Apple Consumption Protects against Acute Ethanol-Induced Liver Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
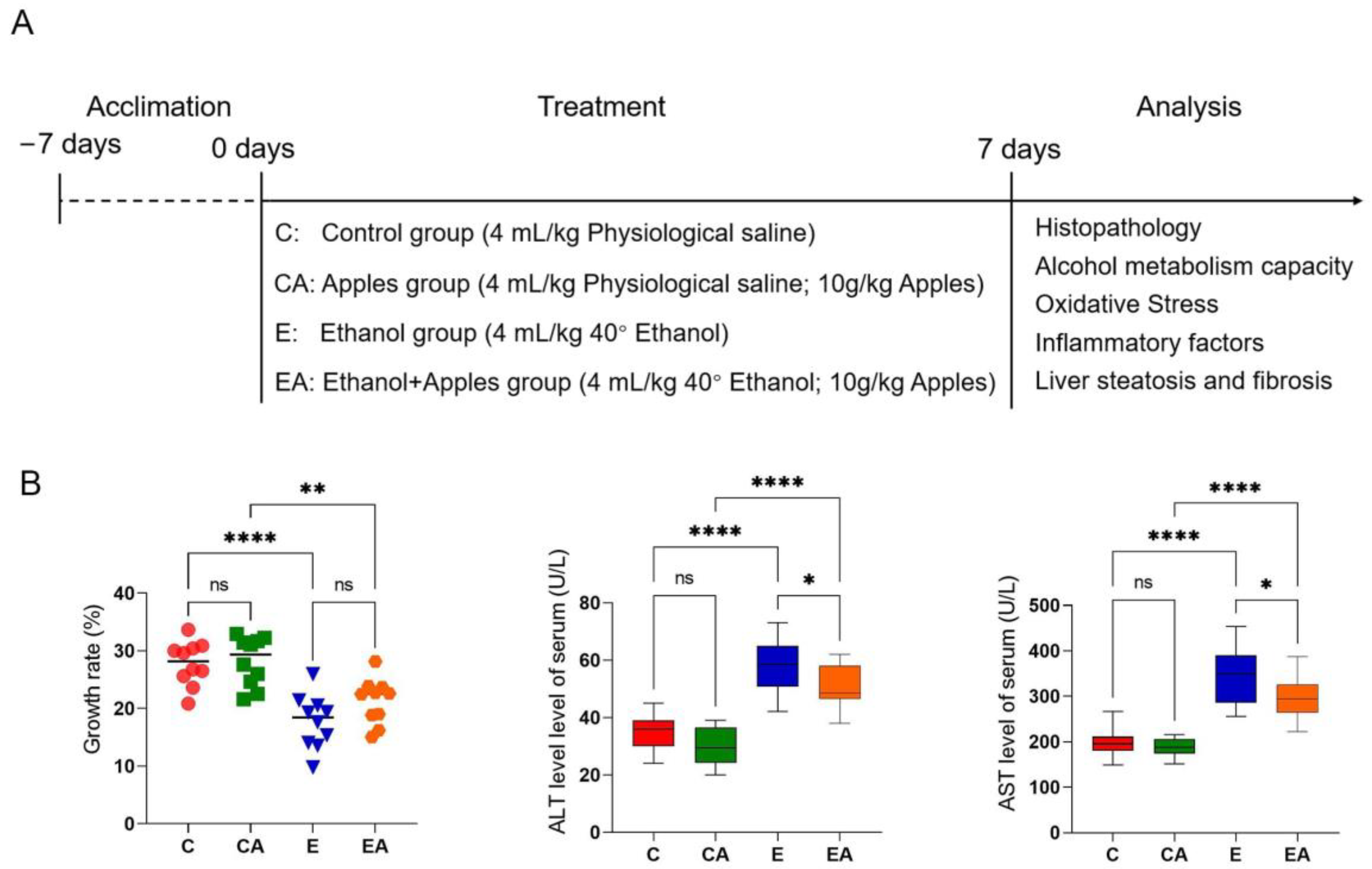
2.1. Animals and Experimental Design
2.2. Measurement of Serum Markers
2.3. Measurement of Enzyme Activity Indicators
2.4. Histopathology
2.5. Enzymelinked Immunosorbent Assay (ELISA) for Immune Factor Levels
2.6. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Semi-Quantitative Real-Time Polymerase Chain Reaction (sPCR)
2.8. Statistical Analysis
3. Results
3.1. Apples Help Slow Weight Growth and Improve the Serum Index in Rats after Alcohol Consumption
3.2. Apples Help Reduce the Effects of Ethanol on Liver Tissue
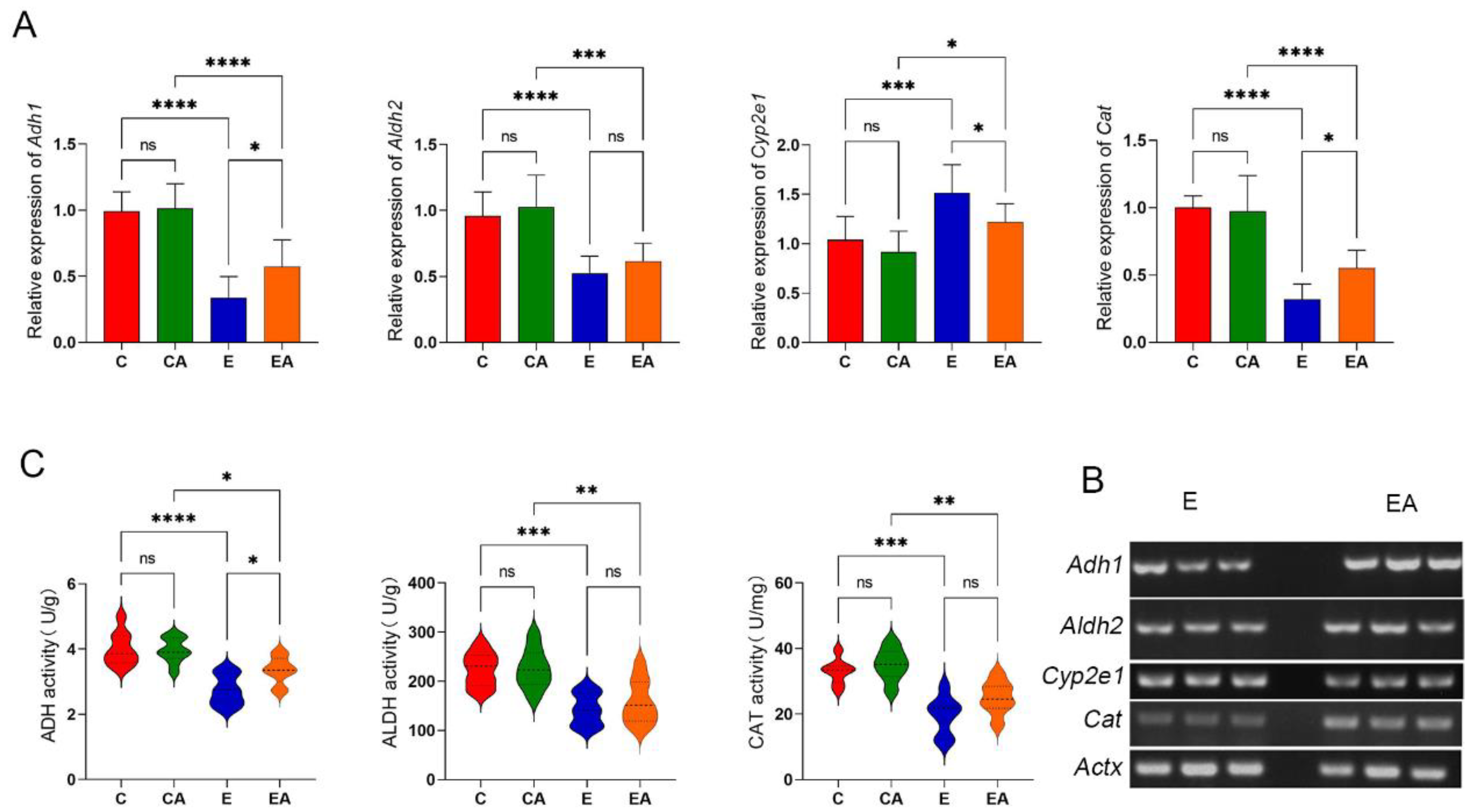
3.3. Apple Intake Has a Positive Effect on the Ability to Metabolize Alcohol
3.4. Apples Slow down the Oxidative Stress Caused by Ethanol
3.5. Apples Reduce the Inflammatory Response Induced by Alcohol
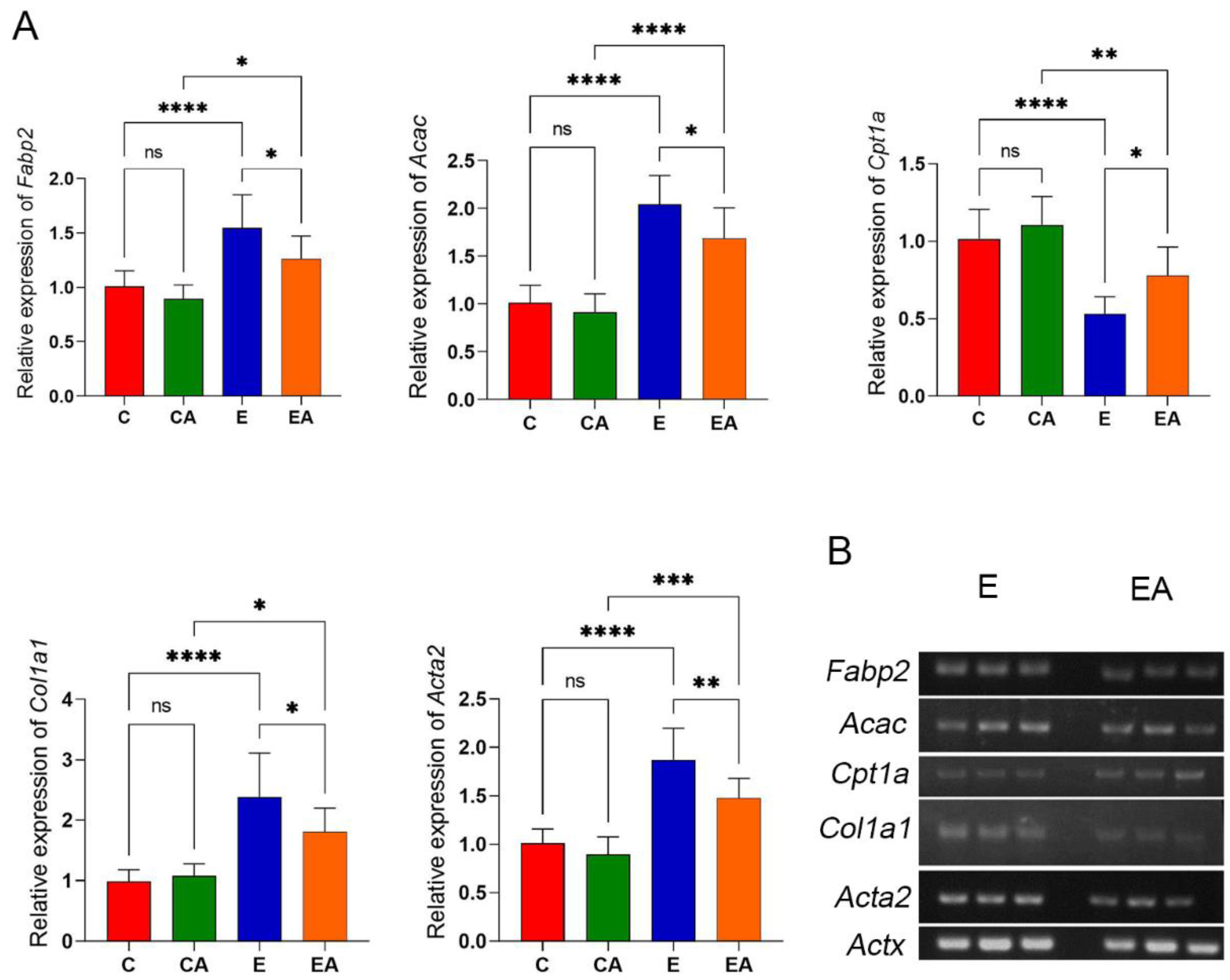
3.6. Apple Improves the Expression of Genes Related to Lipid Metabolism and Liver Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, Y.S.; Sim, J.; Seo, S.; Seo, W. Alcoholic liver disease: A new insight into the pathogenesis of liver disease. Arch. Pharmacal Res. 2022, 45, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef]
- Angireddy, R.; Chowdhury, A.R.; Zielonka, J.; Ruthel, G.; Kalyanaraman, B.; Avadhani, N.G. Alcohol-induced CYP2E1, mitochondrial dynamics and retrograde signaling in human hepatic 3D organoids. Free. Radic. Biol. Med. 2020, 159, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Lach, T.; Cichoż-Lach, H. Oxidative Stress—A Key Player in the Course of Alcohol-Related Liver Disease. J. Clin. Med. 2021, 10, 3011. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Coppens, V.; Morrens, M.; Destoop, M.; Dom, G. The Interplay of Inflammatory Processes and Cognition in Alcohol Use Disorders—A Systematic Review. Front. Psychiatry 2019, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, C.; Thomes, P.G.; Kharbanda, K.K.; Casey, C.A.; McNiven, M.A.; Donohue, T.M. Lipophagy and Alcohol-Induced Fatty Liver. Front. Pharmacol. 2019, 10, 495. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv. Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, I.S.; Otles, S. Effects of Green Apple (Golden Delicious) and Its Three Major Flavonols Consumption on Obesity, Lipids, and Oxidative Stress in Obese Rats. Molecules 2022, 27, 1243. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Valero-Vello, M.; Peris-Martínez, C.; García-Medina, J.J.; Sanz-González, S.M.; Ramírez, A.I.; Fernández-Albarral, J.A.; Galarreta-Mira, D.; Zanón-Moreno, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the Antioxidant, Anti-Inflammatory, and Neuroprotective Potential of Natural Food and Nutritional Supplements for Ocular Health in the Mediterranean Population. Foods 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Mariadoss, V.A.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini-Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef]
- Jia, M.; Ren, D.; Nie, Y.; Yang, X. Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct. 2017, 8, 1282–1292. [Google Scholar] [CrossRef]
- Ren, T.; Mackowiak, B.; Lin, Y.; Gao, Y.; Niu, J.; Gao, B. Hepatic injury and inflammation alter ethanol metabolism and drinking behavior. Food Chem. Toxicol. 2020, 136, 111070. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, X.; Yi, R.; Zhao, X. Antioxidative and Anti-Inflammatory Effects of Lactobacillus plantarum ZS62 on Alcohol-Induced Subacute Hepatic Damage. Oxidative Med. Cell. Longev. 2021, 2021, 7337988. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free. Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Qi, B.; Hao, S.; Ji, R. Camel milk ameliorates inflammatory mechanisms in an alcohol-induced liver injury mouse model. Sci. Rep. 2021, 11, 22811. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Wang, P.; Xia, Y.; Yan, N.; Takahashi, S.; Krausz, K.W.; Hao, H.; Yan, T.; Gonzalez, F.J. Withaferin A alleviates ethanol-induced liver injury by inhibiting hepatic lipogenesis. Food Chem. Toxicol. 2022, 160, 112807. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Woo, M.J.; Ham, J.R.; Lee, M.K. Anti-steatotic and anti-inflammatory effects of Hovenia dulcis Thunb. extracts in chronic alcohol-fed rats. Biomed. Pharmacother. 2017, 90, 393–401. [Google Scholar] [CrossRef]
- Bingül, İ.; Başaran-Küçükgergin, C.; Aydın, A.F.; Çoban, J.; Doğan-Ekici, I.; Doğru-Abbasoğlu, S.; Uysal, M. Betaine treatment decreased oxidative stress, inflammation, and stellate cell activation in rats with alcoholic liver fibrosis. Environ. Toxicol. Pharmacol. 2016, 45, 170–178. [Google Scholar] [CrossRef]
- Cederbaum, A.I.; Lu, Y.; Wu, D. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 2009, 83, 519–548. [Google Scholar] [CrossRef]
- Nunes, P.C.; Barbosa, F.K.S.; de Araújo Silva, A.K.C.; Lima, M.D.S.; Alves, A.F.; de Magalhães Cordeiro, A.M.T.; Alcântara, M.A.; de Albuquerque Meireles, B.R.L.; Melo, N.F.C.B.; de Souza Aquino, J.; et al. Malay apple (Syzygium malaccense) promotes changes in lipid metabolism and a hepatoprotective effect in rats fed a high-fat diet. Food Res. Int. 2022, 155, 110994. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Yang, J.; Lin, F.; Sun, Y.; Li, T.; Wu, C. Hepatoprotective effect of apple polyphenols against concanavalin A-induced immunological liver injury in mice. Chem. Biol. Interact. 2016, 258, 159–165. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Li, L.; Gao, Y.; Wang, F.; Zhang, Y.; Fan, Y.; Wu, C. Protective effect of apple polyphenols on chronic ethanol exposure-induced neural injury in rats. Chem. Biol. Interact. 2020, 326, 109113. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Zhou, J.; Sun, P.; Zhao, L.; Zhou, F. Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods 2022, 11, 3014. [Google Scholar] [CrossRef]
- Xie, K.; Chen, C.H.; Tsai, S.P.; Lu, P.-J.; Wu, H.; Zeng, Y.; Ye, Y.; Tu, H.; Wen, C.; Huang, M.; et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Cai, Z.; Ge, H.; Ma, S.; Yu, Y.; Liu, J.; Zhang, T. Transcriptome analysis reveals the hepatoprotective mechanism of soybean meal peptides against alcohol-induced acute liver injury mice. Food Chem. Toxicol. 2021, 154, 112353. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Zulkawi, N.; Ng, K.H.; Zamberi, R.; Yeap, S.K.; Jaganath, I.B.; Satharasinghe, D.; Yong, C.Y.; Jamaluddin, A.B.; Tan, S.W.; Ho, W.Y.; et al. The in vivo hepato-recovery effects of the polyphenol-rich fermented food XenijiTM on ethanol-induced liver damage. RSC Adv. 2017, 7, 38287–38299. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Wang, X.; Luo, X.; Yang, L.; Zhang, R.; Yin, H.; Xie, D.; Pan, Y.; Chen, Y. Hepatic deletion of Smad7 in mouse leads to spontaneous liver dysfunction and aggravates alcoholic liver injury. PLoS ONE 2011, 6, e17415. [Google Scholar] [CrossRef]
- Thomas, M.; Peters, T.J. Acetaldehyde: Its Role in Alcoholic Toxicity and Dependence. Br. J. Addict. 1981, 76, 375–378. [Google Scholar] [CrossRef]
- Höög, J.O.; Hedberg, J.J.; Strömberg, P.; Svensson, S. Mammalian alcohol dehydrogenase—Functional and structural implications. J. Biomed. Sci. 2001, 8, 71–76. [Google Scholar] [CrossRef]
- Hwang, P.H.; Lian, L.; Zavras, A.I. Alcohol intake and folate antagonism via CYP2E1 and ALDH1: Effects on oral carcinogenesis. Med. Hypotheses 2012, 78, 197–202. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, I.A. Cytochrome P450S and Alcoholic Liver Disease. Curr. Pharm. Des. 2018, 24, 1502–1517. [Google Scholar] [CrossRef]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Shi, L.G.; Hu, G.Y.; Chen, S. Saponins from Panax japonicus Protect Against Alcohol-Induced Hepatic Injury in Mice by Up-regulating the Expression of GPX3, SOD1 and SOD3. Alcohol Alcohol. 2010, 45, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, Z.; Liu, D.; Li, X.; Rehman, R.U.; Wang, H.; Wu, Z. Apple phlorizin attenuates oxidative stress in Drosophila melanogaster. J. Food Biochem. 2019, 43, e12744. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, A.G.; Schulz, M.; Silveira, T.T.; de Oliveira, M.V.; Patrício, M.J.; Gonzaga, L.V.; Fett, R.; da Silva, E.L.; Wazlawik, E. Apple intake improves antioxidant parameters in hemodialysis patients without affecting serum potassium levels. Nutr. Res. 2019, 64, 56–63. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Szabo, G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2016, 64, 955–965. [Google Scholar] [CrossRef]
- Vassallo, G.; Mirijello, A.; Ferrulli, A.; Antonelli, M.; Landolfi, R.; Gasbarrini, A.; Addolorato, G. Review article: Alcohol and gut microbiota—The possible role of gut microbiota modulation in the treatment of alcoholic liver disease. Aliment. Pharmacol. Ther. 2015, 41, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y.; et al. Apple Polyphenols Improve Intestinal Antioxidant Capacity and Barrier Function by Activating the Nrf2/Keap1 Signaling Pathway in a Pig Model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef]
- Yang, G.; Bibi, S.; Du, M.; Suzuki, T.; Zhu, M.J. Regulation of the intestinal tight junction by natural polyphenols: A mechanistic perspective. Crit. Rev. Food Sci. Nutr. 2017, 57, 3830–3839. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Yao, Y.L.; Han, X.; Li, Z.M.; Lian, L.H.; Nan, J.X.; Wu, Y.L. Acanthoic Acid Can Partially Prevent Alcohol Exposure-Induced Liver Lipid Deposition and Inflammation. Front. Pharmacol. 2017, 8, 134. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Fu, L.; Gao, J. n-3 PUFA reduction caused by fabp2 deletion interferes with triacylglycerol metabolism and cholesterolhomeostasis in fish. Appl. Microbiol. Biotechnol. 2020, 104, 2149–2161. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yuan, M.H.; Zhang, C.Y.; Liu, H.M.; Liu, J.R.; Wei, A.L.; Ye, Q.; Zeng, B.; Li, M.F.; Guo, Y.P.; et al. Puerariae Lobatae radix flavonoids and puerarin alleviate alcoholic liver injury in zebrafish by regulating alcohol and lipid metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Parnell, L.D.; Smith, C.E.; Guo, T.; Sayols-Baixeras, S.; Aslibekyan, S.; Tiwari, H.K.; Irvin, M.R.; Bender, C.; Fei, D.; et al. Carbohydrate and fat intake associated with risk of metabolic diseases through epigenetics of CPT1A. Am. J. Clin. Nutr. 2020, 112, 1200–1211. [Google Scholar] [CrossRef]
- Skinner, R.C.; Warren, D.C.; Lateef, S.N.; Benedito, V.A.; Tou, J.C. Apple Pomace Consumption Favorably Alters Hepatic Lipid Metabolism in Young Female Sprague-Dawley Rats Fed a Western Diet. Nutrients 2018, 10, 1882. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Han, H.; Zhang, K.; Ding, X.; Bai, S.; Wang, J.; Zeng, Q. Dietary fibre alleviates hepatic fat deposition via inhibiting lipogenic gene expression in meat ducks. J. Anim. Physiol. Anim. Nutr. 2018, 102, e736–e745. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, D.; Liu, F.; Wang, X.; Cui, Y.; Li, S.; Li, X. The Ameliorating Effects of Apple Polyphenol Extract on High-Fat-Diet-Induced Hepatic Steatosis Are SIRT1-Dependent: Evidence from Hepatic-Specific SIRT1 Heterozygous Mutant C57BL/6 Mice. J. Agric. Food Chem. 2022, 70, 5579–5594. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, S.; Stradiot, L.; Verhulst, S.; Thoen, L.; Mannaerts, I.; van Grunsven, L.A. Inhibitory effect of dietary capsaicin on liver fibrosis in mice. Mol. Nutr. Food Res. 2015, 59, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wei, W.Y.; Li, L.L.; Hu, C.; Tang, Q.Z. Therapeutic Potential of Polyphenols in Cardiac Fibrosis. Front. Pharmacol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Li, M.M.; Zhou, Y.; Zuo, L.; Nie, D.; Li, X.A. Dietary fiber regulates intestinal flora and suppresses liver and systemic inflammation to alleviate liver fibrosis in mice. Nutrition 2021, 81, 110959. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Ma, C.-N.; Liu, X.-L.; Sun, Q.; Zhang, Q.; Lin, Y.-Y.; Yan, C.-Y.; Hu, D.-G. Apple Consumption Protects against Acute Ethanol-Induced Liver Injury in Rats. Appl. Sci. 2023, 13, 5112. https://doi.org/10.3390/app13085112
Wang C, Ma C-N, Liu X-L, Sun Q, Zhang Q, Lin Y-Y, Yan C-Y, Hu D-G. Apple Consumption Protects against Acute Ethanol-Induced Liver Injury in Rats. Applied Sciences. 2023; 13(8):5112. https://doi.org/10.3390/app13085112
Chicago/Turabian StyleWang, Chen, Chang-Ning Ma, Xiao-Long Liu, Quan Sun, Qian Zhang, Ying-Ying Lin, Cheng-Yu Yan, and Da-Gang Hu. 2023. "Apple Consumption Protects against Acute Ethanol-Induced Liver Injury in Rats" Applied Sciences 13, no. 8: 5112. https://doi.org/10.3390/app13085112
APA StyleWang, C., Ma, C.-N., Liu, X.-L., Sun, Q., Zhang, Q., Lin, Y.-Y., Yan, C.-Y., & Hu, D.-G. (2023). Apple Consumption Protects against Acute Ethanol-Induced Liver Injury in Rats. Applied Sciences, 13(8), 5112. https://doi.org/10.3390/app13085112




