A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography
Abstract
:1. Introduction
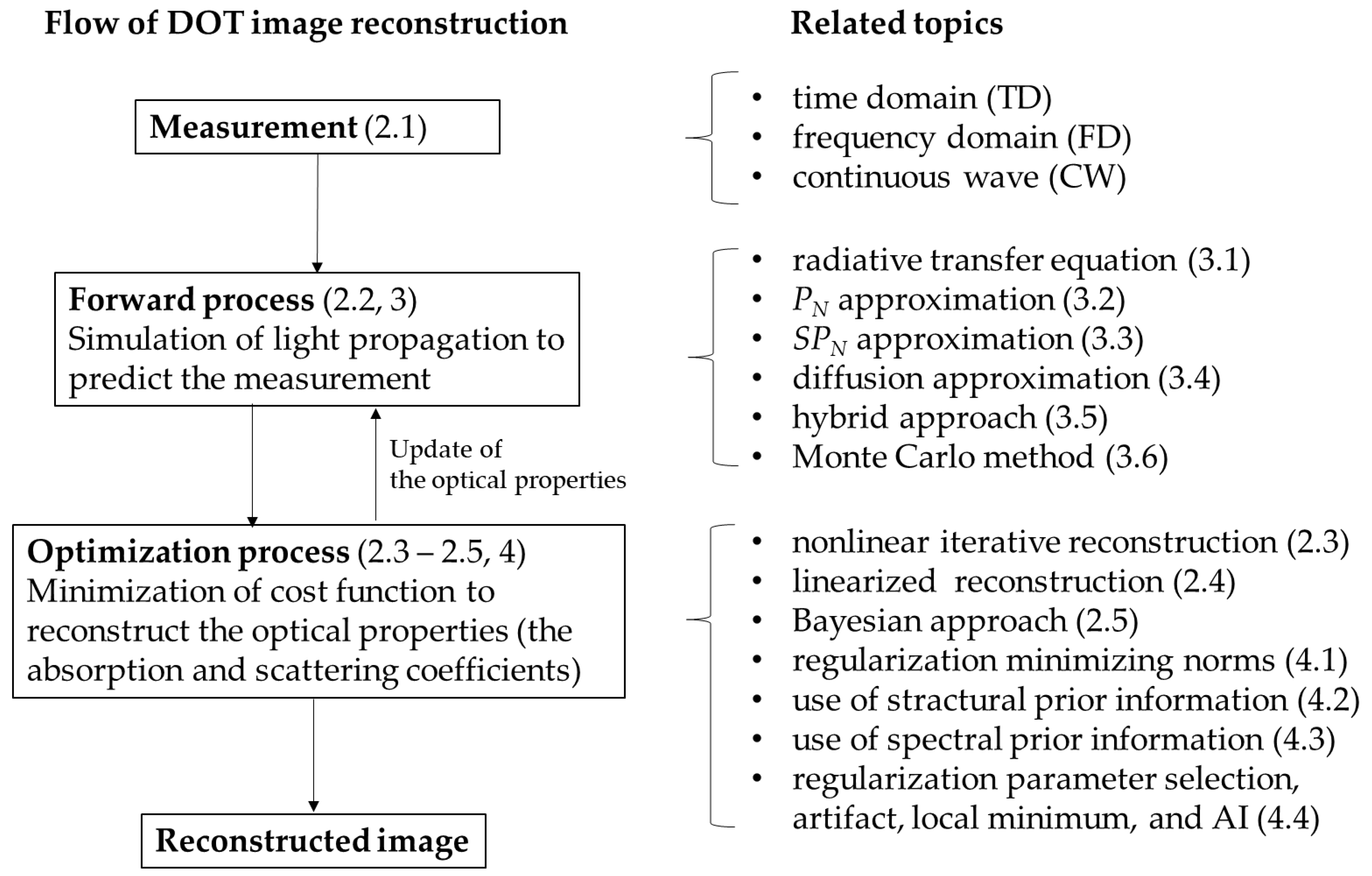
2. Outline of DOT Image Reconstruction
2.1. Measurement
2.2. Prediction of the Measurement: Forward Process
2.3. Optimization Process for Nonlinear Image Reconstruction
2.4. Optimization Process for Linearized Image Reconstruction
2.5. Bayesian Approach
3. Forward Process
3.1. Radiative Transfer Equation
3.2. PN Approximation
3.3. SPN Approximation
3.4. Diffusion Approximation
3.5. Hybrid Approach
3.6. Monte Carlo Simulation
4. Optimization Process
4.1. Use of Regularization Minimizing Norms
4.2. Use of Structural Prior Information
4.3. Use of Spectral Prior Information
4.4. Other Important Topics: Regularization Parameter, Artifacts, Local Minima, and AI
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hebden, J.C.; Arridge, S.R.; Delpy, D.T. Optical imaging in medicine: I. Experimental techniques. Phys. Med. Biol. 1997, 42, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R.; Hebden, J.C. Optical imaging in medicine: II. Modelling and reconstruction. Phys. Med. Biol. 1997, 42, 841–853. [Google Scholar] [CrossRef]
- Boas, D.A.; Brooks, D.H.; Miller, E.L.; DiMarzio, C.A.; Kilmer, M.; Gaudette, R.J.; Zhang, Q. Imaging the body with diffuse optical tomography. IEEE Sig. Process. Mag. 2001, 18, 57–75. [Google Scholar] [CrossRef]
- Hielscher, A.H.; Bluestone, A.Y.; Abdoulaev, G.S.; Klose, A.D.; Lasker, J.; Stewart, M.; Netz, U.; Beuthan, J. Near-infrared diffuse optical tomography. Dis. Markers 2002, 18, 313–337. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.P.; Hebden, J.C.; Arridge, S.R. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1–R43. [Google Scholar] [CrossRef]
- Yamada, Y.; Okawa, S. Diffuse optical tomography: Present status and its future. Opt. Rev. 2014, 21, 185–205. [Google Scholar] [CrossRef]
- Hoshi, Y.; Yamada, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef] [PubMed]
- Hawrysz, D.J.; Sevick-Muraca, E.M. Developments toward diagnostic breast cancer imaging using near-infrared optical measurements and fluorescent contrast agents. Neoplasia 2000, 2, 388–417. [Google Scholar] [CrossRef]
- Tromberg, B.J.; Pogue, B.W.; Paulsen, K.D.; Yodh, A.G.; Boas, D.A.; Cerussi, A.E. Assessing the future of diffuse optical imaging technologies for breast cancer management. Med. Phys. 2008, 35, 2443–2451. [Google Scholar] [CrossRef]
- Leff, D.R.; Warren, O.J.; Enfield, L.C.; Gibson, A.; Athanasiou, T.; Patten, D.K.; Hebden, J.; Yang, G.Z.; Darzi, A. Diffuse optical imaging of the healthy and diseased breast: A systematic review. Breast Cancer Res. Treat. 2008, 108, 9–22. [Google Scholar] [CrossRef]
- Taroni, P. Diffuse optical imaging and spectroscopy of the breast: A brief outline of history and perspectives. Photochem. Photobiol. Sci. 2012, 11, 241–250. [Google Scholar] [CrossRef]
- Grosenick, D.; Rinneberg, H.; Cubeddu, R.; Taroni, P. Review of optical breast imaging and spectroscopy. J. Biomed. Opt. 2016, 21, 091311. [Google Scholar] [CrossRef]
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. NeuroImage 2004, 23, S275–S288. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Cooper, R.J.; Austin, T. Diffuse optical tomography to investigate the newborn brain. Pediatr. Res. 2017, 82, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Sabir, S.; Heo, D.; Kim, K.H.; Wahab, A.; Choi, Y.; Lee, S.-I.; Chae, E.Y.; Kim, H.H.; Bae, Y.M.; et al. Deep Learning Diffuse Optical Tomography. IEEE Trans. Med. Imaging 2020, 39, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Ochoa, M.; Faulkner, D.; Haskins, G.; Intes, X. Deep learning in macroscopic diffuse optical imaging. J. Biomed. Opt. 2022, 27, 020901. [Google Scholar] [CrossRef]
- Balasubramaniam, G.M.; Wiesel, B.; Biton, N.; Kumar, R.; Kupferman, J.; Arnon, S. Tutorial on the use of deep learning in diffuse optical tomography. Electronics 2022, 11, 305. [Google Scholar] [CrossRef]
- Hauptmann, A.; Cox, B.T. Deep learning in photoacoustic tomography: Current approaches and future directions. J. Biomed. Opt. 2020, 25, 112903. [Google Scholar] [CrossRef]
- Deng, H.; Qiao, H.; Dai, Q.; Ma, C. Deep learning in photoacoustic imaging: A review. J. Biomed. Opt. 2021, 25, 040901. [Google Scholar] [CrossRef]
- Yajima, H.; Abe, M.; Umemura, M.; Takamizu, Y.; Hoshi, Y. TRINITY: A three-dimensional time-dependent radiative transfer code for in-vivo near-infrared imaging. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107948. [Google Scholar] [CrossRef]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Wang. L.V.; Fu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R. Optical tomography in medical imaging. Inverse Probl. 1999, 15, R41–R93. [Google Scholar] [CrossRef]
- Arridge, S.R.; Schotland, J.C. Optical tomography: Forward and inverse problems. Inverse Probl. 2009, 25, 123010. [Google Scholar] [CrossRef]
- Dehghani, H.; Srinivasan, S.; Pogue, B.W.; Gibson, A. Numerical modelling and image reconstruction in diffuse optical tomography. Phil. Trans. R. Soc. A 2009, 367, 3073–3093. [Google Scholar] [CrossRef] [PubMed]
- Klose, A.D. The forward and inverse problem in tissue optics based on the radiative transfer equation: A brief review. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1852–1853. [Google Scholar] [CrossRef]
- Arridge, S.R. Methods in diffuse optical imaging. Phil. Trans. R. Soc. A 2011, 369, 4558–4576. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Arridge, S.R.; Delpy, D.T. Application of the finite element method for the forward and inverse models in optical tomography. J. Math. Imaging Vis. 1993, 3, 263–283. [Google Scholar] [CrossRef]
- Arridge, S.R.; Schweiger, M. A gradient-based optimisation scheme for optical tomography. Opt. Express 1998, 2, 213–226. [Google Scholar] [CrossRef]
- Boas, D.A. A fundamental limitation of linearized algorithms for diffuse optical tomography. Opt. Express 1997, 1, 404–413. [Google Scholar] [CrossRef]
- Ye, J.C.; Bouman, A.; Webb, K.J.; Millane, R.P. Bayesian optical diffusion imaging. Proc. SPIE 1999, 3816, 45–56. [Google Scholar]
- Shimokawa, T.; Kosaka, T.; Yamashita, O.; Hiroe, N.; Amita, T.; Inoue, Y.; Sato, M. Hierarchical Bayesian estimation improves depth accuracy and spatial resolution of diffuse optical tomography. Opt. Express 2012, 20, 20427–20446. [Google Scholar] [CrossRef]
- Mozumder, M.; Hauptmann, A.; Nissilä, I.; Arridge, S.R.; Tarvainen, T. A model-based iterative learning approach for diffuse optical tomography. IEEE Trans. Med. Imaging 2022, 41, 1289–1299. [Google Scholar] [CrossRef]
- Fujii, H.; Okawa, S.; Nadamoto, K.; Yamada, Y.; Hoshi, Y.; Watanabe, M. Numerical modeling of photon migration in human neck based on the radiative transport equation. J. Appl. Nonlinear Dyn. 2016, 5, 117–125. [Google Scholar] [CrossRef]
- Mimura, T.; Okawa, S.; Kawaguchi, H.; Tanikawa, Y.; Hoshi, Y. Imaging the human thyroid using three-dimensional diffuse optical tomography: A preliminary study. Appl. Sci. 2021, 11, 1670. [Google Scholar] [CrossRef]
- Klose, A.D.; Netz, U.; Beuthan, J.; Hielscher, A.H. Optical tomography using the time-independent equation of radiative transfer—Part 1: Forward model. J. Quant. Spectrosc. Radiat. Transf. 2002, 72, 691–713. [Google Scholar] [CrossRef]
- Klose, A.D.; Hielscher, A.H. Optical tomography using the time-independent equation of radiative transfer—Part 2: Inverse model. J. Quant. Spectrosc. Radiat. Transf. 2002, 72, 715–732. [Google Scholar] [CrossRef]
- Abdoulaev, G.A.; Hielscher, A.H. Three-dimensional optical tomography with the equation of radiative transfer. J. Electron. Imaging 2003, 12, 594–601. [Google Scholar] [CrossRef]
- Tarvainen, T.; Vauhkonen, M.; Arridge, S.R. Gauss–Newton reconstruction method for optical tomography using the finite element solution of the radiative transfer equation. J. Quant. Spectrosc. Radiat. Transf. 2008, 109, 2767–2778. [Google Scholar] [CrossRef]
- Soloviev, V.Y.; Arridge, S.R. Optical Tomography in weakly scattering media in the presence of highly scattering inclusions. Biomed. Opt. Express 2011, 2, 440–451. [Google Scholar] [CrossRef]
- Machida, M.; Panasyuk, G.Y.; Wang, Z.-M.; Markel, V.A.; Schotland, J.C. Radiative transport and optical tomography with large datasets. J. Opt. Soc. Am. A 2016, 33, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Machida, M. An FN method for the radiative transport equation in three dimensions. J. Phys. A Math. Theor. 2015, 48, 325001. [Google Scholar] [CrossRef]
- Machida, M. Numerical algorithms of the radiative transport equation using rotated reference frames for optical tomography with structured illumination. J. Quant. Spectrosc. Radiat. Transf. 2019, 234, 124–138. [Google Scholar] [CrossRef]
- Hielscher, A.H.; Kim, H.K.; Montejo, L.D.; Blaschke, S.; Netz, U.J.; Zwaka, P.A.; Illing, G.; Müller, G.A.; Beuthan, J. Frequency-domain optical tomographic imaging of arthritic finger joints. IEEE Trans. Med. Imaging 2011, 30, 1725–1736. [Google Scholar] [CrossRef]
- Montejo, L.D.; Jia, J.; Kim, H.K.; Netz, U.J.; Blaschke, S.; Mueller, G.A.; Hielscher, A.H. Computer-aided diagnosis of rheumatoid arthritis with optical tomography, Part 1: Feature extraction. J. Biomed. Opt. 2013, 18, 076001. [Google Scholar] [CrossRef] [PubMed]
- Montejo, L.D.; Jia, J.; Kim, H.K.; Netz, U.J.; Blaschke, S.; Mueller, G.A.; Hielscher, A.H. Computer-aided diagnosis of rheumatoid arthritis with optical tomography, Part 2: Image classification feature extraction. J. Biomed. Opt. 2013, 18, 076002. [Google Scholar] [CrossRef]
- Darne, C.; Lu, Y.; Sevick-Muraca, E.M. Small animal fluorescence and bioluminescence tomography: A review of approaches, algorithms and technology update. Phys. Med. Biol. 2014, 59, R1–R64. [Google Scholar] [CrossRef]
- Klose, A.D.; Ntziachristos, V.; Hielscher, A.H. The inverse source problem based on the radiative transfer equation in optical molecular imaging. J. Comput. Phys. 2005, 202, 323–345. [Google Scholar] [CrossRef]
- Joshi, A.; Rasmussen, J.C.; Sevick-Muraca, E.M.; Wareing, T.A.; McGhee, J. Radiative Transport Based Frequency Domain Fluorescence Tomography. Phys. Med. Biol. 2008, 53, 2069–2088. [Google Scholar] [CrossRef]
- Cox, B.; Laufer, J.G.; Arridge, S.R.; Beard, P.C. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 061202. [Google Scholar] [CrossRef]
- Yao, L.; Sun, Y.; Jiang, H. Quantitative photoacoustic tomography based on the radiative transfer equation. Opt. Lett. 2009, 34, 1765–1767. [Google Scholar] [CrossRef]
- Yao, L.; Sun, Y.; Jiang, H. Transport-based quantitative photoacoustic tomography: Simulations and experiments. Phys. Med. Biol. 2010, 55, 1917–1934. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, T.; Cox, B.T.; Kaipio, J.P.; Arridge, S.R. Reconstructing absorption and scattering distributions in quantitative photoacoustic tomography. Inverse Probl. 2012, 28, 084009. [Google Scholar] [CrossRef]
- Saratoon, T.; Tarvainen, T.; Cox, B.T.; Arridge, S.R. A gradient-based method for quantitative photoacoustic tomography using the radiative transfer equation. Inverse Probl. 2013, 29, 075006. [Google Scholar] [CrossRef]
- Charette, A.; Boulanger, J.; Kim, H.K. An overview on recent radiation transport algorithm development for optical tomography imaging. J. Quant. Spectrosc. Radiat. Transf. 2008, 109, 2743–2766. [Google Scholar] [CrossRef]
- Boas, D.; Liu, H.; O’Leary, M.; Chance, B.; Yodh, A. Photon migration within the P3 approximation. Proc. SPIE 1995, 2389, 240–247. [Google Scholar]
- Jiang, H.; Paulsen, K. Finite-element-based higher order diffusion approximation of light propagation in tissues. Proc. SPIE 1995, 2389, 608–614. [Google Scholar]
- de Oliveria, C.; Tahir, K. Higher-order transport approximations for optical tomography applications. Proc. SPIE 1997, 3194, 212–218. [Google Scholar]
- Jiang, H. Optical image reconstruction based on the third-order diffusion equations. Opt. Express 1999, 4, 241–246. [Google Scholar] [CrossRef]
- Yuan, Z.; Hu, X.-H.; Jiang, H. A higher order diffusion model for three-dimensional photon migration and image reconstruction in optical tomography. Phys. Med. Biol. 2009, 54, 67–90. [Google Scholar] [CrossRef]
- Wright, S.; Schweiger, M.; Arridge, S.R. Reconstruction in optical tomography using the PN approximations. Meas. Sci. Technol. 2007, 18, 79–86. [Google Scholar] [CrossRef]
- Klose, A.D.; Larsen, E.W. Light transport in biological tissue based on the simplified spherical harmonics equations. J. Comput. Phys. 2006, 220, 441–470. [Google Scholar] [CrossRef]
- Chu, M.; Vishwanath, K.; Klose, A.D.; Dehghani, H. Light transport in biological tissue using three-dimensional frequency-domain simplified spherical harmonics equations. Phys. Med. Biol. 2009, 54, 2493–2509. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Dehghani, H. Image reconstruction in diffuse optical tomography based on simplified spherical harmonics approximation. Opt. Express 2009, 17, 24208–24223. [Google Scholar] [CrossRef]
- Domínguez, J.B.; Bérubé-Lauzière, Y. Diffuse light propaga tion in biological media by a time-domain parabolic simplified spherical harmonics approximation with ray-divergence effects. Appl. Opt. 2010, 49, 1414–1429. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.B.; Bérubé-Lauzière, Y. Diffuse optical tomographic imaging of biological media by time-dependent parabolic SPN equations: A two-dimensional study. J. Biomed. Opt. 2012, 17, 086012. [Google Scholar] [CrossRef] [PubMed]
- Klose, A.D.; Pöschinger, T. Excitation-resolved fluorescence tomography with simplified spherical harmonics equations. Phys. Med. Biol. 2011, 56, 1443–1469. [Google Scholar] [CrossRef] [PubMed]
- Naik, N.; Patil, N.; Yadav, Y.; Eriksson, J.; Pradhan, A. Fully nonlinear SP3 approximation based fluorescence optical tomography. IEEE Trans. Med. Imaging 2017, 36, 2308–2318. [Google Scholar] [CrossRef]
- Frederick, C.; Ren, K.; Vallélian, S. Image reconstruction in quantitative photoacoustic tomography with the simplified P2 approximation. SIAM J. Imaging Sci. 2018, 11, 2847–2876. [Google Scholar] [CrossRef]
- Arridge, S.R. Photon measurement density functions. Part 1: Analytical forms. Appl. Opt. 1995, 34, 7395–7409. [Google Scholar] [CrossRef]
- Arridge, S.R.; Schweiger, M. Photon measurement density functions. Part 2: Finite element calculations. Appl. Opt. 1995, 34, 8026–8037. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, H.; Yamada, Y. Improvement of image quality in diffuse optical tomography by use of full time-resolved data. Appl. Opt. 2002, 41, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, F.; Tanikawa, Y.; Homma, K.; Yamada, Y. Time-resolved diffuse optical tomographic imaging for the provision of both anatomical and functional information about biological tissue. Appl. Opt. 2005, 44, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Del Bianco, S.; Ismaelli, A.; Zaccanti, G. Light Propagation through Biological Tissue and Other Diffusive Media Theory, Solutions, and Software; Society of Photo-Optical Instrumentation Engineering (SPIE) Press: Bellingham, WA, USA, 2010. [Google Scholar]
- Pogue, B.W.; Poplack, S.P.; McBride, T.O.; Wells, W.A.; Osterman, K.S.; Osterberg, U.L.; Paulsen, K.D. Quantitative hemoglobin tomography with diffuse near-infrared spectroscopy: Pilot results in the breast. Radiology 2001, 218, 261–266. [Google Scholar] [CrossRef]
- Hebden, J.C.; Gibson, A.; Md Yusof, R.; Everdell, N.; Hillman, E.M.C.; Delpy, D.T.; Arridge, S.R.; Austin, T.; Meek, J.H.; Wyatt, J.S. Three-dimensional optical tomography of the premature infant brain. Phys. Med. Biol. 2002, 47, 4155–4166. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.; Hebden, J.C.; Gibson, A.; Everdell, N.; Arridge, S.R.; Douek, M. Optical tomography of the breast using a multi-channel time-resolved imager. Phys. Med. Biol. 2005, 50, 2503–2517. [Google Scholar] [CrossRef]
- Eggebrecht, A.T.; Ferradal, S.L.; Robichaux-Viehoever, A.; Hassanpour, M.S.; Dehghani, H.; Snyder, A.Z.; Hershey, T.; Culver, J.P. Mapping distributed brain function and networks with diffuse optical tomography. Nat. Photonics 2014, 8, 448–454. [Google Scholar] [CrossRef]
- Frijia, E.M.; Billing, A.; Lloyd-Fox, S.; Rosas, E.V.; Collins-Jones, L.; Crespo-Llado, M.M.; Amadó, M.P.; Austin, T.; Edwards, A.; Dunne, L.; et al. Functional imaging of the developing brain with wearable high-density diffuse optical tomography: A new benchmark for infant neuroimaging outside the scanner environment. NeuroImage 2021, 225, 117490. [Google Scholar] [CrossRef]
- Schweiger, M.; Arridge, S.R. The Toast++ software suite for forward and inverse modeling in optical tomography. J. Boiomed. Opt. 2014, 19, 040801. [Google Scholar] [CrossRef] [PubMed]
- TOAST++ Image Reconstruction in Diffuse Optical Tomography. Available online: http://web4.cs.ucl.ac.uk/research/vis/toast/index.html (accessed on 14 February 2023).
- Dehghani, H.; Eames, M.E.; Yalavarthy, P.K.; Davis, S.C.; Srinivasan, S.; Carpenter, C.M.; Pogue, B.W.; Paulsen, K.D. Near infrared optical tomography using NIRFAST: Algorithm for numerical model and image reconstruction. Commun. Numer. Methods Eng. 2009, 25, 711–732. [Google Scholar] [CrossRef]
- NIRSAST. Open Source Software for Multi-Modal Optical Molecular Imaging. Available online: https://milab.host.dartmouth.edu/nirfast/ (accessed on 14 February 2023).
- Tarvainen, T.; Vauhkonen, M.; Kolehmainen, V.; Kaipio, J.P. Hybrid radiative-transfer–diffusion model for optical tomography. Appl. Opt. 2005, 44, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, T.; Vauhkonen, M.; Kolehmainen, V.; Kaipio, J.P. Finite element model for the coupled radiative transfer equation and diffusion approximation. Int. J. Numer. Methods Eng. 2006, 65, 383–405. [Google Scholar] [CrossRef]
- Fujii, H.; Okawa, S.; Yamada, Y.; Hoshi, Y. Hybrid model of light propagation in random media based on the time-dependent radiative transfer and diffusion equations. J. Quant. Spectrosc. Radiat. Transf. 2014, 147, 145–154. [Google Scholar] [CrossRef]
- Tarvainen, T.; Kolehmainen, V.; Arridge, S.R.; Kaipio, J.P. Image reconstruction in diffuse optical tomography using the coupled radiative transport–diffusion model. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 2600–2608. [Google Scholar] [CrossRef]
- Farlow, S.J. Partial Differential Equations for Scientists and Engineers; Dover Publications, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Periyasamy, V.; Pramanik, M. Advances in Monte Carlo simulation for light propagation in tissue. IEEE Rev. Biomed. Eng. 2017, 10, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Jacques, S.L.; Zheng, L.-Q. MCML—Monte Carlo modeling of photon transport in multi-layered tissues. Comput. Methods Prog. Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Monte Carlo Light Scattering Programs. Monte Carlo Light Scattering Programs. Available online: https://omlc.org/software/mc/ (accessed on 14 February 2023).
- MCX Monte Carlo eXtreme. A GPU-Accelerated Photon Transport Simulator. Available online: http://mcx.space/ (accessed on 14 February 2023).
- Jacques, S.L. History of Monte Carlo modeling of light transport in tissues using mcml.c. J. Biomed. Opt. 2022, 27, 083002. [Google Scholar] [CrossRef]
- Flock, S.T.; Patterson, M.S.; Wilson, B.C.; Wyman, D.R. Monte Carlo modeling of light propagation in highly scattering tissues. I. Model predictions and comparison with diffusion theory. IEEE Trans. Biomed. Eng. 1989, 36, 1162–1168. [Google Scholar] [CrossRef]
- Pogue, B.W.; Testorf, M.; McBride, T.; Osterberg, U.; Paulsen, K. Instrumentation and design of a frequency-domain diffuse optical tomography imager for breast cancer detection. Opt. Express 1997, 1, 391–403. [Google Scholar] [CrossRef]
- Iranmahboob, A.K.; Hillman, E.M.C. Diffusion vs. Monte Carlo for image reconstruction in mesoscopic volumes. In Biomedical Optics; Paper BSuE34; OSA Technical Digest (CD); Optica Publishing Group: Washington, DC, USA, 2008. [Google Scholar]
- Okawa, S.; Hirasawa, T.; Kushibiki, T.; Ishihara, M. Effects of the approximations of light propagation on quantitative photoacoustic tomography using two-dimensional photon diffusion equation and linearization. Opt. Rev. 2017, 24, 705–726. [Google Scholar] [CrossRef]
- Hayakawa, C.K.; Spanier, J.; Bevilacqua, F.; Dunn, A.K.; You, J.S.; Tromberg, B.J.; Venugopalan, V. Perturbation Monte Carlo methods to solve inverse photon migration problems in heterogeneous tissues. Opt. Lett. 2001, 26, 1335–1337. [Google Scholar] [CrossRef]
- Kumar, Y.P.; Vasu, R.M. Reconstruction of optical properties of low-scattering tissue using derivative estimated through perturbation Monte-Carlo method. J. Biomed. Opt. 2004, 9, 1002–1012. [Google Scholar] [CrossRef]
- Boas, D.A.; Culver, J.P.; Stott, J.J.; Dunn, A.K. Three dimensional Monte Carlo code for photon migration through complex heterogeneous media including the adult human head. Opt. Express 2002, 10, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Custo, A.; Boas, D.A.; Tsuzuki, D.; Dan, I.; Mesquita, R.; Fischl, B.; Grimson, W.E.L.; Wells, W., III. Anatomical atlas-guided diffuse optical tomography of brain activation. NeuroImage 2010, 49, 561–567. [Google Scholar] [CrossRef]
- Okawa, S.; Yamada, Y. Reconstruction of fluorescence/bioluminescence sources in biological medium with spatial filter. Opt. Express 2010, 13, 13151–13172. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.L. Image Reconstruction: Applications in Medical Sciences; Walter de Gruyter GmbH: Berlin, Germany, 2017. [Google Scholar]
- Süzen, M.; Giannoula, A.; Durduran, T. Compressed sensing in diffuse optical tomography. Opt. Express 2010, 18, 23676–23690. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. J. Magn. Reson. Imaging 2007, 58, 1182–1195. [Google Scholar] [CrossRef]
- Shaw, C.B.; Yalavarthy, P.K. Effective contrast recovery in rapid dynamic near-infrared diffuse optical tomography using ℓ1-norm-based linear image reconstruction method. J. Biomed. Opt. 2012, 17, 086009. [Google Scholar] [CrossRef] [PubMed]
- YALL1: Your ALgorithms for L1. Available online: https://yall1.blogs.rice.edu/ (accessed on 14 February 2023).
- Yang, J.; Zhang, Y. Alternating direction algorithms for L1-problems in compressive sensing. SIAM J. Sci. Comput. 2011, 33, 250–278. [Google Scholar] [CrossRef]
- Kavuri, V.C.; Lin, Z.-J.; Tian, F.; Liu, H. Sparsity enhanced spatial resolution and depth localization in diffuse optical tomography. Biomed. Opt. Express 2012, 3, 943–957. [Google Scholar] [CrossRef]
- Cao, N.; Nehorai, A.; Jacob, M. Image reconstruction for diffuse optical tomography using sparsity regularization and expectation-mximization algorithm. Opt. Express 2007, 15, 13695–13708. [Google Scholar] [CrossRef]
- Okawa, S.; Hoshi, Y.; Yamada, Y. Improvement of image quality of time-domain diffuse optical tomography with lp sparsity regularization. Biomed. Opt. Express 2011, 2, 3334–3348. [Google Scholar] [CrossRef]
- He, Z.; Cichocki, A.; Zdunek, R.; Xie, S. Improved FOCCUS method with conjugate gradient iterations. IEEE Trans. Signal Process. 2009, 57, 399–404. [Google Scholar]
- Prakash, J.; Shaw, C.B.; Manjappa, R.; Kanhirodan, R.; Yalavarthy, P.K. Sparse Recovery Methods Hold Promise for Diffuse Optical Tomographic Image Reconstruction. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 6800609. [Google Scholar] [CrossRef]
- Chen, C.; Tian, F.; Liu, H.; Huang, J. Diffuse optical tomography by clustered sparsity for functional brain imaging. IEEE. Trans. Med. Imaging 2014, 33, 2323–2331. [Google Scholar] [CrossRef]
- Lu, W.; Lighter, D.; Styles, I.B. L1-norm based nonlinear reconstruction improves quantitative accuracy of spectral diffuse optical tomography. Biomed. Opt. Express 2018, 9, 1423–1444. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.R. Computational Methods for Inverse Problems (Frontiers in Applied Mathematics); Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2002. [Google Scholar]
- Paulsen, K.D.; Jiang, H. Enhanced frequency-domain optical image reconstruction in tissues through total-variation minimization. Appl. Opt. 1996, 35, 3447–3458. [Google Scholar] [CrossRef]
- Douiri, A.; Schweiger, M.; Riley, J.; Arridge, S. Local diffsion regularization method for optical tomography reconstruction by using robust statistics. Opt. Lett. 2005, 30, 2439–2441. [Google Scholar] [CrossRef]
- Douiri, A.; Schweiger, M.; Riley, J.; Arridge, S. Anisotropic diffusion regularization methods for diffuse optical tomography using edge prior information. Meas. Sci. Technol. 2007, 18, 87–95. [Google Scholar] [CrossRef]
- Schweiger, M.; Arridge, S.R. Optical tomographic reconstruction in a complex head model using a priori region boundary information. Phys. Med. Biol. 1999, 44, 2703–2721. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Pogue, B.W.; Shudong, J.; Brooksby, B.; Paulsen, K.D. Three-dimensional optical tomography: Resolution in small-object imaging. Appl. Opt. 2003, 42, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Yodh, A.G.; Schnall, M.D.; Chance, B. MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions. Neoplasia 2002, 4, 347–354. [Google Scholar] [CrossRef]
- Boverman, G.; Miller, E.L.; Li, A.; Zhang, Q.; Chaves, T.; Brooks, D.H.; Boas, D.A. Quantitative spectroscopic diffuse optical tomography of the breast guided by imperfect a priori structural information. Phys. Med. Biol. 2005, 50, 3941–3956. [Google Scholar] [CrossRef]
- Di Sciacca, G.; Maffeis, G.; Frina, A.; Mora, A.D.; Pifferi, A.; Taroni, P.; Arridge, S. Evaluation of a pipline for simulaton, reconstruction, and classification in ultrasound-aided diffuse optical tomography of breast tumors. J. Biomed. Opt. 2022, 27, 036003. [Google Scholar] [CrossRef]
- k-Wave: A MATLAB Toolbox for the Time-Domain Simulation of Acoustic Wave Fields. Available online: http://www.k-wave.org/ (accessed on 14 February 2023).
- Yalavarthy, P.K.; Pogue, B.W.; Dehghani, H.; Carpenter, C.M.; Jiang, S.; Paulsen, K.D. Structural information within regularization matrices improved near infrared diffuse optical tomography. Opt. Express 2007, 15, 8043–8058. [Google Scholar] [CrossRef]
- Yalavarthy, P.K.; Pogue, B.W.; Dehghani, H.; Paulsen, K.D. Weight-matrix structured regularization provides optimal generalized least-squares estimate in diffuse optical tomography. Med. Phys. 2007, 34, 2085–2097. [Google Scholar] [CrossRef]
- Brooksby, B.A.; Dehghani, H.; Pogue, B.W.; Paulsen, K.D. Near-infrared (NIR) tomography breast image reconstruction with a priori structural information from MRI: Algorithm development for reconstructing heterogeneities. IEEE J. Sel. Top. Quantum Electron. 2003, 9, 199–209. [Google Scholar] [CrossRef]
- Li, A.; Boverman, G.; Zhang, Y.; Brooks, D.; Miller, E.L.; Kilmer, M.E.; Zhang, Q.; Hillman, E.M.C.; Boas, D.A. Optimal linear inverse solution with multiple priors in diffuse optical tomography. Appl. Opt. 2005, 44, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Yazici, B.; Intes, X.; Chance, B. Diffuse optical tomography with a priori anatomical information. Phys. Med. Biol. 2005, 50, 2837–2858. [Google Scholar] [CrossRef]
- Panagiotou, C.; Somayajula, S.; Gibson, A.P.; Schweiger, M.; Leahy, R.M.; Arridge, S.R. Information theoretic regularization in diffuse optical tomography. J. Opt. Soc. Am. A 2009, 26, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R.; Lionheart, W.R.B. Nonuniqueness in diffusion-based optcal tomography. Opt. Lett. 1998, 23, 882–884. [Google Scholar] [CrossRef]
- Corlu, A.; Durduran, T.; Choe, R.; Schweiger, M.; Hillman, E.M.C.; Arridge, S.R.; Yodh, A.G. Uniqueness and wavelength optimization in continuous-wave multispectral diffusion optical tomography. Opt. Lett. 2003, 28, 2339–2341. [Google Scholar] [CrossRef]
- Li, A.; Zhang, Q.; Culver, J.P.; Miller, E.L.; Boas, D.A. Reconstruction chromosphere concentration images directly by continuous-wave diffuse optical tomography. Opt. Lett. 2004, 29, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Corlu, A.; Choe, R.; Durduran, T.; Lee, K.; Schweiger, M.; Arridge, S.R.; Hillman, E.M.C.; Yodh, A.G. Diffuse optical tomography with spectral constraints and wavelength optimization. Appl. Opt. 2005, 44, 2082–2092. [Google Scholar] [CrossRef]
- Li, C.; Grobmyer, S.R.; Chen, L.; Zhang, Q.; Fajardo, L.L.; Jiang, H. Multispectral diffuse optical tomography with absorption and scattering spectral constraints. Appl. Opt. 2007, 46, 8229–8235. [Google Scholar] [CrossRef] [PubMed]
- Gaudette, R.J.; Brooks, D.H.; DiMarzio, C.A.; Kilmer, M.E.; Miller, E.L.; Gaudette, T.; Boas, D.A. A comparison study of linear reconstruction techniques for diffuse optical tomographic imaging of absorption coefficient. Phys. Med. Biol. 2000, 45, 1051–1070. [Google Scholar] [CrossRef] [PubMed]
- Habermehl, C.; Steinbrink, J.; Müller, K.-R.; Haufe, S. Optimizing the regularization for image reconstruction of cerebral diffuse optical tomography. J. Biomed. Opt. 2014, 19, 096006. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, R.P.K.; Yalavarthy, P.K. Minimal residual method provides optimal regularization parameter for diffuse optical tomography. J. Biomed. Opt. 2012, 17, 106015. [Google Scholar] [CrossRef]
- Correia, T.; Gibson, A.; Schweiger, M.; Hebden, J. Selection of regularization parameter for optical topography. J. Biomed. Opt. 2009, 14, 034044. [Google Scholar] [CrossRef]
- Prakash, J.; Yalavarthy, P.K. A LSQR-type method provides a computationally efficient automated optimal choice of regularization parameter in diffuse optical tomography. Med. Phys. 2013, 40, 033101. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Jia, K.; Feng, J. Comprehensive study of methods for automatic choice of regularization parameter for diffuse optical tomography. Opt. Eng. 2016, 56, 041310. [Google Scholar] [CrossRef]
- Pogue, B.W.; McBride, T.O.; Prewitt, J.; Österberg, U.L.; Paulsen, K.D. Spatially variant regularization improves diffuse optical tomography. Appl. Opt. 1999, 38, 2950–2961. [Google Scholar] [CrossRef]
- Okawa, S.; Endo, Y.; Hoshi, Y.; Yamada, Y. Reduction of Poisson noise in measured time-resolved data for time-domain diffuse optical tomography. Med. Biol. Eng. Comput. 2012, 50, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Gaudette, T.; Arridge, S.R. Simultaneous imaging and optode calibration with diffuse optical tomography. Opt. Express 2001, 8, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Milstein, A.B.; Millane, R.P.; Bouman, C.A.; Webb, K.J. Source–detector calibration in three-dimensional Bayesian optical diffusion tomography. J. Opt. Soc. Am. A 2002, 19, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, M.; Nissilä, I.; Boas, D.A.; Arridge, S.R. Image reconstruction in optical tomography in the presence of coupling error. Appl. Opt. 2007, 46, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, R.; Okawa, S.; Matsuhashi, S.; Kusaka, T.; Tanikawa, Y.; Hoshi, Y.; Gao, F.; Yamada, Y. Reduction of image artifacts induced by change in the optode coupling in time-resolved diffuse optical tomography. J. Biomed. Opt. 2011, 16, 116022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, H. A calibration method in diffuse optical tomography. J. Opt. A Pure Appl. Opt. 2004, 6, 844–852. [Google Scholar] [CrossRef]
- Tarvainen, T.; Kolehmainen, V.; Vauhkonen, M.; Vanne, A.; Gibson, A.P.; Schweiger, M.; Arridge, S.R.; Kaipio, J.P. Computational calibration method for optical tomography. Appl. Opt. 2005, 44, 1879–1888. [Google Scholar] [CrossRef]
- Li, C.; Liengsawangwong, R.; Choi, H.; Cheung, R. Using a priori structural information from magnetic resonance imaging to investigate the feasibility of prostate diffuse optical tomography and spectroscopy: A simulation study. Med. Phys. 2007, 34, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Whitely, D. A genetic algorithm tutorial. Stat. Comput. 1994, 4, 65–85. [Google Scholar] [CrossRef]
- Jiang, Y.; Machida, M.; Todoroki, N. Diffuse optical tomography by simulated annealing via a spin Hamiltonian. J. Opt. Soc. Am. A. 2021, 38, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hoshi, Y.; Machida, M.; Nakamura, G. A hybrid inversion scheme combining Markov chain Monte Carlo and iterative methods for determining optical properties of random media. Appl. Sci. 2019, 9, 3500. [Google Scholar] [CrossRef]
- Takamizu, Y.; Umemura, M.; Yajima, H.; Abe, M.; Hoshi, Y. Deep learning of diffuse optical tomography based on time-domain radiative transfer equation. Appl. Sci. 2022, 12, 12511. [Google Scholar] [CrossRef]
- Goh, V.; Padhani, A.R.; Rasheed, S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol. 2007, 8, 245–255. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors; a review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef]
- Bauer, A.Q.; Nothdurft, R.E.; Erpelding, T.N.; Wang, L.V.; Culver, J.P. Quantitative photoacoustic imaging: Correcting for heterogenous light fluence distribution using diffuse optical tomography. J. Biomed. Opt. 2011, 16, 096016. [Google Scholar] [CrossRef]
- Kumavor, P.D.; Xu, C.; Aguirre, A.; Gamelin, J.; Ardeshirpour, Y.; Tavakoli, B.; Zanganeh, S.; Alqasemi, U.; Yang, Y.; Zhu, Q. Target detection and quantification using a hybrid hand-held diffuse optical tomography and photoacoustic tomography system. J. Biomed. Opt. 2011, 16, 046010. [Google Scholar] [CrossRef]
- Xi, L.; Jiang, H. Integrated photoacoustic and diffuse optical tomography system for imaging of human finger joints in vivo. J. Biophotonics 2016, 9, 213–217. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Lu, T.; Zhang, L.; Zhou, Z.; Zhao, H.; Gao, F. Combined diffuse optical tomography and photoacoustic tomography for enhanced functional imaging of small animals; a methodological study on phantoms. Appl. Opt. 2017, 56, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Mahmoodkalayeh, S.; Ansari, M.A.; Kratkiewicz, K.; Manwar, R.; Nasiriavanaki, M.R. Simultaneous photoacoustic tomography guided diffuse optical tomography; a numerical study. Proc. SPIE 2019, 10878, 108785U. [Google Scholar]
- Corlu, A.; Choe, R.; Durduran, T.; Rosen, M.A.; Schweiger, M.; Arridge, S.R.; Schnall, M.D.; Yodh, A.G. Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt. Express 2007, 15, 6696–6716. [Google Scholar] [CrossRef]
- Naser, M.A.; Patterson, M.S. Improved bioluminescence and fluorescence reconstruction algorithms using diffuse optical tomography, normalized data, and optimized selection of the permissible source region. Biomed. Opt. Express 2011, 2, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Jeng, G.-S.; Li, M.-L.; Kim, M.W.; Yoon, S.J.; Pitre, J.J., Jr.; Li, D.S.; Pelivanov, I.; O’Donnell, M. Real-time interleaved spectroscopic photoacoustic and ultrasound (PAUS) scanning with simultaneous fluence compensation and motion correction. Nat. Commun. 2021, 12, 716. [Google Scholar] [CrossRef]
- Kirillin, M.; Perekatova, V.; Turchin, I.; Subochev, P. Fluence compensation in raster-scan optoacoustic angiography. Photoacoustics 2017, 8, 59–67. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, M.; Jiang, Y.; Li, C. Optical fluence compensation for handheld photoacoustic probe: An in vivo human study case. J. Innov. Opt. Health Sci. 2017, 10, 1740002. [Google Scholar] [CrossRef]
- Okawa, S.; Sei, K.; Hirasawa, T.; Irisawa, K.; Hirota, K.; Wada, T.; Kushibiki, T.; Furuya, K.; Ishihara, M. In vivo photoacoustic imaging of uterine cervical lesion and its image processing based on light propagation in biological medium. Proc. SPIE 2017, 10064, 100642S. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, S.; Hoshi, Y. A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography. Appl. Sci. 2023, 13, 5016. https://doi.org/10.3390/app13085016
Okawa S, Hoshi Y. A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography. Applied Sciences. 2023; 13(8):5016. https://doi.org/10.3390/app13085016
Chicago/Turabian StyleOkawa, Shinpei, and Yoko Hoshi. 2023. "A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography" Applied Sciences 13, no. 8: 5016. https://doi.org/10.3390/app13085016
APA StyleOkawa, S., & Hoshi, Y. (2023). A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography. Applied Sciences, 13(8), 5016. https://doi.org/10.3390/app13085016






