Integrated Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls: A Comparison of the Effectiveness of Selected Methods on Dried Fruit Matrices
Abstract
1. Introduction
- -
- Solvent extraction using polar and non-polar solvents that are selected depending on the physicochemical properties of the compounds analyzed [25,26,27]. Various methods are used, including Soxhlet extraction, ultrasound-assisted extraction, microwave-assisted extraction (MAE), accelerated solvent extraction (ASE), and supercritical fluid extraction (SFE);
- -
- Sample cleanup to eliminate lipids, sulfur, and other interfering components that hinder analyses with methods such as solid phase extraction (SPE) with silica gel, alumina, Florisil, C18 phases, amine phase adsorbents, or gel chromatography (GPC).
2. Materials and Methods
2.1. Samples and Sample Preparation
2.2. Chemicals
- -
- Deuterated PAHs standards (Semivolatile Internal Standard Mix) Sigma-Aldrich (Germany), naphthalene-D8 (NA D8), acenaphthene-D10 (AC D10), phenanthrene-D10 (PHE D10), chrysene-D12 (CHR D12), benzo[a]pyrene-D12 (BaP D12), and perylene-D12 (Per D12).
- -
- Pesticides Surrogate Spike Mix solutions in acetone (4-8460, SUPELCO, Bellefonte, USA) included 2,4,5,6-Tetrachloro-m-xylene and 2,2′,3,3′,4,4′,5, 5′,6,6′-PCB.
- -
- Standard solutions containing indicators of PCB congeners: PCB 28, PCB 52, PCB 101, PCB 138, PCB 153, PCB 180 (6 PCB–Key Isomers LGC Ltd., NE 5575, Augsburg, Taufkirchen, Germany);
2.3. Extraction Procedures
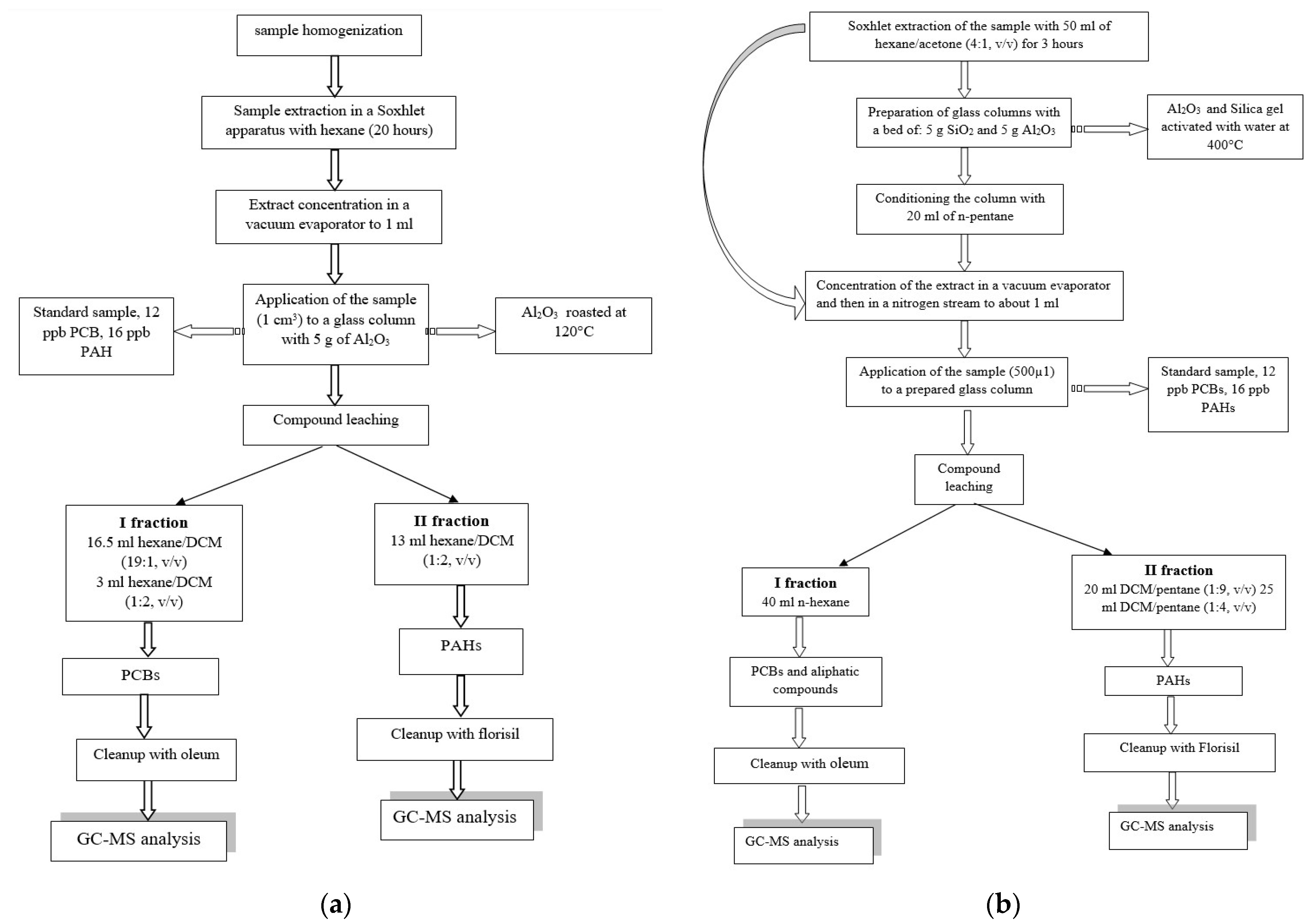
2.3.1. Method 1—Determination of Selected PCBs
2.3.2. Method 2—Determination of Selected PAHs
2.3.3. Method 3—Simultaneous Determinations of PCBs and PAHs, According to Vives and Grimalt [32]
2.3.4. Method 4—Simultaneous Determinations of PCBs and PAHs According to Jaouen-Madulet et al. [33]
2.4. GC-MS Analysis
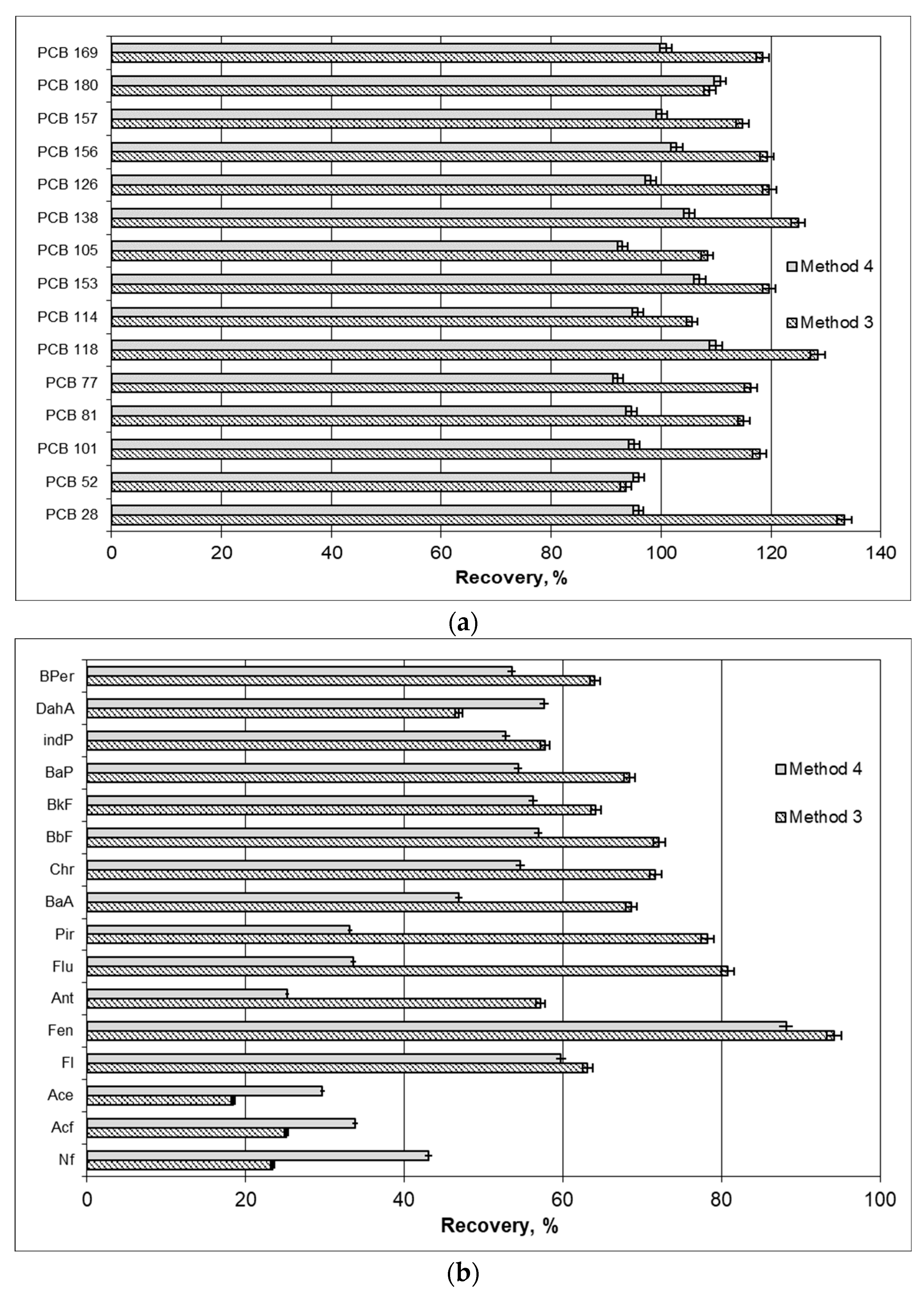
2.5. Analytical Method Validation for Quality Assurance
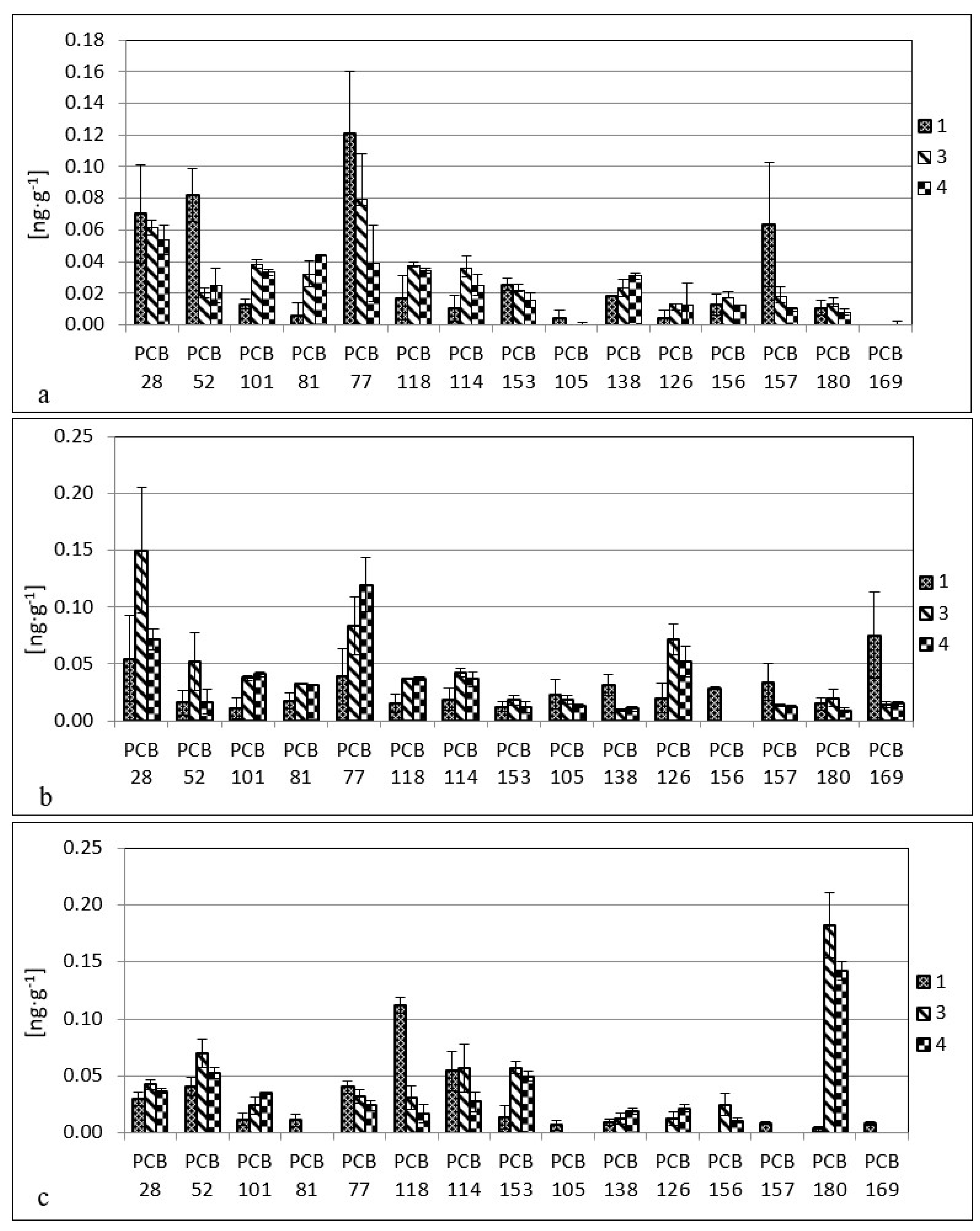
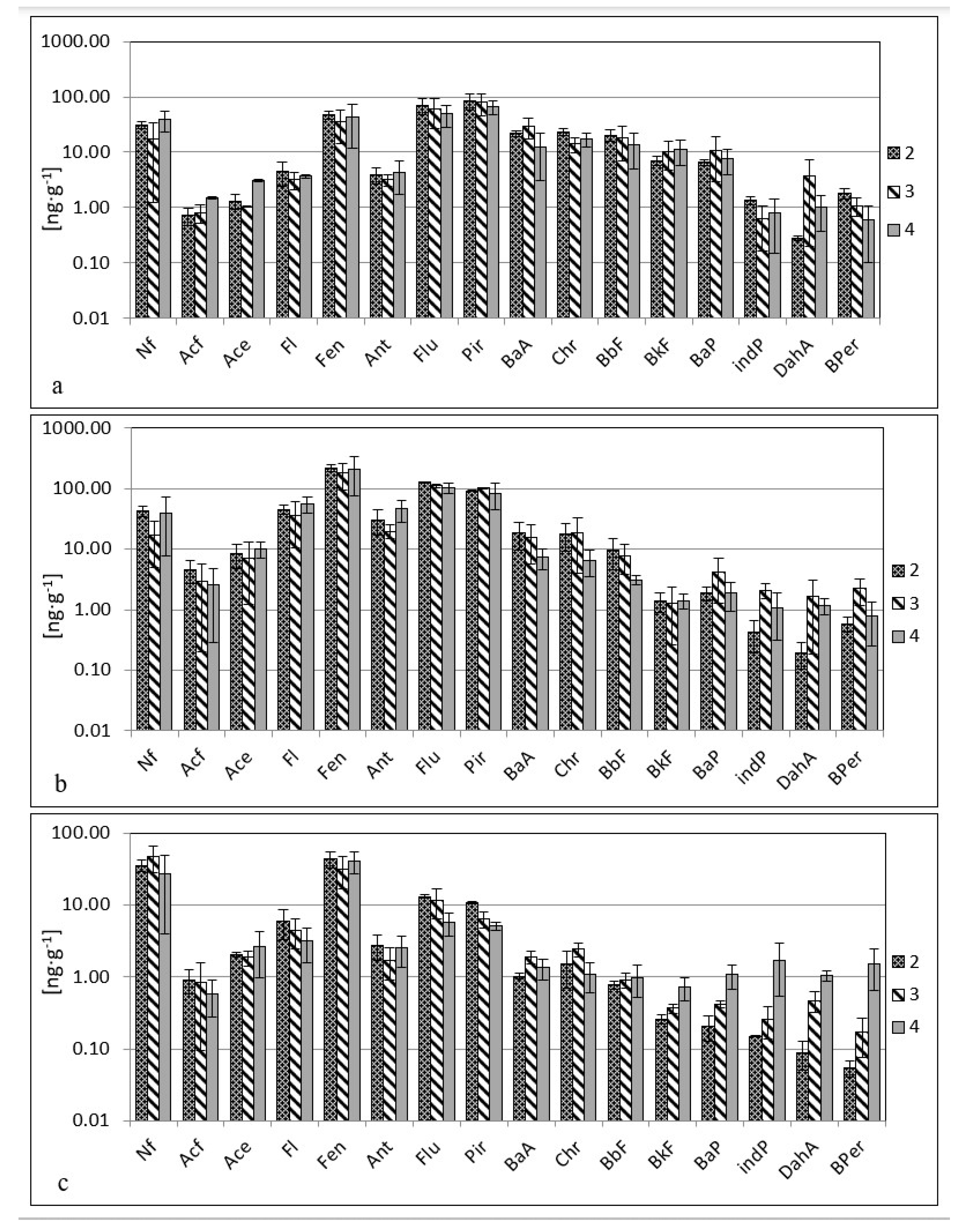
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snedeker, S.M. Pesticides and breast cancer risk: A review of DDT, DDE and dieldrin. Environ. Health Perspect. 2001, 109, 35–47. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J. 2018, 16, 5333. [Google Scholar]
- U.S. Environmental Protection Agency Compendium Method TO-13A. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS). Compendium of Methods for the Determination of Toxic Organic Compounds in Ambient Air. EPA/625/R-96/010b; EPA, Center for Environmental Research Information: Washington, DC, USA, 1999. Available online: https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-13arr.pdf (accessed on 19 July 2022).
- Food and Agriculture Organization of the United Nations; World Health Organization. JECFA 2005, Joint FAO/WHO. In Proceedings of the Summary and Conclusions of Sixty-Fourth Meeting, Expert Committee on Food Additives (JECFA), Rome, Italy, 8–17 February 2005; Available online: https://www.fao.org/3/at877e/at877e.pdf (accessed on 10 July 2022).
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 4–8. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydro-carbons and some related exposures. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Int. Agency Res. Cancer 2010, 92, 868. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321712/ (accessed on 14 August 2022).
- Ciemniak, A.; Witczak, A.; Mocek, K. Assessment of honey contamination with polycyclic aromatic hydrocarbons. J. Environ. Sci. Health B 2013, 48, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Jánska, M.; Hajšlová, J.; Tomaniová, M.; Kocourek, V.; Vávrová, M. Polycyclic aromatic hydrocarbons in fruits and vegetables grown in the Czech republic. Bull. Environ. Contam. Toxicol. 2006, 77, 492–499. [Google Scholar] [CrossRef]
- Paris, A.; Ledauphin, J.; Poinot, P.; Gaillard, J.L. Polycyclic aromatic hydrocarbons in fruits and vegetables: Origin, analysis, and occurrence. Environ. Pollut. 2018, 234, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Murk, A.J.; Rijntjes, E.; Blaauboer, B.J.; Clewell, R.; Crofton, K.M.; Dingemans, M.M.L.; Furlow, J.D.; Kavlock, R.; Köhrle, J.; Opitz, R.; et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. Vitr. 2013, 27, 1320–1346. [Google Scholar] [CrossRef]
- Blanco, L.; Martínez, A.; Ferreira, M.; Juan Vieites, J.; Cabado, A. Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dl-PCBs) in fish, seafood products and fish oil in Spain. Food Addit. Contam. Part B 2013, 6, 218–230. [Google Scholar] [CrossRef]
- Liu, W.; Tao, F.; Zhang, W.; Li, S.; Zheng, M.; Zheng, M.H. Contamination and emission factors of PCDD/Fs, unintentional PCBs, HxCBz, PeCBz and polychlorophenols in chloranil in China. Chemosphere 2012, 86, 248–251. [Google Scholar] [CrossRef]
- Richardson, K.L.; Lopez Castro, M.; Gardner, S.C.; Schlenk, D. Polychlorinated Biphenyls and Biotransformation Enzymes in Three Species of Sea Turtles from the Baja California Peninsula of Mexico. Arch. Environ. Contam. Toxicol. 2010, 58, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.B.; Pessah, I.N. Non-dioxin-like polychlorinated biphenyl neurotoxic equivalents found in environmental and human samples. Regul. Toxicol. Pharmacol. 2021, 120, 104842. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Meijer, M.; Westerink, R.H. Differential effects of 20 non-dioxin-like PCBs on basal and depolarization-evoked intracellular calcium levels in PC12 cells. Toxicol. Sci. 2012, 126, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Min, X.; Weng, M. A review of polychlorinated biphenyls (PCBs)pollution in indoor air environment. J. Air Waste Manag. Assoc. 2016, 66, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Faroon, O.; Ruiz, P. Polychlorinated biphenyls: New evidence from the last decade. Toxicol. Ind. Health 2016, 32, 1825–1847. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Harwell, L.; Summers, J.K. Contaminant concentrations in whole-body fish and shellfish from US estuaries. Environ. Monit. Assess. 2008, 137, 403–412. [Google Scholar] [CrossRef]
- IARC/WHO. Polychlorinated biphenyls and polybrominated biphenyls. Monographs on the evaluation of carcinogenic risk to humans. Lyon, France. Int. Agency Res. Cancer 2016, 107, 1–124. [Google Scholar]
- Adekunle, A.S.; Adekunle, J.; Oyekunle, O.; Ola, I.J.; Obisesan, O.R.; Maxakato, N.W. Determination of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in some personal care products in Nigeria. [published correction appears in Toxicol. Rep. 2020,5, 60–61]. Toxicol. Rep. 2018, 5, 994–1001. [Google Scholar] [CrossRef]
- Taghizadeh, S.F.; Azizi, M.; Rezaee, R.; Giesy, J.P.; Karimi, G. Polycyclic aromatic hydrocarbons, pesticides, and metals in olive: Analysis and probabilistic risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 39723–39741. [Google Scholar] [CrossRef]
- Wolska, L. Miniaturised analytical procedure of determining polycyclic aromatic hydrocarbons and polychlorinated biphenyls in bottom sediments. J. Chromatogr. A 2002, 959, 176. [Google Scholar] [CrossRef]
- Tarawneh, I.; Alawi, M.; Sapah, R.; Abu Shmeis, R. Pesticide Residues in Commonly Consumed Fruits and Vegetables in Jordan and Their Associated Health Risk Assessments. Jordan J. Chem. 2019, 14, 69–80. [Google Scholar]
- Kumari, D.; John, S. Health Risk Assessment of Pesticide Residues in Fruits and Vegetables from Farms and Markets of Western Indian Himalayan Region. Chemosphere 2019, 224, 136–167. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Budzinski, H.; LeMenach, K.; Letellier, M.; Garrigues, P. Multi-residue analysis of polycyclic aromatic hydrocarbons polychlorobiphenyls, and organochlorine pesticides in marine sediments. Anal. Bioanal. Chem. 2002, 372, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Liljelind, P.; Söderström, G.; Hedman, B.; Karlsson, S.; Lundin, L.; Marklund, S. Method for Multiresidue Determination of Halogenated Aromatics and PAHs in Combustion-Related Samples. Environ. Sci. Technol. 2003, 37, 3680–3686. [Google Scholar] [CrossRef]
- Sharma, S.; Chandra, P.; Mishra, C.; Kakkar, P. Microbiological Quality and Organochlorine Pesticide Residue in Commercially Available Ready-To-Eat Raisins. Bull. Environ. Contam. Toxicol. 2008, 81, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.B.; Moniruzzaman, M.; Aminabhavi, T.M. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2018, 358, 1186–1207. [Google Scholar] [CrossRef]
- Witczak, A.; Harada, D.; Aftyka, A.; Cybulski, J. Endocrine-disrupting organochlorine xenobiotics in fish products imported from Asia—An assessment of human health risk. Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef]
- Kuźmicz, K.; Ciemniak, A. Assessing contamination of smoked sprats (Sprattus sprattus) with polycyclic aromatic hydrocarbons (PAHs) and changes in its level during storage in various types of packaging. J. Environ. Sci. Health Part B 2018, 1, 1369306. [Google Scholar] [CrossRef]
- Ciemniak, A.; Kuźmicz, K. Effect of a packaging material type on PAHs contents in oils and water. J. Stored Prod. Res. 2021, 92, 101810. [Google Scholar] [CrossRef]
- Vives, I.; Grimalt, J.O. Method for integrated analysis of polycyclic aromatic hydrocarbons and organochlorine compounds in fish liver. J. Chromatogr. B 2002, 768, 248–254. [Google Scholar] [CrossRef]
- Jaouen-Madoulet, A.; Abarnou, A.; Le Guellec, A.-M.; Loizeau, V.; Leboulenger, F. Validation of an analytical procedure for polychlorinated biphenyls, coplanar polychlorinated biphenyls and polycyclic aromatic hydrocarbons in environmental samples. J. Chromatogr. A 2000, 886, 153–173. [Google Scholar] [CrossRef]
- EU Commission Directive. 2002/63/EC of 11 July 2002 Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing Directive 79/700/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32002L0063&from=EN (accessed on 1 July 2022).
- Castro, I.B.; Costa, P.G.; Primel, E.G.; Fillmann, G. Environmental matrices effect in butyltin determination by GC/MS. Ecotoxicol. Environ. Contam. 2015, 10, 47–53. [Google Scholar] [CrossRef]
- Azis, M.Y.; Asia, L.; Piram, A.; Buchari, B.; Doumenq, P.; Setiyanto, H. Matrix effects on organic pollutants analysis in marine sediment. J. Phys. Conf. Ser. 2018, 1013, 012193. [Google Scholar] [CrossRef]
- Dos Santos, D.M.; de Marchi, M.R.R.; Godoi, A.F.L.; Turra, A.; Montone, A. Matrix effect on butyltin analysis of sediments and fish tissues by GC-PFPD. J. Braz. Chem. Soc. 2013, 24, 998–1005. [Google Scholar] [CrossRef]
- Al-Alam, J.; Baroudi, F.; Chbani, A.; Fajloun, Z.; Millet, M. A multiresidue method for the analysis of pesticides, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls in snails used as environmental biomonitors. J. Chromatogr. A 2020, 1621, 461006. [Google Scholar] [CrossRef] [PubMed]
- Roszko, M.; Szterk, A.; Szymczyk, K.; Waszkiewicz-Robak, B. PAHs, PCBs, PBDEs and Pesticides in Cold-Pressed Vegetable Oils. J. Am. Oil Chem. Soc. 2012, 89, 389–400. [Google Scholar] [CrossRef]
- Yenisoy-Karakaş, S.; Gaga, E.O. Validation of one-step cleanup and separation method of polychlorinated biphenyls, organochlorine pesticides, and polycyclic aromatic hydrocarbons from atmospheric gas- and particle-phasesamples. Talanta 2013, 115, 150–158. [Google Scholar] [CrossRef]
- Lehnik-Habrink, P.; Hein, S.; Win, T.; Bremser, W.; Nehls, I. Multi-residue analysis of PAH, PCB, and OCP optimized for organic matter of forest soil. J. Soils Sediments 2010, 10, 1487–1498. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Lehotay, S.J. Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography–tandem mass spectrometry. Anal. Chim. Acta 2013, 758, 80–92. [Google Scholar] [CrossRef]
- Nácher-Mestre, J.; Serrano, R.; Portolés, T.; Berntssen, M.H.; Pérez-Sánchez, J.; Hernϡndez, F. Screening of Pesticides and Polycyclic Aromatic Hydrocarbons in Feeds and Fish Tissues by Gas Chromatography Coupled to High-Resolution Mass Spectrometry Using Atmospheric Pressure Chemical Ionization. J. Agric. Food Chem. 2014, 62, 2165–2174. [Google Scholar] [CrossRef]
- Stenerson, K.K.; Ye, M.; Shimelis, O.; Barrey, E.; Halpenny, M. Sample Cleanup for the Analysis of Pesticide Residues and Polynuclear Aromatic Hydrocarbons in Fatty Food Matrices. Am. Lab. 2016. Available online: https://www.americanlaboratory.com/914-Application-Notes/185012-Sample-Cleanup-for-the-Analysis-of-Pesticide-Residues-and-Polynuclear-Aromatic-Hydrocarbons-in-Fatty-Food-Matrices. (accessed on 28 September 2021).
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS–SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, Y.; Yang, C.; Liu, W.; Kong, X.; Qin, N.; He, O.; Xu, F. Optimized Multiresidue Analysis of Organic Contaminants of Priority Concern in a Daily Consumed Fish (Grass Carp). J. Anal. Methods Chem. 2017, 2017, 9294024. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, E.; Garcia Sanchez, A.; Ramos Martos, N. Simultaneous Multidetermination of Residues of Pesticides and Polycyclic Aromatic Hydrocarbons in Olive and Olive-pomace Oils by Gas Chromatography/Tandem Mass Spectrometry. J. Chromatogr. A 2006, 1111, 89–96. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Dried Apricot n = 20 | Dried Pear N = 20 | Dried Apple n = 20 |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Fat content [%] | 0.25 ± 0.1 | 0.6 ± 0.1 | 1.5 ± 0.2 |
| Dry matter [%] | 72.5 ± 0.2 | 77.4 ± 0.3 | 75.2 ± 0.1 |
| PCB | PAH | |
|---|---|---|
| Sample injection | 2 µL | 2 µL |
| Column type | ZB-5MS (30 m × 0.25 µm × 250 µm) | ZB-5MS (30 m × 0.25 µm × 250 µm) |
| Carrier gas | He | He |
| Column oven temperature program | 1.1 cm3/min | 1.2 cm3/min |
| 130 °C (0.5 min *) | 80 °C (0.5 min *) | |
| increase rate 7 °C up to 200 °C (10 min *) | increase rate 10 °C up to 230 °C (10 min *) | |
| temperature ramp 14 °C up to 280 °C (10 min *) | temperature ramp 5 °C up to 305 °C (10 min *) | |
| Electron multiplier voltage | 1920 V | 1920 V |
| PAH | LOD | LOQ | PCB | LOD | LOQ |
|---|---|---|---|---|---|
| NA | 0.30 | 0.91 | PCB 28 | 0.031 | 0.094 |
| ACL | 0.19 | 0.59 | PCB 52 | 0.029 | 0.087 |
| AC | 0.23 | 0.70 | PCB 101 | 0.044 | 0.132 |
| FA | 0.11 | 0.34 | PCB 81 | 0.035 | 0.110 |
| PHE | 0.08 | 0.26 | PCB 77 | 0.030 | 0.089 |
| AN | 0.11 | 0.32 | PCB 118 | 0.018 | 0.056 |
| FL | 0.05 | 0.15 | PCB 114 | 0.066 | 0.197 |
| PY | 0.05 | 0.16 | PCB 153 | 0.062 | 0.183 |
| BaA | 0.05 | 0.16 | PCB 105 | 0.060 | 0.179 |
| CHR | 0.06 | 0.18 | PCB 138 | 0.059 | 0.174 |
| BbFA | 0.08 | 0.24 | PCB 126 | 0.053 | 0.158 |
| BkFA | 0.07 | 0.22 | PCB 156 | 0.030 | 0.090 |
| BaP | 0.06 | 0.17 | PCB 157 | 0.030 | 0.091 |
| IP | 0.09 | 0.28 | PCB 180 | 0.009 | 0.028 |
| DbahA | 0.08 | 0.25 | PCB 169 | 0.040 | 0.120 |
| BghiP | 0.10 | 0.32 |
| PCBs | PCB Recovery by Method 1 * (%) | PAHs | PAH Recovery by Method 2 ** (%) | ||
|---|---|---|---|---|---|
| Method 3 | Method 4 | Method 3 | Method 4 | ||
| PCB 52 | 24.7–330.9 | 30.4–127.5 | ACL | 65.7–112.7 | 55.7–211.5 |
| PCB 101 | 202.7–342.3 | 253.0–374.6 | AC | 78.4–92.7 | 63.8–233.2 |
| PCB 81 | 0–559.3 | 0.0–768.4 | FA | 71.3–83.9 | 52.7–124.3 |
| PCB 77 | 35.7–212.9 | 32.2–305.0 | PHE | 60.5–80.0 | 80.1–94.1 |
| PCB 118 | 27.6–220.3 | 14.7–233.7 | AN | 62.1–79.6 | 89.9–150.9 |
| PCB 114 | 105.4–338.3 | 50.2–238.1 | FL | 68.7–88.7 | 42.7–83.5 |
| PCB 153 | 83.9–417.3 | 60.9–361.0 | PY | 59.2–112.1 | 47.0–124.9 |
| PCB 105 | 0.0–78.2 | 0.0–55.9 | BaA | 79.6–187.9 | 39.4–129.9 |
| PCB 138 | 29.6–141.7 | 33.1–215.6 | CHR | 61.5–162.1 | 37.3–75.9 |
| PCB 126 | 330.7–358.1 | 260.6–320.8 | BbFA | 79.2–116.2 | 31.8–125.3 |
| PCB 156 | 0.0–138.3 | 0.0–100.4 | BkFA | 115.8–242.8 | 102.5–280.7 |
| PCB 157 | 0.0–39.8 | 15.7–35.2 | BaP | 70–222.9 | 61.0–513.1 |
| PCB 180 | 123.9–5284.0 | 60.9–4119.1 | IP | 45.9–480.4 | 57.9–1152.2 |
| PCB 169 | 0.0–18.5 | 0.0–19.8 | DBahA | 537.2–1323.0 | 408.1–1177.0 |
| BghiP | 61.3–371.5 | 33.7–2828.7 | |||
| Authors | Extraction Method | Cleanup Method | Target Compounds | ||
|---|---|---|---|---|---|
| Yenisoy-Karakaş and Gaga 2013 [40] | atmospheric gas, particle-phase | Soxhlet extraction: dichloromethane/petroleum ether | aluminum oxide + florisil + anhydrous sodium sulfate | PAHs, PCBs, OCPs | |
| He et al., 2017 [46] | fish | microwave-assisted extraction (MAE) | gel permeation chromatogra-phy (GPC) | neutral alumina + acid silica gel + neutral silicagel | PBDEs, PCBs |
| neutral alumina + wet neutral silica gel + wet alkaline silica gel | PBDEs, PCBs, | ||||
| neutral alumina + wet neutral silica gel + anhydrous sodium sulfate | PAHs, OCPs | ||||
| Lehnik-Habrink et al., 2010 [41] | forest soil | pressurized liquid extraction (PLE), acetone/cyclohexane AC/CH | GPC; silica gel or aluminum oxide | PAH, PCBs, OCPs | |
| Stenerson et al., 2016 [44] | Fatty food | QuEChERS, acetonitrile | PSA/C18, Z-Sep/C18 | Pesticides, PAHs | |
| Sapozhnikva and Lehotay 2013 [42] | fish | QuEChERS, acetonitrile | zirconia-based sorbent (Z-Sep) for d-SPE | PCBs, PAHs, PBDEs. OCPs | |
| Nácher-Mestre et al., 2014 [43] | feeds and fish tissues | QuEChERS, acetonitrile | (primary−secondary amine (PSA) + MgSO4 + C18) | Pesticides, PAHs | |
| Ballesteros et al., 2009 [47] | olive oil | acetonitrile/n-hexane | GPC | Pesticides, PAHs | |
| Jaouen-Madoulet et al., 2000 [33] | environmental samples: blue mussel, cod liver oil | Soxhlet extraction: n-hexane/acetone | Alumina + silica gel | PCBs, PAHs | |
| Thompson et al., 2002 [25] | sediment | microwave-assisted extraction: dichloromethane | sulfuric acid, activated silica + activated copper | PAHs, PCBs, OCPs | |
| Vives and Grimalt 2002 [32] | fish liver | Soxhlet extraction: n-hexane/dichloromethane | aluminium oxide | PAHs, PCBs, OCPs | |
| Wolska 2002 [22] | sediment | dichloromethane | activated silica gel | PAHs | |
| solvent exchanged to pentane + activated silica gel | PCBs | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciemniak, A.; Witczak, A.; Pokorska-Niewiada, K. Integrated Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls: A Comparison of the Effectiveness of Selected Methods on Dried Fruit Matrices. Appl. Sci. 2023, 13, 4047. https://doi.org/10.3390/app13064047
Ciemniak A, Witczak A, Pokorska-Niewiada K. Integrated Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls: A Comparison of the Effectiveness of Selected Methods on Dried Fruit Matrices. Applied Sciences. 2023; 13(6):4047. https://doi.org/10.3390/app13064047
Chicago/Turabian StyleCiemniak, Artur, Agata Witczak, and Kamila Pokorska-Niewiada. 2023. "Integrated Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls: A Comparison of the Effectiveness of Selected Methods on Dried Fruit Matrices" Applied Sciences 13, no. 6: 4047. https://doi.org/10.3390/app13064047
APA StyleCiemniak, A., Witczak, A., & Pokorska-Niewiada, K. (2023). Integrated Analysis of Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls: A Comparison of the Effectiveness of Selected Methods on Dried Fruit Matrices. Applied Sciences, 13(6), 4047. https://doi.org/10.3390/app13064047







