Torulaspora delbrueckii Strain Behaviour within Different Refermentation Strategies for Sparkling Cider Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Micro-Organisms
2.2. Experimental Design
2.2.1. Pét-Nat Method (PN)
2.2.2. Second Fermentation Methods
2.3. Analytical Methods
2.3.1. Microbiological Analysis
2.3.2. Physicochemical Parameters
2.3.3. Enzymatic Assays
2.3.4. Chemical Analysis
2.4. Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
3.2. Fermentation Monitoring
3.3. Physicochemical Parameters
3.4. Polyphenols and Catechins
3.5. Terpenes and Norisoprenoids
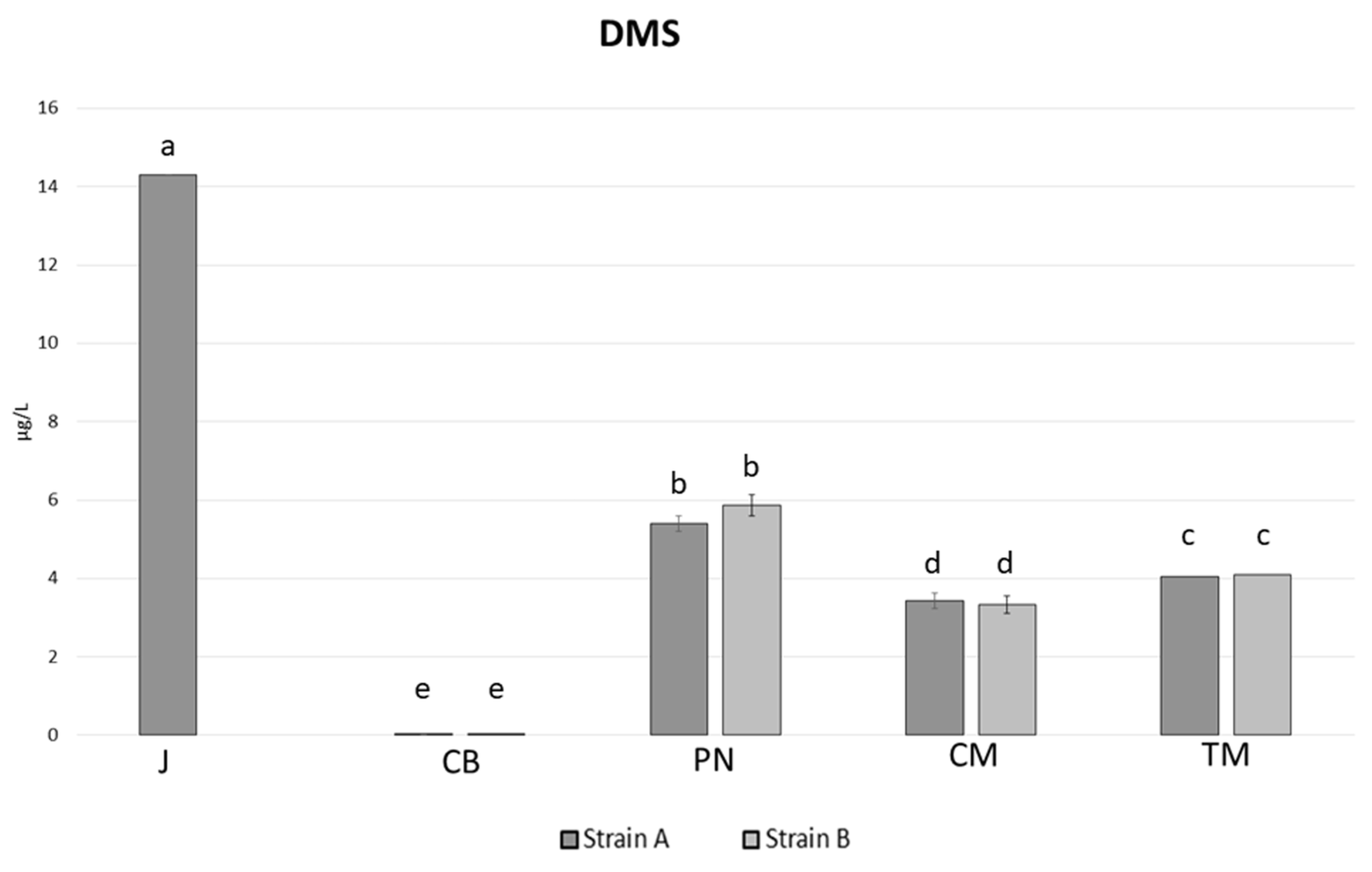
3.6. Volatile Sulphur Compounds
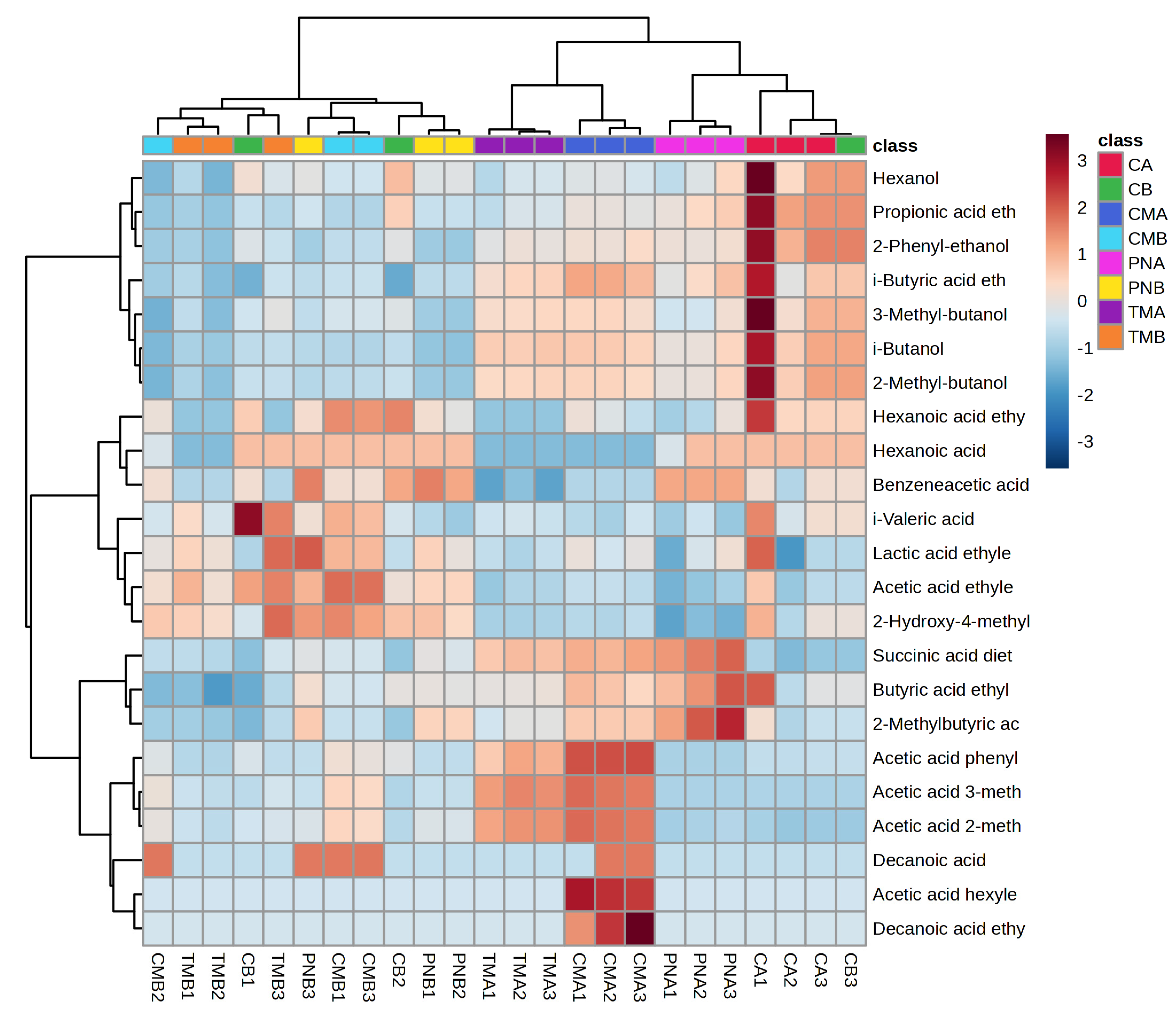
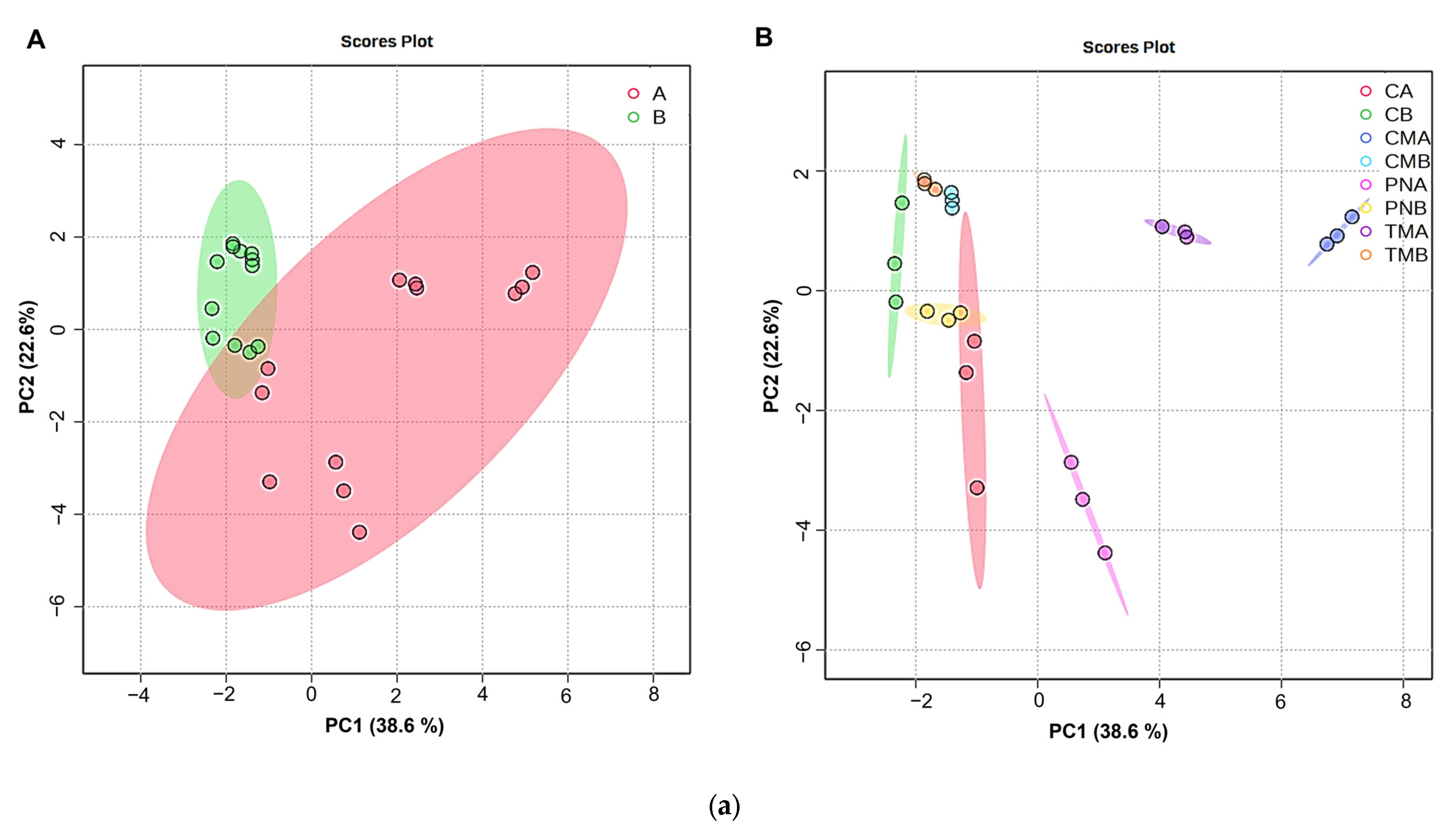
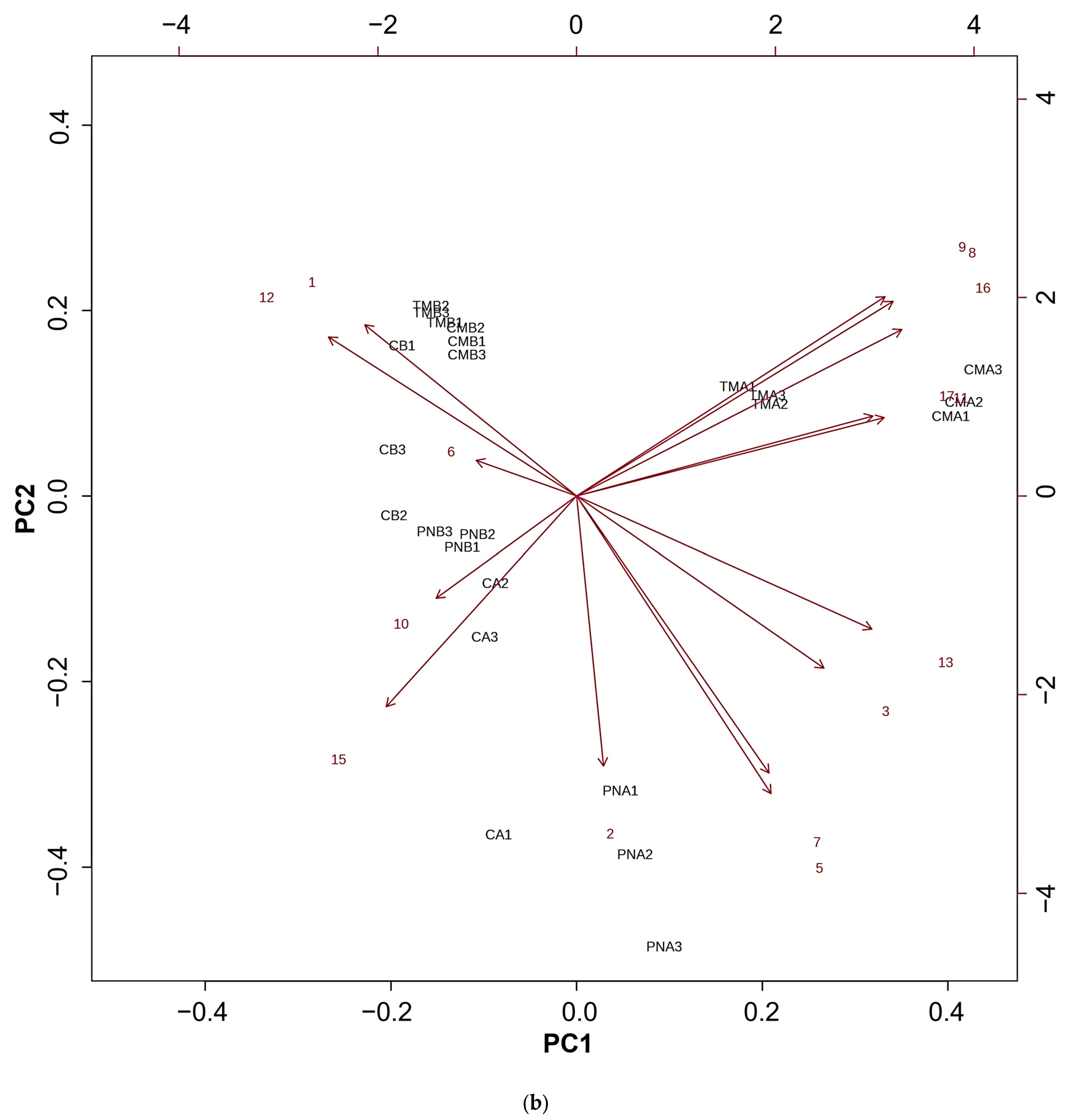
3.7. Volatile Organic Compounds
3.7.1. Higher Alcohols
3.7.2. Volatile Acids
3.7.3. Esters
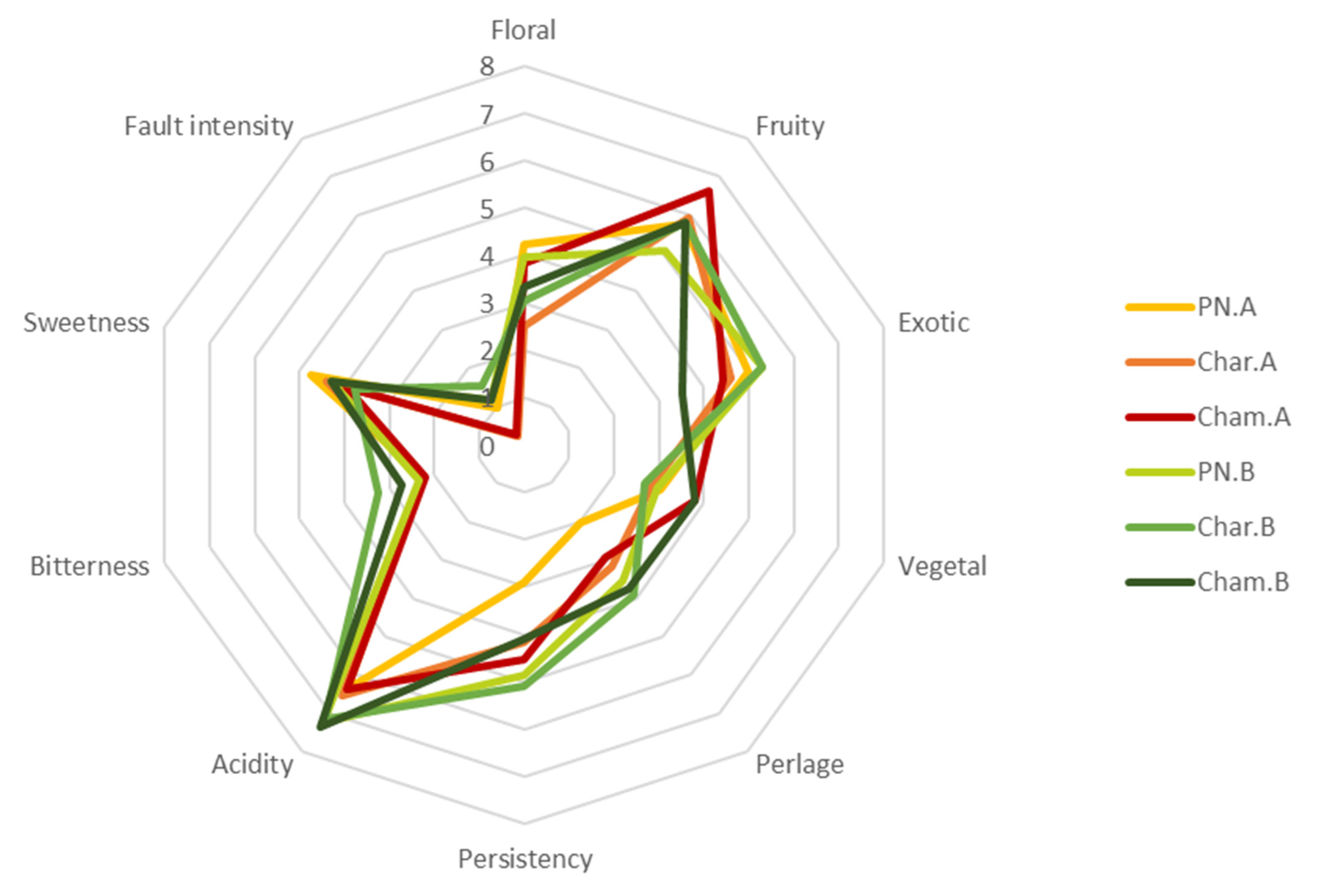
3.8. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, M.; Yue, T.; Yuan, Y. Evolution of polyphenols and organic acids during the fermentation of apple cider. J. Sci. Food. Agric. 2014, 94, 2951–2957. [Google Scholar] [CrossRef] [PubMed]
- Plotkowski, D.J.; Cline, J.A. Evaluation of selected cider apple (Malus domestica Borkh.) cultivars grown in Ontario. II. Juice attributes. Can. J. Plant Sci. 2021, 101, 836–852. [Google Scholar] [CrossRef]
- Miles, C.A.; Alexander, T.R.; Peck, G.; Galinato, S.P.; Gottschalk, C.; van Nocker, S. Growing apples for hard cider production in the United States—Trends and research opportunities. HortTechnology 2020, 30, 148–155. [Google Scholar] [CrossRef]
- Cousin, F.J.; Le Guellec, R.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in fermented apple beverages: Current knowledge and future directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef]
- Morrissey, W.F.; Davenport, B.; Querol, A.; Dobson, A.D.W. The role of indigenous yeasts in traditional Irish cider fermentations. J. Appl. Microbiol. 2004, 97, 647–655. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.R.; Pretorius, I.S. The effect of non-Saccharomyces yeasts on fermentation and wine quality. S. Afr. J. Enol. Vitic. 2003, 24, 55–62. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Wei, J.; Wang, S.; Zhang, Y.; Yuan, Y.; Yue, T. Characterization and screening of non-Saccharomyces yeasts used to produce fragrant cider. LWT 2019, 107, 191–198. [Google Scholar] [CrossRef]
- Gschaedler, A.; Iñiguez-Muñoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of non-Saccharomyces yeasts in cider fermentation: Importance of the nutrients addition to obtain an efficient fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Al Daccache, M.; Salameh, D.; Chamy, L.E.; Koubaa, M.; Maroun, R.G.; Vorobiev, E.; Louka, N. Evaluation of the fermentative capacity of an indigenous Hanseniaspora sp. strain isolated from Lebanese apples for cider production. FEMS Microbiol. Lett. 2020, 367, fnaa093. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Wang, Y.; Ju, H.; Niu, C.; Song, Z.; Youan, Y.; Yue, T. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Qiu, Y.; Guo, H.; Ju, H.; Wang, Y.; Youan, Y.; Yue, T. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co-and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, Y.; Zhu, R.; Bai, J.; Qiu, J.; Wu, Y.; Zhong, K.; Gao, H. Insight into the characteristics of cider fermented by single and co-culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe based on metabolomic and transcriptomic approaches. LWT 2022, 163, 113538. [Google Scholar] [CrossRef]
- Junior, W.J.L.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89, 103446. [Google Scholar] [CrossRef]
- Zhang, Z.; Lan, Q.; Yu, Y.; Zhou, J.; Lu, H. Comparative metabolome and transcriptome analyses of the properties of Kluyveromyces marxianus and Saccharomyces yeasts in apple cider fermentation. Food Chem. Mol. Sci. 2022, 4, 100095. [Google Scholar] [CrossRef] [PubMed]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Ogawa, M.; Vararu, F.; Moreno-Garcia, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Analyzing the minor volatilome of Torulaspora delbrueckii in an alcoholic fermentation. Eur. Food Res. Technol. 2022, 248, 613–624. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. App. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult to-use tool for winemaking. Fermentation 2018, 4, 94. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- OIV: Compendium of International Methods of Wine and Must Analysis, 2022 ed.; International Organisation of Vine and Wine: Paris, France, 2022.
- Jung, R.; Kumar, K.; Patz, C.; Rauhut, D.; Tarasov, A.; Schüßler, C. Influence of transport temperature profiles on wine quality. Food Packag. Shelf Life 2021, 29, 100706. [Google Scholar] [CrossRef]
- Brandt, M. The Influence of Abiotic Factors on the Composition of Berries, Juice, and Wine in Vitis vinifera L. cv. Riesling. Ph.D. Thesis, Hochschule Geisenheim University, Geisenheim, Germany, Justus-Liebig-University Giessen, Gießen, Germany, 2021. [Google Scholar]
- ISO 3591 (1977); Sensory analysis-Apparatus-Wine-Tasting glass. International Organization for Standardization: Geneva, Switzerland.
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gautiere, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2022, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Fejzullahu, F.; Kiss, Z.; Kun-Farkas, G.; Kun, S. Influence of Non-Saccharomyces Strains on Chemical Characteristics and Sensory Quality of Fruit Spirit. Foods 2021, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Using Torulaspora delbrueckii killer yeasts in the elaboration of base wine and traditional sparkling wine. Int. J. Food Microbiol. 2019, 289, 134–144. [Google Scholar] [CrossRef]
- Porras-Agüera, J.A.; Román-Camacho, J.J.; Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Effect of endogenous CO2 overpressure on the yeast “stressome” during the “prise de mousse” of sparkling wine. Food Microbiol. 2020, 89, 103431. [Google Scholar] [CrossRef]
- Porras–Agüera, J.A.; Moreno–García, J.; García–Martínez, T.; Moreno, J.; Mauricio, J.C. Impact of CO2 overpressure on yeast mitochondrial associated proteome during the “prise de mousse” of sparkling wine production. Int. J. Food Microbiol. 2021, 348, 109226. [Google Scholar] [CrossRef]
- Ignacio, B.; Navascués, E.; Marquina, D.; Santos, A.; Calderon, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar]
- Chua, J.Y.; Huang, A.; Liu, S.Q. Comparing the effects of isoleucine and leucine supplementation at different dosage on the growth and metabolism of Torulaspora delbrueckii Biodiva during soy whey fermentation. Food Biosci. 2022, 50, 101963. [Google Scholar] [CrossRef]
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Cusano, E.; Cagliani, L.R.; Consonni, R.; Simonato, B.; Zapparoli, G. NMR-based metabolic profiling of different yeast fermented apple juices. LWT 2020, 118, 108771. [Google Scholar] [CrossRef]
- Ivit, N.N.; Longo, R.; Kemp, B. The effect of non-Saccharomyces and Saccharomyces non-cerevisiae yeasts on ethanol and glycerol levels in wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Benvenutti, L.; Mottin Demiate, I.; Nogueira, A.; Alberti, A.; Ferreira Zielinski, A.A. A new approach to the use of apple pomace in cider making for the recovery of phenolic compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Millet, M.; Poupard, P.; Le Quéré, J.M.; Bauduin, R.; Guyot, S. Haze in apple-based beverages: Detailed polyphenol, polysaccharide, protein, and mineral compositions. J. Agric. Food Chem. 2017, 65, 6404–6414. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, P.P.; Ntushelo, N.; Ngqumba, Z.; Van Breda, V.; Jolly, N.P. Effect of Torulaspora delbrueckii yeast on the anthocyanin and flavanol concentrations of Cabernet franc and Pinotage wines. S. Afr. J. Enol. Vitic. 2015, 36, 50–58. [Google Scholar] [CrossRef]
- Ferreira, D.D.F.; Garruti, D.D.S.; Barin, J.S.; Cichoski, A.J.; Wagner, R. Characterization of Odor-Active Compounds in Gabiroba Fruits (Campomanesia xanthocarpa O. Berg). J. Food Qual. 2016, 39, 90–97. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Magalhães, F.; Krogerus, K.; Vidgren, V.; Sandell, M.; Gibson, B. Improved cider fermentation performance and quality with newly generated Saccharomyces cerevisiae × Saccharomyces eubayanus hybrids. J. Ind. Microbiol. Biotechnol. 2017, 44, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Ségurel, M.; Dagan, L.; Sommerer, N.; Marin, T.; Baumes, R. Identification of S-methylmethionine in Petit Manseng grapes as dimethyl sulphide precursor in wine. Anal. Chim. Acta 2008, 621, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kebede, B.; Ting, V.; Eyres, G.; Oey, I. Volatile changes during storage of shelf stable apple juice: Integrating GC-MS fingerprinting and chemometrics. Foods 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Toh, D.W.K.; Chua, J.Y.; Liu, S.Q. Impact of simultaneous fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii on volatile and non-volatile constituents in beer. LWT 2018, 91, 26–33. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Pereira, R.; Câmara, J.S. Evaluation of volatilomic fingerprint from apple fruits to ciders: A useful tool to find putative biomarkers for each apple variety. Foods 2020, 9, 1830. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Morris, A.K.R.; Rush, M.; Parris, R.; Sheridan, S.; Ringrose, T.; Wright, I.P.; Morgan, G.H. Quantification of ethyl acetate using FAIMS. In Proceedings of the Analytical Research Forum, Loughborough, UK, 26–28 July 2010. [Google Scholar]
- Buglass, A.J. (Ed.) Handbook of Alcoholic Beverages, 2 Volume Set: Technical, Analytical and Nutritional Aspects; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 1. [Google Scholar]
- Guerrini, L.; Masella, P.; Angeloni, G.; Sacconi, A.; Calamai, L.; Parenti, A. Effects of a Small Increase in Carbon Dioxide Pressure during Fermentation on Wine Aroma. Foods 2020, 9, 1496. [Google Scholar] [CrossRef]
- Martínez-García, R.; Roldán-Romero, Y.; Moreno, J.; Puig-Pujol, A.; Mauricio, J.C.; García-Martínez, T. Use of a flor yeast strain for the second fermentation of sparkling wines: Effect of endogenous CO2 over-pressure on the volatilome. Food Chem. 2020, 308, 125555. [Google Scholar] [CrossRef]


| Significance | Strain A | Strain B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | M | SxM | Base cider | Pet-Nat | Charmat | Champenoise | Base cider | Pét-Nat | Charmat | Champenoise | |
| °Brix | ns | ns | ns | 4.53 ± 0.05 a | 4.33 ± 0.60 b | 4.70 ± 0.08 a | 4.47 ± 1.11 a | 4.47 ± 0.05 a | 4.80 ± 0.00 a | 5.47 ± 0.66 a | 5.33 ± 0.05 a |
| pH | *** | *** | *** | 3.42 ± 0.02 a | 3.80 ± 0.00 b | 3.80 ± 0.00 b | 3.75 ± 0.03 b | 3.85 ± 0.03 b | 3.70 ± 0.03 c | 3.76 ± 0.03 bc | 3.73 ± 0.00 bc |
| Total acidity [g/L] | n | *** | *** | 8.19 ± 0.08 a | 4.45 ± 0.14 b | 4.50 ± 0.05 b | 5.24 ± 0.58 c | 4.82 ± 0.72 b | 6.20 ± 0.11 c | 5.60 ± 0.48 c | 6.16 ± 0.06 c |
| Glucose & Fructose [g/L] | *** | *** | *** | 0.02 ± 0.02 a | 0.26 ± 0.16 b | 0.09 ± 0.02 b | 0.14 ± 0.01 b | 0.66 ± 0.15 c | 0.14 ± 0.04 b | 0.04 ± 0.01 a | 0.18 ± 0.03 b |
| Sucrose [g/L] | *** | *** | *** | 0.44 ± 0.03 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.03 ± 0.04 b | 0.03 ± 0.05 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Malic acid [g/L] | *** | *** | *** | 6.71 ± 0.12 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.32 ± 0.45 c | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| D-lactic acid [g/L] | *** | *** | ** | 0.11 ± 0.00 a | 3.47 ± 0.21 b | 3.42 ± 0.13 b | 4.22 ± 0.18 c | 3.09 ± 0.47 b | 5.02 ± 0.79 c | 4.02 ± 0.23 b | 5.11 ± 0.49 c |
| L-lactic acid [g/L] | Ns | *** | *** | 0.00 ± 0.00 a | 3.30 ± 0.16 b | 4.21 ± 1.13 b | 3.40 ± 0.11 b | 2.75 ± 0.19 c | 1.94 ± 0.12 d | 2.70 ± 0.10 c | 2.65 ± 0.16 c |
| Acetic acid [g/L] | *** | *** | *** | 0.16 ± 0.00 a | 0.36 ± 0.10 b | 0.37 ± 0.01 b | 0.50 ± 0.01 c | 0.44 ± 0.11 c | 1.66 ± 0.15 d | 1.49 ± 0.13 d | 1.69 ± 0.15 d |
| α–amino nitrogen [mg/L] | * | *** | ** | 3.33 ± 0.47 a | 29.33 ± 2.2 b | 17.33 ± 4.50 c | 12.67 ± 2.87 c | 3.33 ± 2.62 a | 13.33 ± 2.62 c | 16.00 ± 3.74 c | 13.00 ± 1.63 c |
| Ammonium nitrogen [mg/L] | ns | *** | Ns | 17.00 ± 0.00 a | 79.33 ± 2.87 b | 83.67 ± 13.02 b | 82.33 ± 20.75 b | 22.33 ± 8.95 a | 71.67 ± 20.33 b | 72.33 ± 22.81 b | 85.67 ± 30.92 b |
| Polyphenols [mg/L] | ns | *** | Ns | 151.67 ± 23.81 a | 27.00 ± 20.61 b | 14.00 ± 10.42 b | 10.00 ± 10.20 b | 131.67 ± 51.98 a | 2.00 ± 2.83 c | 15.33 ± 18.93 b | 16.33 ± 22.40 b |
| Catechins [mg/L] | * | ** | Ns | 41.00 ± 4.32 a | 39.67 ± 1.25 a | 25.67 ± 3.30 b | 33.67 ± 6.02 ab | 41.33 ± 3.09 a | 22.33 ± 11.32 ab | 24.00 ± 1.41 b | 29.33 ± 1.70 b |
| Glycerol [g/L] | * | ns | * | 5.56 ± 2.31 ab | 4.65 ± 0.47 a | 2.86 ± 2.06 a | 4.65 ± 0.26 a | 3.23 ± 0.09 a | 6.48 ± 1.32 ab | 5.62 ± 1.94 ab | 9.89 ± 2.60 b |
| Ethyl Alcohol [%] | ** | *** | *** | 6.30 ± 0.08 a | 6.35 ± 0.03 a | 7.04 ± 0.03 b | 7.37 ± 0.34 b | 6.21 ± 0.16 a | 6.00 ± 0.03 c | 7.14 ± 0.32 b | 7.43 ± 0.02 b |
| Significance | Strain A | Strain B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | M | SxM | Juice | Cider | Pét-Nat | Cider AC | Cider | Pét-Nat | Cider AC | |
| Linalool oxide 1 (µg/L) | * | *** | * | 3.70 ± 0.22 a | 4.41 ± 0.12 a | 6.17 ± 0.55 b | 4.11 ± 0.05 a | 4.31 ± 0.02 a | 5.17 ± 0.13 c | 4.11 ± 0.15 a |
| Linalool (µg/L) | *** | *** | *** | nd | 2.32 ± 0.03 a | 1.69 ± 0.03 b | 2.09 ± 0.02 c | 1.78 ± 0.02 b | 1.39 ± 0.05 d | 1.58 ± 0.0 dc |
| Hotrienol (µg/L) | ns | *** | *** | nd | 9.14 ± 0.69 b | 3.95 ± 0.08 c | 3.77 ± 0.04 c | 7.17 ± 0.30 b | 3.02 ± 0.06 d | 6.07 ± 0.16 e |
| α-Terpineol (µg/L) | ns | ns | ns | 7.30 ± 0.03 a | nd | nd | nd | nd | nd | nd |
| Citronellol (µg/L) | ns | *** | ns | nd | 4.53 ± 0.73 b | nd | 1.55 ± 0.01 c | 3.77 ± 0.20 b | nd | 1.29 ± 0.04 d |
| β-Damascenone (µg/L) | ** | *** | *** | 0.17 ± 0.01 a | 1.87 ± 0.01 b | 0.97 ± 0.14 c | 1.55 ± 0.01 d | 1.55 ± 0.01 d | 1.18 ± 0.01 c | 1.29 ± 0.04 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocci, N.; Egger, M.; Hoellrigl, P.; Sanoll, C.; Beisert, B.; Brezina, S.; Fritsch, S.; Schnell, S.; Rauhut, D.; Conterno, L. Torulaspora delbrueckii Strain Behaviour within Different Refermentation Strategies for Sparkling Cider Production. Appl. Sci. 2023, 13, 4015. https://doi.org/10.3390/app13064015
Tocci N, Egger M, Hoellrigl P, Sanoll C, Beisert B, Brezina S, Fritsch S, Schnell S, Rauhut D, Conterno L. Torulaspora delbrueckii Strain Behaviour within Different Refermentation Strategies for Sparkling Cider Production. Applied Sciences. 2023; 13(6):4015. https://doi.org/10.3390/app13064015
Chicago/Turabian StyleTocci, Noemi, Magdalena Egger, Philipp Hoellrigl, Christof Sanoll, Beata Beisert, Silvia Brezina, Stefanie Fritsch, Sylvia Schnell, Doris Rauhut, and Lorenza Conterno. 2023. "Torulaspora delbrueckii Strain Behaviour within Different Refermentation Strategies for Sparkling Cider Production" Applied Sciences 13, no. 6: 4015. https://doi.org/10.3390/app13064015
APA StyleTocci, N., Egger, M., Hoellrigl, P., Sanoll, C., Beisert, B., Brezina, S., Fritsch, S., Schnell, S., Rauhut, D., & Conterno, L. (2023). Torulaspora delbrueckii Strain Behaviour within Different Refermentation Strategies for Sparkling Cider Production. Applied Sciences, 13(6), 4015. https://doi.org/10.3390/app13064015







