Influence of Oxygen Impurity on Nitrogen Atmospheric-Pressure Plasma Jet
Abstract
:1. Introduction
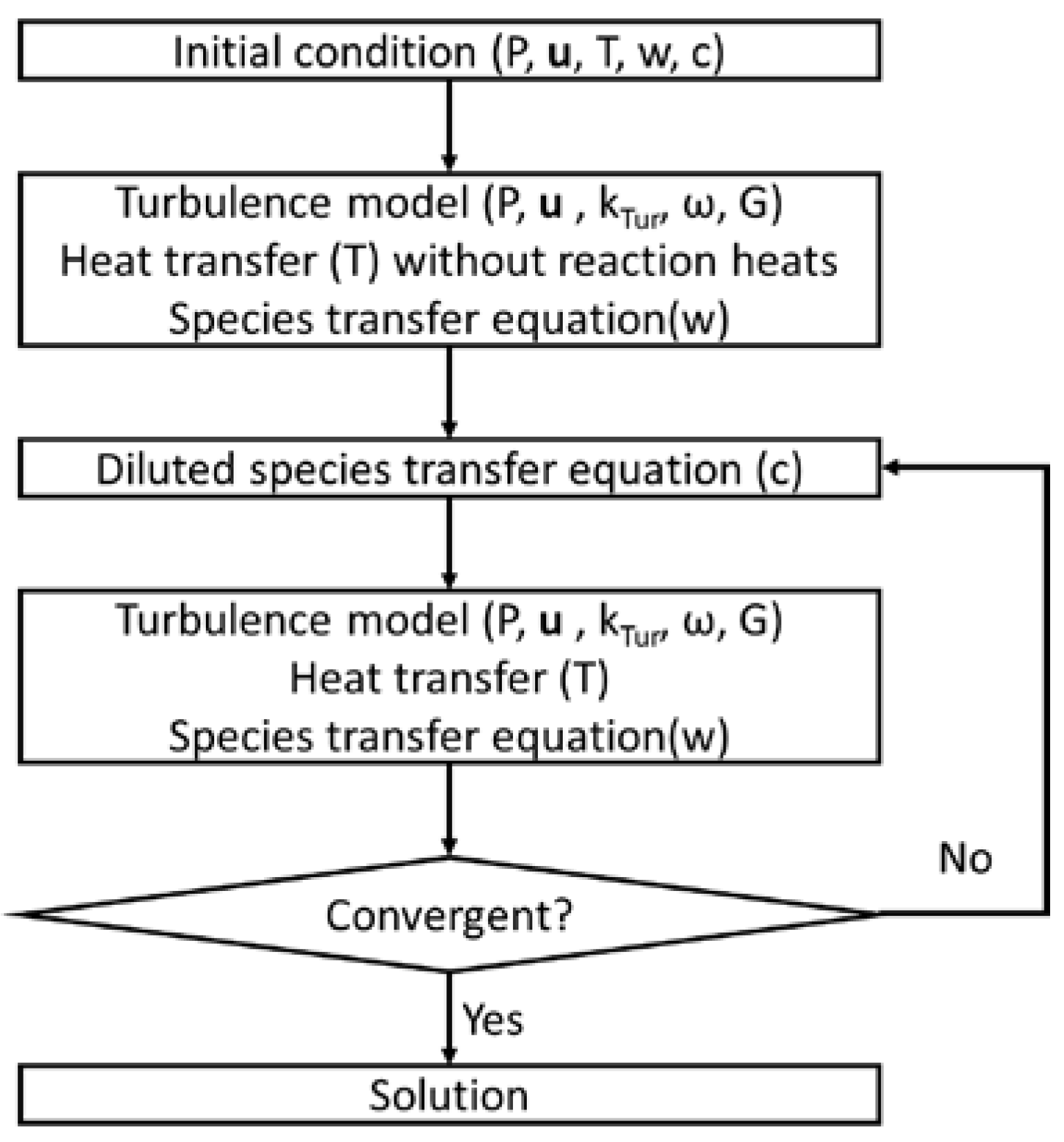
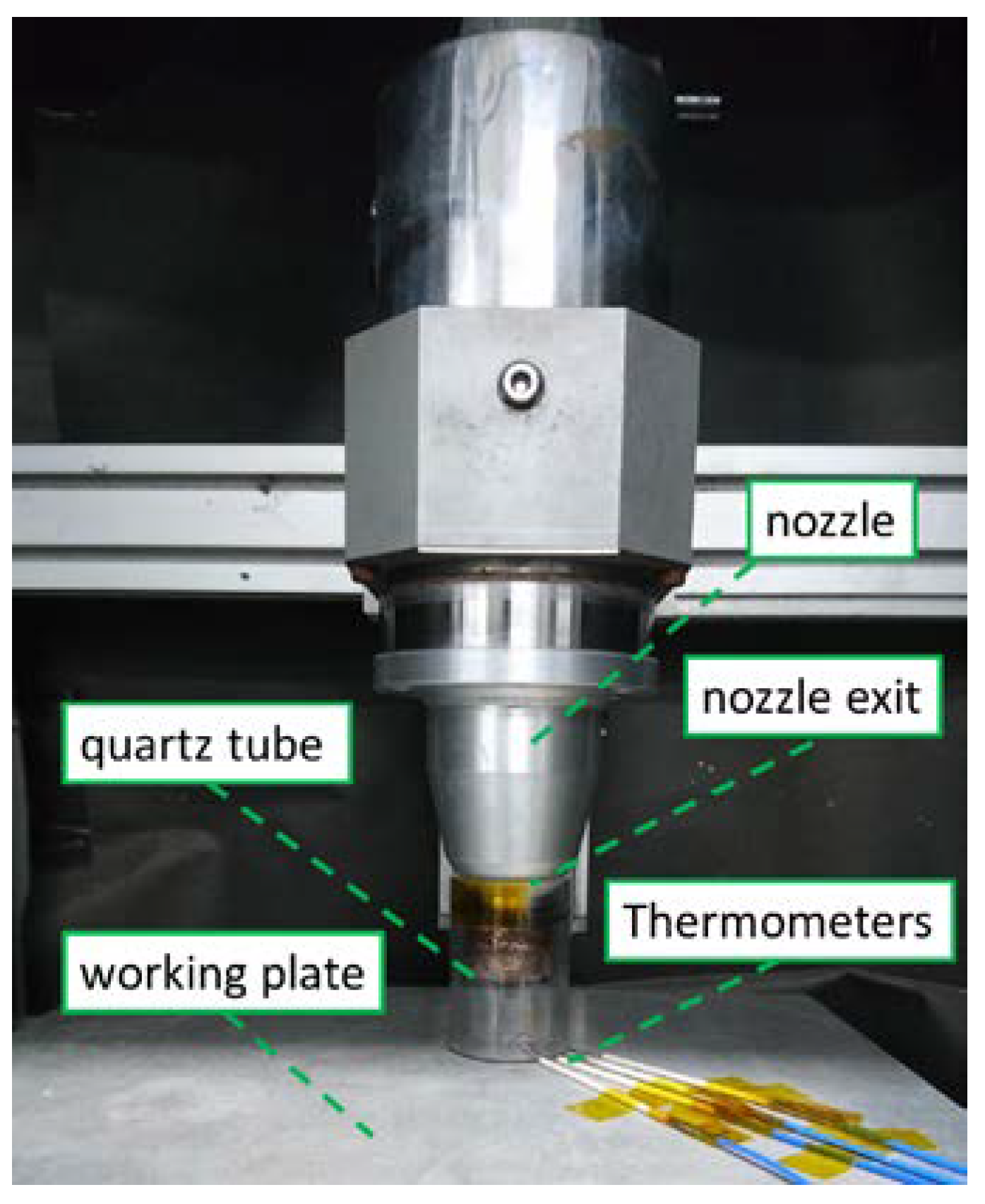
2. Materials and Methods
3. Results and Discussion
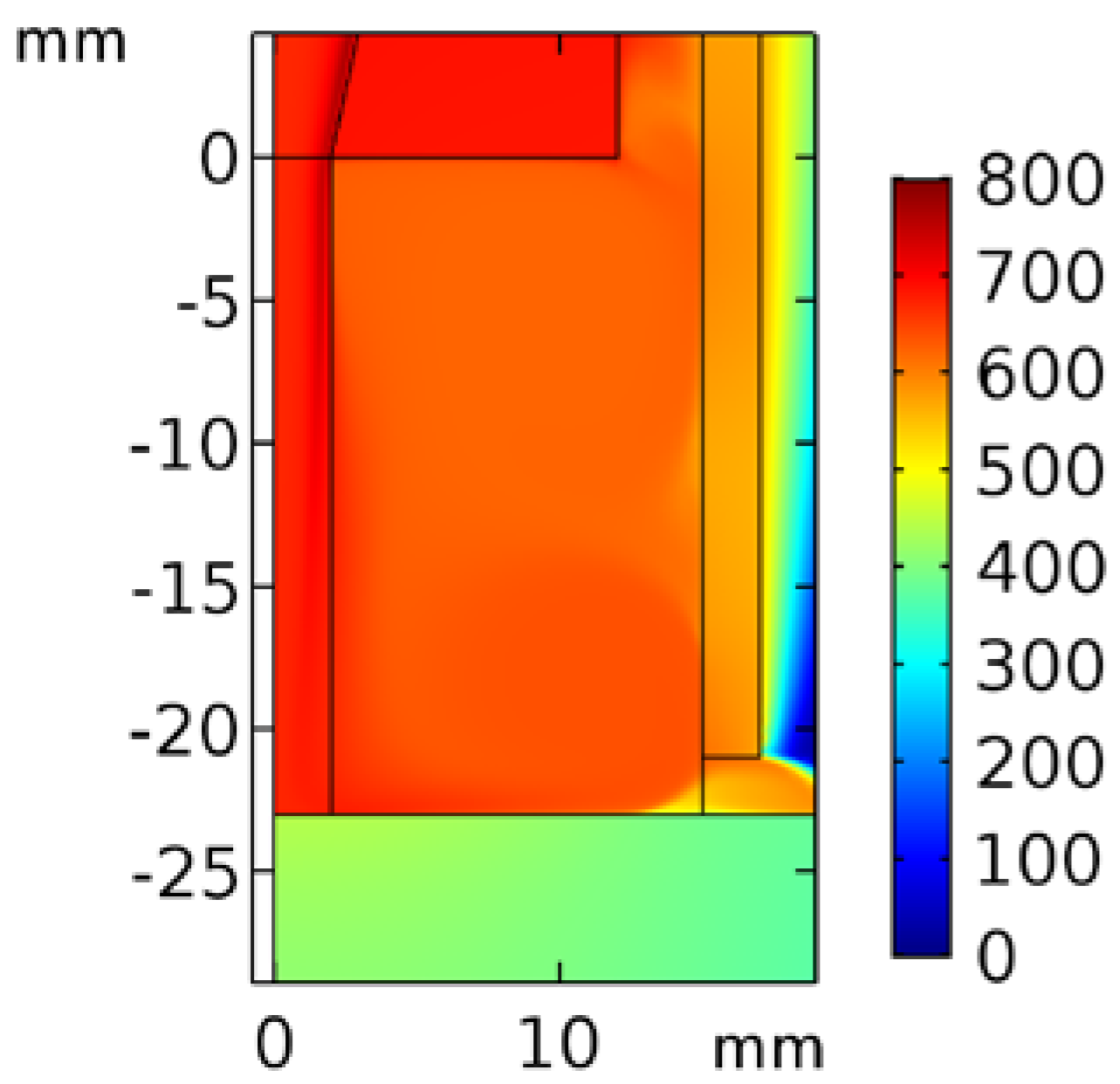
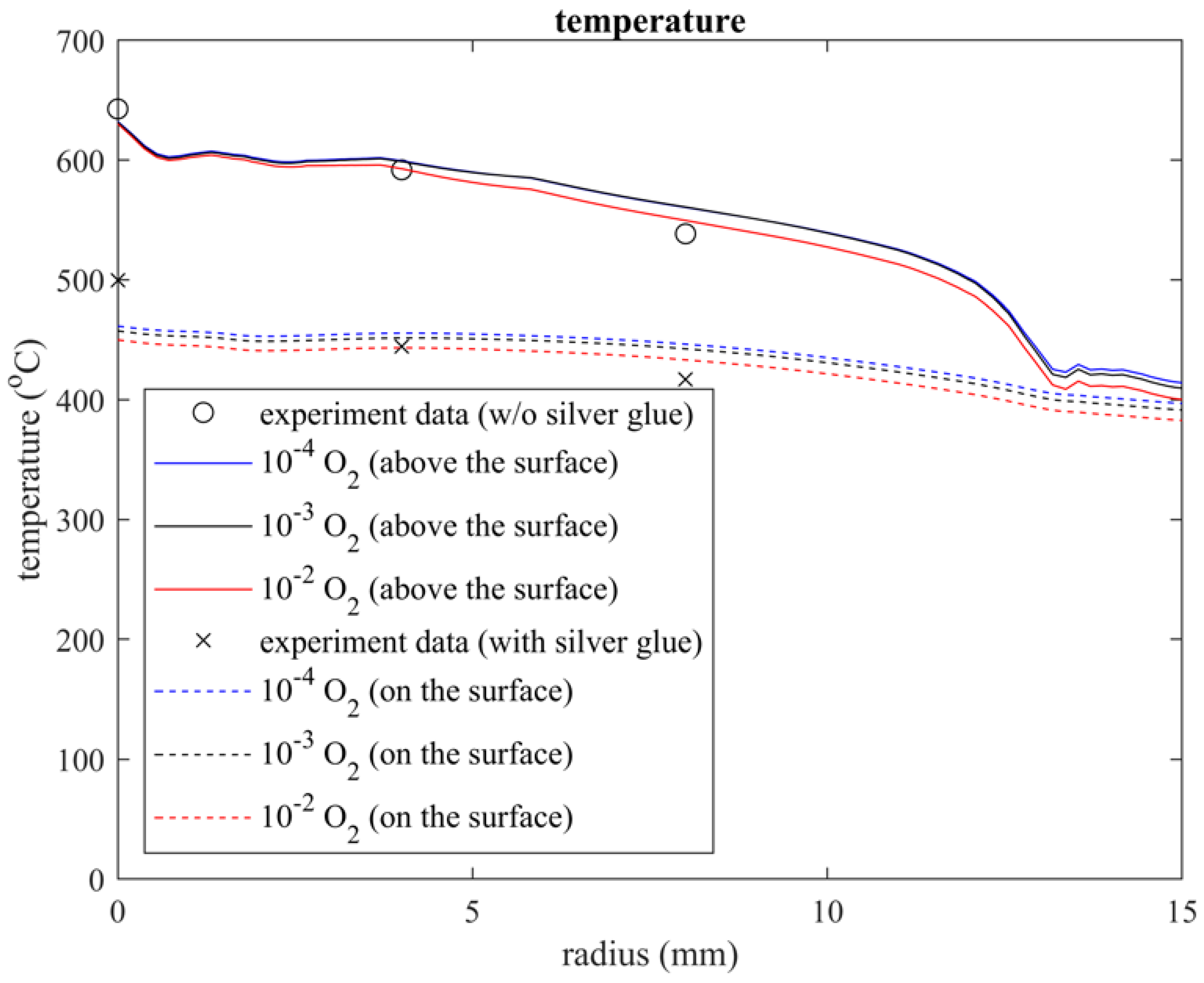
3.1. Temperature Profile
3.1.1. Temperature Profile inside the Quartz Tube
3.1.2. Comparison with Experimental Data
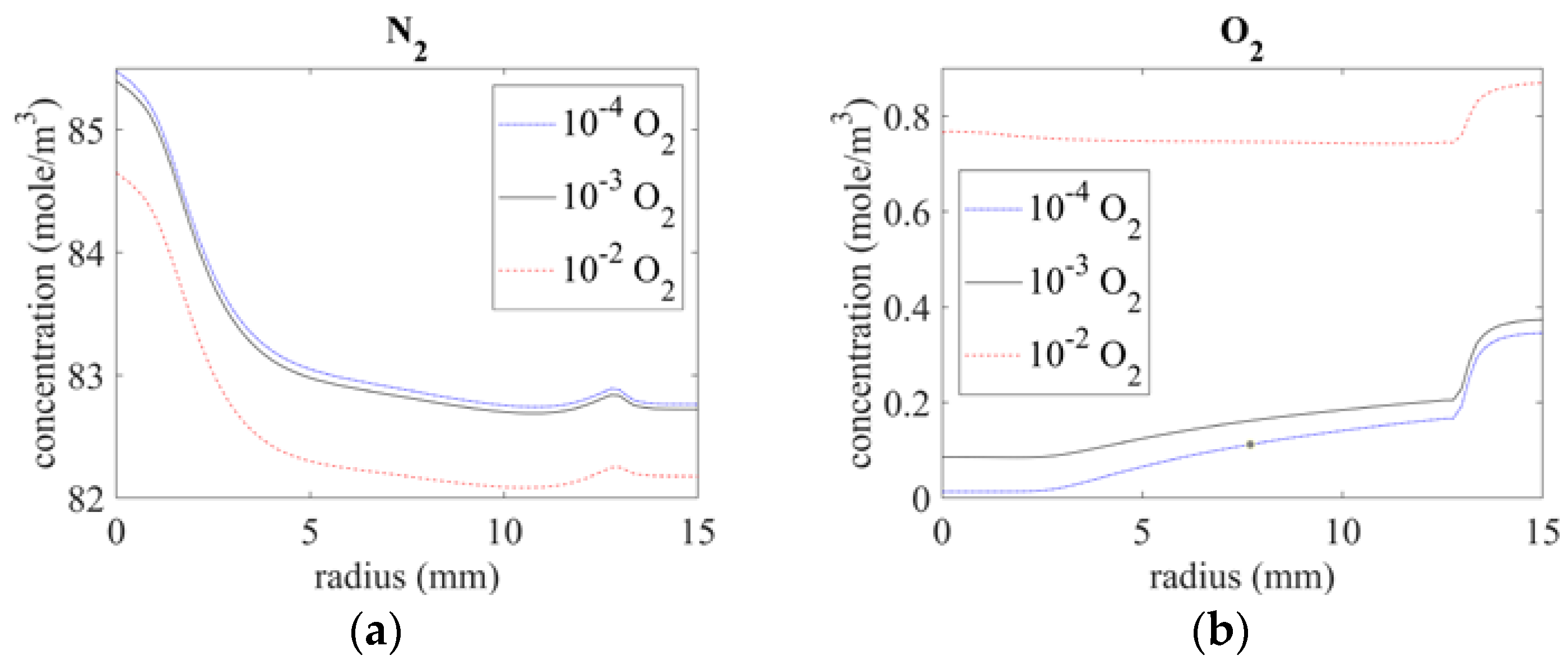
3.2. Species Distribution
3.2.1. Distribution of Major Molecules (N2, O2)
3.2.2. N-series Species Distribution (N2(A), N2(B), N2(C), N2(a), N)
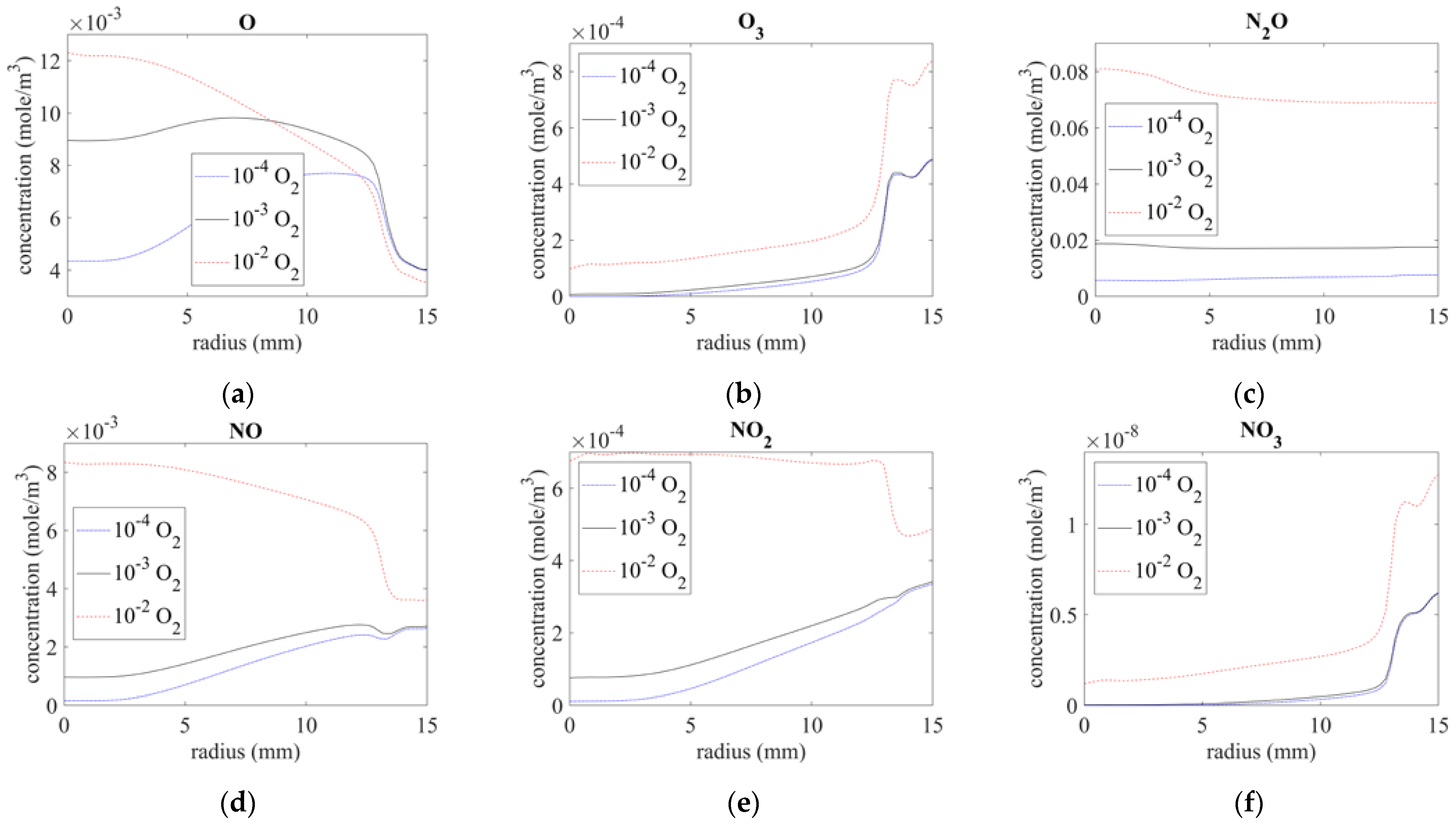
3.2.3. Distribution of O-Series and NOx Components (O, O3, N2O, NO, NO2, and NO3)
4. Discussion & Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Jaeyoung, P.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef] [Green Version]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Yáñez-Pacios, A.; Martín-Martínez, J. Comparative Adhesion, Ageing Resistance, and Surface Properties of Wood Plastic Composite Treated with Low Pressure Plasma and Atmospheric Pressure Plasma Jet. Polymers 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Du, D.; Song, A.; Zhang, F.; Huang, W. Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment. Materials 2020, 13, 4775. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-H.; Cheng, I.C.; Hsu, C.-C.; Chen, J.-Z. DC-pulse atmospheric-pressure plasma jet and dielectric barrier discharge surface treatments on fluorine-doped tin oxide for perovskite solar cell application. J. Phys. D Appl. Phys. 2018, 51, 025502. [Google Scholar] [CrossRef]
- Cheng, P.-Y.; Lu, N.-H.; Lu, Y.-S.; Chen, C.-H.; Lee, Y.-L.; Chen, J.-Z. Surface Modification of FeCoNiCr Medium-Entropy Alloy (MEA) Using Octadecyltrichlorosilane and Atmospheric-Pressure Plasma Jet. Polymers 2020, 12, 788. [Google Scholar] [CrossRef] [Green Version]
- Stryczewska, H.D.; Boiko, O. Applications of Plasma Produced with Electrical Discharges in Gases for Agriculture and Biomedicine. Appl. Sci. 2022, 12, 4405. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, Y.; Shi, M.; Liu, Y. Effect of an Atmospheric Pressure Plasma Jet on the Structure and Physicochemical Properties of Waxy and Normal Maize Starch. Polymers 2018, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Cordaro, L.; De Masi, G.; Fassina, A.; Gareri, C.; Pimazzoni, A.; Desideri, D.; Indolfi, C.; Martines, E. The Role of Thermal Effects in Plasma Medical Applications: Biological and Calorimetric Analysis. Appl. Sci. 2019, 9, 5560. [Google Scholar] [CrossRef] [Green Version]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Greb, A.; Niemi, K.; O’Connell, D.; Ennis, G.J.; Macgearailt, N.; Gans, T. Improved fluid simulations of radio-frequency plasmas using energy dependent ion mobilities. Phys. Plasmas 2013, 20, 053502. [Google Scholar] [CrossRef]
- Naidis, G.V.; Babaeva, N.Y. Modeling of streamer dynamics in helium plasma jets driven by modulated voltage pulses. Phys. Plasmas 2019, 26, 094501. [Google Scholar] [CrossRef]
- Tochikubo, F.; Shirai, N.; Uchida, S. Simulation of Atmospheric Pressure Direct Current Glow Discharge along a Miniature Helium Flow in Nitrogen. Appl. Phys. Express 2011, 4, 056001. [Google Scholar] [CrossRef]
- Logothetis, D.K.; Papadopoulos, P.K.; Svarnas, P.; Vafeas, P. Numerical simulation of the interaction between helium jet flow and an atmospheric-pressure “plasma jet”. Comput. Fluids 2016, 140, 11–18. [Google Scholar] [CrossRef]
- Passaras, D.N. Simulation of Atmospheric Pressure Plasma Jets with a Global Model. Master’s Thesis, National and Kapodistrian University of Athens, Zografou, Greece, 2016. [Google Scholar]
- Tsai, J.-H.; Hsu, C.-M.; Hsu, C.-C. Numerical Simulation of Downstream Kinetics of an Atmospheric Pressure Nitrogen Plasma Jet Using Laminar, Modified Laminar, and Turbulent Models. Plasma Chem. Plasma Process. 2013, 33, 1121–1135. [Google Scholar] [CrossRef]
- Lin, K.M.; Hu, M.H.; Hung, C.T.; Wu, J.S.; Hwang, F.N.; Chen, Y.S.; Cheng, G. A parallel hybrid numerical algorithm for simulating gas flow and gas discharge of an atmospheric-pressure plasma jet. Comput. Phys. Commun. 2012, 183, 2550–2560. [Google Scholar] [CrossRef]
- Kuzenov, V.V.; Ryzhkov, S.V.; Starostin, A.V. Pulsed Jets for Dense Plasma Generation in an External Magnetic Field. Russian Phys. J. 2020, 62, 2041–2045. [Google Scholar] [CrossRef]
- Kuzenov, V.V.; Ryzhkov, S.V. Evaluation of the possibility of ignition of a hydrogen-oxygen mixture by erosive flame of the impulse laser. Laser Phys. 2019, 29, 096001. [Google Scholar] [CrossRef]
- Asghar, A.H.; Galaly, A.R. The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics. Appl. Sci. 2021, 11, 6870. [Google Scholar] [CrossRef]
- Komin, O.V.; Pelipasov, O.V. Effect of O2 in plasma gas on parameters of nitrogen MIP-OES. J. Anal. Atomic Spectrom. 2022, 37, 1573–1577. [Google Scholar] [CrossRef]
- Krumpolec, R.; Jurmanová, J.; Zemánek, M.; Kelar, J.; Kováčik, D.; Černák, M. Selective Plasma Etching of Polymer-Metal Mesh Foil in Large-Area Hydrogen Atmospheric Pressure Plasma. Appl. Sci. 2020, 10, 7356. [Google Scholar] [CrossRef]
- Tochikubo, F.; Uchida, S.; Yasui, H.; Sato, K. Numerical Simulation of NO Oxidation in Dielectric Barrier Discharge with Microdischarge Formation. Jpn. J. Appl. Phys. 2009, 48, 076507. [Google Scholar] [CrossRef]
- Cheng, K.-W. Fluid Modeling of Parallel-Plate Nitrogen-based Dielectric Barrier Discharge Driven by a Realistic Distorted Sinusoidal AC Power Source. Master’s Thesis, National Chiao Tung University, Hsinchu, Taiwan, 2012. [Google Scholar]
- Sakiyama, Y.; Graves, D.B.; Chang, H.-W.; Shimizu, T.; Morfill, G.E. Plasma chemistry model of surface microdischarge in humid air and dynamics of reactive neutral species. J. Phys. D Appl. Phys. 2012, 45, 425201. [Google Scholar] [CrossRef]
- Nikiforov, A.Y.; Sarani, A.; Leys, C. The influence of water vapor content on electrical and spectral properties of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2011, 20, 015014. [Google Scholar] [CrossRef]
- Lin, K.-M. Development of Parallel Hybrid Simulation of Gas Discharge and Gas Flow and Its Application in the Modeling of Atmospheric-Pressure Helium Dielectric Barrier Discharge Jet Considering Impurities. Master’s Thesis, National Chiao Tung University, Hsinchu, Taiwan, 2012. [Google Scholar]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Chemical kinetics and reactive species in atmospheric pressure helium-oxygen plasmas with humid-air impurities. Plasma Sources Sci. Technol. 2013, 22, 015003. [Google Scholar] [CrossRef]
- Schröter, S.; Gibson, A.R.; Kushner, M.J.; Gans, T.; O’Connell, D. Numerical study of the influence of surface reaction probabilities on reactive species in an rf atmospheric pressure plasma containing humidity. Plasma Phys. Control. Fusion 2018, 60, 014035. [Google Scholar] [CrossRef]
- Itikawa, Y. Cross sections for electron collisions with nitrogen molecules. J. Phys. Chem. Ref. Data 2006, 35, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Tsai, I.H.; Hsu, C.C. Numerical Simulation of Downstream Kinetics of an Atmospheric-Pressure Nitrogen Plasma Jet. IEEE Trans. Plasma Sci. 2010, 38, 3387–3392. [Google Scholar] [CrossRef]
- Moravej, M.; Yang, X.; Barankin, M.; Penelon, J.; Babayan, S.E.; Hicks, R.F. Properties of an atmospheric pressure radio-frequency argon and nitrogen plasma. Plasma Sources Sci. Technol. 2006, 15, 204–210. [Google Scholar] [CrossRef]
- Capitelli, M.; Ferreira, C.M.; Gordiets, B.F.; Osipov, A.I. Plasma Kinetics in Atmospheric Gases; Springer: Berlin/Heidelberg, Germany, 2000; p. 300. [Google Scholar]
- Babayan, S.E.; Ding, G.; Nowling, G.R.; Yang, X.; Hicks, R.F. Characterization of the active species in the afterglow of a nitrogen and helium atmospheric-pressure plasma. Plasma Chem. Plasma Process. 2002, 22, 255–269. [Google Scholar] [CrossRef]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- Piper, L.G. State-to-state N2(A 3Σ+u) energy-pooling reactions. I. The formation of N2(C 3Πu) and the Herman infrared system. J. Chem. Phys. 1988, 88, 231–239. [Google Scholar] [CrossRef]
- Uhm, H.S.; Ki, S.H.; Baik, K.Y.; Choi, E.H. Influence of oxygen on generation of reactive chemicals from nitrogen plasma jet. Sci. Rep. 2018, 8, 9318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herron, J.T. Evaluated Chemical Kinetics Data for Reactions of N(2D), N(2P), and N2(A3Σu+) in the Gas Phase. J. Phys. Chem. Ref. Data 1999, 28, 1453–1483. [Google Scholar] [CrossRef]
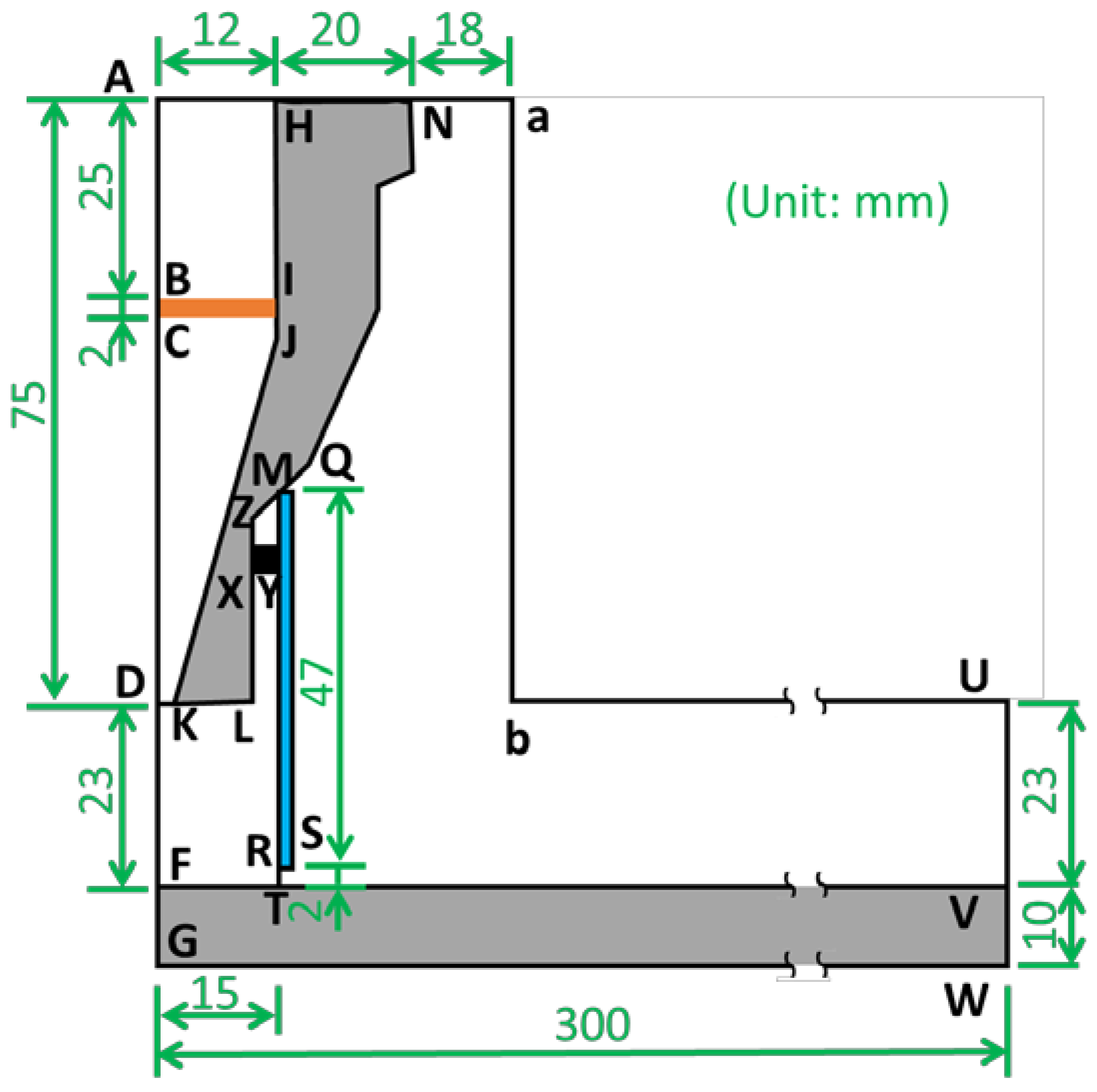

| Region | Material | Model/Equation (Parameters) |
|---|---|---|
| HKLN | Steel | Heat transfer equation () |
| FGWV | Steel | |
| MRSQ | Quartz (SiO2) | |
| BCJI | N2 with electron and heat source | Turbulence model (, , , , and ) Species transfer equation () Diluted species transfer equation () Heat transfer equation () |
| Others | N2 |
| Boundary | Turbulent Flow | Species Transfer Equation | Diluted Species Transfer Equation | Heat Transfer Equation |
|---|---|---|---|---|
| AF | Axis symmetry | Axis symmetry | ||
| FG | N/A | |||
| AH | Inflow (45 slm) | Inflow (wN2/wO2) | Concentration (ci = 0) | Inflow (T = 20 °C) |
| VUbaN | Outflow | Open boundary | Open boundary | Open boundary |
| GWV and HN | N/A | |||
| HIJKL and FTV and LXYRSQMN | Wall (No slip) | No flux | No flux | Heat flux continuity |
| MZXY | N/A | Thermal insulation | ||
| BI | N/A | Boundary heat source (1500 W) | ||
| k | Reaction | Reaction Rate * | Reference |
|---|---|---|---|
| k1 | e+N2→2N+e | [31,32] | |
| k2 | e+N2→N2(A)+e | [31,32] | |
| k3 | e+N2→N2(B)+e | [31,32] | |
| k4 | e+N2→N2(C)+e | [31,32] | |
| k5 | e+N2→N2(a)+e | [31,32] | |
| k6 | N2+N→N2(A)+N | [33] | |
| k7 | N+N+M→N2+M, M=N2, 100–600 K | [34] | |
| k8 | N+N+M→N2+M, M=N2, 600–6300 K | [34] | |
| k9 | N+N+M→N2+M, M=O2, NO, 290–400 K | [34] | |
| k10 | N+N+M→N2+M, M=O, N | [34] | |
| k11 | N2+M→N+N+M, M=O2, NO, N2 | [34] | |
| k12 | N2+M→N+N+M, M=O, N | [34] | |
| k13 | N2+N+N→N2(A)+N2 | [32] | |
| k14 | N2(A)+N2→N2+N2 | [32,33,35] | |
| k15 | N2(A)+N→N2+N | [32,33,35,36] | |
| k16 | N2+N+N→N2(B)+N2 | [35] | |
| k17 | N2(A)+N2(A)→N2(B)+N2 | [32,33] | |
| k18 | N2(B)→N2(A)+hv | [32,33,35,36] | |
| k19 | N2(B)+N2→N2(A)+N2 | [32,35,36] | |
| k20 | N2(A)+N2(A)→N2(C)+N2 | [32,33,35,37] | |
| k21 | N2(C)→N2(B)+hv | [32,33,35,36] | |
| k22 | N2(C)+N2→N2(a)+N2 | [36] | |
| k23 | N2(a)+N2→N2(B)+N2 | [32,36] | |
| k24 | N+O2→O+NO, 200 K < T < 300 K | [36] | |
| k25 | N+O2→O+NO, T > 300 K | [36] | |
| k26 | N+O+M→NO+M, M=N2, O2 | [34] | |
| k27 | N+O+M→NO+M, M=N, O, NO | [34] | |
| k28 | N+O→NO | [38] | |
| k29 | N+O3→NO+O2 | [34,36] | |
| k30 | N+NO→O+N2 | [32] | |
| k31 | N+NO2→2O+N2 | [32] | |
| k32 | N+NO2→N2+O2 | [32] | |
| k33 | N+NO2→2NO | [32] | |
| k34 | N+NO2→N2O+O | [32] | |
| k35 | N2+O2→O+N2O | [34] | |
| k36 | N2(A)+O→N2+O | [39] | |
| k37 | N2(A)+O2→N2+O+O | [32,36] | |
| k38 | N2(A)+O2→N2O+O | [32,36] | |
| k39 | N2(A)+NO→N2+NO | [32,36] | |
| k40 | N2(A)+N2O→N2+N+NO | [32,36] | |
| k41 | N2(B)+O2→N2+O+O | [32,36] | |
| k42 | N2(B)+NO→N2(A)+NO | [32,36] | |
| k43 | N2(C)+O2→N2+O+O | [36] | |
| k44 | N2(a)+O2→N2+O+O | [32,36] | |
| k45 | N2(a)+NO→N2+N+O | [32,36] | |
| k46 | O+O+M→O2+M, M=N2, 190–500 K | [34] | |
| k47 | O+O+M→O2+M, M=N2, 500–4000 K | [34] | |
| k48 | O+O+M→O2+M, M=O2, 290–4000 K | [34] | |
| k49 | O+O+M→O2+M, M=N | [34] | |
| k50 | O+O+M→O2+M, M=O | [34] | |
| k51 | O+O+M→O2+M, M=NO | [34] | |
| k52 | O+O2+N2→O3+N2 | [34] | |
| k53 | O+O2+O2→O3+O2 | [34] | |
| k54 | O+O2+N→O3+N | [34] | |
| k55 | O+O3→O2+O2 | [32,36] | |
| k56 | O+N2+M→N2O+M, M=any | [34] | |
| k57 | O+NO→NO2+hv | [32,34] | |
| k58 | O+NO+M→NO2+M, M=N2 | [34] | |
| k59 | O+NO+M→NO2+M, M=O2,NO | [34] | |
| k60 | O+NO2→NO+O2 | [32,34] | |
| k61 | O+NO3→O2+NO2 | [34,36] | |
| k62 | O+N2O→NO+NO | [32,34] | |
| k63 | O2+O2→O+O3 | [34] | |
| k64 | O2+NO→O+NO2 | [34] | |
| k65 | O2+NO2→NO+O3 | [34] | |
| k66 | O2+NO3→NO2+O3 | [34] | |
| k67 | O2+M→O+O+M, M=N2, N, NO | [34] | |
| k68 | O2+M→O+O+M, M=O2 | [34] | |
| k69 | O2+M→O+O+M, M=O | [34] | |
| k70 | O3+NO→O2+NO2 | [32,36] | |
| k71 | O3+NO2→O2+NO3 | [34,36] | |
| k72 | O3+M→O2+O+M, M=N2 | [34] | |
| k73 | O3+M→O2+O+M, M=O2 | [34] | |
| k74 | O3+M→O2+O+M, M=O, N | [34] | |
| k75 | NO+NO→O+NO2 | [34] | |
| k76 | NO+NO3→NO2+NO2 | [34,36] | |
| k77 | NO2+NO2→2NO+O2 | [32,34] | |
| k78 | NO2+NO3→NO+NO2+O2 | [34,36] | |
| k79 | NO2+M→NO+O+M, M=N2 | [34] | |
| k80 | NO2+M→NO+O+M, M=O2 | [34] | |
| k81 | NO2+M→NO+O+M, M=NO | [34] | |
| k82 | NO2+M→NO+O+M, M=NO2 | [34] | |
| k83 | NO3+NO3→O2+NO2+NO2 | [36] | |
| k84 | NO3+M→NO2+O+M, M=N2, O2, NO | [34] | |
| k85 | NO3+M→NO2+O+M, M=N, O | [34] | |
| k86 | NO3+M→NO+O2+M, M=N2, O2, NO | [34] | |
| k87 | NO3+M→NO+O2+M, M=N, O | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, J.-S.; Chen, J.-Z. Influence of Oxygen Impurity on Nitrogen Atmospheric-Pressure Plasma Jet. Appl. Sci. 2023, 13, 4199. https://doi.org/10.3390/app13074199
Tsai J-S, Chen J-Z. Influence of Oxygen Impurity on Nitrogen Atmospheric-Pressure Plasma Jet. Applied Sciences. 2023; 13(7):4199. https://doi.org/10.3390/app13074199
Chicago/Turabian StyleTsai, Jia-Shiuan, and Jian-Zhang Chen. 2023. "Influence of Oxygen Impurity on Nitrogen Atmospheric-Pressure Plasma Jet" Applied Sciences 13, no. 7: 4199. https://doi.org/10.3390/app13074199
APA StyleTsai, J.-S., & Chen, J.-Z. (2023). Influence of Oxygen Impurity on Nitrogen Atmospheric-Pressure Plasma Jet. Applied Sciences, 13(7), 4199. https://doi.org/10.3390/app13074199







