Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water
Abstract
1. Introduction
2. Characteristics of Laccases
3. Occurrence and Function of Laccases
4. Biochemical Properties of Laccase
5. Applications of Laccases
6. Types and Mechanisms of Removing Xenobiotics from Water
6.1. Types of Laccase- Assisted Removing Xenobiotics from Water
6.1.1. Synthetic Dyes
6.1.2. PAHs (Polycyclic Aromatic Hydrocarbons)
6.1.3. Pharmaceutical
6.1.4. Plasticizers
6.2. Mechanism of Removing Xenobiotics from Water
6.3. Challenges of Enzymatic Bioremediation
7. Methods of Increasing the Productivity and Activity of Laccases
7.1. Improvement of Catalytic Activity of Laccases
7.2. Improvement of Laccase Productivity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, Y.; Ali, S.; Liu, S.; Zhou, J.; Tang, Y. Characterization of Plant Laccase Genes and Their Functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef]
- Asano, T.; Seto, Y.; Hashimoto, K.; Kurushima, H. Mini-Review an Insect-Specific System for Terrestrialization: Laccase-Mediated Cuticle Formation. Insect Biochem. Mol. Biol. 2019, 108, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect Cuticular Sclerotization: A Review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, X.; Korzhev, M.; Schröder, H.C.; Link, T.; Tahir, M.N.; Diehl-Seifert, B.; Müller, W.E.G. Potential Biological Role of Laccase from the Sponge Suberites Domuncula as an Antibacterial Defense Component. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 118–128. [Google Scholar] [CrossRef]
- Lisov, A.V.; Zavarzina, A.G.; Zavarzin, A.A.; Leontievsky, A.A. Laccases Produced by Lichens of the Order Peltigerales. FEMS Microbiol. Lett. 2007, 275, 46–52. [Google Scholar] [CrossRef]
- Jeon, J.R.; Chang, Y.S. Laccase-Mediated Oxidation of Small Organics: Bifunctional Roles for Versatile Applications. Trends Biotechnol. 2013, 31, 335–341. [Google Scholar] [CrossRef]
- Daâssi, D.; Prieto, A.; Zouari-Mechichi, H.; Martínez, M.J.; Nasri, M.; Mechichi, T. Degradation of Bisphenol A by Different Fungal Laccases and Identification of Its Degradation Products. Int. Biodeterior. Biodegrad. 2016, 110, 181–188. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic Organic Dyes as Contaminants of the Aquatic Environment and Their Implications for Ecosystems: A Review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of Azo Dyes in the Context of Environmental Processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive Review of Polycyclic Aromatic Hydrocarbons in Water Sources, Their Effects and Treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef] [PubMed]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments-Occurrence, Fate and Bioremediation Prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef]
- Krupinski, M.; Długoński, J. Biodegradation of nonylphenols by some microorganisms. Post. Mikrobiol. 2011, 50, 313–319. [Google Scholar]
- Wróblewska-Krepsztula, J.; Michalska-Pożogaa, I.; Szczypiński, M.; Szczypiński, M.M.; Rydzkowski, T. Biodegradacja—Atrakcyjna alternatywa dla obecnych technik utylizacji odpadów tworzyw polimerowych. Przetwórstwo Tworzyw 2017, 23, 579–584. [Google Scholar]
- Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Singh, A.; Yadav, V.K.; Yadav, K.K.; Lee, C.; et al. Bacterial Laccases as Biocatalysts for the Remediation of Environmental Toxic Pollutants: A Green and Eco-Friendly Approach—A Review. Water 2022, 14, 4068. [Google Scholar] [CrossRef]
- Siroosi, M.; Amoozegar, M.A.; Khajeh, K.; Dabirmanesh, B. Decolorization of Dyes by a Novel Sodium Azide-Resistant Spore Laccase from a Halotolerant Bacterium, Bacillus Safensis Sp. Strain S31. Water Sci. Technol. 2018, 77, 2867–2875. [Google Scholar] [CrossRef]
- Chandra, R.; Chowdhary, P. Properties of Bacterial Laccases and Their Application in Bioremediation of Industrial Wastes. Environ. Sci. Process. Impacts 2015, 17, 326–342. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase: A Multi-Purpose Biocatalyst at the Forefront of Biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467. [Google Scholar] [CrossRef]
- Valles, M.; Kamaruddin, A.F.; Wong, L.S.; Blanford, C.F. Inhibition in Multicopper Oxidases: A Critical Review. Catal. Sci. Technol. 2020, 10, 5386–5410. [Google Scholar] [CrossRef]
- Dey, B.; Dutta, T. Laccases: Thriving the Domain of Bio-Electrocatalysis. Bioelectrochemistry 2022, 146, 108144. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Legerská, B.; Chmelová, D.; Ondrejovič, M. Decolourization and Detoxification of Monoazo Dyes by Laccase from the White-Rot Fungus Trametes Versicolor. J. Biotechnol. 2018, 285, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzym. Res. 2011, 2011, 217861. [Google Scholar] [CrossRef]
- Olbrich, A.C.; Schild, J.N.; Urlacher, V.B. Correlation between the T1 Copper Reduction Potential and Catalytic Activity of a Small Laccase. J. Inorg. Biochem. 2019, 201, 110843. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawabata, T.; Yura, K.; Go, N. Novel Types of Two-Domain Multi-Copper Oxidases: Possible Missing Links in the Evolution. FEBS Lett. 2003, 553, 239–244. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Silva, C.; Cavaco-Paulo, A. Can Laccase-Assisted Processing Conditions Influence the Structure of the Reaction Products? Trends Biotechnol. 2019, 37, 683–686. [Google Scholar] [CrossRef]
- Mayolo-Deloisa, K.; González-González, M.; Rito-Palomares, M. Laccases in Food Industry: Bioprocessing, Potential Industrial and Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 8, 222. [Google Scholar] [CrossRef]
- Polaina, J.; MacCabe, A.P. Industrial Enzymes: Structure, Function and Applications; Springer: Dordrecht, The Netherlands, 2007; ISBN 9781402053771. [Google Scholar]
- Sheng, S.; Farinas, E.T. Laccase and Its Mutant Displayed on the Bacillus Subtilis Spore Coat for Oxidation of Phenolic Compounds in Organic Solvents. Catalysts 2021, 11, 606. [Google Scholar] [CrossRef]
- Jia, H.; Lee, F.S.; Farinas, E.T. Bacillus Subtilis Spore Display of Laccase for Evolution under Extreme Conditions of High Concentrations of Organic Solvent. ACS Comb. Sci. 2014, 16, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Niladevi, K.N.; Sukumaran, R.K.; Jacob, N.; Anisha, G.S.; Prema, P. Optimization of Laccase Production from a Novel Strain-Streptomyces Psammoticus Using Response Surface Methodology. Microbiol. Res. 2009, 164, 105–113. [Google Scholar] [CrossRef]
- Ali, N.S.; Huang, F.; Qin, W.; Yang, T.C. Identification and Characterization of a New Serratia Proteamaculans Strain That Naturally Produces Significant Amount of Extracellular Laccase. Front. Microbiol. 2022, 13, 878360. [Google Scholar] [CrossRef]
- Sharma, V.; Upadhyay, L.S.B.; Vasanth, D. Extracellular Thermostable Laccase-Like Enzymes from Bacillus Licheniformis Strains: Production, Purification and Characterization. Appl. Biochem. Microbiol. 2020, 56, 420–432. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase Engineering: From Rational Design to Directed Evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Bhalla, A.; Kaur, P.; Capalash, N.; Sharma, P. Laccase from Prokaryotes: A New Source for an Old Enzyme. Rev. Environ. Sci. Biotechnol. 2011, 10, 309–326. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C. Laccase-Catalyzed Formation of Cinnabarinic Acid Is Responsible for Antibacterial Activity of Pycnoporus Cinnabarinus. Microbiol. Res. 1997, 152, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Hölker, U.; Dohse, J.; Höfer, M. Extracellular laccases in ascomycetes Trichoderma atroviride and Trichoderma harzianum. Folia Microbiol. 2002, 47, 423–427. [Google Scholar] [CrossRef]
- Dwivedi, U.N.; Singh, P.; Pandey, V.P.; Kumar, A. Structure-Function Relationship among Bacterial, Fungal and Plant Laccases. J. Mol. Catal. B Enzym. 2011, 68, 117–128. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal laccases: Production, function, and applications in food processing. Enzym. Res. 2010, 2010, 149748. [Google Scholar] [CrossRef]
- Berthet, S.; Thévenin, J.; Baratiny, D.; Demont-Caulet, N.; Debeaujon, I.; Bidzinski, P.; Leplé, J.-C.; Huis, R.; Hawkins, S.; Gomez, L.D. Role of Plant Laccases in Lignin Polymerization. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 61, pp. 145–172. [Google Scholar]
- Gavnholt, B.; Larsen, K. Molecular Biology of Plant Laccases in Relation to Lignin Formation. Physiol. Plant. 2002, 116, 273–280. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, G.; Singh, J.; Khan, A.; Fazio, G.; Saltzgiver, M.; Xia, R. Laccase Directed Lignification Is One of the Major Processes Associated With the Defense Response Against Pythium Ultimum Infection in Apple Roots. Front. Plant Sci. 2021, 12, 629776. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Jia, W.; Chang, S.; Li, S.; Li, Y. Lignin Engineering through Laccase Modification: A Promising Field for Energy Plant Improvement. Biotechnol. Biofuels 2015, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Elias-Neto, M.; Soares, M.P.M.; Simões, Z.L.P.; Hartfelder, K.; Bitondi, M.M.G. Developmental Characterization, Function and Regulation of a Laccase2 Encoding Gene in the Honey Bee, Apis Mellifera (Hymenoptera, Apinae). Insect Biochem. Mol. Biol. 2010, 40, 241–251. [Google Scholar] [CrossRef]
- Łukasiewicz, K.; Wegrzyn, G. Changes Is Genes Coding for Laccases 1 and 2 May Contribute to Deformation and Reduction of Wings in Apollo Butterfly (Parnassius Apollo, Lepidoptera: Papilionidae) from the Isolated Population in Pieniny National Park (Poland). Acta Biochim. Pol. 2016, 63, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Du, M.H.; Yan, Z.W.; Hao, Y.J.; Yan, Z.T.; Si, F.L.; Chen, B.; Qiao, L. Suppression of Laccase 2 Severely Impairs Cuticle Tanning and Pathogen Resistance during the Pupal Metamorphosis of Anopheles Sinensis (Diptera: Culicidae). Parasites Vectors 2017, 10, 171. [Google Scholar] [CrossRef]
- Ni, J.; Tokuda, G. Lignocellulose-Degrading Enzymes from Termites and Their Symbiotic Microbiota. Biotechnol. Adv. 2013, 31, 838–850. [Google Scholar] [CrossRef]
- Jeon, J.R.; Baldrian, P.; Murugesan, K.; Chang, Y.S. Laccase-Catalysed Oxidations of Naturally Occurring Phenols: From in Vivo Biosynthetic Pathways to Green Synthetic Applications. Microb. Biotechnol. 2012, 5, 318–332. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Gorman, M.J.; Kanost, M.R. Characterization of Endogenous and Recombinant Forms of Laccase-2, a Multicopper Oxidase from the Tobacco Hornworm, Manduca Sexta. Insect Biochem. Mol. Biol. 2009, 39, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Go, N. Function and Molecular Evolution of Multicopper Blue Proteins. Cell Mol. Life Sci. 2005, 62, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Diamantidis, G.; Effosse, A.; Potier, P.; Bally, R.J. Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol. Biochem. 2000, 32, 919–927. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Goradia, B.; Saxena, A. Bacterial Laccase: Recent Update on Production, Properties and Industrial Applications. 3 Biotech 2017, 7, 323. [Google Scholar] [CrossRef]
- Muthukumarasamy, N.P.; Jackson, B.; Joseph Raj, A.; Sevanan, M. Production of Extracellular Laccase from Bacillus Subtilis MTCC 2414 Using Agroresidues as a Potential Substrate. Biochem. Res. Int. 2015, 2015, 765190. [Google Scholar] [CrossRef]
- Cao, L.; Lin, L.; Sui, H.; Wang, H.; Zhang, Z.; Jiao, N.; Zhou, J. Efficient Extracellular Laccase Secretionviabio-Designed Secretory Apparatuses to Enhance Bacterial Utilization of Recalcitrant Lignin. Green Chem. 2021, 23, 2079–2094. [Google Scholar] [CrossRef]
- Martins, L.O.; Soares, C.M.; Pereira, M.M.; Teixeira, M.; Costa, T.; Jones, G.H.; Henriques, A.O. Molecular and Biochemical Characterization of a Highly Stable Bacterial Laccase That Occurs as a Structural Component of the Bacillus Subtilis Endospore Coat. J. Biol. Chem. 2002, 277, 18849–18859. [Google Scholar] [CrossRef]
- Margot, J.; Bennati-Granier, C.; Maillard, J.; Blánquez, P.; Barry, D.A.; Holliger, C. Bacterial versus Fungal Laccase: Potential for Micropollutant Degradation. AMB Express 2013, 3, 63. [Google Scholar] [CrossRef]
- Galai, S.; Limam, F.; Marzouki, M.N. A New Stenotrophomonas Maltophilia Strain Producing Laccase. Use in Decolorization of Synthetics Dyes. Appl. Biochem. Biotechnol. 2009, 158, 416–431. [Google Scholar] [CrossRef]
- Hildén, K.; Hakala, T.K.; Lundell, T. Thermotolerant and Thermostable Laccases. Biotechnol. Lett. 2009, 31, 1117–1128. [Google Scholar] [CrossRef]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal Laccases-Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Aishy, A.; Samra, Z.Q.; Gull, I.; Athar, M.A. Identification, Purification and Characterization of a Novel Extracellular Laccase from Cladosporium Cladosporioides. Biotechnol. Biotechnol. Equip. 2012, 26, 3345–3350. [Google Scholar] [CrossRef]
- Yang, C.H.; Guo, J.Y.; Chu, D.; DIng, T.B.; Wei, K.K.; Cheng, D.F.; Wan, F.H. Secretory Laccase 1 in Bemisia Tabaci MED Is Involved in Whitefly-Plant Interaction. Sci. Rep. 2017, 7, 3623. [Google Scholar] [CrossRef]
- Omura, T. Studies on laccases of lacquer trees. I. Comparison of laccases obtained from Rhus vernicifera and Rhus succedanea. J. Biochem. 1961, 50, 264–272. [Google Scholar] [CrossRef]
- Sugumaran, M.; Giglio, L.; Kundzicz, H.; Saul, S.; Semensi, V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 1992, 19, 271–283. [Google Scholar] [CrossRef]
- Gorman, M.J.; Sullivan, L.I.; Nguyen, T.D.T.; Dai, H.; Arakane, Y.; Dittmer, N.T.; Syed, L.U.; Li, J.; Hua, D.H.; Kanost, M.R. Kinetic Properties of Alternatively Spliced Isoforms of Laccase-2 from Tribolium Castaneum and Anopheles Gambiae. Insect Biochem. Mol. Biol. 2012, 42, 193–202. [Google Scholar] [CrossRef]
- Otto, B.; Schlosser, D. First Laccase in Green Algae: Purification and Characterization of an Extracellular Phenol Oxidase from Tetracystis Aeria. Planta 2014, 240, 1225–1236. [Google Scholar] [CrossRef]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Purification of a Thermostable Laccase from Leucaena Leucocephala Using a Copper Alginate Entrapment Approach and the Application of the Laccase in Dye Decolorization. Process Biochem. 2014, 49, 1196–1204. [Google Scholar] [CrossRef]
- ElyasiGhahfarokhi, A.; Hashemi, S.; Saeedi, M.; Khanavi, M.; Faramarzi, M.A. Phytocatalytic and Cytotoxic Activity of the Purified Laccase from Bled Resin of Pistacia Atlantica Desf. Int. J. Biol. Macromol. 2021, 176, 394–403. [Google Scholar] [CrossRef]
- Xiong, D.; Wen, J.; Lu, G.; Li, T.; Long, M. Isolation, Purification, and Characterization of a Laccase-Degrading Aflatoxin B1 from Bacillus Amyloliquefaciens B10. Toxins 2022, 14, 250. [Google Scholar] [CrossRef]
- Birge, A.; Alcicek, E.A.; Baltaci, M.O.; Sisecioglu, M.; Adiguzel, A. Purification and Biochemical Characterization of a New Thermostable Laccase from Enterococcus Faecium A2 by a Three-Phase Partitioning Method and Investigation of Its Decolorization Potential. Arch. Microbiol. 2022, 204, 533. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Selvankumar, T.; Kamala-Kannan, S.; Mythili, R.; Sengottaiyan, A.; Govarthanan, M.; Senthilkumar, B.; Selvam, K. Production and Purification of Laccase by Bacillus Sp. Using Millet Husks and Its Pesticide Degradation Application. 3 Biotech 2019, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Mahmoud, M.; Abdel Karim, G.S.A.; Abdelraof, M.; Othman, A.M. Purification and Biochemical Characterization of Two Laccase Isoenzymes Isolated from Trichoderma Harzianum S7113 and Its Application for Bisphenol A Degradation. Microb. Cell Factories 2023, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Ahmed, S. Optimization, Purification and Characterization of Laccase from Ganoderma Leucocontextum along with Its Phylogenetic Relationship. Sci. Rep. 2022, 12, 2416. [Google Scholar] [CrossRef] [PubMed]
- González-González, P.; Gómez-Manzo, S.; Tomasini, A.; Martínez y Pérez, J.L.; García Nieto, E.; Anaya-Hernández, A.; Ortiz Ortiz, E.; Castillo Rodríguez, R.A.; Marcial-Quino, J.; Montiel-González, A.M. Laccase Production from Agrocybe Pediades: Purification and Functional Characterization of a Consistent Laccase Isoenzyme in Liquid Culture. Microorganisms 2023, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Wang, H.-H.; Xue, C.-B. Molecular Identification and Enzymatic Properties of Laccase2 from the Diamondback Moth Plutella Xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 2018, 17, 2310–2319. [Google Scholar] [CrossRef]
- Miyake, K.; Baba, Y. De Novo Transcriptome Assembly of the Midgut Glands of Herbivorous Land Crabs, Chiromantes Haematocheir, and Identification of Laccase Genes Involved in Lignin Degradation. J. Comp. Physiol. B 2022, 192, 247–261. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Velasco-Ayala, C.; Leal-Isla Flores, P.; de Jesús Rostro-Alanis, M.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Biotransformation of Lignocellulosic Biomass into Industrially Relevant Products with the Aid of Fungi-Derived Lignocellulolytic Enzymes. Int. J. Biol. Macromol. 2020, 161, 1099–1116. [Google Scholar] [CrossRef]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Cost Analysis in Laccase Production. J. Environ. Manag. 2011, 92, 2907–2912. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Nong, Y.; Wang, P.; Yu, Y.; Deng, C.; Yuan, J.; Wang, Q. Laccase-Mediated Construction of Flexible Double-Network Hydrogels Based on Silk Fibroin and Tyramine-Modified Hyaluronic Acid. Int. J. Biol. Macromol. 2020, 160, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The Production of Laccases by White-Rot Fungi under Solid-State Fermentation Conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.S.; Gill, P.K. Laccase Production by Some White Rot Fungi under Different Nutritional Conditions. Bioresour. Technol. 2000, 73, 283–285. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Biotransformation of Lignocellulosic Materials into Value-Added Products—A Review. Int. J. Biol. Macromol. 2017, 98, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Biko, O.D.V.; Viljoen-Bloom, M.; van Zyl, W.H. Microbial Lignin Peroxidases: Applications, Production Challenges and Future Perspectives. Enzym. Microb. Technol. 2020, 141, 109669. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Naturally-Derived Biopolymers: Potential Platforms for Enzyme Immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Singh, A.K.; Bilal, M.; Iqbal, H.M.N.; Meyer, A.S.; Raj, A. Bioremediation of Lignin Derivatives and Phenolics in Wastewater with Lignin Modifying Enzymes: Status, Opportunities and Challenges. Sci. Total Environ. 2021, 777, 145988. [Google Scholar] [CrossRef]
- Jafari, N.; Rezaei, S.; Rezaie, R.; Dilmaghani, H.; Khoshayand, M.R.; Faramarzi, M.A. Improved Production and Characterization of a Highly Stable Laccase from the Halophilic Bacterium Chromohalobacter Salexigens for the Efficient Delignification of Almond Shell Bio-Waste. Int. J. Biol. Macromol. 2017, 105, 489–498. [Google Scholar] [CrossRef]
- Rezaei, S.; Shahverdi, A.R.; Faramarzi, M.A. Isolation, One-Step Affinity Purification, and Characterization of a Polyextremotolerant Laccase from the Halophilic Bacterium Aquisalibacillus Elongatus and Its Application in the Delignification of Sugar Beet Pulp. Bioresour. Technol. 2017, 230, 67–75. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Larsson, S.; Cassland, P.; Jönsson, L.J. Development of a Saccharomyces Cerevisiae Strain with Enhanced Resistance to Phenolic Fermentation Inhibitors in Lignocellulose Hydrolysates by Heterologous Expression of Laccase. Appl. Environ. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Graz, M.; Wlizło, K.; Szałapata, K.; Kapral-Piotrowska, J.; Paduch, R.; Jarosz-Wilkołazka, A. Bioactive Properties of a Novel Antibacterial Dye Obtained from Laccase-Mediated Oxidation of 8-Anilino-1-Naphthalenesulfonic Acid. Molecules 2022, 27, 487. [Google Scholar] [CrossRef] [PubMed]
- Debnath, R.; Saha, T. An Insight into the Production Strategies and Applications of the Ligninolytic Enzyme Laccase from Bacteria and Fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent Advances on the Removal of Dyes from Wastewater Using Various Adsorbents: A Critical Review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Singh, J.; Saharan, V.; Kumar, S.; Gulati, P.; Kapoor, R.K. Laccase Grafted Membranes for Advanced Water Filtration Systems: A Green Approach to Water Purification Technology. Crit. Rev. Biotechnol. 2018, 38, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Kumar, V.; Shah, M. Bioremediation for Environmental Sustainability: Toxicity, Mechanisms of Contaminants Degradation, Detoxification and Challenges, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128205259. [Google Scholar]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and Fate of Emerging Contaminants in Water Environment: A Review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Couto, S.R.; Luis Toca-Herrera, J. Lacasses in the Textile Industry. Biotechnol. Mol. Biol. Rev. 2006, 1, 115–120. [Google Scholar]
- Kumar, D.; Bhardwaj, R.; Jassal, S.; Goyal, T.; Khullar, A.; Gupta, N. Application of Enzymes for an Eco-Friendly Approach to Textile Processing. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Pereira, J.C.V.; Serbent, M.P.; Skoronski, E. Application of Immobilized Mycelium-Based Pellets for the Removal of Organochlorine Compounds: A Review. Water Sci. Technol. 2021, 83, 1781–1796. [Google Scholar] [CrossRef]
- Narnoliya, L.K.; Agarwal, N.; Patel, S.N.; Singh, S.P. Kinetic Characterization of Laccase from Bacillus Atrophaeus, and Its Potential in Juice Clarification in Free and Immobilized Forms. J. Microbiol. 2019, 57, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Patel, V.; Patel, R.; Trivedi, U.; Patel, K. Fungal Laccases: Versatile Green Catalyst for Bioremediation of Organopollutants. In Emerging Technologies in Environmental Bioremediation, 1st ed.; Shah, M.P., Rodriguez-Couto, S., Sevinç Şengör, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 85–129. [Google Scholar]
- Berger, R.G.; Ersoy, F. Improved Foods Using Enzymes from Basidiomycetes. Processes 2022, 10, 726. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Kwiatos, N.; Szczęsna-Antczak, M.; Bielecki, S. Laccases—Enzymes with an unlimited potential. Biotechnol. Food Sci. 2017, 81, 41–70. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Held, C.; Kandelbauer, A.; Schroeder, M.; Cavaco-Paulo, A.; Guebitz, G.M. Biotransformation of Phenolics with Laccase Containing Bacterial Spores. Environ. Chem. Lett. 2005, 3, 74–77. [Google Scholar] [CrossRef]
- Amin, R.; Khorshidi, A.; Bensch, W.; Senkale, S.; Faramarzi, M.A. Degradation of Sesame Oil Phenolics Using Magnetic Immobilized Laccase. Catal. Lett. 2020, 150, 3086–3095. [Google Scholar] [CrossRef]
- Catherine, H.; Penninckx, M.; Frédéric, D. Product Formation from Phenolic Compounds Removal by Laccases: A Review. Environ. Technol. Innov. 2016, 5, 250–266. [Google Scholar] [CrossRef]
- Kilara, A.; Shahani, K.M.; Shukla, T.P. The Use of Immobilized Enzymes in the Food Industry: A Review. C R C Crit. Rev. Food Sci. Nutr. 1979, 12, 161–198. [Google Scholar] [CrossRef]
- de Andrade, B.C.; Gennari, A.; Renard, G.; Benvenutti, E.V.; Chies, J.M.; Volpato, G.; Volken de Souza, C.F. Nickel-Functionalized Chitosan for the Oriented Immobilization of Histidine-Tagged Enzymes: A Promising Support for Food Bioprocess Applications. Catal. Lett. 2022, 152, 2956–2970. [Google Scholar] [CrossRef]
- Wlizło, K.; Polak, J.; Jarosz-Wilkołazka, A.; Pogni, R.; Petricci, E. Novel Textile Dye Obtained through Transformation of 2-Amino-3-Methoxybenzoic Acid by Free and Immobilised Laccase from a Myceliophthora Thermophila Strain. Enzym. Microb. Technol. 2020, 132, 109398. [Google Scholar] [CrossRef]
- Polak, J.; Wlizło, K.; Pogni, R.; Petricci, E.; Grąz, M.; Szałapata, K.; Osińska-jaroszuk, M.; Kapral-piotrowska, J.; Pawlikowska-pawlęga, B.; Jarosz-wilkołazka, A. Structure and Bioactive Properties of Novel Textile Dyes Synthesised by Fungal Laccase. Int. J. Mol. Sci. 2020, 21, 2052. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Tringali, C. Laccase-Mediated Synthesis of Bioactive Natural Products and Their Analogues. RSC Chem. Biol. 2022, 3, 617–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, A.; Sondhi, S.; Sharma, P.; Gupta, N. An Alkaline Bacterial Laccase for Polymerization of Natural Precursors for Hair Dye Synthesis. 3 Biotech 2018, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue Enzymes for Green Chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Yassein, M.A.; Aboshanab, K.M. Proteome Analysis and In Vitro Antiviral, Anticancer and Antioxidant Capacities of the Aqueous Extracts of Lentinulaedodes and Pleurotus Ostreatus Edible Mushrooms. Molecules 2021, 26, 4623. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B. Purification of a Laccase from Fruiting Bodies of the Mushroom Pleurotus Eryngii. Appl. Microbiol. Biotechnol. 2006, 69, 521–525. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Q.J.; Cao, Q.Q.; Wu, Y.Y.; Xu, L.J.; Zhu, M.J.; Ng, T.B.; Wang, H.X.; Zhang, G.Q. A Laccase with Antiproliferative and HIV-I Reverse Transcriptase Inhibitory Activities from the Mycorrhizal Fungus Agaricus Placomyces. J. Biomed. Biotechnol. 2012, 2012, 736472. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kumarasamy, M.; Sosnik, A.; Danino, D. Enhanced Thermostability and Anticancer Activity in Breast Cancer Cells of Laccase Immobilized on Pluronic-Stabilized Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39436–39448. [Google Scholar] [CrossRef]
- Shnyreva, A.V.; Shnyreva, A.A.; Espinoza, C.; Padrón, J.M.; Trigos, Á. Antiproliferative Activity and Cytotoxicity of Some Medicinal Wood-Destroying Mushrooms from Russia. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, R.Y.; Zhang, G.Q.; Wang, H.X.; Ng, T.B. A Laccase with Antiproliferative Activity against Tumor Cells from an Edible Mushroom, White Common Agrocybe Cylindracea. Phytomedicine 2011, 18, 374–379. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Tian, T.; Liu, Y.P.; Wang, H.X.; Chen, Q.J. A Laccase with Anti-Proliferative Activity against Tumor Cells from a White Root Fungus Abortiporus Biennis. Process Biochem. 2011, 46, 2336–2340. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Wang, H.; Ng, T. Purification and Characterization of a Laccase from the Edible Wild Mushroom Tricholoma Mongolicum. J. Microbiol. Biotechnol. 2010, 20, 1069–1076. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Bae, J.H.; Sohn, J.H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Laccase and Tyrosinase Biosensors Used in the Determination of Hydroxycinnamic Acids. Int. J. Mol. Sci. 2021, 22, 4811. [Google Scholar] [CrossRef]
- Sezgintrk, M.K.; Odaci, D.; Pazarliolu, N.; Pilloton, R.; Dinçkaya, E.; Telefoncu, A.; Timur, S. Construction and Comparison of Trametes Versicolor Laccase Biosensors Capable of Detecting Xenobiotics. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 192–199. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Calandra, P.; Antonacci, A.; Scognamiglio, V. The Convergence of Forefront Technologies in the Design of Laccase-Based Biosensors—An Update. TrAC—Trends Anal. Chem. 2019, 119, 115615. [Google Scholar] [CrossRef]
- Zeng, Z.; Tian, L.; Li, Z.; Jia, L.; Zhang, X.; Xia, M.; Hu, Y. Whole-Cell Method for Phenol Detection Based on the Color Reaction of Phenol with 4-Aminoantipyrine Catalyzed by CotA Laccase on Endospore Surfaces. Biosens. Bioelectron. 2015, 69, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Yu, C.H.; Chang, B.V. Biodegradation of Nonylphenol in River Sediment. Environ. Pollut. 2004, 127, 425–430. [Google Scholar] [CrossRef]
- Mao, Z.; Zheng, X.F.; Zhang, Y.Q.; Tao, X.X.; Li, Y.; Wang, W. Occurrence and Biodegradation of Nonylphenol in the Environment. Int. J. Mol. Sci. 2012, 13, 491–505. [Google Scholar] [CrossRef]
- Garcia-Morales, R.; Rodríguez-Delgado, M.; Gomez-Mariscal, K.; Orona-Navar, C.; Hernandez-Luna, C.; Torres, E.; Parra, R.; Cárdenas-Chávez, D.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation of Endocrine-Disrupting Compounds in Groundwater: Bisphenol A, Nonylphenol, Ethynylestradiol and Triclosan by a Laccase Cocktail from Pycnoporus Sanguineus CS43. Water Air Soil Pollut. 2015, 226, 251. [Google Scholar] [CrossRef]
- Junghanns, C.; Moeder, M.; Krauss, G.; Martin, C.; Schlosser, D. Degradation of the Xenoestrogen Nonylphenol by Aquatic Fungi and Their Laccases. Microbiology 2005, 151, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, S.; Yu, J.; Gong, R.; Si, Y.; Liu, X.; Chu, G. Cu2+-Assisted Laccase from Trametes Versicolor Enhanced Self-Polyreaction of Triclosan. Chemosphere 2019, 225, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Si, Y.; Wu, X.; Li, F.; Zhang, B. Triclosan Removal by Laccase Immobilized on Mesoporous Nanofibers: Strong Adsorption and Efficient Degradation. Chem. Eng. J. 2014, 255, 63–70. [Google Scholar] [CrossRef]
- Asadgol, Z.; Forootanfar, H.; Rezaei, S.; Mahvi, A.H.; Faramarzi, M.A. Removal of Phenol and Bisphenol-a Catalyzed by Laccase in Aqueous Solution. J. Environ. Health Sci. Eng. 2014, 12, 93. [Google Scholar] [CrossRef]
- Nicolucci, C.; Rossi, S.; Menale, C.; Godjevargova, T.; Ivanov, Y.; Bianco, M.; Mita, L.; Bencivenga, U.; Mita, D.G.; Diano, N. Biodegradation of Bisphenols with Immobilized Laccase or Tyrosinase on Polyacrylonitrile Beads. Biodegradation 2011, 22, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Grelska, A.; Noszczyńska, M. White Rot Fungi Can Be a Promising Tool for Removal of Bisphenol A, Bisphenol S, and Nonylphenol from Wastewater. Environ. Sci. Pollut. Res. Int. 2020, 27, 39958–39976. [Google Scholar] [CrossRef]
- Gassara, F.; Brar, S.K.; Verma, M.; Tyagi, R.D. Bisphenol A Degradation in Water by Ligninolytic Enzymes. Chemosphere 2013, 92, 1356–1360. [Google Scholar] [CrossRef]
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A Promising Laccase Immobilization Approach for Bisphenol A Removal from Aqueous Solutions. Bioresour. Technol. 2019, 271, 360–367. [Google Scholar] [CrossRef]
- Přenosilová, L.; Křesinová, Z.; Amemori, A.S.; Cajthaml, T.; Svobodová, K. Transcriptional Response of Lignin-Degrading Enzymes to 17α-Ethinyloestradiol in Two White Rots. Microb. Biotechnol. 2013, 6, 300–306. [Google Scholar] [CrossRef]
- Rózalska, S.; Bernat, P.; Michnicki, P.; Długoński, J. Fungal Transformation of 17α-Ethinylestradiol in the Presence Ofvarious Concentrations of Sodium Chloride. Int. Biodeterior. Biodegrad. 2015, 103, 77–84. [Google Scholar] [CrossRef]
- Tüzün, S.C.; Karapınar, I. A Review on Diclofenac Degradation, Transformation Products and Their Fate in the Environment. Pamukkale Univ. J. Eng. Sci. 2022, 28, 937–952. [Google Scholar] [CrossRef]
- Lonappan, L.; Rouissi, T.; Laadila, M.A.; Brar, S.K.; Hernandez Galan, L.; Verma, M.; Surampalli, R.Y. Agro-Industrial-Produced Laccase for Degradation of Diclofenac and Identification of Transformation Products. ACS Sustain. Chem. Eng. 2017, 5, 5772–5781. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust Biodegradation of Naproxen and Diclofenac by Laccase Immobilized Using Electrospun Nanofibers with Enhanced Stability and Reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar] [CrossRef] [PubMed]
- Neelkant, K.S.; Shankar, K.; Jayalakshmi, S.K.; Sreeramulu, K. Purification, Biochemical Characterization, and Facile Immobilization of Laccase from Sphingobacterium Ksn-11 and Its Application in Transformation of Diclofenac. Appl. Biochem. Biotechnol. 2020, 192, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Hahn, V.; Meister, M.; Hussy, S.; Cordes, A.; Enderle, G.; Saningong, A.; Schauer, F. Enhanced Laccase-Mediated Transformation of Diclofenac and Flufenamic Acid in the Presence of Bisphenol A and Testing of an Enzymatic Membrane Reactor. AMB Express 2018, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Limon, A.; García Suárez, P.C.; Arellano-García, E.; Contreras, O.E.; Aguila, S.A. Enhanced Degradation of Pesticide Dichlorophen by Laccase Immobilized on Nanoporous Materials: A Cytotoxic and Molecular Simulation Investigation. Bioconjugate Chem. 2018, 29, 1073–1080. [Google Scholar] [CrossRef]
- Mtibaà, R.; Ezzanad, A.; Aranda, E.; Pozo, C.; Ghariani, B.; Moraga, J.; Nasri, M.; Manuel Cantoral, J.; Garrido, C.; Mechichi, T. Biodegradation and Toxicity Reduction of Nonylphenol, 4-Tert-Octylphenol and 2,4-Dichlorophenol by the Ascomycetous Fungus Thielavia Sp HJ22: Identification of Fungal Metabolites and Proposal of a Putative Pathway. Sci. Total Environ. 2020, 708, 135129. [Google Scholar] [CrossRef]
- Bhandari, G.; Bagheri, A.R.; Bhatt, P.; Bilal, M. Occurrence, Potential Ecological Risks, and Degradation of Endocrine Disrupter, Nonylphenol, from the Aqueous Environment. Chemosphere 2021, 275, 130013. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous Contaminants in the Environment and Their Laccase-Assisted Degradation—A Review. J. Environ. Manag. 2019, 234, 253–264. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Bilal, M.; Parra-Saldivar, R.; Barceló, D. Greening the 21st Century Environmental Engineering—A Robust Platform to Mitigate Contaminants of Emerging Concern. Case Stud. Chem. Environ. Eng. 2022, 5, 100209. [Google Scholar] [CrossRef]
- Ahsan, Z.; Kalsoom, U.; Bhatti, H.N.; Aftab, K.; Khalid, N.; Bilal, M. Enzyme-Assisted Bioremediation Approach for Synthetic Dyes and Polycyclic Aromatic Hydrocarbons Degradation. J. Basic Microbiol. 2021, 61, 960–981. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rizwan, K.; Adeel, M.; Barceló, D.; Awad, Y.A.; Iqbal, H.M.N. Robust Strategies to Eliminate Endocrine Disruptive Estrogens in Water Resources. Environ. Pollut. 2022, 306, 119373. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqdi, K.A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S.S.; Shah, I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials 2021, 11, 3124. [Google Scholar] [CrossRef]
- Zdarta, J.; Jesionowski, T.; Pinelo, M.; Meyer, A.S.; Iqbal, H.M.N.; Bilal, M.; Nguyen, L.N.; Nghiem, L.D. Free and Immobilized Biocatalysts for Removing Micropollutants from Water and Wastewater: Recent Progress and Challenges. Bioresour. Technol. 2022, 344 Pt B, 126201. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, S.; Capalash, N.; Gupta, N.; Sharma, P. Production of Thermo-Alkali-Stable Laccase and Xylanase by Co-Culturing of Bacillus Sp. and B. Halodurans for Biobleaching of Kraft Pulp and Deinking of Waste Paper. Bioprocess Biosyst. Eng. 2015, 38, 947–956. [Google Scholar] [CrossRef]
- Nathan, V.K.; Kanthimathinathan, S.R.; Rani, M.E.; Rathinasamy, G.; Kannan, N.D. Biobleaching of Waste Paper Using Lignolytic Enzyme from Fusarium Equiseti VKF2: A Mangrove Isolate. Cellulose 2018, 25, 4179–4192. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Rajarathinam, R.; Selvaraj, R.N.; Rajendren, V.B. A Novel Method for Improving Laccase Activity by Immobilization onto Copper Ferrite Nanoparticles for Lignin Degradation. Int. J. Biol. Macromol. 2020, 152, 1098–1107. [Google Scholar] [CrossRef]
- Li, Y.; Noro, J.; Martins, M.; Jing, S.; Silva, C.; Cavaco-Paulo, A. Changing the Shape of Wool Yarns via Laccase-Mediated Grafting of Tyrosine. J. Biotechnol. 2021, 339, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Sheikh, J.N.; Dutta, T. Sustainable Denim Bleaching by a Novel Thermostable Bacterial Laccase. Appl. Biochem. Biotechnol. 2020, 192, 1238–1254. [Google Scholar] [CrossRef]
- Unuofin, J.O. Treasure from Dross: Application of Agroindustrial Wastes-Derived Thermo-Halotolerant Laccases in the Simultaneous Bioscouring of Denim Fabric and Decolorization of Dye Bath Effluents. Ind. Crops Prod. 2020, 147, 112251. [Google Scholar] [CrossRef]
- Chen, H.; Ji, A.; Qiu, S.; Liu, Y.; Zhu, Q.; Yin, L. Covalent Conjugation of Bovine Serum Album and Sugar Beet Pectin through Maillard Reaction/Laccase Catalysis to Improve the Emulsifying Properties. Food Hydrocoll. 2018, 76, 173–183. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Guo, Y.; Qiao, Y.; Ma, Q.; Ji, C.; Zhao, L. Degradation of Zearalenone and Aflatoxin B1 by Lac2 from Pleurotus Pulmonarius in the Presence of Mediators. Toxicon 2021, 201, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, S.; Rostami, A.; Salimi, A.; Pourshiani, O. Graphene Oxide/CuFe2O4 Nanocomposite as a Novel Scaffold for the Immobilization of Laccase and Its Application as a Recyclable Nanobiocatalyst for the Green Synthesis of Arylsulfonyl Benzenediols. Biochem. Eng. J. 2018, 133, 1–11. [Google Scholar] [CrossRef]
- Gigli, V.; Piccinino, D.; Avitabile, D.; Antiochia, R.; Capecchi, E.; Saladino, R. Laccase Mediator Cocktail System as a Sustainable Skin Whitening Agent for Deep Eumelanin Decolorization. Int. J. Mol. Sci. 2022, 23, 6238. [Google Scholar] [CrossRef]
- Panwar, V.; Dey, B.; Sheikh, J.N.; Dutta, T. Thermostable Bacterial Laccase for Sustainable Dyeing Using Plant Phenols. RSC Adv. 2022, 12, 18168–18180. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena Unicolor as an Effective Source of New Antiviral, Immunomodulatory, and Anticancer Compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef]
- Sondhi, S.; Kaur, R.; Madan, J. Purification and Characterization of a Novel White Highly Thermo Stable Laccase from a Novel Bacillus Sp. MSK-01 Having Potential to Be Used as Anticancer Agent. Int. J. Biol. Macromol. 2021, 170, 232–238. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, G.; Guo, Y.; Ke, D.; Yin, J.; Wang, D.; Fan, X.; Liu, Z.; Ruan, L.; Hu, Y. Engineered Glyphosate Oxidase Coupled to Spore-Based Chemiluminescence System for Glyphosate Detection. Anal. Chim. Acta 2020, 1133, 39–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Fei, R.; Liu, X.; Zhou, Y.; Chen, J.; Chen, H.; Zhou, R.; Hu, Y. Sensitive Chemiluminescence Immunoassay for E. Coli O157:H7 Detection with Signal Dual-Amplification Using Glucose Oxidase and Laccase. Anal. Chem. 2014, 86, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, J.R.; Lin, S.B.; Chen, L.C.; Lou, S.N.; Chen, S.H.; Chen, H.H. Development of Immobilized Laccase-Based Time Temperature Indicator by Electrospinning Zein Fiber. Food Packag. Shelf Life 2020, 23, 100436. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Li, Y.; Ma, J.; Wang, S.; Zhang, Y.; Ge, X.; Wang, N.; Lu, F.; Liu, Y. Characterization and Application of a Novel Laccase Derived from Bacillus Amyloliquefaciens. Int. J. Biol. Macromol. 2020, 150, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, H.; Heidary Tabar, R.; Hamedi, J. Assessing the Biodegradation of Polycyclic Aromatic Hydrocarbons and Laccase Production by New Fungus Trematophoma Sp. UTMC 5003. World J. Microbiol. Biotechnol. 2017, 33, 136. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Godheja, J.; SK, S.; Siddiqui, S.A.; DR, M. Xenobiotic Compounds Present in Soil and Water: A Review on Remediation Strategies. J. Environ. Anal. Toxicol 2016, 6, 1000392. [Google Scholar] [CrossRef]
- Kong, L.; Kadokami, K.; Wang, S.; Duong, H.T.; Chau, H.T.C. Monitoring of 1300 Organic Micro-Pollutants in Surface Waters from Tianjin, North China. Chemosphere 2015, 122, 125–130. [Google Scholar] [CrossRef]
- Kupski, L.; Salcedo, G.M.; Caldas, S.S.; de Souza, T.D.; Furlong, E.B.; Primel, E.G. Optimization of a Laccase-Mediator System with Natural Redox-Mediating Compounds for Pesticide Removal. Environ. Sci. Pollut. Res. 2019, 26, 5131–5139. [Google Scholar] [CrossRef]
- Chiadò, A.; Bosco, F.; Bardelli, M.; Simonelli, L.; Pedotti, M.; Marmo, L.; Varani, L. Rational Engineering of the Lccβ T. Versicolor Laccase for the Mediator-Less Oxidation of Large Polycyclic Aromatic Hydrocarbons. Comput. Struct. Biotechnol. J. 2021, 19, 2213–2222. [Google Scholar] [CrossRef]
- Longe, L.F.; Couvreur, J.; Leriche Grandchamp, M.; Garnier, G.; Allais, F.; Saito, K. Importance of Mediators for Lignin Degradation by Fungal Laccase. ACS Sustain. Chem. Eng. 2018, 6, 10097–10107. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Paraschiv, G.; Ferdes, M.; Ionescu, M.; Moiceanu, G.; Zabava, B.S.; Dinca, M.N. Laccases—Versatile Enzymes Used to Reduce Environmental Pollution. Energies 2022, 15, 1835. [Google Scholar] [CrossRef]
- Itoh, T.; Takagi, Y. Laccase-Catalyzed Reactions in Ionic Liquids for Green Sustainable Chemistry. ACS Sustain. Chem. Eng. 2021, 9, 1443–1458. [Google Scholar] [CrossRef]
- Aleksejeva, O.; Mateljak, I.; Ludwig, R.; Alcalde, M.; Shleev, S. Electrochemistry of a High Redox Potential Laccase Obtained by Computer-Guided Mutagenesis Combined with Directed Evolution. Electrochem. Commun. 2019, 106, 106511. [Google Scholar] [CrossRef]
- Shleev, S.; Tkac, J.; Christenson, A.; Ruzgas, T.; Yaropolov, A.I.; Whittaker, J.W.; Gorton, L. Direct Electron Transfer between Copper-Containing Proteins and Electrodes. Biosens. Bioelectron. 2005, 20, 2517–2554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–29. [Google Scholar]
- Patel, N.; Shahane, S.; Shivam; Majumdar, R.; Mishra, U. Mode of Action, Properties, Production, and Application of Laccase: A Review. Recent Pat. Biotechnol. 2018, 13, 19–32. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Pinheiro, L.R.S.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A.V. Degradation of Azo Dyes: Bacterial Potential for Bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Purification and Biochemical Characterization of Two Isolated Laccase Isoforms from Agaricus Bisporus CU13 and Their Potency in Dye Decolorization. Int. J. Biol. Macromol. 2018, 113, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Jawale, J.P.; Nandre, V.S.; Latpate, R.V.; Kulkarni, M.V.; Doshi, P.J. Isolation, Characterization and Optimizations of Laccase Producer from Soil: A Comprehensive Study of Application of Statistical Approach to Enhance Laccase Productivity in Myrothecium Verrucaria NFCCI 4363. Bioresour. Technol. Rep. 2021, 15, 100751. [Google Scholar] [CrossRef]
- Iark, D.; Buzzo, A.J.D.R.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Peralta Muniz Moreira, R.d.F.; Bracht, A.; Peralta, R.M. Enzymatic Degradation and Detoxification of Azo Dye Congo Red by a New Laccase from Oudemansiella Canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.T. Polycyclic Aromatic Hydrocarbons. A Review. Cogent Environ. Sci. 2017, 3, 1339841. [Google Scholar] [CrossRef]
- Imam, A.; Kumar Suman, S.; Kanaujia, P.K.; Ray, A. Biological Machinery for Polycyclic Aromatic Hydrocarbons Degradation: A Review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y.; Ju, H. Polycyclic Aromatic Hydrocarbons (PAHs) in Water and Sediment from a River Basin: Sediment–Water Partitioning, Source Identification and Environmental Health Risk Assessment. Environ. Geochem. Health 2017, 39, 63–74. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Berrojalbiz, N.; Wollgast, J.; Dachs, J. Polycyclic Aromatic Hydrocarbons (PAHs) in the Mediterranean Sea: Atmospheric Occurrence, Deposition and Decoupling with Settling Fluxes in the Water Column. Environ. Pollut. 2012, 166, 40–47. [Google Scholar] [CrossRef]
- Waszak, I.; Jonko-Sobuś, K.; Ożarowska, A.; Zaniewicz, G. Estimation of Native and Alkylated Polycyclic Aromatic Hydrocarbons (PAHs) in Seabirds from the South Coast of the Baltic Sea. Environ. Sci. Pollut. Res. 2021, 28, 4366–4376. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Singh, G.; Arya, S.K. Utility of Laccase in Pulp and Paper Industry: A Progressive Step towards the Green Technology. Int. J. Biol. Macromol. 2019, 134, 1070–1084. [Google Scholar] [CrossRef]
- Singh, Y.; Saxena, M.K. Insights into the Recent Advances in Nano-Bioremediation of Pesticides from the Contaminated Soil. Front. Microbiol. 2022, 13, 982611. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Singh, G.; Shukla, R. Impact of Xenobiotics under a Changing Climate Scenario in Climate Change and Agricultural Ecosystems; Woodhead Publishing: Sawston, UK, 2019; pp. 133–151. [Google Scholar]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent Advanced Technologies for the Characterization of Xenobiotic-Degrading Microorganisms and Microbial Communities. Front. Bioeng. Biotechnol. 2021, 9, 632059. [Google Scholar] [CrossRef]
- Mishra, B.; Varjani, S.; Agrawal, D.C.; Mandal, S.K.; Ngo, H.H.; Taherzadeh, M.J.; Chang, J.S.; You, S.; Guo, W. Engineering Biocatalytic Material for the Remediation of Pollutants: A Comprehensive Review. Environ. Technol. Innov. 2020, 20, 101063. [Google Scholar] [CrossRef]
- Ike, P.T.L.; Birolli, W.G.; dos Santos, D.M.; Porto, A.L.M.; Souza, D.H.F. Biodegradation of Anthracene and Different PAHs by a Yellow Laccase from Leucoagaricus Gongylophorus. Environ. Sci. Pollut. Res. 2019, 26, 8675–8684. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; Amin, H.M.; Shebl, R.I. Modulation of Laccase Transcriptome during Biodegradation of Naphthalene by White Rot Fungus Pleurotus Ostreatus. Int. Microbiol. 2019, 22, 217–225. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, Fate and Risk Assessment of BPA and Its Substituents in Wastewater Treatment Plant: A Review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef] [PubMed]
- Torres-Farradá, G.; Manzano León, A.M.; Rineau, F.; Ledo Alonso, L.L.; Sánchez-López, M.I.; Thijs, S.; Colpaert, J.; Ramos-Leal, M.; Guerra, G.; Vangronsveld, J. Diversity of Ligninolytic Enzymes and Their Genes in Strains of the Genus Ganoderma: Applicable for Biodegradation of Xenobiotic Compounds? Front. Microbiol. 2017, 8, 898. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, C.; Liu, H.; Jia, W.; Sun, H. Enzyme Activities during Benzo[a]Pyrene Degradation by the Fungus Lasiodiplodia Theobromae Isolated from a Polluted Soil. Sci. Rep. 2020, 10, 865. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Schlosser, D.; Dubrovskaya, E.; Balandina, S.; Sigida, E.; Grinev, V.; Turkovskaya, O. The Degradative Activity and Adaptation Potential of the Litter-Decomposing Fungus Stropharia Rugosoannulata. World J. Microbiol. Biotechnol. 2018, 34, 133. [Google Scholar] [CrossRef]
- Alajärvi, L.; Timonen, J.; Lavikainen, P.; Martikainen, J. Attitudes and Considerations towards Pharmaceuticals-related Environmental Issues among Finnish Population. Sustainability 2021, 13, 12930. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lai, W.W.P.; Tung, H.H.; Lin, A.Y.C. Occurrence of Pharmaceuticals, Hormones, and Perfluorinated Compounds in Groundwater in Taiwan. Environ. Monit. Assess 2015, 187, 256. [Google Scholar] [CrossRef] [PubMed]
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Porretti, M.; Arrigo, F.; di Bella, G.; Faggio, C. Impact of Pharmaceutical Products on Zebrafish: An Effective Tool to Assess Aquatic Pollution. Comp. Biochem. Physiol. Part—C Toxicol. Pharmacol. 2022, 261, 109439. [Google Scholar] [CrossRef]
- Jijie, R.; Mihalache, G.; Balmus, I.M.; Strungaru, S.A.; Baltag, E.S.; Ciobica, A.; Nicoara, M.; Faggio, C. Review Zebrafish as a Screening Model to Study the Single and Joint Effects of Antibiotics. Pharmaceuticals 2021, 14, 578. [Google Scholar] [CrossRef]
- da Silva, M.C.G.; da Silva, J.F.; Santos, T.P.; da Silva, N.P.C.; dos Santos, A.R.; de Andrade, A.L.C.; da Silva Souza, E.H.L.; Cadena, M.R.S.; de Sá, F.B.; da Silva Junior, V.A.; et al. The Complexation of Steroid Hormones into Cyclodextrin Alters the Toxic Effects on the Biological Parameters of Zebrafish (Danio Rerio). Chemosphere 2019, 214, 330–340. [Google Scholar] [CrossRef] [PubMed]
- García-Cambero, J.P.; Corpa, C.; Lucena, M.A.; Méndez, P.; Sierra, P.; Galán-Madruga, D.; Aguayo, S. Presence of diclofenac, estradiol, and ethinylestradiol in Manzanares River (Spain) and their toxicity to zebrafish embryo development. Environ. Sci. Pollut. Res. Int. 2021, 28, 49921–49935. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Helmreich, B.; Škrbić, B.; Carballa, M.; Papa, M.; Pastore, C.; Emre, Z.; Oehmen, A.; Langenhoff, A.; Molinos, M.; et al. Status of Hormones and Painkillers in Wastewater Effluents across Several European States—Considerations for the EU Watch List Concerning Estradiols and Diclofenac. Environ. Sci. Pollut. Res. 2016, 23, 12835–12866. [Google Scholar] [CrossRef]
- Senthivelan, T.; Kanagaraj, J.; Panda, R.C.; Narayani, T. Screening and Production of a Potential Extracellular Fungal Laccase from Penicillium Chrysogenum: Media Optimization by Response Surface Methodology (RSM)and Central Composite Rotatable Design (CCRD). Biotechnol. Rep. 2019, 23, e00344. [Google Scholar] [CrossRef]
- Navada, K.K.; Kulal, A. Enhanced Production of Laccase from Gamma Irradiated Endophytic Fungus: A Study on Biotransformation Kinetics of Aniline Blue and Textile Effluent Decolourisation. J. Environ. Chem. Eng. 2020, 8, 103550. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Mekkawy, R.M.; Hassan, N.S. Detoxification and Bioremediation of Sulfa Drugs and Synthetic Dyes by Streptomyces Mutabilis A17 Laccase Produced in Solid State Fermentation. J. Pure Appl. Microbiol. 2019, 13, 85–96. [Google Scholar] [CrossRef]
- Herbert, J.; Beckett, A.H.; Robson, S.C. A Review of Cross-Disciplinary Approaches for the Identification of Novel Industrially Relevant Plastic-Degrading Enzymes. Sustainability 2022, 14, 15898. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Jankiewicz, U. Endocrine Disrupting Compounds (Nonylphenol and Bisphenol A)–Sources, Harmfulness and Laccase-Assisted Degradation in the Aquatic Environment. Microorganisms 2022, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, A.; Chen, B.; Wang, L. Adsorption of Phenol and Bisphenol A on River Sediments: Effects of Particle Size, Humic Acid, PH and Temperature. Ecotoxicol. Environ. Saf. 2020, 204, 111093. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, Toxicity and Ecological Risk of Bisphenol A Analogues in Aquatic Environment—A Review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef]
- Janda, R.; Ukić, Š.; Mikulec, N.; Vitaler, K. Bisphenol A-an Environmental and Human Threat. Agric. Conspec. Sci. 2021, 86, 295–304. [Google Scholar]
- Rocha, P.R.S.; Oliveira, V.D.; Vasques, C.I.; dos Reis, P.E.D.; Amato, A.A. Exposure to Endocrine Disruptors and Risk of Breast Cancer: A Systematic Review. Crit. Rev. Oncol./Hematol. 2021, 161, 103330. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, A.; Siedlewicz, G.; Szymczycha, B.; Dzierzbicka-Głowacka, L.; Pazdro, K. Legacy and Emerging Pollutants in the Gulf of Gdańsk (Southern Baltic Sea)—Loads and Distribution Revisited. Mar. Pollut. Bull. 2019, 139, 238–255. [Google Scholar] [CrossRef]
- Noszczyńska, M.; Piotrowska-Seget, Z. Bisphenols: Application, Occurrence, Safety, and Biodegradation Mediated by Bacterial Communities in Wastewater Treatment Plants and Rivers. Chemosphere 2018, 201, 214–223. [Google Scholar] [CrossRef]
- Das, R.; Li, G.; Mai, B.; An, T. Spore Cells from BPA Degrading Bacteria Bacillus Sp. GZB Displaying High Laccase Activity and Stability for BPA Degradation. Sci. Total Environ. 2018, 640–641, 798–806. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Ahmad, W.; Ahmad, A.; Vatin, N.I.; Mohamed, A.M.; Deifalla, A.F.; Mehmood, I. Plastic Waste Management Strategies and Their Environmental Aspects: A Scientometric Analysis and Comprehensive Review. Int. J. Environ. Res. Public Health 2022, 19, 4556. [Google Scholar] [CrossRef]
- Sowmya, H.V.; Ramalingappa; Krishnappa, M.; Thippeswamy, B. Degradation of Polyethylene by Trichoderma Harzianum—SEM, FTIR, and NMR Analyses. Environ. Monit. Assess 2014, 186, 6577–6586. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Gou, H.; Feng, X.; Zhang, X.; Yang, L. Screening of Polyethylene-Degrading Bacteria from Rhyzopertha Dominica and Evaluation of Its Key Enzymes Degrading Polyethylene. Polymers 2022, 14, 5127. [Google Scholar] [CrossRef]
- Santo, M.; Weitsman, R.; Sivan, A. The Role of the Copper-Binding Enzyme—Laccase—In the Biodegradation of Polyethylene by the Actinomycete Rhodococcus Ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Sharma, A.; Jain, K.K.; Jain, A.; Kidwai, M.; Kuhad, R.C. Bifunctional in Vivo Role of Laccase Exploited in Multiple Biotechnological Applications. Appl. Microbiol. Biotechnol. 2018, 102, 10327–10343. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, S.; Si, Y.; Huang, Q. Advances in Laccase-Triggered Anabolism for Biotechnology Applications. Crit. Rev. Biotechnol. 2021, 41, 969–993. [Google Scholar] [CrossRef] [PubMed]
- Bankole, P.O.; Adekunle, A.A.; Govindwar, S.P. Demethylation and Desulfonation of Textile Industry Dye, Thiazole Yellow G by Aspergillus Niger LAG. Biotechnol. Rep. 2019, 23, e00327. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhao, H.; Cao, H.; Huang, Q. Polymerization of Micropollutants in Natural Aquatic Environments: A Review. Sci. Total Environ. 2019, 693, 133751. [Google Scholar] [CrossRef]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive Insight into Triclosan—From Widespread Occurrence to Health Outcomes. Environ. Sci. Pollut. Res. 2023, 30, 25119–25140. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Catalano, A.; Mariconda, A.; Pellegrino, M.; Saturnino, C.; Longo, P.; Aquaro, S. Triclosan: A Small Molecule with Controversial Roles. Antibiotics 2022, 11, 735. [Google Scholar] [CrossRef]
- Wang, C.F.; Tian, Y. Reproductive Endocrine-Disrupting Effects of Triclosan: Population Exposure, Present Evidence and Potential Mechanisms. Environ. Pollut. 2015, 206, 195–201. [Google Scholar] [CrossRef]
- Wang, K.; Huang, K.; Jiang, G. Enhanced Removal of Aqueous Acetaminophen by a Laccase-Catalyzed Oxidative Coupling Reaction under a Dual-PH Optimization Strategy. Sci. Total Environ. 2018, 616–617, 1270–1278. [Google Scholar] [CrossRef]
- Fukuda, T.; Uchida, H.; Takashima, Y.; Uwajima, T.; Kawabata, T.; Suzuki, M. Degradation of Bisphenol A by Purified Laccase from Trametes Villosa. Biochem. Biophys. Res. Commun. 2001, 284, 704–706. [Google Scholar] [CrossRef]
- Galliker, P.; Hommes, G.; Schlosser, D.; Corvini, P.F.X.; Shahgaldian, P. Laccase-Modified Silica Nanoparticles Efficiently Catalyze the Transformation of Phenolic Compounds. J. Colloid Interface Sci. 2010, 349, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hongyan, L.; Zexiong, Z.; Shiwei, X.; He, X.; Yinian, Z.; Haiyun, L.; Zhongsheng, Y. Study on Transformation and Degradation of Bisphenol A by Trametes Versicolor Laccase and Simulation of Molecular Docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The Effect of Operational Parameters on the Biodegradation of Bisphenols by Trametes Versicolor Laccase Immobilized on Hippospongia Communis Spongin Scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Eltoukhy, A.; Jia, Y.; Nahurira, R.; Abo-Kadoum, M.A.; Khokhar, I.; Wang, J.; Yan, Y. Biodegradation of Endocrine Disruptor Bisphenol A by Pseudomonas Putida Strain YC-AE1 Isolated from Polluted Soil, Guangdong, China. BMC Microbiol. 2020, 20, 11. [Google Scholar] [CrossRef]
- Zeng, S.; Qin, X.; Xia, L. Degradation of the Herbicide Isoproturon by Laccase-Mediator Systems. Biochem. Eng. J. 2017, 119, 92–100. [Google Scholar] [CrossRef]
- Vaithyanathan, V.K.; Vaidyanathan, V.K.; Cabana, H. Laccase-Driven Transformation of High Priority Pesticides Without Redox Mediators: Towards Bioremediation of Contaminated Wastewaters. Front. Bioeng. Biotechnol. 2022, 9, 770435. [Google Scholar] [CrossRef]
- Blánquez, A.; Rodríguez, J.; Brissos, V.; Mendes, S.; Martins, L.O.; Ball, A.S.; Arias, M.E.; Hernández, M. Decolorization and Detoxification of Textile Dyes Using a Versatile Streptomyces Laccase-Natural Mediator System. Saudi J. Biol. Sci. 2019, 26, 913–920. [Google Scholar] [CrossRef]
- Apriceno, A.; Astolfi, M.L.; Girelli, A.M.; Scuto, F.R. A New Laccase-Mediator System Facing the Biodegradation Challenge: Insight into the NSAIDs Removal. Chemosphere 2019, 215, 535–542. [Google Scholar] [CrossRef]
- Ashe, B.; Nguyen, L.N.; Hai, F.I.; Lee, D.J.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Impacts of Redox-Mediator Type on Trace Organic Contaminants Degradation by Laccase: Degradation Efficiency, Laccase Stability and Effluent Toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176. [Google Scholar] [CrossRef]
- Atacag Erkurt, H. Biodegradation and Detoxification of BPA: Involving Laccase and a Mediator. Clean 2015, 43, 932–939. [Google Scholar] [CrossRef]
- Jankowska, K.; Su, Z.; Zdarta, J.; Jesionowski, T.; Pinelo, M. Synergistic Action of Laccase Treatment and Membrane Filtration during Removal of Azo Dyes in an Enzymatic Membrane Reactor Upgraded with Electrospun Fibers. J. Hazard. Mater. 2022, 435, 129071. [Google Scholar] [CrossRef] [PubMed]
- Spindola Vilela, C.L.; Damasceno, T.L.; Thomas, T.; Peixoto, R.S. Global Qualitative and Quantitative Distribution of Micropollutants in the Deep Sea. Environ. Pollut. 2022, 307, 119414. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hao Ngo, H.; Guo, W.; Woong Chang, S.; Duc Nguyen, D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and Applications of Enzymes for Resistant Pollutants Removal in Wastewater Treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M.O. A Mini-Review on Wastewater Treatment through Bioremediation towards Enhanced Field Applications of the Technology. AIMS Environ. Sci. 2022, 9, 403–431. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Iman Moezzi, S.M.; Ravan, N.; Gholami, A.; Lai, C.W.; Chiang, W.H.; Omidifar, N.; Yousefi, K.; Behbudi, G. Recent Advances in Enzymes for the Bioremediation of Pollutants. Biochem. Res. Int. 2021, 2021, 5599204. [Google Scholar] [CrossRef]
- Sai Preethi, P.; Hariharan, N.M.; Vickram, S.; Rameshpathy, M.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Yadav, V.; Ravindran, B.; Chang, S.W.; et al. Advances in bioremediation of emerging contaminants from industrial wastewater by oxidoreductase enzymes. Bioresour. Technol. 2022, 359, 127444. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, H.; Zhu, S.; Tian, K.; Qiu, Q.; Huo, H. Degradation of 17β-Estradiol and Products by a Mixed Culture of Rhodococcus Equi DSSKP-R-001 and Comamonas Testosteroni QYY20150409. Biotechnol. Biotechnol. Equip. 2019, 33, 268–277. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, C.; Niu, L.; Cai, W. Occurrence of Endocrine Disrupting Compounds in Aqueous Environment and Their Bacterial Degradation: A Review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1–59. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent Developments in Hazardous Pollutants Removal from Wastewater and Water Reuse within a Circular Economy. NPJ Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Singh, A.K.; Chandra, R. Pollutants Released from the Pulp Paper Industry: Aquatic Toxicity and Their Health Hazards. Aquatic Toxicology 2019, 211, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Degórska, O.; Kaźmierczak, K.; Nguyen, L.N.; Nghiem, L.D.; Jesionowski, T. Enhanced Wastewater Treatment by Immobilized Enzymes. Curr. Pollut. Rep. 2021, 7, 167–179. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Cui, J.; Lou, W.Y.; Franco, M.; Mulla, S.I.; Iqbal, H.M.N. Harnessing the Biocatalytic Attributes and Applied Perspectives of Nanoengineered Laccases—A Review. Int. J. Biol. Macromol. 2021, 166, 352–373. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Multi-Faceted Strategy Based on Enzyme Immobilization with Reactant Adsorption and Membrane Technology for Biocatalytic Removal of Pollutants: A Critical Review. Biotechnol. Adv. 2019, 37, 107401. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive Removal of Bisphenol A (BPA) from Aqueous Solution: A Review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef]
- Mohammadi, M.; As’habi, M.A.; Salehi, P.; Yousefi, M.; Nazari, M.; Brask, J. Immobilization of Laccase on Epoxy-Functionalized Silica and Its Application in Biodegradation of Phenolic Compounds. Int. J. Biol. Macromol. 2018, 109, 443–447. [Google Scholar] [CrossRef]
- Varsha, Y.M.; Deepthi CH, N.; Chenna, S. An Emphasis on Xenobiotic Degradation in Environmental Clean Up. J. Bioremediat. Biodegrad. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Natural Carriers in Bioremediation: A Review. Electron. J. Biotechnol. 2016, 23, 28–36. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary Enzyme Based Technologies for Bioremediation: A Review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.A.; Scelza, R.; Scotti, R.; Gianfreda, L. Role of enzyme in the remediation of polluted environments. J. Soil Sci. Plant Nutr. 2010, 10, 333–353. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A Review on Catalytic-Enzyme Degradation of Toxic Environmental Pollutants: Microbial Enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef] [PubMed]
- Karigar, C.S.; Rao, S.S. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation Characteristics of Pharmaceutical Substances by Whole Fungal Culture Trametes Versicolor and Its Laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef]
- Becker, D.; Varela Della Giustina, S.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of Antibiotics in Wastewater by Enzymatic Treatment with Fungal Laccase—Degradation of Compounds Does Not Always Eliminate Toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Stadlmair, L.F.; Letzel, T.; Drewes, J.E.; Grassmann, J. Enzymes in Removal of Pharmaceuticals from Wastewater: A Critical Review of Challenges, Applications and Screening Methods for Their Selection. Chemosphere 2018, 205, 649–661. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, S.; Miao, S.; Suo, H.; Xu, H.; Hu, Y. Co-Immobilization of Laccase and ABTS onto Amino-Functionalized Ionic Liquid-Modified Magnetic Chitosan Nanoparticles for Pollutants Removal. J. Hazard. Mater. 2021, 401, 123353. [Google Scholar] [CrossRef]
- Mao, G.; Wang, K.; Wang, F.; Li, H.; Zhang, H.; Xie, H.; Wang, Z.; Wang, F.; Song, A. An Engineered Thermostable Laccase with Great Ability to Decolorize and Detoxify Malachite Green. Int. J. Mol. Sci. 2021, 22, 11755. [Google Scholar] [CrossRef]
- Sharma, A.; Balda, S.; Capalash, N.; Sharma, P. Engineering Multifunctional Enzymes for Agro-Biomass Utilization. Bioresour. Technol. 2022, 347, 126706. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Purchase, D.; Saxena, G.; Mulla, S.I. Applications of Metagenomics in Microbial Bioremediation of Pollutants: From Genomics to Environmental Cleanup. From Genomics to Environmental Cleanup. In Microbial Diversity in the Genomic Era; Elsevier: Amsterdam, The Netherlands, 2018; pp. 459–477. ISBN 9780128148501. [Google Scholar]
- Bharagava, R.N.; Chowdhary, P. Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2018; ISBN 9789811086694. [Google Scholar]
- Arora, P.K.; Kumar, A.; Srivastava, A.; Garg, S.K.; Singh, V.P. Current Bioinformatics Tools for Biodegradation of Xenobiotic Compounds. Front. Environ. Sci. 2022, 10, 980284. [Google Scholar] [CrossRef]
- Rylott, E.L.; Bruce, N.C. How Synthetic Biology Can Help Bioremediation. Curr. Opin. Chem. Biol. 2020, 58, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Serban, S. Industrial Applications of Immobilized Enzymes—A Review. Mol. Catal. 2019, 479. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.A.; Díaz, S.A.; Medintz, I.L. Enhancing Enzymatic Performance with Nanoparticle Immobilization: Improved Analytical and Control Capability for Synthetic Biochemistry. Curr. Opin. Biotechnol. 2021, 71, 77–90. [Google Scholar] [CrossRef]
- Cabana, H.; Alexandre, C.; Agathos, S.N.; Jones, J.P. Immobilization of Laccase from the White Rot Fungus Coriolopsis Polyzona and Use of the Immobilized Biocatalyst for the Continuous Elimination of Endocrine Disrupting Chemicals. Bioresour. Technol. 2009, 100, 3447–3458. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Laccase Immobilization for Water Purification: A Comprehensive Review. Chem. Eng. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Adeel, M.; Yang, Y.S.; Wang, Y.Y.; Song, X.M.; Ahmad, M.A.; Rogers, H.J. Uptake and Transformation of Steroid Estrogens as Emerging Contaminants Influence Plant Development. Environ. Pollut. 2018, 243, 1487–1497. [Google Scholar] [CrossRef]
- Rahmani, K.; Faramarzi, M.A.; Mahvi, A.H.; Gholami, M.; Esrafili, A.; Forootanfar, H.; Farzadkia, M. Elimination and Detoxification of Sulfathiazole and Sulfamethoxazole Assisted by Laccase Immobilized on Porous Silica Beads. Int. Biodeterior. Biodegrad. 2015, 97, 107–114. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous Adsorption and Biotransformation of Micropollutants by Granular Activated Carbon-Bound Laccase in a Packed-Bed Enzyme Reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef]
- Robert, B.; Chenthamara, D.; Subramaniam, S. Fabrication and Biomedical Applications of Arabinoxylan, Pectin, Chitosan, Soy Protein, and Silk Fibroin Hydrogels via Laccase—Ferulic Acid Redox Chemistry. Int. J. Biol. Macromol. 2022, 201, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Pandey, V.P.; Dwivedi, U.N. Immobilization of Papaya Laccase in Chitosan Led to Improved Multipronged Stability and Dye Discoloration. Int. J. Biol. Macromol. 2016, 86, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Silva-Torres, O.; Bojorquez-Vazquez, L.; Simakov, A.; Vazquez-Duhalt, R. Enhanced Laccase Activity of Biocatalytic Hybrid Copper Hydroxide Nanocages. Enzym. Microb. Technol. 2019, 128, 59–66. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Q.; Wang, Y.; Zhang, Y.; Zhang, B.; Zhang, H. Immobilization of Laccase on Chitosan–Halloysite Hybrid Porous Microspheres for Phenols Removal. Desalination Water Treat. 2015, 55, 1293–1301. [Google Scholar] [CrossRef]
- Ding, R. Removal of Organic Micro-Pollutants from Water Using Sewage Removal of Organic Micro-Pollutants from Water Using Sewage Sludge Based Composite Adsorbents Sludge Based Composite Adsorbents. City University of New York (CUNY). 2017. Available online: https://academicworks.cuny.edu/gc_etds/1903/ (accessed on 1 February 2017).
- Qiao, W.; Liu, H. Enhanced Decolorization of Malachite Green by a Magnetic Graphene Oxide-CotA Laccase Composite. Int. J. Biol. Macromol. 2019, 138, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in Support Materials for Immobilization of Oxidoreductases: A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef]
- Corvini, P.F.X.; Shahgaldian, P. LANCE: Laccase-Nanoparticle Conjugates for the Elimination of Micropollutants (Endocrine Disrupting Chemicals) from Wastewater in Bioreactors. Rev. Environ. Sci. Biotechnol. 2010, 9, 23–27. [Google Scholar] [CrossRef]
- Xu, H.; Boeuf, G.; Jia, Z.; Kanaev, A.; Azouani, R.; Amamra, M.; Elm’selmi, A.; Traore, M. Novel Synthesis of Oxidoreductase Immobilized Biocatalyst for Effective Anthraquinone Dye Treatment in Bioreactor. Chem. Eng. Trans. 2021, 84, 157–162. [Google Scholar] [CrossRef]
- Teerapatsakul, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Repeated Batch for Dye Degradation in an Airlift Bioreactor by Laccase Entrapped in Copper Alginate. Int. Biodeterior. Biodegrad. 2017, 120, 52–57. [Google Scholar] [CrossRef]
- Katibi, K.K.; Yunos, K.F.; Man, H.C.; Aris, A.Z.; bin Mohd Nor, M.Z.; binti Azis, R.S. Recent Advances in the Rejection of Endocrine-Disrupting Compounds from Water Using Membrane and Membrane Bioreactor Technologies: A Review. Polymers 2021, 13, 392. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Shah, M.P. A Comprehensive Review on Application of Bioreactor for Industrial Wastewater Treatment. Lett. Appl. Microbiol. 2022, 74, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current State and Challenges of Full-Scale Membrane Bioreactor Applications: A Critical Review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and Biotechnological Applications of Fungal Laccase. 3 Biotech 2016, 6, 15. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Vivek, N.; Kumar, V.; Chandel, N.; Thakur, M.; Kumar, D.; Yang, Y.H.; Pugazendhi, A.; Kumar, G. Molecular Biology Interventions for Activity Improvement and Production of Industrial Enzymes. Bioresour. Technol. 2021, 324, 124596. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Han, X.; Wang, Y.; Rao, S.; Zhang, Q.; Zhou, J.; Li, J.; Liu, S.; Du, G. Recent Progress in Key Lignocellulosic Enzymes: Enzyme Discovery, Molecular Modifications, Production, and Enzymatic Biomass Saccharification. Bioresour. Technol. 2022, 363, 127986. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Morsy, S.A.G.Z.; Ahmad Tajudin, A.; Ali, M.S.M.; Shariff, F.M. Current Development in Decolorization of Synthetic Dyes by Immobilized Laccases. Front. Microbiol. 2020, 11, 572309. [Google Scholar] [CrossRef]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and Characterization of an Immobilized Laccase Biocatalyst and Its Application to Eliminate Organic Micropollutants in Wastewater. New Biotechnol. 2013, 30, 814–823. [Google Scholar] [CrossRef]
- Rai, R.; Bibra, M.; Chadha, B.S.; Sani, R.K. Enhanced Hydrolysis of Lignocellulosic Biomass with Doping of a Highly Thermostable Recombinant Laccase. Int. J. Biol. Macromol. 2019, 137, 232–237. [Google Scholar] [CrossRef]
- González-González, R.B.; Parra-Arroyo, L.; Parra-Saldívar, R.; Ramirez-Mendoza, R.A.; Iqbal, H.M.N. Nanomaterial-Based Catalysts for the Degradation of Endocrine-Disrupting Chemicals—A Way Forward to Environmental Remediation. Mater. Lett. 2022, 308, 131217. [Google Scholar] [CrossRef]
- Rizwan; Singh, M.; Mitra, C.K.; Morve, R.K. Ecofriendly Application of Nanomaterials: Nanobioremediation. J. Nanoparticles 2014, 2014, 431787. [Google Scholar] [CrossRef]
- Luo, H.; Liu, X.; Yu, D.; Yuan, J.; Tan, J.; Li, H. Research Progress on Lignocellulosic Biomass Degradation Catalyzed by Enzymatic Nanomaterials. Chem. Asian J. 2022, 17, e202200566. [Google Scholar] [CrossRef]
- Wehaidy, H.R.; Abdel-Naby, M.A.; El-Hennawi, H.M.; Youssef, H.F. Nanoporous Zeolite-X as a New Carrier for Laccase Immobilization and Its Application in Dyes Decolorization. Biocatal. Agric. Biotechnol. 2019, 19, 101135. [Google Scholar] [CrossRef]
- Xu, K.Z.; Wang, H.R.; Wang, Y.J.; Xia, J.; Ma, H.; Cai, Y.J.; Liao, X.R.; Guan, Z.B. Enhancement in Catalytic Activity of CotA-Laccase from Bacillus Pumilus W3 via Site-Directed Mutagenesis. J. Biosci. Bioeng. 2020, 129, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Wang, J.; Yin, Q.; Fang, W.; Xiao, Y.; Fang, Z. Expression of a Thermo- and Alkali-Philic Fungal Laccase in Pichia Pastoris and Its Application. Protein Expr. Purif. 2019, 154, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, Y.; Liu, M.; Wang, S.; Wang, Q. Improving the Thermostability of a Thermostable Endoglucanase from Chaetomium Thermophilum by Engineering the Conserved Noncatalytic Residue and N-Glycosylation Site. Int. J. Biol. Macromol. 2020, 164, 3361–3368. [Google Scholar] [CrossRef]
- Pande, V.; Joshi, T.; Pandey, S.C.; Sati, D.; Mathpal, S.; Pande, V.; Chandra, S.; Samant, M. Molecular Docking and Molecular Dynamics Simulation Approaches for Evaluation of Laccase-Mediated Biodegradation of Various Industrial Dyes. J. Biomol. Struct. Dyn. 2022, 40, 12461–12471. [Google Scholar] [CrossRef]
- Bertrand, B.; Martínez-Morales, F.; Trejo-Hernández, M.R. Upgrading Laccase Production and Biochemical Properties: Strategies and Challenges. Biotechnol. Prog. 2017, 33, 1015–1034. [Google Scholar] [CrossRef]
- Kembaren, R.; Westphal, A.H.; Kamperman, M.; Kleijn, J.M.; Borst, J.W. Charged Polypeptide Tail Boosts the Salt Resistance of Enzyme-Containing Complex Coacervate Micelles. Biomacromolecules 2022, 23, 1195–1204. [Google Scholar] [CrossRef]
- Othman, A.M.; Elsayed, M.A.; Elshafei, A.M.; Hassan, M.M. Application of Central Composite Design as a Strategy to Maximize the Productivity of Agaricus Bisporus CU13 Laccase and Its Application in Dye Decolorization. Biocatal. Agric. Biotechnol. 2018, 14, 72–79. [Google Scholar] [CrossRef]
- Thakur, S.; Gupte, A. Optimization and Hyper Production of Laccase from Novel Agaricomycete Pseudolagarobasidium Acaciicola AGST3 and Its Application in in Vitro Decolorization of Dyes. Ann. Microbiol. 2015, 65, 185–196. [Google Scholar] [CrossRef]
- Atilano-Camino, M.M.; Álvarez-Valencia, L.H.; García-González, A.; García-Reyes, R.B. Improving Laccase Production from Trametes Versicolor Using Lignocellulosic Residues as Cosubstrates and Evaluation of Enzymes for Blue Wastewater Biodegradation. J. Environ. Manag. 2020, 275, 111231. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Tepper, K.; Petroll, K.; Kumar, S.; Sunna, A.; Maselko, M. Bioremediation of Industrial Pollutants by Insects Expressing a Fungal Laccase. ACS Synth. Biol. 2022, 11, 308–316. [Google Scholar] [CrossRef] [PubMed]
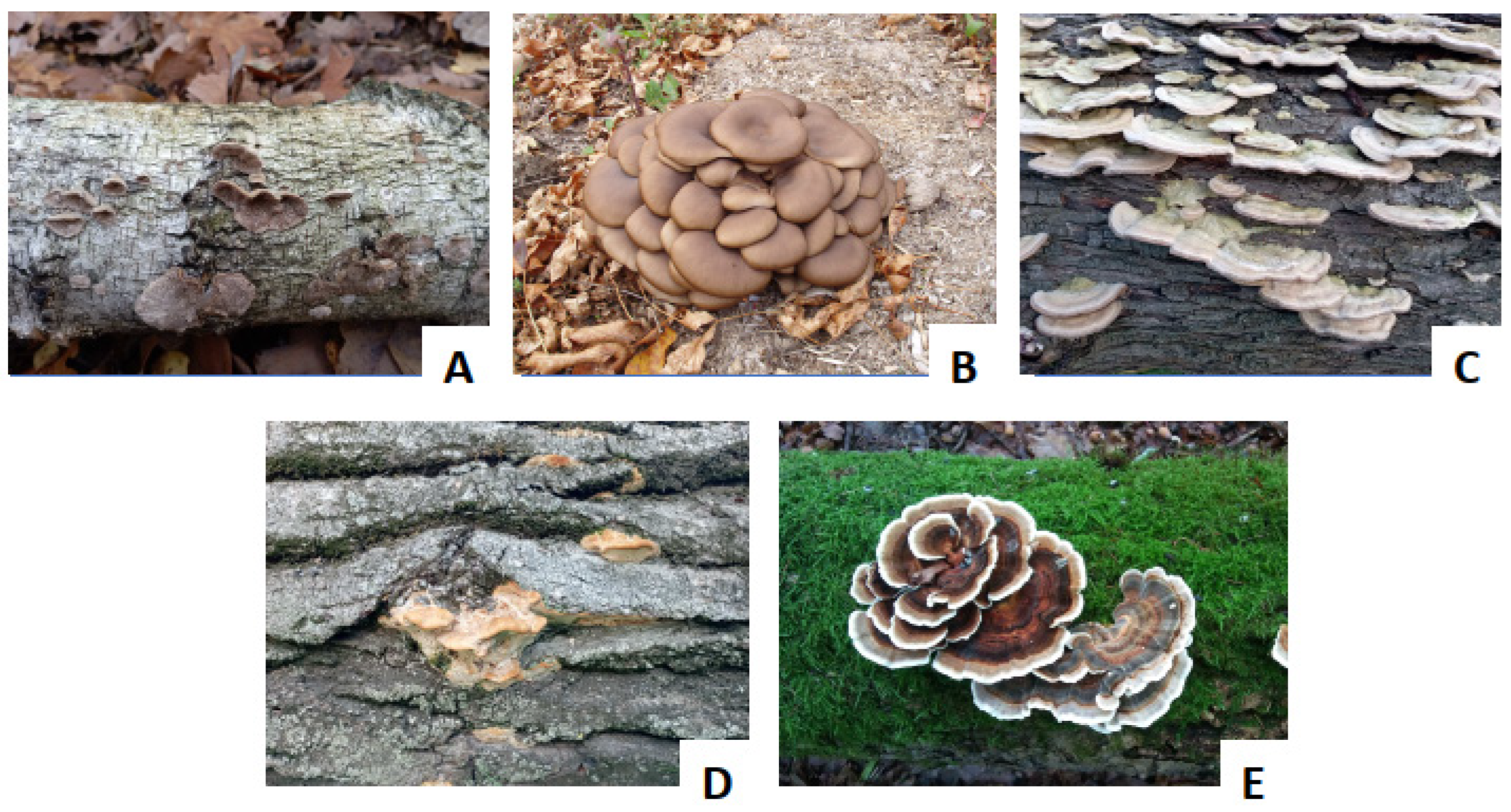

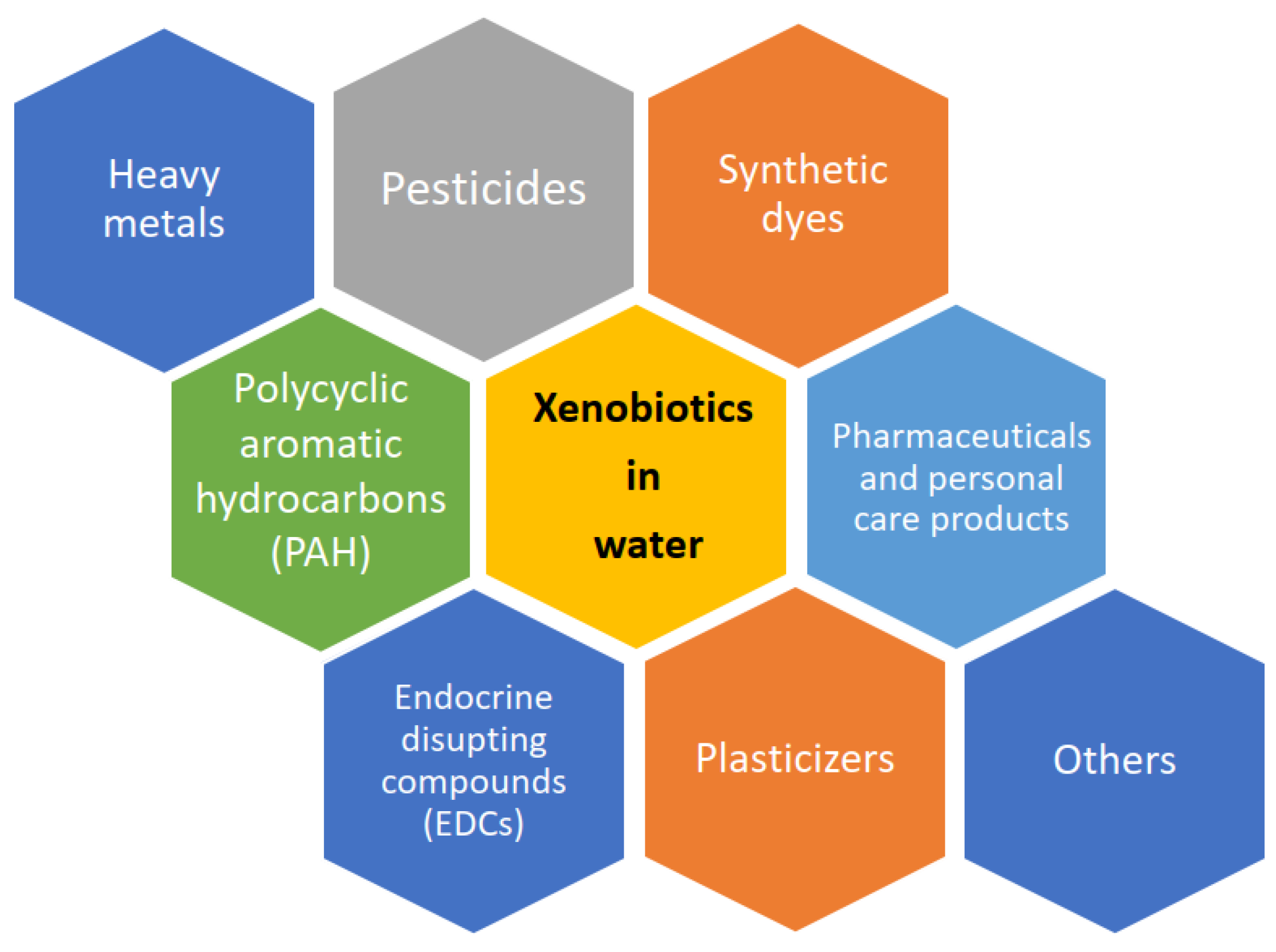

| Type of Source Organism | Source/Organism | MW, Subunit Structure | Optimum pH and Temperature | Substrate (Most Efficient) | Regulation of Activity/Synthesis | References |
|---|---|---|---|---|---|---|
| Plant | Tetracystis aeria SAG 89.80 | 220 kDa, heterooligomer: two polypeptides highly N-glycosylated: 110 and 71 kDa | pH 2.5 | ABTS | Not mentioned | [71] |
| Leucaena leucocephala | ∼220 kDa, heterodimer: two subunits of 100 and 120 kDa | pH 7.0, 80 °C | Catechol |
Mn2+, Cd2+, Fe2+, Cu2+, Ca2+ and Na+ activation laccase;
Co2+, Hg2+, DTT, SDS and EDTA inhibition laccase | [72] | |
| Pistacia atlantica Desf. | 60 kDa | pH 7.5, 45 °C | DMP | Cu2+ activation laccase; Zn2+ and Mg2+ have a stabilizing effect; Hg2+ Fe2+ inhibition laccase | [73] | |
| Bacteria | Bacillus amyloliquefaciens B10 | 30.9 kDa | pH 6.0–8.0, 40 °C | Aflatoxin B1 (AFB1) |
Significant reduction in activity in the presence of metal ions: Cu2+, Co2+, Fe3+, Mn2+, and Zn2+;
Slight reduction in activity in the presence Na+ and K+ | [74] |
| Enterococcus faecium A2 | 50.11 kDa. | pH 6.0, 80 °C | ABTS | Cr2+, Cu2+, Fe and Ag+ activation laccase | [75] | |
| Bacillus sp. | 63–75 kDa | pH 7 40 °C | ABTS | Not mentioned | [76] | |
| Fungi | Trichoderma harzianum S7113 | Two isoenzymes: 63 kDa (LacA) and 48 kDa (LacB) | pH 3.0, 50 °C (LacA) pH 2.5, 50 °C (LacB) | ABTS | Mg2+, Zn2+, K+, and Ni2+ activation laccase; Hg2+ and Pb2+, β-mercaptoethanol, EDTA and SDS, sodium azide inhibition laccase | [77] |
| Ganoderma leucocontextum | 65.0 kDa/monomer | pH 3.0, 70 °C | Guaiacol | Ca2+, Cu2+ and Zn2+ activation laccase | [78] | |
| Agrocybe pediades | 55–60 kDa/monomer | pH 5.0 45 °C | 2,6-DMP | Not mentioned | [79] | |
| Insect | Plutella xylostella | 66.09 kDa | pH 3.0, 35 °C | ABTS | Cu2+ increased laccase activity | [80] |
| Crabs | Chiromantes haematocheir | 67.708 kDa (deduced)/monomer | 2,6-DMP | Presence of CuSO4 in culture medium was essential for laccase activity | [81] |
| Application | Source Organism | Activity | References |
|---|---|---|---|
| Pulp and paper industry | T. versicolor | Demethylated and delignified the sulphate pulp | [161] |
| Bacillus sp. | Decolorization of old newsprint and the biological bleaching of eucalyptus kraft pulp | [162] | |
| Fusarium equiseti VKF2 | Biological bleaching of newspaper waste | [163] | |
| T. versicolor | Immobilized laccase on copper ferrite magnetic nanoparticles (CuMNPs) improve the delignification | [164] | |
| Textile industry | Myceliophthora thermophila | Altered the shape of wool yarn by laccase-assisted tyrosine grafting | [165] |
| Cerrena unicolor | Biocatalyst to transform 8-anilino-1-naphthalenesulfonic acid into a water-soluble green dye with antibacterial and antiallergic properties and high dyeing efficiency of wool fibers | [95] | |
| Brevibacillus agri | Bleaching denim and decolorizing water-soluble azo dyes | [166] | |
| Achromobacter xylosoxidans HWN16 Citrobacter freundii LLJ16 | Biocleaned and discolored jeans discolored the wastewater after washing the fabrics | [167] | |
| Food industry | Bacillus atrophaeus | Recombinant laccase covalently immobilized on magnetic iron nanoparticles and then used them to remove phenols and clarify plant juice samples | [105] |
| T. versicolor | Improving the stability of emulsifying beet pectin | [168] | |
| Recombinant laccase from Pleurotus pulmonarius expressed in Pichia pastoris X33 | Degrade mycotoxins zearalenone and aflatoxin B1 | [169] | |
| Synthetic chemistry | Laccase P. ostreatus immobilized on the CuFe2O4 nanocomposite | Synthesize arylsulfonylbenzenediols through oxygen oxidative coupling between benzenediols and sodium benzenesulfinates | [170] |
| P. ostreatus | Synthesize new organic orange textile dye (N15) by transforming 2-amino-3-methoxybenzoic acid | [115] | |
| Cosmetic industry | T. versicolor | Bleaching agents in whitening cream that degraded up to 87% eumelanin | [171] |
| C. unicolor | Green compound, a result of the 8-anilino-1-naphthalenesulfonic acid (ANS) oxidation reaction, with antibacterial activity against the growth of bacterial strains Staphylococcus aureus ATCC®25923TM and Staphylococcus epidermidis ATCC®14990TM commonly found on the skin; antioxidant properties and low cytotoxicity (cosmetic additive) | [95] | |
| Brevibacillus agri | Hair dyeing | [172] | |
| Biomedicine | T. versicolor | Producing laccase cross-linked hydrogels based on silk fibroin and hyaluronic acid modified with tyramine | [84] |
| C. unicolor | Antiviral effect against human herpes virus type 1 (HHV-1) and encephalomyocarditis virus (EMCV) anticancer effect against cell lines derived from primary cervical cancer (SiHa) and its metastases to the small intestine (CaSki) | [173] | |
| Bacillus sp. MSK-01 | Antiproliferative activity against a lung cancer cell line A549 | [174] | |
| Biosensor | Bacillus subtilis | Detection of glyphosate; CotA laccase effectively catalyze the luminol-H2O2 reaction, creating a chemiluminescent (CL) signal | [175] |
| T. versicolor | The chemiluminescent (CL) immunoassay for the detection of Escherichia coli O157:H7 in synthetic samples (spring water, apple juice, skimmed milk) | [176] | |
| Laccase T. versicolor immobilized on an electrospun zein fiber (ceZL) | Producing smart packaging systems can provide food quality information and the time and temperature index, TTI | [177] | |
| Bioremediation | Recombinant Bacillus amyloliquefaciens TCCC 111018 laccase expressed in E. coli | Decolorize synthetic azodyes (azofloxine, congo red and adisole black B), anthraquinones (reactive blue 19, reactive blue 5 and remazol brilliant blue R) and triphenylmethane (crystal violet, indigo carmine and malachite green) | [178] |
| Trematophoma sp. UTMC5003 | Pyrene, anthracene and phenanthrene degrading | [179] | |
| Sphingobacterium ksn-11 | Transformation of diclofenac | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałązka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water. Appl. Sci. 2023, 13, 4394. https://doi.org/10.3390/app13074394
Gałązka A, Jankiewicz U, Szczepkowski A. Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water. Applied Sciences. 2023; 13(7):4394. https://doi.org/10.3390/app13074394
Chicago/Turabian StyleGałązka, Agnieszka, Urszula Jankiewicz, and Andrzej Szczepkowski. 2023. "Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water" Applied Sciences 13, no. 7: 4394. https://doi.org/10.3390/app13074394
APA StyleGałązka, A., Jankiewicz, U., & Szczepkowski, A. (2023). Biochemical Characteristics of Laccases and Their Practical Application in the Removal of Xenobiotics from Water. Applied Sciences, 13(7), 4394. https://doi.org/10.3390/app13074394





