Roles of Marine Shellfish Proteins with High Contents of Angiotensin-Converting Enzyme (ACE)-Binding Peptides in Nutrition Support for Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Silico Proteolysis
2.2. Molecular Docking
2.3. In Vitro ACE Activity
2.4. Statistical Analysis
3. Results
3.1. In Silico Gastrointestinal Enzyme Hydrolysis
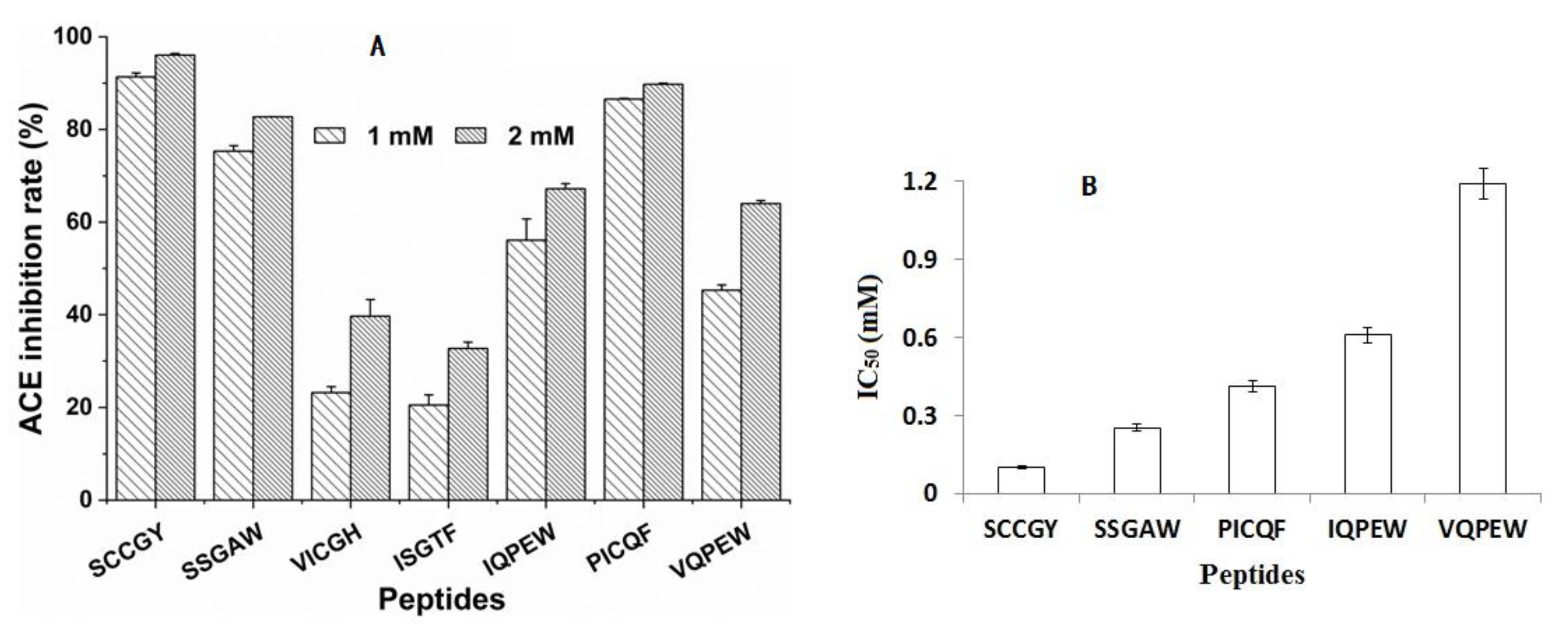
3.2. Binding Ability of the Digested Peptides to Angiotensin-Converting Enzyme
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Ferreira-Santos, P.; Carron, R.; Recio, I.; Sevilla, M.Á.; Montero, M.J. Effects of milk casein hydrolyzate supplemented with phytosterols on hypertension and lipid profile in hypercholesterolemic hypertensive rats. J. Funct. Food 2017, 28, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Aluko, R.E. Food protein-derived renin-inhibitory peptides: In vitro and in vivo properties. J. Food Biochem. 2019, 43, e12648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Gao, X.; Wei, Y.; Liu, Q.; Jiang, Y.; Zhao, L.; Ulaah, S. Isolation, purification and the anti-hypertensive effect of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from Ruditapes philippinarum fermented with Ba-cillus natto. Food Funct. 2018, 9, 5230–5237. [Google Scholar] [CrossRef]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Ann. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Sheih, I.; Fang, T.; Wu, T. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Q.; Xue, B.; Chen, L.; Wang, Y.; Ou, S.; Peng, X. Angiotensin-i-converting enzyme inhibitory activities and in vivo antihypertensive effects of sardine protein hydrolysate. J. Food Sci. 2016, 81, H2831–H2840. [Google Scholar] [CrossRef]
- Ngo, D.; Vo, T.; Ryu, B.; Kim, S. Angiotensin-I-converting enzyme (ACE) inhibitory peptides from Pacific cod skin gel-atin using ultrafiltration membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.B.; Jeon, Y.J.; Kim, Y.T.; Je, J.Y. Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochem. 2012, 47, 2240–2245. [Google Scholar] [CrossRef]
- Vishkaei, M.S.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I converting enzyme (ACE) Inhibitory and anti-hypertensive effect of protein hydrolysate from actinopyga lecanora (sea cucumber) in rats. Mar. Drugs 2016, 14, 176. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs. 2018, 16, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Lan, X.; Yaseen, M.; Wu, S.; Feng, X.; Zhou, L.; Sun, J.; Liao, A.; Liao, D.; Sun, L. Purification, Characterization and Evaluation of Inhibitory Mechanism of ACE Inhibitory Peptides from Pearl Oyster (Pinctada fucata martensii) Meat Protein Hydrolysate. Mar. Drugs. 2019, 17, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenigwe, C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Tech. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Lafarga, T.; O’Connor, P.; Hayes, M. Identification of novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides 2014, 59, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’Shea, N.; Gallagher, E.; Lafarga, T. Predicted release and analysis of novel ACE-I, renin, and DPP-IV inhibitory peptides from common oat (Avena sativa) protein hydrolysates using in silico analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, K.; Liu, Q.; Zhang, X. Discovery of monoamine oxidase A inhibitory peptides from hairtail (Trichiurus japonicus) using in vitro simulated gastrointestinal digestion and in silico studies. Bioorg. Chem. 2020, 101, 104032. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.Y. HPEPDOCK: A web server for blind peptide-protein docking based on a hierarchical al-gorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, S.; Xie, X.N.; Tan, Z.R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 2017, 11, 3511–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, J.P.; Brayden, D.J.; Ryan, S.M. Evaluation of PepT1 transport of food-derived antihypertensive peptides, Ile-Pro-Pro and Leu-Lys-Pro using in vitro, ex vivo and in vivo transport models. Eur. J. Pharm. Biopharm. 2017, 115, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chothe, P.; Singh, N.; Ganapathy, V. Evidence for two different broadspecificity oligopeptide transporters in intesti-nal cell line Caco-2 and colonic cell line CCD841. Am. J. Physiol. Cell Physiol. 2011, 300, C1260–C1269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Sun, S.S.; Li, Y.S.; Liu, R. Proteolysis of tilapia skin collagen: Identification and release behavior of ACE-inhibitory peptides. LWT Food Sci. Technol. 2021, 139, 110502. [Google Scholar] [CrossRef]
- Ding, Q.; Sheikh, A.R.; Chen, Q.; Hu, Y.; Sun, N.; Su, X.; Luo, L.; Ma, H.; He, R. Understanding the Mechanism for the Structure-Activity Relationship of Food-Derived ACEI Peptides. Food Rev. Int. 2021, 1936005. [Google Scholar] [CrossRef]
- Li, G.; Le, G.; Shi, Y.; Shrestha, S. Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Moskowitz, D.W. Is ‘somatic’ angiotensin I-converting enzyme a mechanosensor? Diabetes Technol. Ther. 2002, 4, 841–858. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Chen, F.; Ning, D.; Wang, Y.; Du, J.; Liu, F.; Zhao, L.; Cao, Y. Application and structure-activity relationship of antihy-pertensive peptides derived from milk protein. China Dairy Ind. 2015, 43, 29–33. [Google Scholar]
- Miralles, B.; Amigo, L.; Recio, I. Critical review and perspectives on food-derived antihypertensive peptides. J. Agric. Food Chem. 2018, 66, 9384–9390. [Google Scholar] [CrossRef]
- Je, J.-G.; Kim, H.-S.; Lee, H.-G.; Oh, J.-Y.; Lu, Y.A.; Wang, L.; Rho, S.; Jeon, Y.-J. Low-molecular weight peptides isolated from seahorse (Hippocampus abdominalis) improve vasodilation via inhibition of angiotensin-converting enzyme in vivo and in vitro. Process Biochem. 2020, 95, 30–35. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.C.; Kim, H.S.; Yang, H.W.; Ahn, G.; Lee, S.C.; Lee, T.G.; Lee, J.S.; Jeon, Y.J. Structural Evidence for Antihypertensive Effect of an Antioxidant Peptide Purified from the Edible Marine Animal Styela clava. J. Med. Food. 2020, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, J.; Cui, J.; Bai, X.; Du, Z.; Miyaguchi, Y.; Lin, B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 2008, 111, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Lin, T.; Chen, J.; Pan, B. The inhibitory effects of freshwater clam (Corbicula fluminea, Muller) muscle protein hy-drolysates on angiotensin I converting enzyme. Process Biochem. 2006, 41, 2276–2281. [Google Scholar] [CrossRef]
- Regulska, K.; Stanisz, B.; Regulski, M.; Murias, M. How to design a potent, specific, and stable angiotensin-converting enzyme inhibitor. Drug Discov. Today 2014, 19, 1731–1743. [Google Scholar] [CrossRef]
- Jimsheena, V.; Gowda, L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: Structure−activity relationship. Peptides 2010, 31, 1165–1176. [Google Scholar] [CrossRef]
- Bravo, R.K.D.; Angelia, M.R.N.; Uy, L.Y.C.; Garcia, R.N.; Torio, M.A.O. Isolation, purification and characterization of the antibac-terial, antihypertensive and antioxidative properties of the bioactive peptides in the purified and proteolyzed major storage protein of pigeon pea (Cajanus cajan) seeds. Food Chem. 2021, 4, 100062. [Google Scholar]



| Protein Names Amino Acids Length | Accession Number | Sequences of High-Affinity Oligopeptides (1 < Length < 6 and Docking Score < −110) Binding to Angiotensin-Converting Enzyme Binding Score | Frequency of High-Affinity Oligopeptide in a Protein (F) |
|---|---|---|---|
| Razor clam | |||
| Cytochrome b 381 | ACF41613.1 | AGTCL-129, VIQIL-163.2, SVSH-145.6, VGVSM-151.6, GIAF-158.5, VVVH-161.6, STGSN-138.9, GIVL-128.6, PDIF-171.3, DPVN-119.9, PADPM-154.1, TPTH-155.7, IQPEW-201.7, SIPN-134.8, GAGF-142.8, PICQF-206, VGSF-150.7, EVSIL-137.9, ASVIY-177.2, SPVIF-190.5, GDSY-136 | 0.0604 |
| NADH dehydrogenase subunit 6 176 | ACF41614.1 | CCW-160.8, ACIAL-144.9, ISVY-161.7, VGGVM-146.8, VGSSK-143.8, SDSTF-160.2, DSSIF-155.1, SEGW-161.5, SAVL-118.7, SGCSK-141.1, VPVDF-189.4 | 0.0625 |
| ATP synthase F0 subunit 6 232 | ACF41615.1 | GASIF-159.1, SCCGY-187.7, EAGY-145.2, SSVF-161.8, TTAAH-169.2, SIAL-137.4, QSIY-170.4, SIQGF-186, VAEGF-155.9, EIISY-172.7, CVVM-142, QVY-153.4, ATTR-152.7 | 0.0603 |
| Ferritin 171 | ACZ65230.1 | AETM-113.1, DDVAL-118.3, QPISK-157.9, GSGL-111.9, VADSH-148.3, GDAQM-135.7, EGEY-125.5, EEQV-112.7, EAIK-117.9 | 0.0526 |
| Lysozyme 2 141 | AYC12388.1 | IAVF-163.1, ESGCN-113.8, DVGSL-130.8, SCGY-157.8, SSTL-119.6 | 0.0355 |
| Clam | |||
| Cytochrome b 397 | YP_003934253.1 | PVPM-170.6, SIQL-137.8, ISGL-128.9, SVVH-152.3, SSGAW-184.5, SGVVL-139.3, GIAF-158.5, GTSN-115.2, DPIN-114.2, IQPEW201.7, SIPSK-150.2, IGDF-150.1, ASVVY-176.7, ASSSV-131.9 | 0.0352 |
| NADH dehydrogenase subunit 6 170 | YP_003934258.1 | TVGM-141, SIAL-137.4, IAQEN-136.8, GGSM-112.7, QGVL-122.7, SDDW-153.4 | 0.0353 |
| ATP synthase F0 subunit 6 245 | YP_003934254.1 | GSTSF-169.6, AGTPH-158.4, ICEM-130.8, SIPF-169.3, PVSM-175.4, PVTL-160.7, IISSL-159.9, SITL-130.9, SGVL-120.2, CSVF-152.9, VSY-149.7 | 0.0449 |
| Scallop | |||
| Cytochrome b 390 | YP_002640515.1 | SVSF-179.9, EVEM-111.7, SGVVL-139.3, DIAIW-180.6, VGVL-119.9, VVAF-162.7, VACDY-162.9, SVPSK-140.4, CSIL-132.3, CQCVF-187.5, SISK-135.4, DSEAK-116.4, GPTR-146.7, GSGR-143.1 | 0.0359 |
| NADH dehydrogenase subunit 1 315 | ABQ96662.1 | DTIF-141.7, SISF-168.3, VTVL-139.2, SVAY-165.8, GPVM-131.6, ADGIK-117.2, GPCF-155.6, GCGVY-164.3, SVGVY-180.2, GVVL-123.4, VISY-170.9, IVPF-168.8, SDVIF-166.2, VVPL-135.7, GSGGF-152.2, IAEY-144.4, TSVL-125.5, VISPF-177.6 | 0.0571 |
| ATP synthase F0 subunit 6 262 | ABQ96658.1 | SVSF-179.9, GAAF-144.2, SAAK-111.4, CSPF-156.3, A GGAH-139.3, GCTVL-139.9, SVEGF-165.6, SGIW-170.2, VVVL-132.7, SEVL-112.7, PVVL-153.2, VICGH-181.9, SSSPK-156.1 | 0.0496 |
| Superoxide dismutase 262 | QDX46961.1 | TTVM-138, SSIQN-147.5, GGGY-139.5, EPVN-117.6, TQEAL-145.7, GSGCY-164.1, PIEN-150, VISK-131.7, QDSPL-129.5, VIDVW-192.7, VSDW-170.6 | 0.0420 |
| 5’ AMP-activated protein kinase beta subunit 258 | QFR39801.1 | TTPK-126.7, GPSL-115, TAGR-141.4, DSASF-152, PTVF-180, ISGTF-182.3, ITIL-140.6, PEGEH-149.9, VDGQW-178.1, TVGTL-151.6, PGEK-117.8, QVIL-134.6, DTPAH-159.7, CEPTL-158.3, PEPN-134.5, SGTH-155.9, VTTL-131.5 | 0.0659 |
| Mussel | |||
| Cytochrome b 397 | AAT98404.1 | SVGPW-192.2, PCPVN-165.9, VIQL-139.6, DSVVH-154.1, GSSM-130.6, ICIY-180.2, TVCN-128.7, VAVVF-175.4, CVPF-160.2, VQPEW-199.6, SIPH-156.2, AGGVY-160.6, SIVVL-150.4, IPTL-145, QVVF-167.4, VGSF-150.7, IGAR-154.8, GSAF-147.4, GCEM-111.3 | 0.0479 |
| NADH dehydrogenase subunit 1 305 | AAV68406.1 | VGVL-119.9, AVGF-150.9, SIIM-148.9, GPSK-114.1, VSY-149.7, IVPTR-181.7, VGPF-153.4, APAVM-148.3, IISL-159.8, SAEVF-161.2, GVIL-125.8, GAVR-138.1, EIPM-130.6, CAGL-114, QEISM-149.3, QQGL-139.9, CGAL-114.4, VSGY-153.1, SGGGF-152.5, IAEY-144.4, SSIL-127.5, AVAIF-177.8 | 0.0721 |
| ATP synthase F0 subunit 6 238 | AAT98403.1 | DVF-123, SDVH-126.8, EQGL-123.2, VVVEL-140.5, ISGM-135.8, CSSEL-130.5, VVGW-176.6, SSSL-122.6, GGGVF-152.2, TEDH-142 | 0.0420 |
| Peptides | Sources | Protein | Docking Score |
|---|---|---|---|
| IQPEW | Razor clam, Clam | Cytochrome b | −201.7 |
| PICQF | Razor clam | Cytochrome b | −206 |
| SPVIF | Razor clam | Cytochrome b | −190.5 |
| VPVDF | Razor clam | NADH dehydrogenase | −189.4 |
| SCCGY | Razor clam | ATP synthase | −187.7 |
| SIQGF | Razor clam | ATP synthase | −186 |
| SSGAW | Clam | Cytochrome b | −184.5 |
| DIAIW | Scallop | Cytochrome b | −180.6 |
| CQCVF | Scallop | Cytochrome b | −187.5 |
| SVGVY | Scallop | NADH dehydrogenase | −180.2 |
| VICGH | Scallop | ATP synthase | −181.9 |
| VIDVW | Scallop | Superoxide dismutase | −192.7 |
| PTVF | Scallop | AMP-activated protein kinase | −180 |
| ISGTF | Scallop | AMP-activated protein kinase | −182.3 |
| SVGPW | Mussel | Cytochrome b | −192.2 |
| ICIY | Mussel | Cytochrome b | −180.2 |
| VQPEW | Mussel | Cytochrome b | −199.6 |
| IVPTR | Mussel | NADH dehydrogenase | −181.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Shu, T.; Wang, K.; Yuan, Z.; Zhang, X. Roles of Marine Shellfish Proteins with High Contents of Angiotensin-Converting Enzyme (ACE)-Binding Peptides in Nutrition Support for Hypertension. Appl. Sci. 2023, 13, 4654. https://doi.org/10.3390/app13084654
Yang L, Shu T, Wang K, Yuan Z, Zhang X. Roles of Marine Shellfish Proteins with High Contents of Angiotensin-Converting Enzyme (ACE)-Binding Peptides in Nutrition Support for Hypertension. Applied Sciences. 2023; 13(8):4654. https://doi.org/10.3390/app13084654
Chicago/Turabian StyleYang, Li, Tianyu Shu, Kai Wang, Zhen Yuan, and Xuewu Zhang. 2023. "Roles of Marine Shellfish Proteins with High Contents of Angiotensin-Converting Enzyme (ACE)-Binding Peptides in Nutrition Support for Hypertension" Applied Sciences 13, no. 8: 4654. https://doi.org/10.3390/app13084654
APA StyleYang, L., Shu, T., Wang, K., Yuan, Z., & Zhang, X. (2023). Roles of Marine Shellfish Proteins with High Contents of Angiotensin-Converting Enzyme (ACE)-Binding Peptides in Nutrition Support for Hypertension. Applied Sciences, 13(8), 4654. https://doi.org/10.3390/app13084654






