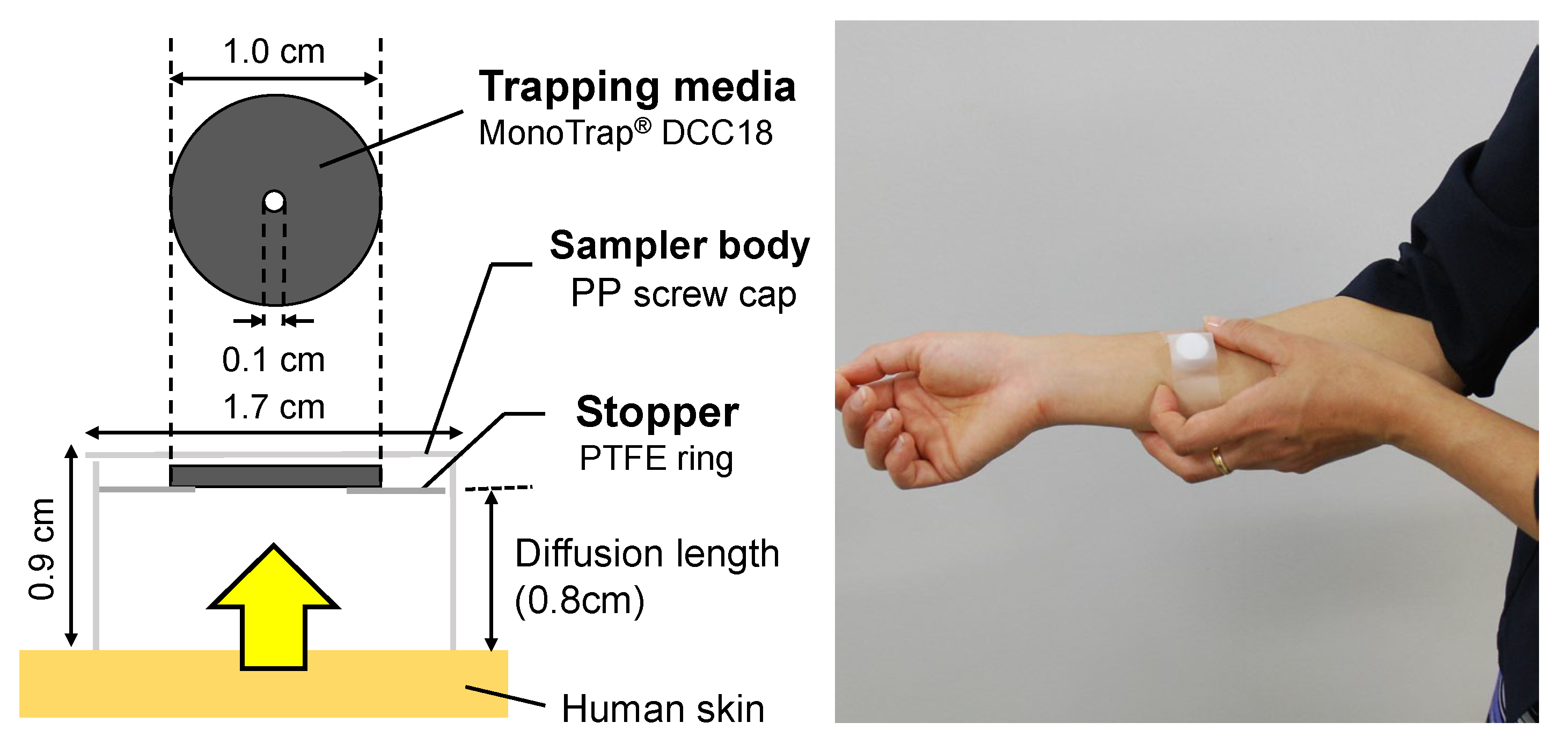
2.2. Skin Gas Sampling and Analysis
2.3. Determination of Faecal Bifidobacterial Numbers
2.4. Statistical Analyses
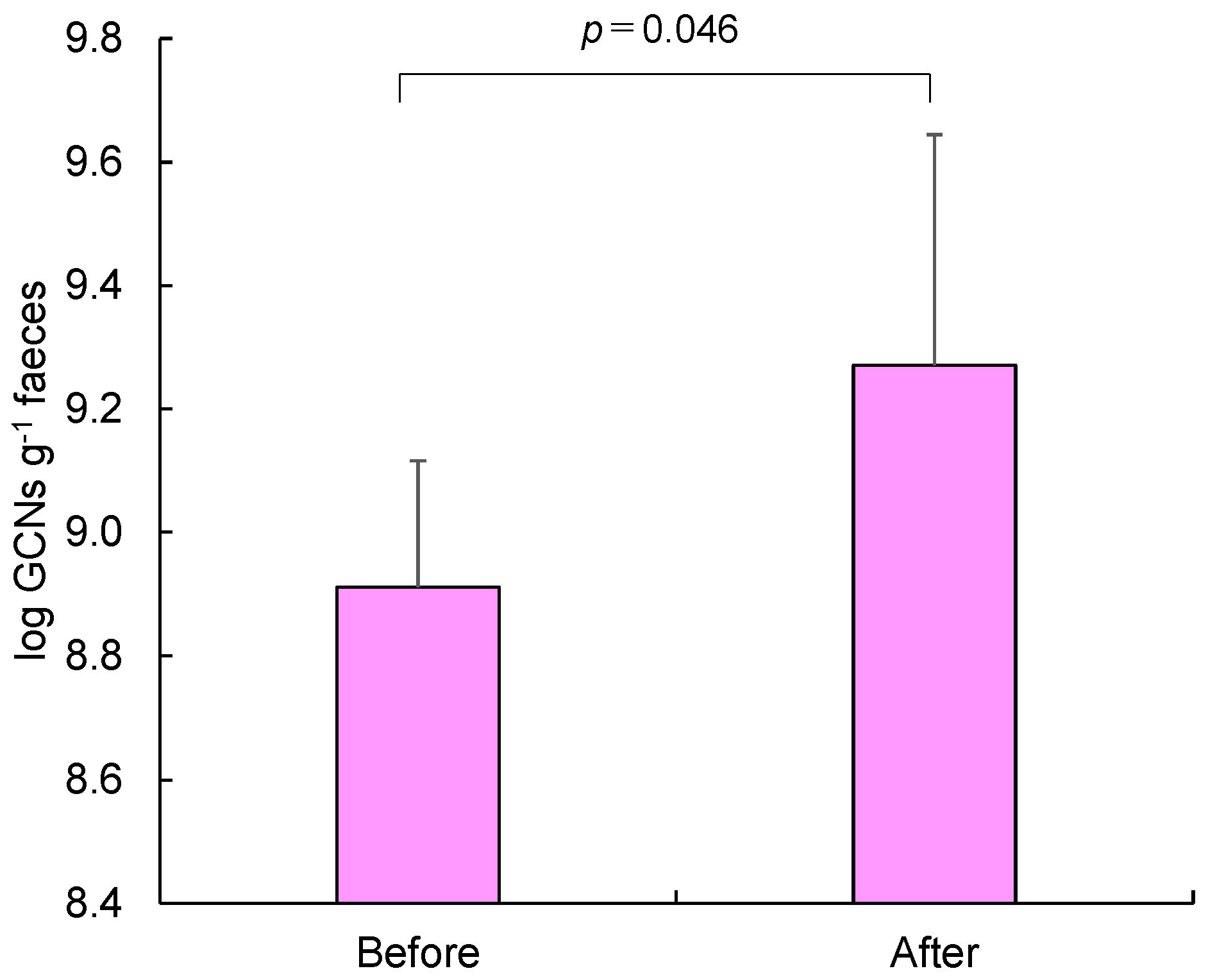
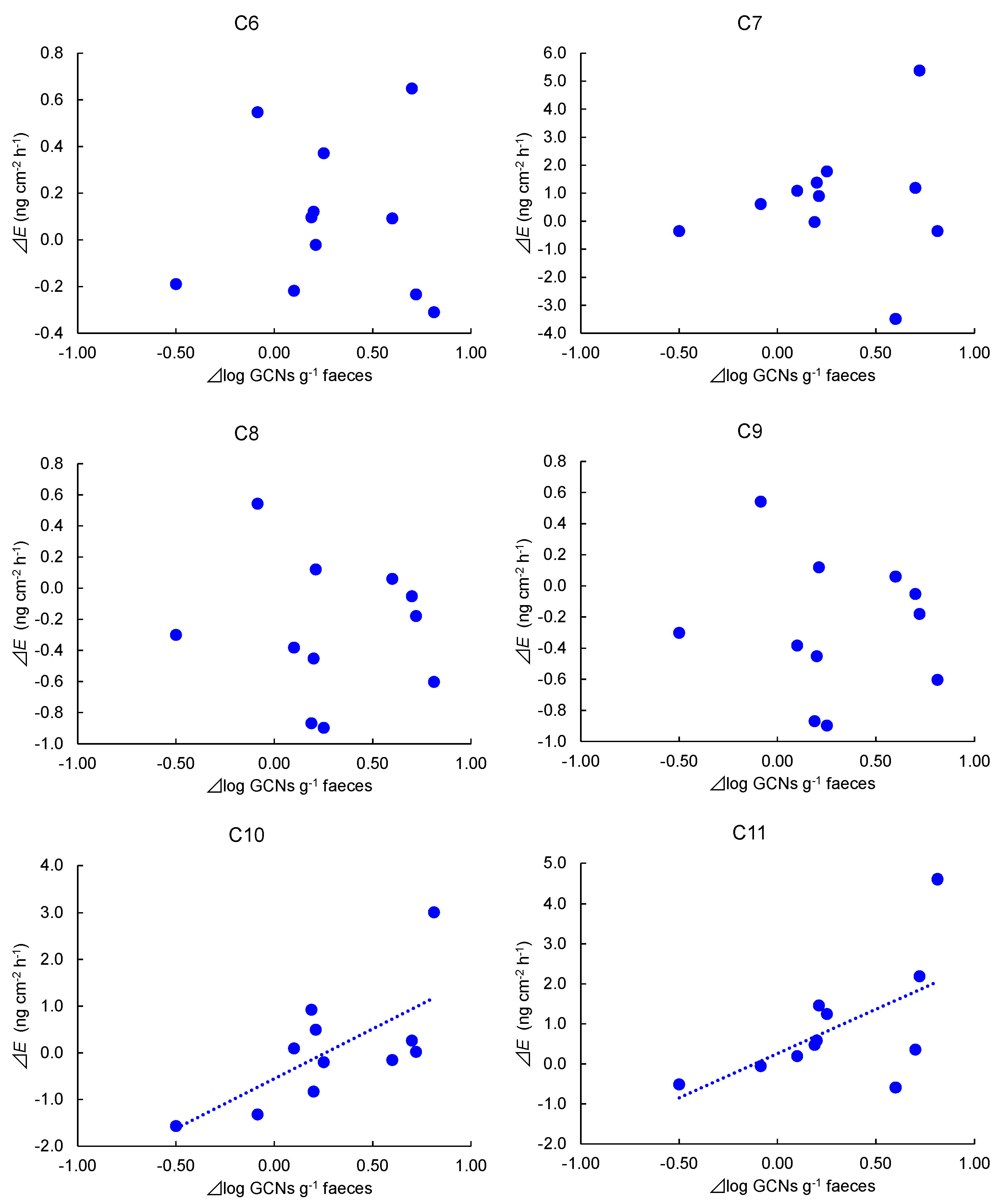
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakai, Y.; Seki, N.; Hamano, H.; Ochi, H.; Abe, F.; Shimizu, F.; Masuda, K.; Iino, H. A study of the prebiotic effect of lactulose at low dosages in healthy Japanese women. Biosci. Microb. Food Health 2019, 38, 69–72. [Google Scholar] [CrossRef]
- Bothe, M.K.; Maathuis, A.J.M.; Bellmann, S.; van der Vossen, J.M.B.M.; Berressem, D.; Koehler, A.; Schwejda-Guettes, S.; Gaigg, B.; Kuchinka-Koch, A.; Stover, J.F. Dose-dependent prebiotic effect of lactulose in a computer-controlled in vitro model of the human large intestine. Nutrients 2017, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Shukla, A.; Mehboob, S.; Guha, S. Meta-analysis: The effects of gut flora modulation using prebiotics, probiotics and synbiotics on minimal hepatic encephalopathy. Alim. Pharm. Therap. 2011, 33, 662–671. [Google Scholar] [CrossRef]
- Vince, A.; Killingley, H.; Wrong, O.M. Effect of lactulose on ammonia production in a faecal incubation system. Gastroenterology 1978, 74, 544–549. [Google Scholar] [CrossRef]
- Vince, A.J.; McNeil, N.I.; Wager, J.D.; Wrong, O.M. The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br. J. Nutr. 1990, 63, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Uchiyama, S.; Todaka, M.; Sakai, Y.; Sakiyama, R.; Ochi, H.; Abe, F.; Asai, S.; Umezawa, K. Prebiotic effect of lactulose on ammonia emanating from human skin surface. J. Jpn. Assoc. Odor Environ. 2020, 51, 338–345. [Google Scholar] [CrossRef]
- Furukawa, S.; Sekine, Y.; Kimura, K.; Umezawa, K.; Asai, S.; Miyachi, H. Simultaneous and multi-point measurement of ammonia emanating from human skin surface for the estimation of whole body dermal emission rate. J. Chromatogr. B 2017, 1053, 60–64. [Google Scholar] [CrossRef]
- Ikeda, S.; Asai, S.; Umezawa, K.; Miyachi, H.; Nakamura, A.; Kaifuku, Y.; Sekine, Y. Development of a wristband-type wearable device for the colorimetric detection of ammonia emanating from the human skin surface. Results Chem. 2022, 4, 100502. [Google Scholar] [CrossRef]
- Silva, R.; Aguiar, T.Q.; Coelho, E.; Jiménez, A.; Revuelta, J.L.; Domingues, J. Metabolic engineering of Ashbya gossypii for deciphering the de novo biosynthesis of γ-lactones. Microb. Cell Fact. 2019, 18, 62. [Google Scholar] [CrossRef]
- Sánchez, G.; Venegas-Calerón, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An integrative “omics” approach identifies new candidate genes to impact aroma volatiles in peach fruit. BMC Genom. 2013, 23, 343. [Google Scholar] [CrossRef]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering gamma-decalactone biosynthesis in strawberry fruit using a combination of genetic mapping, RNA-Seq and eQTL analyses. BMC Genom. 2014, 15, 218. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Greger, V.; Schieberle, P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Sekine, Y.; Uchiyama, S.; Todaka, M. Clinical study on γ-lactone emanating from the surface of human skin. Koryo 2021, 289, 21–27. (In Japanese) [Google Scholar]
- Willems, M.E.T.; Todaka, M.; Banic, M.; Cook, M.D.; Sekine, Y. Intake of New Zealand Blackcurrant Powder affects skin-borne volatile organic compounds in middle-aged and older adults. J. Diet. Suppl. 2022, 19, 603–620. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The role of short-chain fatty acids of gut microbiota origin in hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I. Host energy regulation via SCFAs receptors, as dietary nutrition sensors, by gut microbiota. Yakugaku Zasshi 2014, 134, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Sato, S.; Kimura, K.; Sato, H.; Nakai, S.; Yanagisawa, Y. Detection of tobacco smoke emanating from human skin surface of smokers employing passive flux sampler-GCMS system. J. Chromatogr. B 2018, 1092, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Kado, Y.; Takada, T.; Matsumoto, K.; Tanaka, R. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 2004, 70, 167–173. [Google Scholar] [CrossRef]
- Satoh, T.; Odamaki, T.; Namura, M.; Shimizu, T.; Iwatsuki, K.; Nishimoto, M.; Kitaoka, M.; Xiao, J.Z. In vitro comparative evaluation of the impact of lacto-N-biose I, a major building block of human milk oligosaccharides, on the fecal microbiota of infants. Anaerobe 2013, 19, 50–57. [Google Scholar] [CrossRef]
- Labows, J.N.; McGinley, K.J.; Leyden, J.J.; Webster, G.F. Characteristic γ-lactone odor production of the genus Pityrosporum. Appl. Environ. Microbiol. 1979, 38, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Nikaido, N.; Sato, S.; Todaka, M. Trace bi-gases emanating from human skin surface-Acetic acid as a possible biomarker for sweating. Frag. J. 2018, 46, 19–25. (In Japanese) [Google Scholar]
- Den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-chain fatty acids protect against high-fat diet–induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, H.J.; Chang, J.W.; Hurh, B.S.; Kim, Y.S. Investigation on the formations of volatile compounds, fatty acids, and γ-lactones in white and brown rice during fermentation. Food Chem. 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Marella, E.R.; Dahlin, J.; Dam, M.I.; Ter Horst, J.; Christensen, H.B.; Sudarsan, S.; Wang, G.; Holkenbrink, C.; Borodina, I. A single-host fermentation process for the production of flavor lactones from non-hydroxylated fatty acids. Metab. Eng. 2020, 1, 427–436. [Google Scholar] [CrossRef]
- Muramatsu, M.; Sekine, Y.; Todaka, M. Relationship between dermal emission of γ-lactone and menstrual cycle. In Proceedings of the 2022 Annual Meeting of Society of Indoor Environment Japan (SIEJ), Tokyo, Japan, 1 December 2022; pp. 64–65. (In Japanese). [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, Y.; Uchiyama, S.; Todaka, M.; Sakai, Y.; Sakiyama, R.; Ochi, H.; Muramatsu, M.; Asai, S.; Umezawa, K. Influence of Ingestion of Lactulose on γ-Lactones Emanating from Human Skin Surface. Appl. Sci. 2023, 13, 3930. https://doi.org/10.3390/app13063930
Sekine Y, Uchiyama S, Todaka M, Sakai Y, Sakiyama R, Ochi H, Muramatsu M, Asai S, Umezawa K. Influence of Ingestion of Lactulose on γ-Lactones Emanating from Human Skin Surface. Applied Sciences. 2023; 13(6):3930. https://doi.org/10.3390/app13063930
Chicago/Turabian StyleSekine, Yoshika, Shiori Uchiyama, Michihito Todaka, Yohei Sakai, Ryo Sakiyama, Hiroshi Ochi, Maho Muramatsu, Satomi Asai, and Kazuo Umezawa. 2023. "Influence of Ingestion of Lactulose on γ-Lactones Emanating from Human Skin Surface" Applied Sciences 13, no. 6: 3930. https://doi.org/10.3390/app13063930
APA StyleSekine, Y., Uchiyama, S., Todaka, M., Sakai, Y., Sakiyama, R., Ochi, H., Muramatsu, M., Asai, S., & Umezawa, K. (2023). Influence of Ingestion of Lactulose on γ-Lactones Emanating from Human Skin Surface. Applied Sciences, 13(6), 3930. https://doi.org/10.3390/app13063930







