A Case Series on Intraoral Blue Nevi with a Literature Review-Based Clinico-Pathologic Comparison of Intraoral Blue Nevi with Other Oral Melanocytic Nevi
Abstract
1. Introduction
2. Materials and Method
2.1. Acquisition of New Cases of OMN including Blue Nevi
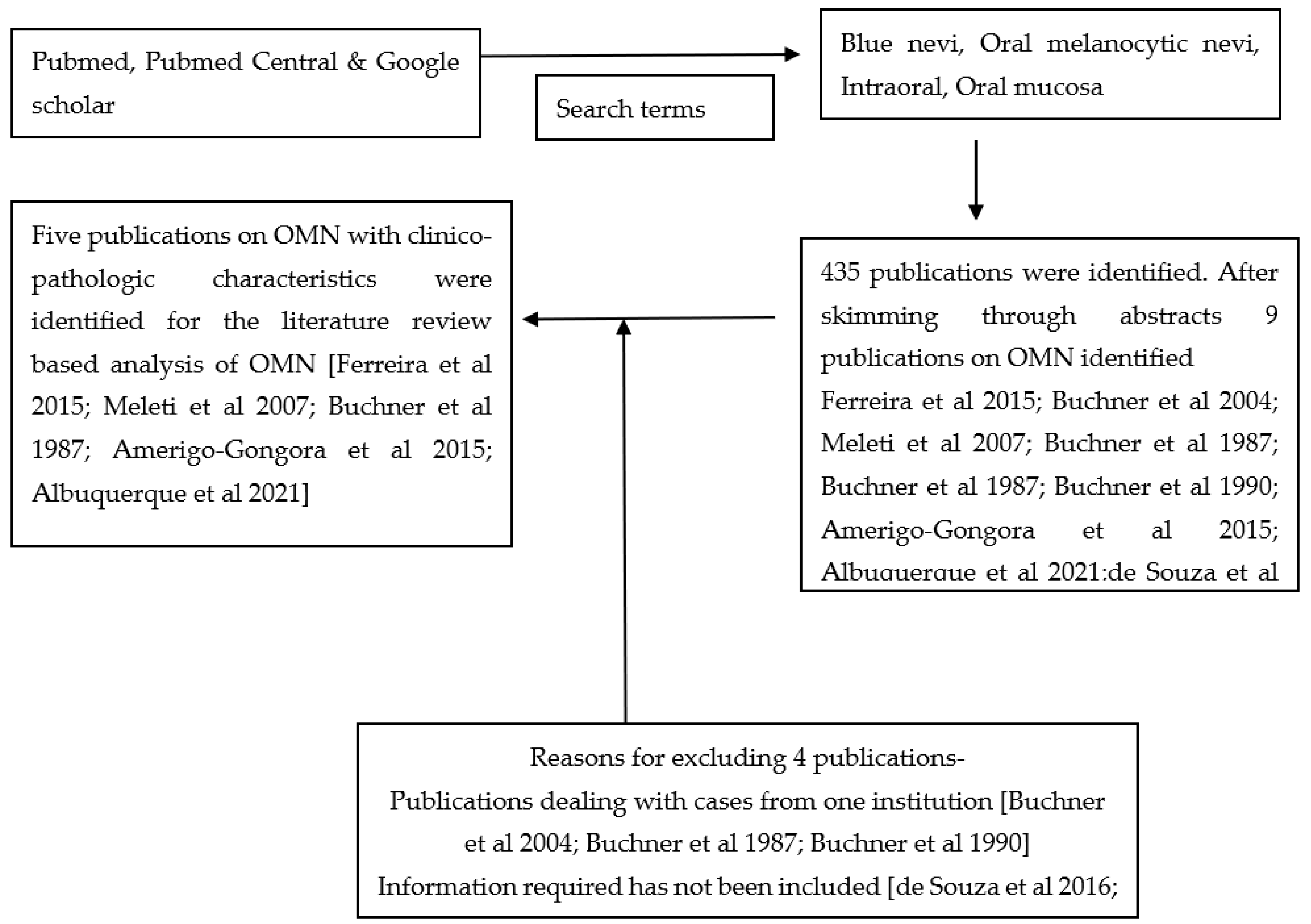
2.2. Literature Review
3. Results
Clinico-Pathologic Characteristics of New Case Series of Intraoral Common Blue Nevi
4. Discussion
4.1. How Do We Clinically Differentiate Nevi from Melanoma?
4.2. Where Do Dendritic Blue Nevi Commonly Occur in the Oral Cavity?
4.3. Are Females More Prone to Developing Oral Melanocytic Nevi?
4.4. Why Do General Dental Practitioners Need to Be Aware of Oral Melanocytic Nevi?
4.5. How Do Oral Melanocytic Nevi Occur?
4.6. What Types Other Than Common Blue Nevi Occur in the Oral Mucosa and What Are Their Diagnostic Challenges?
4.7. How Do We Manage Oral Melanocytic Nevi?
4.8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murali, R.; McCarthy, S.W.; Scolyer, R.A. Blue nevi and related lesions: A review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv. Anat. Pathol. 2009, 16, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Tieche, M. Uber benigne Melanome (“Chromatophorome”) der Haut- “blaue Naevi”. Virchows. Arch. Pathol. Anat. 1906, 186, 212–229. [Google Scholar]
- Allen, A.C. A reorientation on the histogenesis and clinical significance of cutaneous nevi and melanomas. Cancer 1949, 2, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.R.; Silverman, R.A.; Harrist, T.J.; Perez-Atayde, A.R. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: The “LAMB” syndrome. J. Am. Acad. Dermatol. 1984, 10, 72–82. [Google Scholar] [CrossRef]
- Atherton, D.J.; Pitcher, D.W.; Wells, R.S.; Macdonald, D.M. A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: The NAME syndrome. Br. J. Dermatol. 1980, 103, 421–429. [Google Scholar] [CrossRef]
- Carney, J.A. The Carney Complex (Myxomas, Spotty Pigmentation, Endocrine Overactivity, and Schwannomas). Dermatol. Clin. 1995, 13, 19–26. [Google Scholar] [CrossRef]
- Carney, J.A. Carney complex: The complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin. Dermatol. 1995, 14, 90–98. [Google Scholar] [CrossRef]
- Carney, J.A.; Stratakis, C.A. Epithelioid blue nevus and psammomatous melanotic schwannoma: The unusual pigmented skin tumors of the Carney complex. Semin. Diagn. Pathol. 1998, 15, 216–224. [Google Scholar]
- Ferreira, L.; Jham, B.; Assi, R.; Readinger, A.; Kessler, H.P. Oral melanocytic nevi: A clinicopathologic study of 100 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 358–367. [Google Scholar] [CrossRef]
- Buchner, A.; Merrell, P.W.; Carpenter, W.M. Relative frequency of solitary melanocytic lesions of the oral mucosa. J. Oral Pathol. Med. 2004, 33, 550–557. [Google Scholar] [CrossRef]
- Meleti, M.; Mooi, W.J.; Casparie, M.K.; van der Waal, I. Melanocytic nevi of the oral mucosa–No evidence of increased risk for oral malignant melanoma: An analysis of 119 cases. Oral Oncol. 2007, 43, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.; Hansen, L.S. Pigmented nevi of the oral mucosa: A clinicopathologic study of 36 new cases and review of 155 cases from the literature: Part I: A clinicopathologic study of 36 new cases. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Buchner, A.; Hansen, L.S. Pigmented nevi of the oral mucosa: A clinicopathologic study of 36 new cases and review of 155 cases from the literature: Part II: Analysis of 191 cases. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 676–682. [Google Scholar] [CrossRef]
- Buchner, A.; Leider, A.S.; Merrell, P.W.; Carpenter, W.M. Melanocytic nevi of the oral mucosa: A clinicopathologic study of 130 cases from northern California. J. Oral Pathol. Med. 1990, 19, 197–201. [Google Scholar] [CrossRef]
- Amérigo-Góngora, M.; Machuca-Portillo, G.; Torres-Lagares, D.; Lesclous, P.; Amérigo-Navarro, J.; González-Cámpora, R. Clinicopathological and immunohistochemical analysis of oral melanocytic nevi and review of the literature. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 151–155. [Google Scholar] [CrossRef]
- Albuquerque, D.M.; Cunha, J.L.; Roza, A.L.; Arboleda, L.P.; Santos-Silva, A.R.; Lopes, M.A.; Vargas, P.A.; Jorge, J.; de Almeida, O.P.; Abrahão, A.C.; et al. Oral pigmented lesions: A retrospective analysis from Brazil. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e284–e291. [Google Scholar] [CrossRef]
- de Souza do Nascimento, J.; Carlos, R.; Delgado-Azañero, W.; Mosqueda Taylor, A.; De Almeida, O.P.; Romañach, M.J.; De Andrade, B.A.B. Immunohistochemical expression of cyclooxygenase-2 (COX-2) in oral nevi and melanoma. J. Oral Pathol. Med. 2016, 45, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Gondak, R.O.; Da Silva-Jorge, R.; Jorge, J.; Lopes, M.A.; Vargas, P.A. Oral pigmented lesions: Clinicopathologic features and review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e919–e924. [Google Scholar] [CrossRef]
- MacKie, R.M.; English, J.; Aitchison, T.C.; Fitzsimons, C.P.; Wilson, P. The number and distribution of benign pigmented moles (melanocytic naevi) in a healthy Britishpopulation. Br. J. Dermatol. 1985, 113, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Biesbrock, A.R.; Aguirre, A. Multiple Focal Pigmented Lesions in the Maxillary Tuberosity and Hard Palate: A Unique Display of Intraoral Junctional Nevi. J. Periodontol. 1992, 63, 718–721. [Google Scholar] [CrossRef]
- Horlick, H.P.; Walther, R.R.; Zegarelli, D.J.; Silvers, D.N.; Eliezri, Y.D. Mucosal melanotic macule, reactive type: A simulation of melanoma. J. Am. Acad. Dermatol. 1988, 19, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Mader, C.L.; Konzelman, J.L. Blue nevus of the oral cavity. Gen. Dent. 1978, 26, 66–67. [Google Scholar] [PubMed]
- Lovas, G.L.; Wysocki, G.P.; Daley, T.D. The oral blue nevus: Histogenetic implications of its ultrastructural features. Oral Surg. Oral Med. Oral Pathol. 1983, 55, 145–150. [Google Scholar] [CrossRef]
- Esguep, A.; Solar, M.; Encina, A.M.; Fuentes, G. Primary melanotic alterations in the oral cavity. J. Oral Med. 1983, 38, 141–146. [Google Scholar] [PubMed]
- Papanicolaou, S.J.; Pierrakou, E.D.; Patsakas, A.J. Intraoral blue nevus. J. Oral Med. 1985, 40, 32–35. [Google Scholar]
- Brener, M.D.; Harrison, B.D. Intraoral blue nevus. Report of a case. Oral Surg. Oral Med. Oral Pathol. 1969, 28, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Laskaris, G.; Kittas, C.; Triantafyllou, A. Unpigmented nevus of the palate. An unusual clinical presentation. Int. J. Oral Maxillofac. Surg. 1994, 23, 39–40. [Google Scholar] [CrossRef]
- Fistarol, S.K.; Itin, P.H. Plaque-Type Blue Nevus of the Oral Cavity. Dermatology 2005, 211, 224–233. [Google Scholar] [CrossRef]
- Santos, T.D.S.; Frota, R.; Martins-Filho, P.R.S.; Cavalcante, J.R.; Raimundo, R.D.C.; Andrade, E.S.D.S. Extenso nevo azul intraoral: Relato de caso. An. Bras. de Dermatol. 2011, 86, 61–65. [Google Scholar] [CrossRef]
- Flaitz, C.M.; McCandless, G. Palatal blue nevus in a child. Pediatr. Dent. 2001, 23, 354–355. [Google Scholar]
- Ojha, J.; Akers, J.L.; O Akers, J.; Hassanein, A.M.; Islam, N.M.; Cohen, D.M.; Bhattacharyya, I. Intraoral cellular blue nevus: Report of a unique histopathologic entity and review of the literature. Cutis 2007, 80, 189–192. [Google Scholar] [PubMed]
- Pinto, A.; Raghavendra, S.; Lee, R.; DeRossi, S.; Alawi, F. Epithelioid blue nevus of the oral mucosa: A rare histologic variant. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.T.; Pereira, A.; Pimentel, J.; Prates, M.; Fonseca, L.; Marques, M.R.; Proença, F. Blue Nevus of the Hard Palate: The Importance of a Careful Examination in an Emergency Setting. Case Rep. Dermatol. Med. 2022, 2022, 6329334. [Google Scholar] [CrossRef] [PubMed]
- Shumway, B.S.; Rawal, Y.B.; Allen, C.M.; Kalmar, J.R.; Magro, C.M. Oral Atypical Cellular Blue Nevus: An Infiltrative Melanocytic Proliferation. Head Neck Pathol. 2013, 7, 171–177. [Google Scholar] [CrossRef][Green Version]
- Hasse, C.D.; Zoutendam, G.L.; Gombas, O.F. Intraoral blue (Jadassohn-Tieche) nevus. Oral Surg. Oral Med. Oral Pathol. 1978, 45, 755–761. [Google Scholar] [CrossRef]
- Soltani-Arabshahi, R.; Sweeney, C.; Jones, B.; Florell, S.R.; Hu, N.; Grossman, D. Predictive value of biopsy specimens suspicious for melanoma: Support for 6-mm criterion in the ABCD rule. J. Am. Acad. Dermatol. 2015, 72, 412–418. [Google Scholar] [CrossRef]
- Hicks, M.J.; Flaitz, C.M. Oral mucosal melanoma: Epidemiology and pathobiology. Oral Oncol. 2000, 36, 152–169. [Google Scholar] [CrossRef]
- Notani, K.; Shindoh, M.; Yamazaki, Y.; Nakamura, H.; Watanabe, M.; Kogoh, T.; Ferguson, M.; Fukuda, H. Amelanotic malignant melanomas of the oral mucosa. Br. J. Oral Maxillofac. Surg. 2002, 40, 195–200. [Google Scholar] [CrossRef]
- Regezi, J.A. Oral mucosal melanomas. In Oral Cancer; Silverman, S., Ed.; B.C. Decker Inc.: Hamilton, ON, Canada, 1998; pp. 155–158. [Google Scholar]
- Van der Waal, R.I.; Snow, G.B.; Karim, A.B.; van der Waal, I. Primary malignant melanoma of the oral cavity: A review of eight cases. Br. Dent. J. 1994, 176, 185–188. [Google Scholar]
- Rapini, R.P. Oral melanoma: Diagnosis and treatment. Semin. Cutan. Med. Surg. 1997, 16, 320–322. [Google Scholar] [CrossRef]
- Rowland, H.N.; Schnetler, J.F. Primary malignant melanoma arising in the dorsum of the tongue. Br. J. Oral Maxillofac. Surg. 2003, 41, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.G.; Prasad, M.L.; Escrig, M.; Singh, B.; Shaha, A.R.; Kraus, D.H.; Boyle, J.O.; Huvos, A.G.; Busam, K.; Shah, J.P. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002, 24, 247–257. [Google Scholar] [CrossRef]
- Umeda, M.; Komatsubara, H.; Shibuya, Y.; Yokoo, S.; Komori, T. Premalignant melanocytic dysplasia andmalignant melanoma of the oral mucosa. Oral Oncol. 2002, 38, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Cordero, D.R.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. Part A 2011, 155, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; Van Der Horst, C.M.A.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Granter, S.R.; McKee, P.H.; Calonje, E.; Mihm, M.C., Jr.; Busam, K. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: A clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus’. Am. J. Surg. Pathol. 2001, 25, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Na, S.Y.; Son, Y.M.; Kang, H.K.; Baek, J.O.; Lee, J.R.; Roh, J.Y. A Malignant Melanoma Associated with a Blue Nevus of the Lip. Ann. Dermatol. 2010, 22, 119–124. [Google Scholar] [CrossRef]
- Moreno, C.; Requena, L.; Kutzner, H.; De La Cruz, A.; Jaqueti, G.; Yus, E.S. Epithelioid blue nevus: A rare variant of blue nevus not always associated with the Carney complex. J. Cutan. Pathol. 2000, 27, 218–223. [Google Scholar] [CrossRef]
- O’Grady, T.C.; Barr, R.J.; Billman, G.; Cunningham, B.B. Epithelioid Blue Nevus Occurring in Children With No Evidence of Carney Complex. Am. J. Dermatopathol. 1999, 21, 483–486. [Google Scholar] [CrossRef]
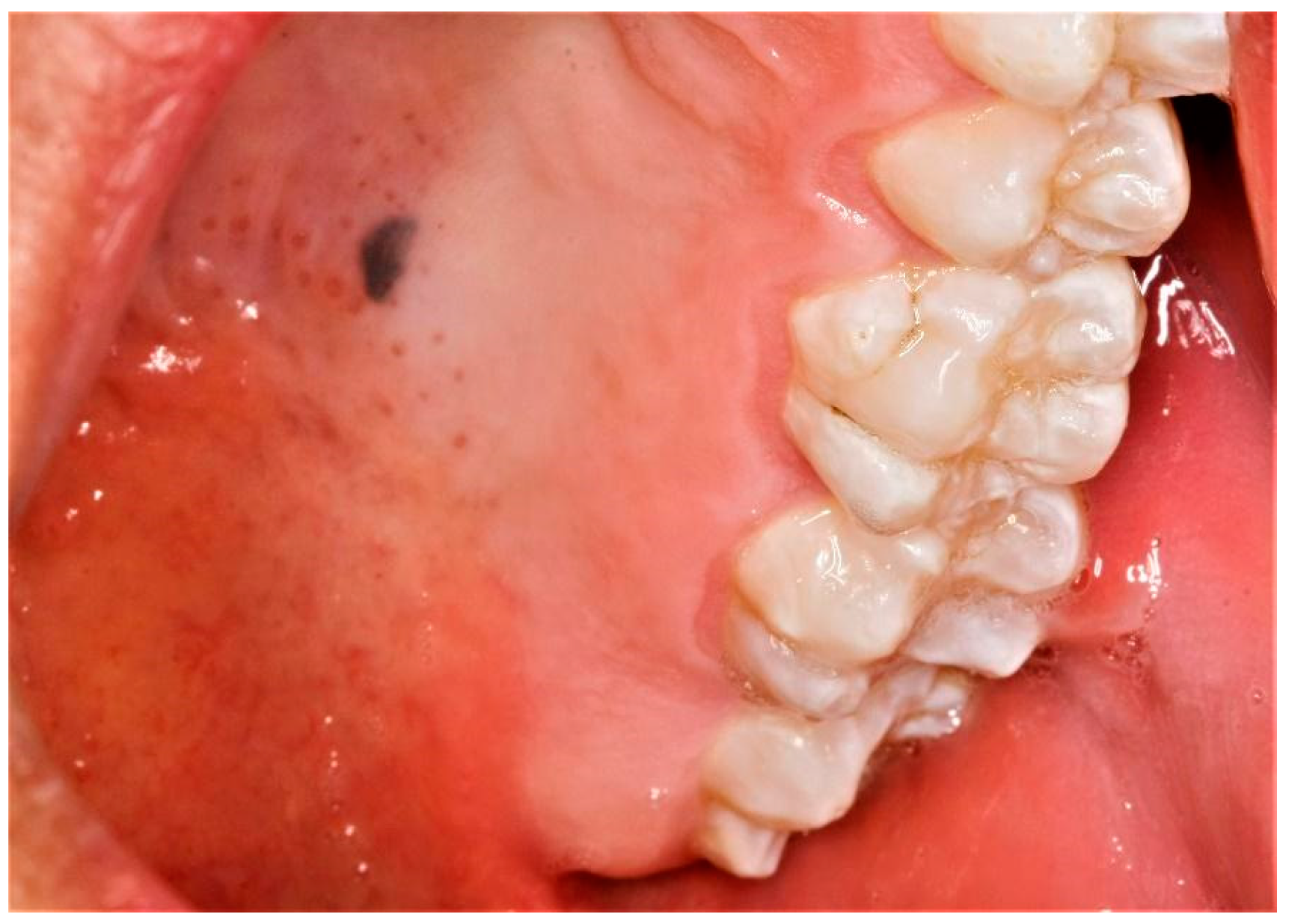
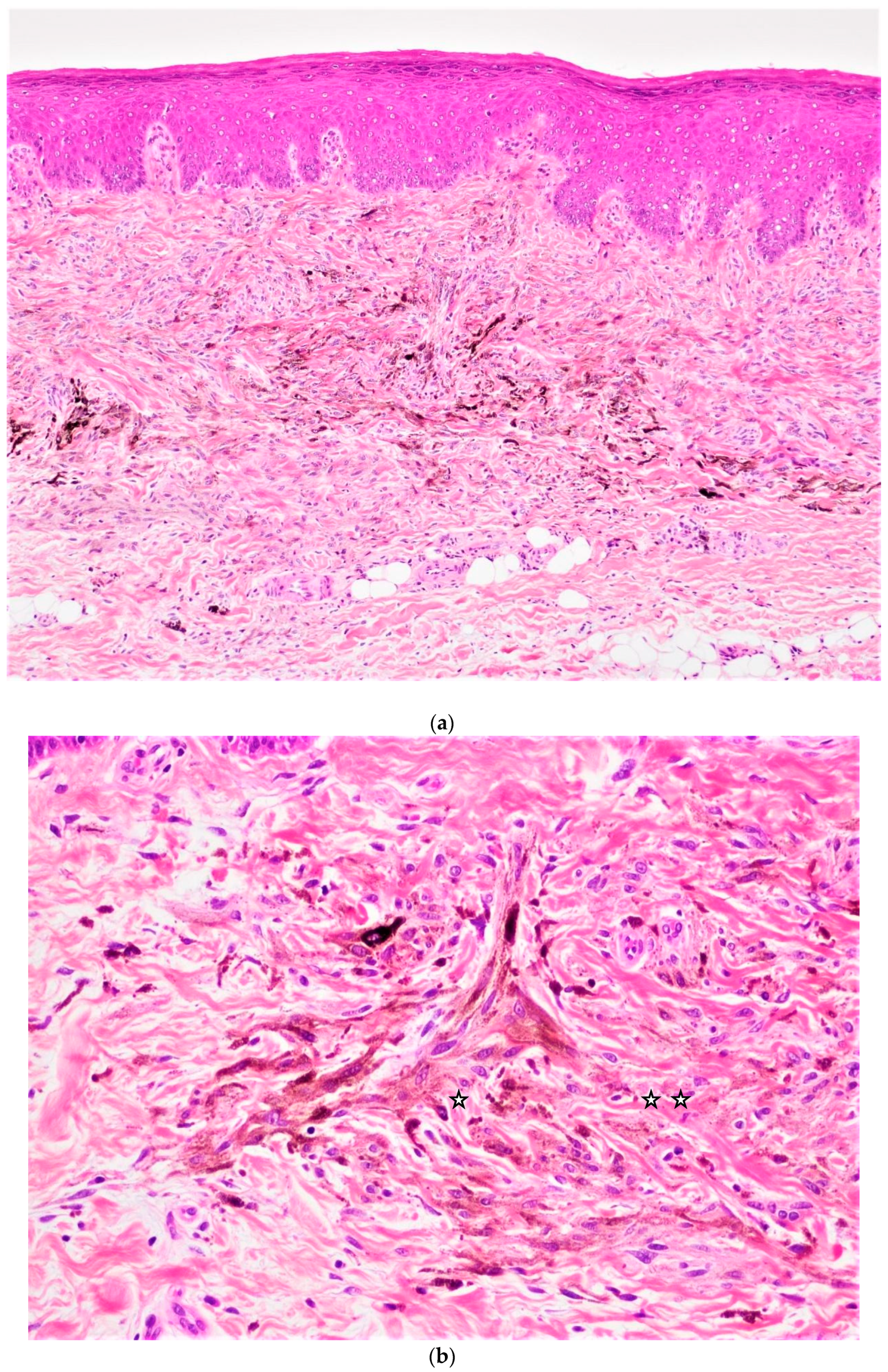

| No. | Age | Sex | Location | Clinical Presentation | Size | Gross | Recurrence /Follow-Up |
|---|---|---|---|---|---|---|---|
| 1 | 52 | Male | Palate, left side distal 25 | Blackish-grey patch | - | 0.6 × 0.4 × 0.5 cm | No/15 years |
| 2 | 26 | Female | Palate, median | - | - | 0.6 × 0.4 × 0.1 cm | No/13 years |
| 3 | 44 | Female | Palate, right side facing 17–18 | Blackish patch | 4 × 2 mm | 0.8 × 0.6 × 0.2 cm | No/11 years |
| 4 | 25 | Female | Palate, ant., right side | Blackish patch | 2 mm | 0.6 × 0.3 × 0.1 cm | No/11 years |
| 5 | 19 | Female | Palate, left side, facing 25 | - | - | 0.6 × 0.5 × 0.2 cm | No/5 years |
| 6 | 39 | Female | Palate, post, right side | Black patch | 5 × 1 mm | 0.5 × 0.3 × 0.2 cm | No/4 years |
| 7 | 22 | Male | Palate, left side | Bluish patch | 4 × 3 mm | 0.6 × 0.6 × 0.2 cm | - |
| 8 | 32 | Female | Palate | Bluish patch | 3 × 4 mm | 0.5 × 0.6 cm | No/8 years |
| Article (Total Number of Cases) [Reference] | Intramucosal Nevi | Junctional Nevi | Compound Nevi | DBN | CBN | ABN | EBN | Dysplastic Nevi | Spitz Nevi | Combined |
|---|---|---|---|---|---|---|---|---|---|---|
| Buchner et al. (191) [13] | 105 | 10 | 12 | 61 | 0 | 0 | 0 | 0 | 0 | 03 |
| Meleti et al. (119) [11] | 96 | 05 | 07 | 10 | 0 | 0 | 0 | 0 | 0 | 01 |
| Ferreira et al. (100) [9] | 61 | 03 | 07 | 23 | 02 | 0 | 0 | 02 | 0 | 02 |
| Amérigo-Góngora M et al. (10) [15] | 04 | 01 | 02 | 03 | 0 | 0 | 0 | 0 | 0 | 0 |
| Albuquerque DM et al. (71) [16] | 30 | 03 | 09 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Jayasooriya et al. (30) present study | 19 | 0 | 03 | 08 | 0 | 0 | 0 | 0 | 0 | 0 |
| Feature | Intramucosal Nevi (n = 282) (%) | Common Blue Nevi (n = 128) (%) | X2 Test |
|---|---|---|---|
| Age in years | p = 0.007 | ||
| <20 | 20 (7.1) | 15 (11.7) | |
| 21–50 | 203 (72) | 72 (56.2) | |
| >51 | 59 (20.9) | 41 (32.1) | |
| Gender | p = 0.019 | ||
| Male | 82 (31.8) | 47 (44.8) | |
| Female | 176 (68.2) | 58 (55.2) | |
| * Unknown | 24 | 23 | |
| Site | p = 0.000 | ||
| Hard palate | 88 (31.4) | 85 (68.0) | |
| Gingiva | 63 (22.5) | 02 (1.6) | |
| Buccal mucosa | 59 (21.1) | 16 (12.8) | |
| Vermillion border/labial mucosa | 70 (25.0) | 22 (17.6) | |
| Other | 02 | 03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasooriya, P.R.; Mendis, B.R.R.N.; Lombardi, T. A Case Series on Intraoral Blue Nevi with a Literature Review-Based Clinico-Pathologic Comparison of Intraoral Blue Nevi with Other Oral Melanocytic Nevi. Appl. Sci. 2023, 13, 4420. https://doi.org/10.3390/app13074420
Jayasooriya PR, Mendis BRRN, Lombardi T. A Case Series on Intraoral Blue Nevi with a Literature Review-Based Clinico-Pathologic Comparison of Intraoral Blue Nevi with Other Oral Melanocytic Nevi. Applied Sciences. 2023; 13(7):4420. https://doi.org/10.3390/app13074420
Chicago/Turabian StyleJayasooriya, Primali. R., B. Ranjit. R. N. Mendis, and Tommaso Lombardi. 2023. "A Case Series on Intraoral Blue Nevi with a Literature Review-Based Clinico-Pathologic Comparison of Intraoral Blue Nevi with Other Oral Melanocytic Nevi" Applied Sciences 13, no. 7: 4420. https://doi.org/10.3390/app13074420
APA StyleJayasooriya, P. R., Mendis, B. R. R. N., & Lombardi, T. (2023). A Case Series on Intraoral Blue Nevi with a Literature Review-Based Clinico-Pathologic Comparison of Intraoral Blue Nevi with Other Oral Melanocytic Nevi. Applied Sciences, 13(7), 4420. https://doi.org/10.3390/app13074420