Influence of L-PRF Topical Application on Bone Tissue Healing after Surgical Extraction of Impacted Mandibular Third Molars: Randomized Split-Mouth Clinical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Randomization
2.2. Preparation of Leukocyte- and Platelet-Rich Fibrin
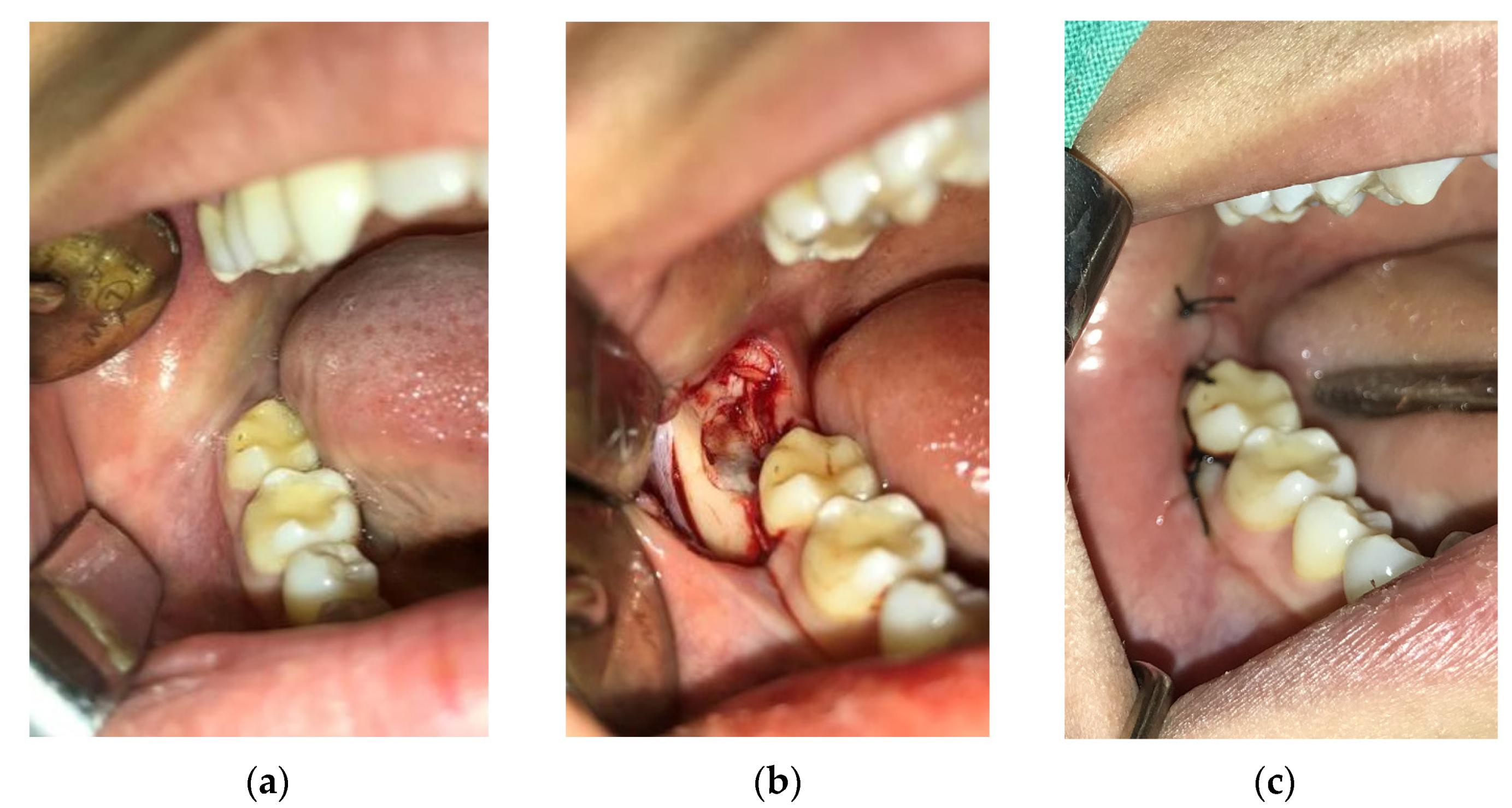
2.3. Surgical Procedure
2.4. Postoperative Period and Control Check-Ups of Patients
2.5. Analysis and Processing of Data from CBCT Images
2.6. Statistical Analysis
3. Results
3.1. Reconstruction of Surface 3D Models of Cavites Based on CBCT Data
3.2. Extraction of Surface 3D Cavity Model
3.3. Measurement of Bone Density in Cavities
3.4. Statistical Analysis
3.4.1. Analysis of Parameters in the I Measurement
3.4.2. Analysis of Parameters in the II Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srinivas, B.; Das, P.; Rana, M.M.; Qureshi, A.Q.; Vaidya, K.C.; Raziuddin, S.J.A. Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann. Maxillo-Facial Surg. 2018, 8, 28–34. [Google Scholar]
- Araújo, M.G.; da Silva, J.C.C.; de Mendonça, A.F.; Lindhe, J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin. Oral Implants Res. 2015, 26, 407–412. [Google Scholar] [CrossRef]
- Ivanova, V.; Chenchev, I.; Zlatev, S.; Mijiritsky, E. Comparison Study of the Histomorphometric Results after Socket Preservation with PRF and Allograft Used for Socket Preservation—Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 7451. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Bone Recontouring in Fresh Sockets with Buccal Bone Loss: A Cone Beam Computed Tomography Study. Int. J. Oral Maxillofac. Implants 2014, 29, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Omran, M.; Min, S.; Abdelhamid, A.; Liu, Y.; Zadeh, H.H. Alveolar ridge dimensional changes following ridge preservation procedure: Part-2—CBCT 3D analysis in non-human primate model. Clin. Oral Implants Res. 2016, 27, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.; Scarfe, W.; Vaughn, V.; Jacobs, R. Cone Beam Computed Tomography in Implant Dentistry: A Systematic Review Focusing on Guidelines, Indications, and Radiation Dose Risks. Int. J. Oral Maxillofac. Implants 2014, 29, 55–77. [Google Scholar] [CrossRef]
- Chen, Y.W.; Finkelman, M.; Papaspirisdakos, P.; César-Neto, J.B.; Weber, H.P.; de Souza, A.B. Comparative analysis of dimensional alterations following extraction of maxillary molars using three-dimensional images’ superimposition: A CBCT study. Odontology 2021, 109, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Sokac, M.; Budak, I.; Puskar, T.; Mirkovic, S.; Santosi, Z.; Kuzmanovic, M.; Vukelic, D. Investigation of radiation level and assessment of dimensional accuracy of acquired CBCT images. Measurement 2020, 155, 107551. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge Alterations Post-extraction in the Esthetic Zone. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef] [Green Version]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. J. Prosthet. Dent. 2003, 23, 313–323. [Google Scholar]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar socket healing: What can we learn? Periodontology 2000, 68, 122–134. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chi, L.Y.; Lee, O.K. Revisit incidence of complications after impacted mandibular third molar extraction: A nationwide population-based cohort study. PLoS ONE 2021, 22, e0246625. [Google Scholar] [CrossRef] [PubMed]
- Couso-Queiruga, E.; Stuhr, S.; Tattan, M.; Chambrone, L.; Avila-Ortiz, G. Post-extraction dimensional changes: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 127–145. [Google Scholar] [CrossRef]
- Leblebicioglu, B.; Hegde, R.; Yildiz, V.O.; Tatakis, D.N. Immediate effects of tooth extraction on ridge integrity and dimensions. Clin. Oral Investig. 2015, 19, 1777–1784. [Google Scholar] [CrossRef]
- Farmer, M.; Darby, I. Ridge dimensional changes following single-tooth extraction in the aesthetic zone. Clin. Oral Implants Res. 2014, 25, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Araújo, M.G.; Simion, M. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implants Res. 2012, 23, 80–82. [Google Scholar] [CrossRef]
- Thoma, D.S.; Benić, G.I.; Zwahlen, M.; Hämmerle, C.H.F.; Jung, R.E. A systematic review assessing soft tissue augmentation techniques. Clin. Oral Implants Res. 2009, 20, 146–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of Postextraction Sockets Preserved with Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Girish Kumar, N.; Chaudhary, R.; Kumar, I.; Arora, S.S.; Kumar, N.; Singh, H. To assess the efficacy of socket plug technique using platelet rich fibrin with or without the use of bone substitute in alveolar ridge preservation: A prospective randomised controlled study. Oral Maxillofac. Surg. 2018, 22, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L. Relationship between the Buccal Bone Plate Thickness and the Healing of Postextraction Sockets with/without Ridge Preservation. Int. J. Periodontics Restor. Dent. 2014, 34, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Salama, M.; Chrepa, V.; Hinrichs, J.E.; Gaillard, P. A Randomized, Blinded, Controlled Clinical Study of Particulate Anorganic Bovine Bone Mineral and Calcium Phosphosilicate Putty Bone Substitutes for Socket Preservation. Int. J. Oral Maxillofac. Implants 2014, 29, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohl, S.; Binderman, I.; Tomac, J. Maintenance of Alveolar Ridge Dimensions Utilizing an Extracted Tooth Dentin Particulate Autograft and Platelet-Rich fibrin: A Retrospective Radiographic Cone-Beam Computed Tomography Study. Materials 2020, 13, 1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, I.A.; Monje, A. Guided Bone Regeneration in Alveolar Bone Reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López del Amo, F.; Monje, A. Efficacy of biologics for alveolar ridge preservation/reconstruction and implant site development: An American Academy of Periodontology best evidence systematic review. J. Periodontol. 2022, 93, 1827–1847. [Google Scholar] [CrossRef]
- Yoshpe, M.; Ruparel, N.; Einy, S.; Ganatra, S.; Kaufman, A.Y. Treatment of Necrotic Anterior and Posterior Teeth with Regenerative Endodontic Procedures Using PRF as a Scaffold: A Retrospective Study. Appl. Sci. 2022, 12, 6774. [Google Scholar] [CrossRef]
- Nair, U.P.; Shivamurthy, R.; Nagate, R.R.; Chaturvedi, S.; Al-Qahtani, S.M.; Magbol, M.A.; Gokhale, S.T.; Tikare, S.; Chaturvedi, M. Effect of Injectable Platelet-Rich Fibrin with a Nano-Hydroxyapatite Bone Graft on the Treatment of a Grade II Furcation. Defect. Bioeng. 2022, 9, 602. [Google Scholar] [CrossRef]
- Agrawal, A.A. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J. Clin. Cases 2017, 5, 159. [Google Scholar] [CrossRef]
- Hwan Jung, M.; Hun Lee, J.; Wadhwa, P.; Bo Jiang, H.; Jang, H.S.; Seok Lee, E. Bone Regeneration in Peri-Implant Defect Using Autogenous Tooth Biomaterial Enriched with Platelet-Rich Fibrin in Animal Model. Appl. Sci. 2020, 10, 1939. [Google Scholar] [CrossRef] [Green Version]
- Francisco, I.; Fernandes, M.H.; Vale, F. Platelet-Rich Fibrin in Bone Regenerative Strategies in Orthodontics: A Systematic Review. Materials 2020, 13, 1866. [Google Scholar] [CrossRef] [Green Version]
- Anitua, E.; Tejero, R.; Zalduendo, M.M.; Orive, G. Plasma Rich in Growth Factors Promotes Bone Tissue Regeneration by Stimulating Proliferation, Migration, and Autocrine Secretion in Primary Human Osteoblasts. J. Periodontol. 2013, 84, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, L. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, J.R. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef]
- Baca-Gonzalez, L.; Serrano Zamora, R.; Rancan, L.; González Fernández-Tresguerres, F.; Fernández-Tresguerres, I.; López-Pintor, R.M.; López-Quiles, J.; Leco, I.; Jesús Torres, J. Plasma rich in growth factors (PRGF) and leukocyte-platelet rich fibrin (L-PRF): Comparative release of growth factors and biological effect on osteoblasts. Int. J. Implant Dent. 2022, 8, 39. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Marques, J.A.; Esteves, M.; Sousa, V.; Palma, P.J.; Matos, S. Intentional Replantation as a Starting Approach for a Multidisciplinary Treatment of a Mandibular Second Molar: A Case Report. J. Clin. Med. 2022, 11, 5111. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Oh, J.H. New Resorbable Membrane Materials for Guided Bone Regeneration. Appl. Sci. 2018, 8, 2157. [Google Scholar] [CrossRef] [Green Version]
- Mozzati, M.; Gallesio, G.; Tumedei, M.; Del Fabbro, M. Concentrated Growth Factors vs. Leukocyte-and-Platelet-Rich Fibrin for Enhancing Postextraction Socket Healing. A Longitudinal Comparative Study. Appl. Sci. 2020, 10, 8256. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, Y.; Mo, A. Lateral Ridge Augmentation with Guided Bone Regeneration Using Particulate Bone Substitutes and Injectable Platelet-Rich Fibrin in a Digital Workflow: 6 Month Results of a Prospective Cohort Study Based on Cone-Beam Computed Tomography Data. Materials 2021, 14, 6430. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gyulai-Gaál, S.; Kivovics, M.; Jákob, N.P.; Hegedűs, C.; Szabó, B.T.; Dobó-Nagy, C.; Szabó, G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials 2021, 14, 1810. [Google Scholar] [CrossRef]
- Pirpir, C.; Yilmaz, O.; Candirli, C.; Balaban, E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int. J. Implant Dent. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trybek, G.; Rydlińska, J.; Aniko-Włodarczyk, M.; Jaroń, A. Effect of Platelet-Rich Fibrin Application on Non-Infectious Complications after Surgical Extraction of Impacted Mandibular Third Molars. Int. J. Environ. Res. Public Health 2021, 18, 8249. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.T.; de Almeida Barros Mourão, C.F.; Mello-Machado, R.C.; Montemezzi, P.; de Lima Barbosa, R.; Sartoretto, S.C.; Leite, P.E.C.; Javid, K.; Kawase, T.; Alves, G.G.; et al. Effects of Leukocyte-Platelet-Rich Fibrin (L–PRF) on Pain, Soft Tissue Healing, Growth Factors, and Cytokines after Third Molar Extraction: A Randomized, Split-Mouth, Double-Blinded Clinical Trial. Appl. Sci. 2021, 11, 1666. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Machut, K.; Żółtowska, A. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: 3-Dimensional Analysis Using Cone-Beam Computed Tomography on the Outcomes of Non-Surgical Endodontic Treatment Using A-PRF+ and Calcium Hydroxide: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 6092. [Google Scholar] [PubMed]
- Al-Hamed, F.S.; Tawfik, M.A.M.; Abdelfadil, E.; Al-Saleh, M.A.Q. Efficacy of Platelet-Rich Fibrin After Mandibular Third Molar Extraction: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1124–1135. [Google Scholar] [CrossRef]
- Yu, H.Y.; Chang, Y.C. A Bibliometric Analysis of Platelet-Rich Fibrin in Dentistry. Int. J. Environ. Res. Public Health 2022, 19, 12545. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Y.; Fu, Y.; Zou, D.; Lu, J.; Lyu, C. Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry. APL Bioeng. 2022, 6, 031503. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Yuan, X.; He, T.; Liu, H.; Wang, J.; Xu, B. Effect of platelet-rich fibrin on the control of alveolar osteitis, pain, trismus, soft tissue healing, and swelling following mandibular third molar surgery: An updated systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2021, 50, 398–406. [Google Scholar] [CrossRef]
- Xiang, X.; Shi, P.; Zhang, P.; Shen, J.; Kang, J. Impact of platelet-rich fibrin on mandibular third molar surgery recovery: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 163. [Google Scholar] [CrossRef] [Green Version]
- Starzyńska, A.; Kaczoruk-Wieremczuk, M.; Lopez, M.A.; Passarelli, P.C.; Adamska, P. The Growth Factors in Advanced Platelet-Rich Fibrin (A-PRF) Reduce Postoperative Complications after Mandibular Third Molar Odontectomy. Int. J. Environ. Res. Public Health 2021, 18, 13343. [Google Scholar] [CrossRef]
- Kwak, S.G.; Kim, J.H. Central limit theorem: The cornerstone of modern statistics. Korean J. Anesthesiol. 2017, 70, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Nikolidakis, D.; Mulder, J.; Jansen, J.A.; Creugers, N.H.J. Effect of platelet-rich plasma on bone regeneration in dentistry: A systematic review. Clin. Oral Implants Res. 2008, 19, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.; Ritto, F.; Canellas, J.V.; Junger, B.; Cruz, M.; Medeiros, P.J. Re: Randomized double-blind clinical trial evaluation of bone healing after third molar surgery with the use of leukocyte- and platelet-rich fibrin. Int. J. Oral Maxillofac. Surg. 2020, 49, 692. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: Sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Machut, K.; Zoltowska, A.; Pawlowska, E.; Derwich, M. Plasma Rich in Growth Factors in the Treatment of Endodontic Periapical Lesions in Adult Patients: Case Reports. Int. J. Mol. Sci. 2021, 22, 9458. [Google Scholar] [CrossRef] [PubMed]
- MacBeth, N.; Trullenque-Eriksson, A.; Donos, N.; Mardas, N. Hard and soft tissue changes following alveolar ridge preservation: A systematic review. Clin. Oral Implants Res. 2016, 28, 982–1004. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Karanxha, L.; Panda, S.; Bucchi, C.; Nadathur Doraiswamy, J.; Sankari, M.; Ramamoorthi, S.; Varghese, S.; Taschieri, S. Autologous platelet concentrates for treating periodontal infrabony defects. Cochrane Database Syst. Rev. 2018, 11, CD011423. [Google Scholar] [CrossRef]
- Zwittnig, K.; Kirnbauer, B.; Jakse, N.; Schlenke, P.; Mischak, I.; Ghanaati, S.; Al-Maawi, S.; Végh, D.; Payer, M.; Zrnc, T.A. Growth Factor Release within Liquid and Solid PRF. J. Clin. Med. 2022, 11, 5070. [Google Scholar] [CrossRef] [PubMed]
- Njokanma, A.R.; Fatusi, O.A.; Ogundipe, O.K.; Arije, O.O.; Akomolafe, A.G.; Kuye, O.F. Does platelet-rich fibrin increase bone regeneration in mandibular third molar extraction sockets? J. Korean Assoc. Oral Maxillofac. Surg. 2022, 48, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khare, G.; Arun Kumar, K.V. A Comparative Study of Platelet-Rich Fibrin and Platelet-Rich Fibrin with Hydroxyapatite to Promote Healing of Impacted Mandibular Third Molar Socket. J. Oral Maxillofac. Surg. 2020, 21, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, I.; Ciapiński, D.; Bąk, M.; Niedzielski, D. The Assessment of the Usefulness of Platelet-Rich Fibrin in the Healing Process Bone Resorption. Coatings 2022, 12, 247. [Google Scholar] [CrossRef]








| Bone Density (HU) | Volume (mm3) | Periodontal Pocket (mm) | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| I Measurement | −297.76 | −163.36 | 408.49 | 397.92 | 6.34 | 6.23 |
| II Measurement | 130.57 | 58.25 | 319.44 | 346.33 | 6.72 | 6.69 |
| Mean Value | std.d | Min | Max | c.var | conf.inter. | sk | ku | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | VOL (I) | 430.97 | 92.88 | 251.5 | 608.7 | 21.55 | 396.28 | 465.66 | −0.05 | −0.67 | 0.823 |
| density (I) | −89.23 | 71.86 | −297.8 | 44.9 | 80.53 | −116.07 | −62.39 | −0.48 | 0.87 | 0.977 | |
| pocket (I) | 6.27 | 2.21 | 3.0 | 11.0 | 35.29 | 5.44 | 7.09 | 0.49 | −0.88 | 0.510 | |
| Experimental Group | VOL (I) | 417.57 | 93.77 | 197.8 | 574.5 | 22.45 | 382.55 | 452.59 | −0.38 | −0.43 | 1.000 |
| density (I) | −94.00 | 71.36 | −251.2 | 76.0 | 75.92 | −120.65 | −67.34 | 0.25 | −0.10 | 0.783 | |
| pocket (I) | 6.67 | 2.52 | 3.0 | 13.0 | 37.85 | 5.72 | 7.61 | 0.58 | −0.32 | 0.622 | |
| Analysis | F | p |
|---|---|---|
| MANOVA | 0.299 | 0.826 |
| VOL (I) | 0.309 | 0.580 |
| Density (I) | 0.066 | 0.798 |
| Periodontal pocket (I) | 0.426 | 0.516 |
| Mean Value | std.d | Min | Max | c.var | conf.inter. | sk | ku | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | VOL (II) | 296.02 | 115.73 | 112.2 | 538.7 | 39.09 | 252.80 | 339.24 | 0.44 | −0.82 | 0.574 |
| density (II) | 134.69 | 63.59 | 23.9 | 310.2 | 47.22 | 110.93 | 158.44 | 0.67 | 0.92 | 0.565 | |
| pocket (II) | 4.32 | 1.76 | 1.0 | 9.0 | 40.87 | 3.66 | 4.98 | 0.69 | 0.48 | 0.516 | |
| Experimental Group | VOL (II) | 261.47 | 90.31 | 120.8 | 460.4 | 34.54 | 227.74 | 295.20 | 0.39 | −0.37 | 0.641 |
| density (II) | 131.33 | 74.96 | −55.2 | 319.5 | 57.08 | 103.33 | 159.33 | 0.24 | 1.17 | 0.778 | |
| pocket (II) | 3.83 | 1.60 | 1.0 | 8.0 | 41.72 | 3.24 | 4.43 | 0.84 | 0.51 | 0.095 | |
| Analysis | F | p |
|---|---|---|
| MANOVA | 0.800 | 0.499 |
| VOL (I) | 1.662 | 0.203 |
| Density (I) | 0.035 | 0.852 |
| Periodontal pocket (I) | 0.426 | 0.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadic, A.; Bajkin, B.; Mijatov, I.; Mirnic, J.; Vukoje, K.; Sokac, M.; Vukelic, D. Influence of L-PRF Topical Application on Bone Tissue Healing after Surgical Extraction of Impacted Mandibular Third Molars: Randomized Split-Mouth Clinical Study. Appl. Sci. 2023, 13, 4823. https://doi.org/10.3390/app13084823
Tadic A, Bajkin B, Mijatov I, Mirnic J, Vukoje K, Sokac M, Vukelic D. Influence of L-PRF Topical Application on Bone Tissue Healing after Surgical Extraction of Impacted Mandibular Third Molars: Randomized Split-Mouth Clinical Study. Applied Sciences. 2023; 13(8):4823. https://doi.org/10.3390/app13084823
Chicago/Turabian StyleTadic, Ana, Branislav Bajkin, Ivana Mijatov, Jelena Mirnic, Karolina Vukoje, Mario Sokac, and Djordje Vukelic. 2023. "Influence of L-PRF Topical Application on Bone Tissue Healing after Surgical Extraction of Impacted Mandibular Third Molars: Randomized Split-Mouth Clinical Study" Applied Sciences 13, no. 8: 4823. https://doi.org/10.3390/app13084823





