Novel Highly Dispersed Additive for Proton-Conducting Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
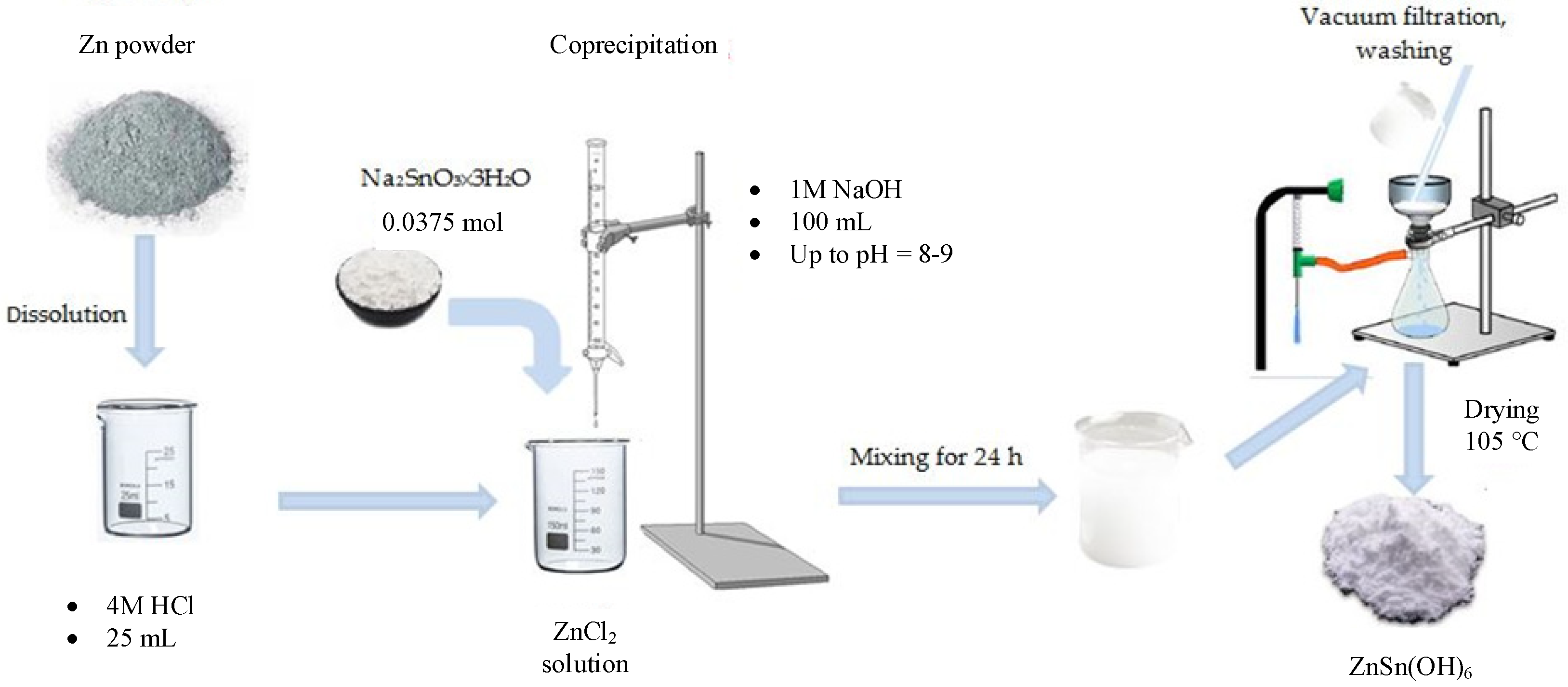
2.2. Synthesis of the Precursor ZnSn(OH)6
2.3. Characterization
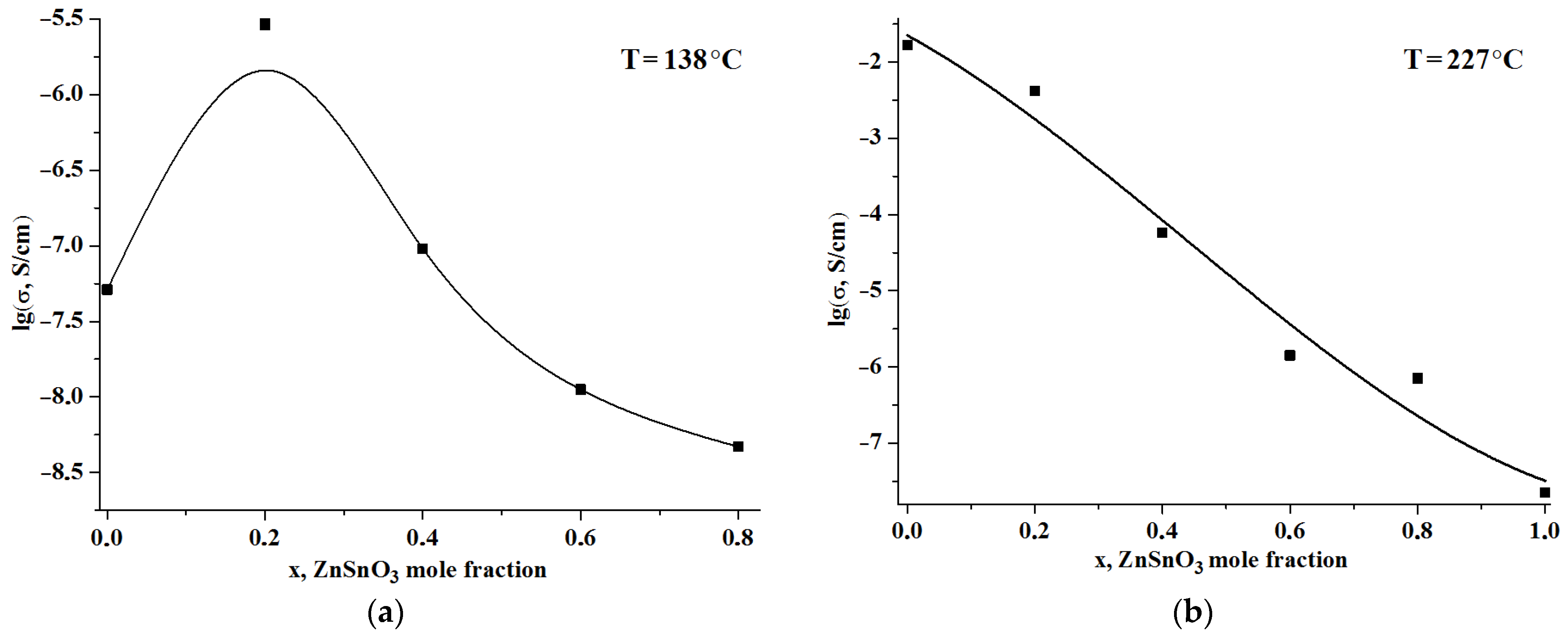
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Li, D.; Liu, Y.; Zhang, J. Fabrication of novel rugby-like ZnSnO3/reduced graphene oxide composites as a high-performance anode material for lithium-ion batteries. Mater. Lett. 2016, 167, 222–225. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, Q.; Liu, X.; Zhao, Y.; Zeng, D.; Wang, L.; Zheng, Y.; Peng, D.L. Synthesis of amorphous ZnSnO3 double-shell hollow microcubes as advanced anode materials for lithium ion batteries. Electrochim. Acta 2015, 182, 327–333. [Google Scholar] [CrossRef]
- Wei, J.L.; Jin, X.Y.; Yu, M.C.; Wang, L.; Guo, Y.H.; Dong, S.T.; Zhang, Y.M. Electrospun ZnSnO3/C nanofibers as an anode material for lithium-ion batteries. J. Electron. Mater. 2021, 50, 4945–4953. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Maksimov, A.I.; Matyushkin, L.B.; Moshnikov, V.A. Current state of studies on synthesis and application of zinc stannate. Glas. Phys. Chem. 2019, 45, 251–260. [Google Scholar] [CrossRef]
- Sim, C.K.; Majid, S.R.; Mahmood, N.Z. Synthesis of highly porous carbon/ZnSnO3 composite and its electrochemical properties. J. Energy Storage 2020, 32, 101843. [Google Scholar] [CrossRef]
- Sim, C.K.; Majid, S.R.; Mahmood, N.Z. ZnSnO3/mesoporous biocarbon composite towards sustainable electrode material for energy storage device. Microchem. J. 2021, 164, 105968. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.Z.; Xie, W.F.; Gai, L.Y.; Yuan, H.M.; Zhang, D.; Zhang, H.; Liu, X.; Yang, W.; Chi, Z.T. Chemiresistive ethanol sensors based on In2O3/ZnSnO3 nanocubes. Sens. Actuators Rep. 2022, 4, 100099. [Google Scholar] [CrossRef]
- Feng, G.; Che, Y.; Song, C.; Xiao, J.; Fan, X.; Sun, S.; Huang, G.; Ma, Y. Morphology-controlled synthesis of ZnSnO3 hollow spheres and their n-butanol gas-sensing performance. Ceram. Int. 2021, 47, 2471–2482. [Google Scholar] [CrossRef]
- Guo, W.; Zhao, B.; Huang, L.; He, Y. One-step synthesis of ZnWO4/ZnSnO3 composite and the enhanced gas sensing performance to formaldehyde. Mater. Lett. 2020, 277, 4–7. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, K.; Wang, X.; Sui, Y.; Zou, B.; Zheng, W.; Zou, G. Rapid and selective H2S detection of hierarchical ZnSnO3 nanocages. Sens. Actuators B Chem. 2011, 159, 245–250. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Punegova, K.N.; Ryabko, A.A.; Maksimov, A.I. Synthesis and study of gas-sensitive nanostructures of the Zn-Sn-O system. Phys. Chem. Asp. Study Clust. Nanostruct. Nanomater. 2021, 13, 910–918. [Google Scholar] [CrossRef]
- Sá, B.S.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Porous ZnSnO3 nanocubes as a triethylamine sensor. Sens. Actuators B Chem. 2021, 338, 129869. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wang, Q.; Wu, X.; Zhang, W.; Luo, C. Facile synthesis of hollow cube-like ZnSnO3 wrapped by nitrogen-doped graphene: As a high-performance and enhanced synergistic microwave absorber. J. Magn. Magn. Mater. 2019, 486, 165251. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zhang, T.; Zhang, R.; Lou, Z.; Deng, J.N.; Wang, L.L. Hollow ZnSnO3 cubes with controllable shells enabling highly efficient chemical sensing detection of formaldehyde vapors. ACS Appl. Mater. Interfaces. 2017, 9, 14525–14533. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zheng, Z.; Xu, J.; Zhang, C. Enhanced visible light-excited ZnSnO3 for room temperature ppm-level CO2 detection. J. Alloys Compd. 2022, 907, 164440. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Lian, D.; Yang, J.; Wang, S.; Li, Y.; Song, H. Enhanced selective acetone gas sensing performance by fabricating ZnSnO3/SnO2 concave microcube. Appl. Surf. Sci. 2021, 542, 148555. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, Y.; Zhou, P.; Lu, R.; Li, A.; Zhao, S.; Liu, W.; Wei, D.; Wei, K. Fabrication, characterization and n-propanol sensing properties of perovskite-type ZnSnO3 nanospheres based gas sensor. Appl. Surf. Sci. 2020, 509, 145335. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, X.; Zheng, W. Synthesis of novel hollow ZnSnO3 cubic nanocages and their HCHO sensing properties. J. Nanosci. Nanotechnol. 2013, 13, 1286–1290. [Google Scholar] [CrossRef]
- Song, P.; Wang, Q.; Yang, Z. Ammonia gas sensor based on PPy/ZnSnO3 nanocomposites. Mater. Lett. 2011, 65, 430–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, L.; Zhou, H.; Meng, W.; Li, Y.; He, Z.; Dai, L. A mixed-potential type NH3 sensors based on spinel Zn2SnO4 sensing electrode. Sens. Actuators B Chem. 2022, 367, 132154. [Google Scholar] [CrossRef]
- Arora, I.; Praveen, K. Effect of annealing temperature on structure-property correlations in Zn2SnO4 nanostructured films for optoelectronics. Mater. Res. Express. 2020, 7, 035023. [Google Scholar] [CrossRef]
- Rembeza, S.I.; Belousov, S.A.; Kosheleva, N.N.; Rembeza, E.S.; Svistova, T.V.; Suvaci, E.; Özel, E.; Tuncolu, G.; Açiksari, C. Amorphous films of ternary zinc and tin oxides for transparent electronics. Tech. Phys. Lett. 2018, 44, 984–987. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Latha, D.; Karthikeyan, V.; Narayanan, V. Enhanced photocatalytic performance of ZnSnO3/rGO nanocomposite. Chem. Phys. Lett. 2020, 739, 137050. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhang, W.; Xia, L.; Zhou, S.; Russell, C.K.; Fan, M.; Feng, J.; Sun, J. Double-shelled ZnSnO3 hollow cubes for efficient photocatalytic degradation of antibiotic wastewater. Chem. Eng. J. 2020, 384, 123279. [Google Scholar] [CrossRef]
- Habibi, M.H.; Mardani, M. Synthesis and characterization of bi-component ZnSnO3/Zn2SnO4 (perovskite/spinel) nano-composites for photocatalytic degradation of Intracron Blue: Structural, opto-electronic and morphology study. J. Mol. Liq. 2017, 238, 397–401. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.; Chen, Z.; Ren, H.; Li, M.; Chen, L. MoS2 nanosheets anchored on porous ZnSnO3 cubes as an efficient visible-light-driven composite photocatalyst for the degradation of tetracycline and mechanism insight. J. Hazard. Mater. 2020, 390, 122158. [Google Scholar] [CrossRef]
- Kumar, A.; Mathur, L.; Bae, H.; Sim, U.K.; Song, S.J. Design of Novel transition metal based multiphase stannate: An efficient electrocatalyst for oxygen evolution reaction. Mater. Chem. Phys. 2022, 279, 125613. [Google Scholar] [CrossRef]
- Anisimov, V.V.; Saprykin, A.V.; Artemkina, I.M.; Makarov, N.A. Influence of the anionic composition of initial salts on obtaining zinc orthostannate by the sol-gel method. Glas. Ceram. 2021, 78, 148–152. [Google Scholar] [CrossRef]
- Venkatesan, R.; Zhang, Y.; Chen, G. Preparation of poly(butylene adipate-co-terephthalate)/ZnSnO3 composites with enhanced antimicrobial activity. Compos. Commun. 2020, 22, 100469. [Google Scholar] [CrossRef]
- Wang, D.; Pu, X.; Yu, X.; Bao, L.; Cheng, Y.; Xu, J.; Han, S.; Ma, Q.; Wang, X. Controlled preparation and gas sensitive properties of two-dimensional and cubic structure ZnSnO3. J. Colloid Interface Sci. 2022, 608, 1074–1085. [Google Scholar] [CrossRef]
- Ge, Q.; Liu, C.; Zhao, Y.; Wang, N.; Zhang, X.; Feng, C.; Zhang, S.; Wang, H.; Jiang, W.; Liu, S.; et al. Phase evolution in preparing ZnSnO3 powders by precipitation method. Appl. Phys. A Mater. Sci. Process. 2021, 127, 89. [Google Scholar] [CrossRef]
- Sidorak, A.V.; Shubin, A.A.; Ivanov, V.V.; Nikolayeva, N.S. Some Aspects of Zn2SnO4 Powders Synthesis by Thermotreatment of Coprecipitated Compounds. J. Siberian Federal Univ. Chem. 2010, 26, 1343–1348. [Google Scholar]
- Loginov, A.V.; Bagavieva, S.K.; Aparnev, A.I.; Uvarov, N.F. Synthesis of oxide materials in the Mg–Sn–O system for use in composite solid electrolytes. Russ. J. Appl. Chem. 2017, 90, 496–498. [Google Scholar] [CrossRef]
- Loginov, A.V.; Mateyshina, Y.G.; Aparnev, A.I.; Uvarov, N.F. Synthesis of BaSnO3/SnO2 nanocomposites as heterogeneous additive for composite solid electrolytes. Russ. J. Appl. Chem. 2018, 91, 1660–1664. [Google Scholar] [CrossRef]
- Loginov, A.V.; Aparnev, A.I.; Uvarov, N.F. Synthesis of SrSnO3/SnO2 composites via thermal decomposition of a precursor. Inorg. Mater. 2022, 58, 420–424. [Google Scholar] [CrossRef]
- Loginov, A.V.; Aparnev, A.I.; Uvarov, N.F. Nanocomposites Prepared via Thermal Decomposition of Calcium Hydroxystannate CaSn(OH)6. Inorg. Mater. 2022, 58, 814–821. [Google Scholar] [CrossRef]
- Jiang, L.; Xue, K.; Chen, Z.; Cui, Q.; Xu, S. Hydrothermal synthesis of ZnSnO3/rGO composite material and highly gas sensing performance to acetone. Mater. Sci. Semicond. Process. 2021, 134, 106051. [Google Scholar] [CrossRef]
- He, Q.; Zi, J.; Huang, B.; Yan, L.; Fa, W.; Li, D.; Zhang, Y.; Gao, Y.; Zheng, Z. Controlled growth and thermal decomposition of well-dispersed and uniform ZnSn(OH)6 submicrocubes. J. Alloys Compd. 2014, 607, 193–197. [Google Scholar] [CrossRef]
- Fu, X.; Huang, D.; Qin, Y.; Li, L.; Jiang, X.; Chen, S. Effects of preparation method on the microstructure and photocatalytic performance of ZnSn(OH)6. Appl. Catal. B Environ. 2014, 148–149, 532–542. [Google Scholar] [CrossRef]
- Baranov, A.I.; Khiznichenko, V.P.; Sandur, V.A.; Shuvalov, L.A. Frequency dielectric dispersion in the ferroelectric and superionic phases of CsH2PO4. Ferroelectrics 1988, 81, 183–186. [Google Scholar] [CrossRef]
- Matsunaga, H.; Itoh, K.; Nakamura, E. X-ray structural study of CDP at room temperature. J. Phys. Soc. Japan 1980, 48, 2011–2014. [Google Scholar] [CrossRef]
- Baranov, A.I. Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Rev. Crystallogr. Rep. 2003, 48, 1012–1037. [Google Scholar] [CrossRef]
- Taninouchi, Y.K.; Uda, T.; Awakura, Y.; Ikeda, A.; Haile, S.M. Dehydration behavior of the superprotonic conductor CsH2PO4 at moderate temperatures: 230 to 260 °C. J. Mater. Chem. 2007, 17, 3182–3189. [Google Scholar] [CrossRef]
- Boysen, D.A.; Uda, T.; Chisholm, C.R.I.; Haile, S.M. High-performance solid acid fuel cells through humidity stabilization. Sci. 2004, 303, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Uda, T.; Haile, S.M. Thin-membrane solid-acid fuel cell. Electrochem. Solid-State Lett. 2005, 8, 245–246. [Google Scholar] [CrossRef]
- Baranov, A.I.; Grebenev, V.V.; Khodan, A.N.; Dolbinina, V.V.; Efremova, E.P. Optimization of superprotonic acid salts for fuel cell applications. Solid State Ionics. 2005, 176, 2871–2874. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Lavrova, G.V. Controlling the proton transport properties of solid acids via structural and microstructural modification. J. Solid State Electrochem. 2011, 15, 213–221. [Google Scholar] [CrossRef]
- Anfimova, T.; Jensen, A.H.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J.; Li, Q. CsH2PO4/NdPO4 composites as proton conducting electrolytes for intermediate temperature fuel cells. J. Electrochem. Soc. 2015, 162, F436–F441. [Google Scholar] [CrossRef]
- Qing, G.; Kikuchi, R.; Takagaki, A.; Sugawara, T.; Oyama, S.T. CsH2PO4/Polyvinylidene Fluoride Composite Electrolytes for Intermediate Temperature Fuel Cells. J. Electrochem. Soc. 2014, 161, 451–457. [Google Scholar] [CrossRef]
- Muroyama, H.; Matsui, T.; Kikuchi, R.; Eguchi, K. Electrochemical hydrogen production from carbon monoxide and steam with a cell employing CsH2PO4/SiP2O7 composite electrolyte. J. Electrochem. Soc. 2009, 156, 1389. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Lavrova, G.V. New type of composite proton electrolytes based on CsH2PO4 synthesized by mechanical activation. Mater. Today Proc. 2019, 12, 9–12. [Google Scholar] [CrossRef]
- Lavrova, G.V.; Shutova, E.S.; Ponomareva, V.G.; Dunyushkina, L.A. Proton conductivity and interphase interaction in CsH2PO4-SrZrO3 composites. Russ. J. Electrochem. 2013, 49, 718–724. [Google Scholar] [CrossRef]
- Bagryantseva, I.N.; Ponomareva, V.G.; Lazareva, N.P. Proton-conductive membranes based on CsH2PO4 and ultra-dispersed polytetrafluoroethylene. Solid State Ion. 2019, 329, 61–66. [Google Scholar] [CrossRef]
- Bagryantseva, I.N.; Gaydamaka, A.A.; Ponomareva, V.G. Intermediate temperature proton electrolytes based on cesium dihydrogen phosphate and Butvar polymer. Ionics 2020, 26, 1813–1818. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Bagryantseva, I.N.; Shutova, E.S. Hybrid systems based on nanodiamond and cesium dihydrogen phosphate. Mater. Today Proc. 2020, 25, 521–524. [Google Scholar] [CrossRef]
- Bagryantseva, I.N.; Ponomareva, V.G.; Khusnutdinov, V.R. Intermediate temperature proton electrolytes based on cesium dihydrogen phosphate and poly (vinylidene fluoride-co-hexafluoropropylene). J. Mater. Sci. 2021, 56, 14196–14206. [Google Scholar] [CrossRef]
- Bagryantseva, I.N.; Kungurtsev, Y.E.; Dormidonova, D.O.; Ponomareva, V.G. Composite electrolytes based on cesium dihydrophosphate and fluoropolymers. Russ. J. Electrochem. 2022, 58, 611–616. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Shutova, E.S.; Kovalenko, K.A.; Fedin, V.P. New type of nanocomposite CsH2PO4-UiO-66 Electrolyte with High Proton Conductivity. Molecules 2022, 27, 8387. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Zhao, Y.; Wu, Y.; Xia, L.; Su, X.; Zhang, C.; Wang, D.; Guo, W.; Sun, J. Crystal structure and photocatalytic properties of perovskite MSn(OH)6 (M = Cu and Zn) composites with d10 -d10 configuration. Appl. Surf. Sci. 2019, 463, 659–667. [Google Scholar] [CrossRef]
- Faust, G.T.; Schaller, W.T. Schoenfliesite, MgSn(OH)6. Schoenfliesite 1971, 141, 116–141. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1997; Volume 479. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies Contents, Tables and Charts; John Wiley & Sons. Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Yasser, H.; Rodriguez-Paez, J.E.; Gutierrez, R.M. Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route. Mater. Chem. Phys. 2021, 266, 124557. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. One-pot in-situ hydrothermal synthesis of ternary In2S3/Nb2O5/Nb2C Schottky/S-scheme integrated heterojunction for efficient photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 628 Pt B, 500–512. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Min, S.; Irfan, R.M.; Zhu, Q.; Zhou, L.; Lei, J.; Zhang, J. Simultaneous hydrogen production with the selective oxidation of benzyl alcohol to benzaldehyde by a noble-metal-free photocatalyst VC/CdS nanowires. Chin. J. Catal. 2022, 43, 1165–1175. [Google Scholar] [CrossRef]
- Liu, G.; Feng, M.; Tayyab, M.; Gong, J.; Zhang, M.; Yang, M.; Lin, K. Direct and efficient reduction of perfluorooctanoic acid using bimetallic catalyst supported on carbon. J. Hazard. Mater. 2021, 412, 125224. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Tayyab, M.; Akhtar, A.; Kausar, S.; Ullah, S.; Iqbal, M. Effect of soft template variation on the synthesis, physical, and electrochemical properties of Mn3O4 nanomaterial. Inorg. Nano-Met. Chem. 2021, 51, 359–365. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. A new breakthrough in photocatalytic hydrogen evolution by amorphous and chalcogenide enriched cocatalysts. Chem. Eng. J. 2023, 455, 140601. [Google Scholar] [CrossRef]
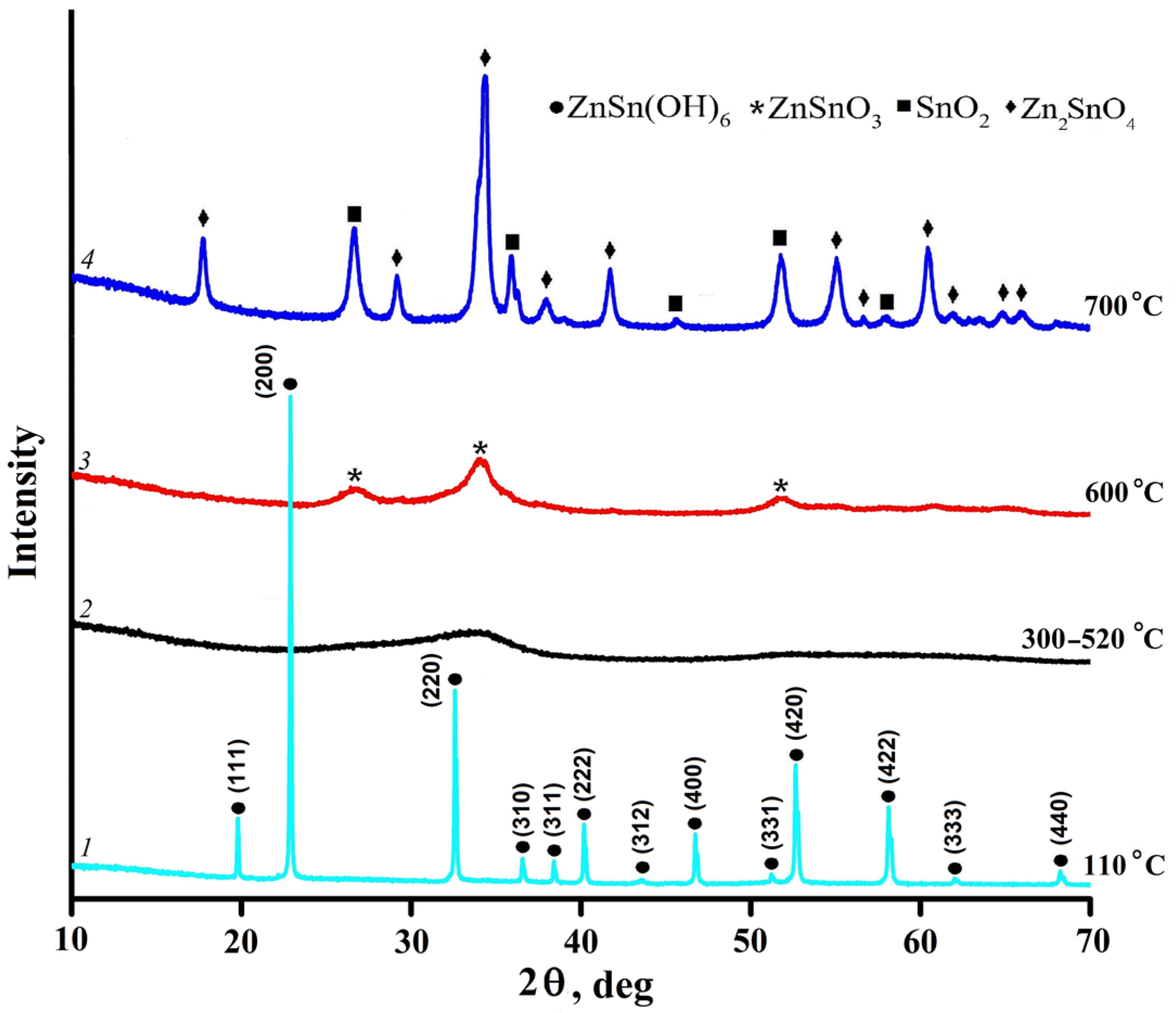


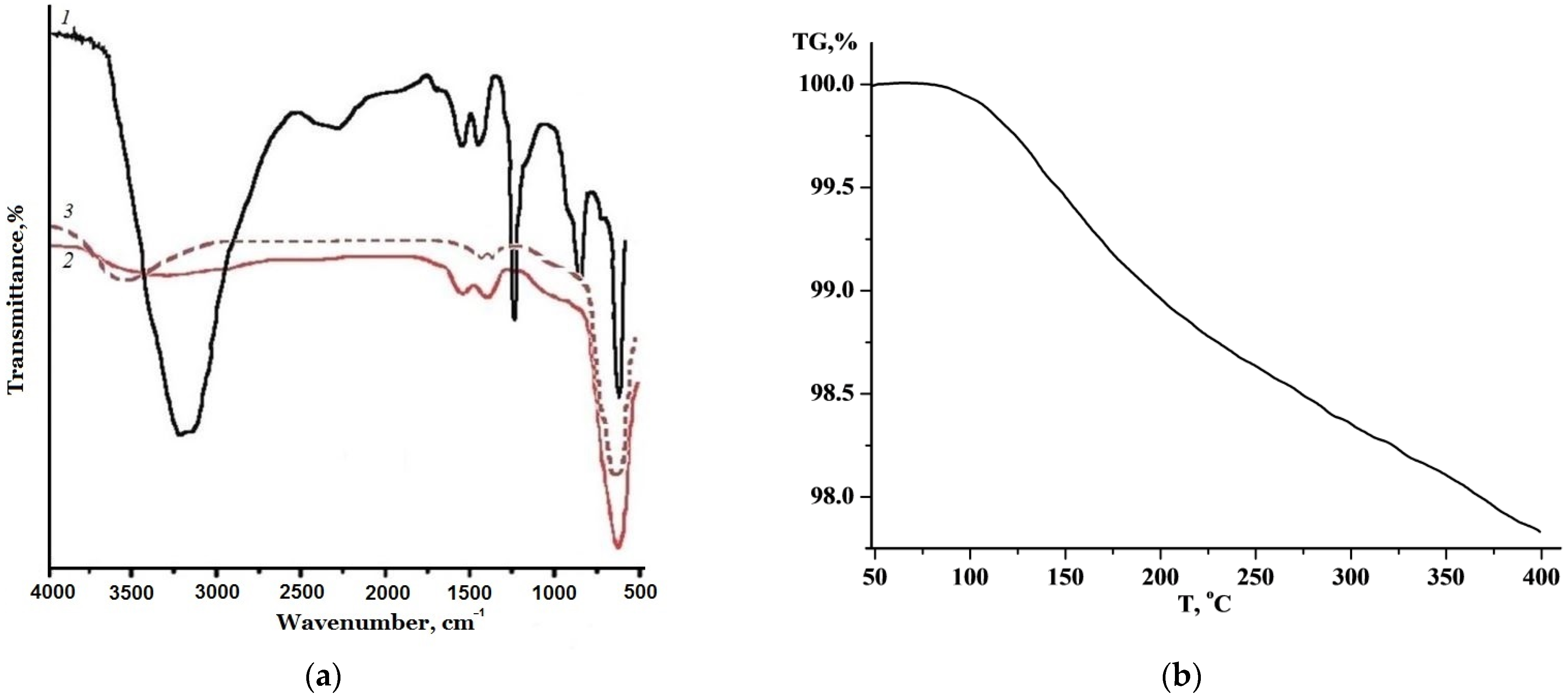
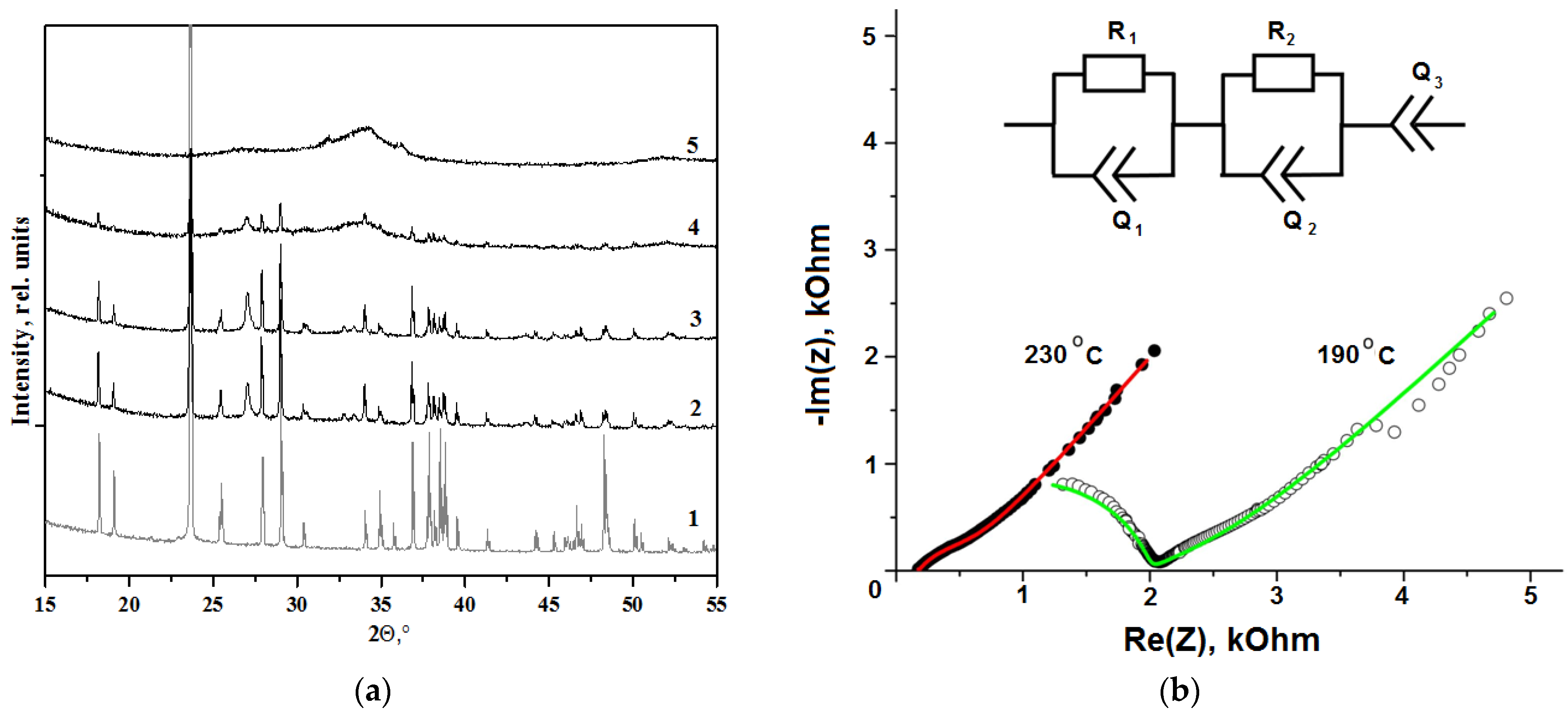


| Heating Temperature, °C | 110 | 300 | 520 | 600 | 700 |
|---|---|---|---|---|---|
| Phase composition | ZnSn(OH)6 | amorphous | ZnSnO3 | Zn2SnO4–SnO2 | |
| Specific surface area, m2·g−1 | 18 | 80 | 85 | 36 | 15 |
| Pore size, nm | ~1 | 3 | 6 | 4 | 10 |
| Parameter | 190 °C | 230 °C |
|---|---|---|
| R1, kΩ | 1.97 ± 0.09 | 0.176 ± 0.004 |
| Y1, | 7.0 × 10−10 ± 0.8 × 10−10 | - |
| n1 | 0.882 ± 0.013 | - |
| R2, kΩ | 0.96 ± 0.02 | 0.360 ± 0.005 |
| Y2, | 4.0 × 10−4 ± 0.6 × 10−4 | 1.6 × 10−4 ± 0.1 × 10−4 |
| n2 | 0.335 ± 0.008 | 0.669 ± 0.012 |
| Y3, | 4.2 × 10−4 ± 0.9 × 10−4 | 5.11 × 10−4 ± 0.07 × 10−4 |
| n3 | 0.546 ± 0.011 | 0.594 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparnev, A.I.; Loginov, A.V.; Uvarov, N.; Ponomareva, V.; Bagryantseva, I.; Manakhov, A.; Al-Qasim, A.S.; Golovakhin, V.V.; Bannov, A.G. Novel Highly Dispersed Additive for Proton-Conducting Composites. Appl. Sci. 2023, 13, 5038. https://doi.org/10.3390/app13085038
Aparnev AI, Loginov AV, Uvarov N, Ponomareva V, Bagryantseva I, Manakhov A, Al-Qasim AS, Golovakhin VV, Bannov AG. Novel Highly Dispersed Additive for Proton-Conducting Composites. Applied Sciences. 2023; 13(8):5038. https://doi.org/10.3390/app13085038
Chicago/Turabian StyleAparnev, Aleksandr I., Anton V. Loginov, Nikolai Uvarov, Valentina Ponomareva, Irina Bagryantseva, Anton Manakhov, Abdulaziz S. Al-Qasim, Valeriy V. Golovakhin, and Alexander G. Bannov. 2023. "Novel Highly Dispersed Additive for Proton-Conducting Composites" Applied Sciences 13, no. 8: 5038. https://doi.org/10.3390/app13085038
APA StyleAparnev, A. I., Loginov, A. V., Uvarov, N., Ponomareva, V., Bagryantseva, I., Manakhov, A., Al-Qasim, A. S., Golovakhin, V. V., & Bannov, A. G. (2023). Novel Highly Dispersed Additive for Proton-Conducting Composites. Applied Sciences, 13(8), 5038. https://doi.org/10.3390/app13085038







