Sorption Behaviour of Ibuprofen Using Activated Carbon Derived from Rose Geranium (Pelargonium graveolens L.) Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Carbon
2.3. Adsorption Studies
2.4. Reusability Tudies
2.5. Characterization Analysis
3. Results and Discussions
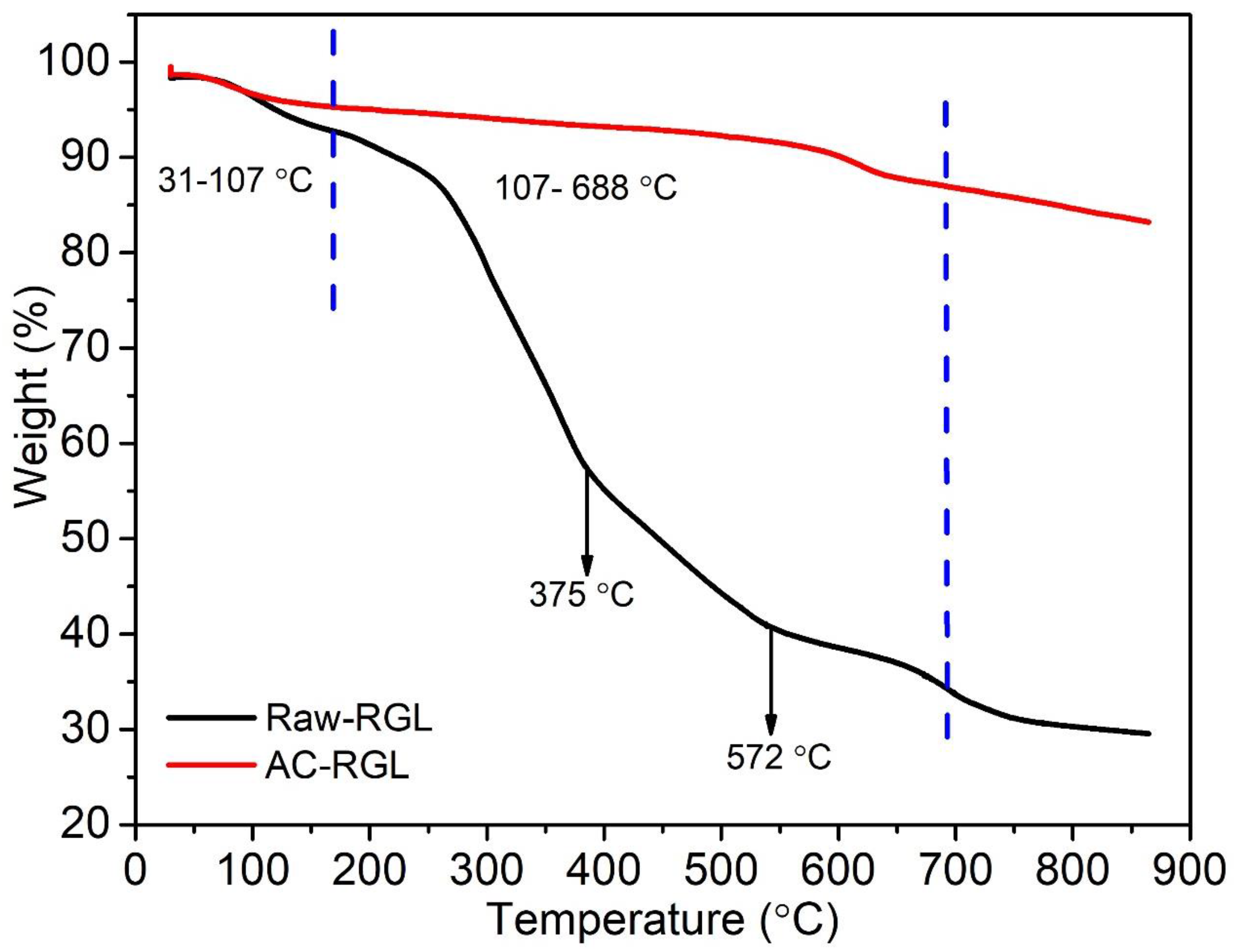
3.1. Thermal Determination
3.2. Textural Characterization
3.3. Surface Element Composition
3.4. Surface Characterization
3.5. Physicochemical Characterization
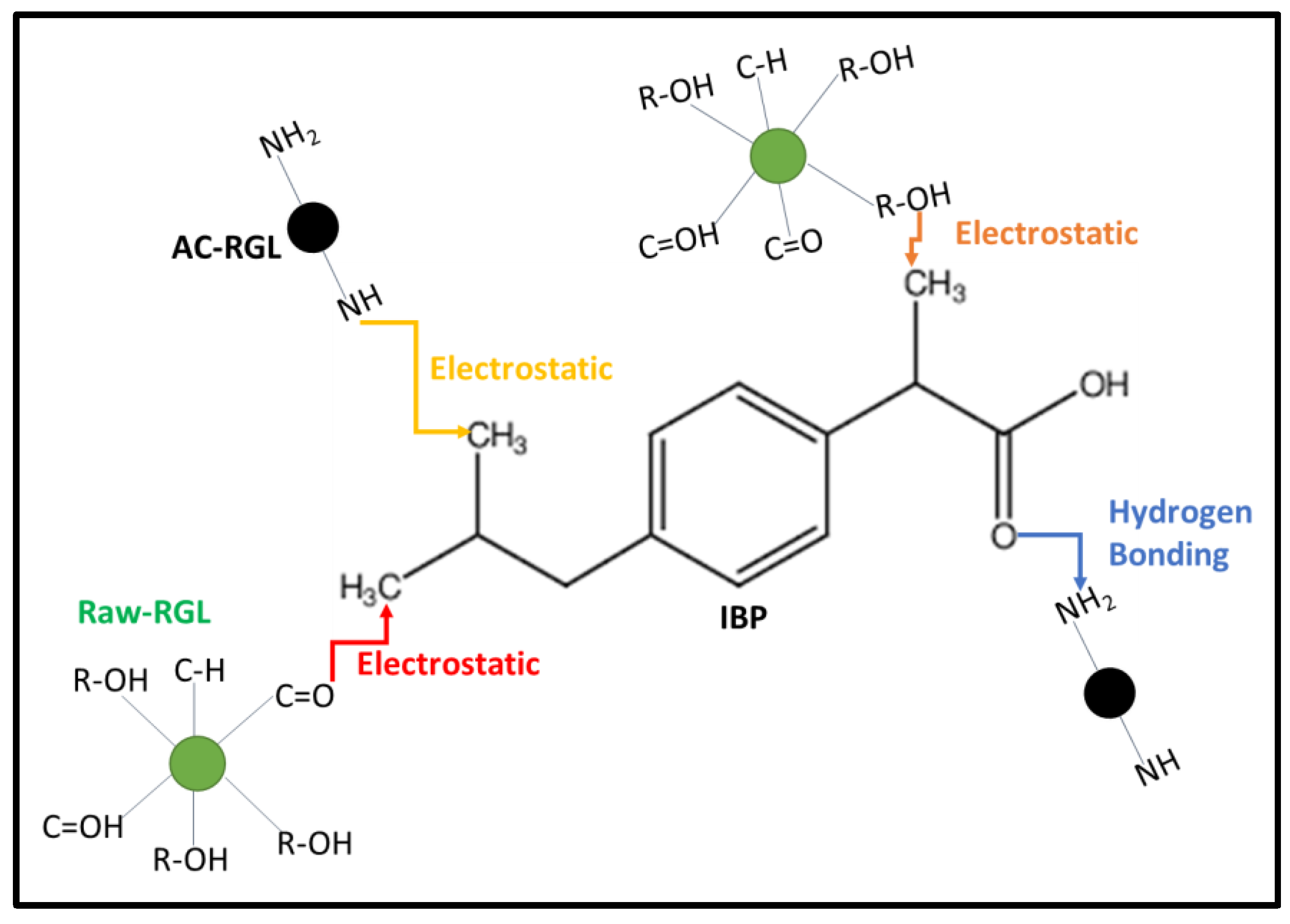
3.6. Proposed Adsorption Mechanism of IBP
3.7. Effect of Concentration and Adsorption Models
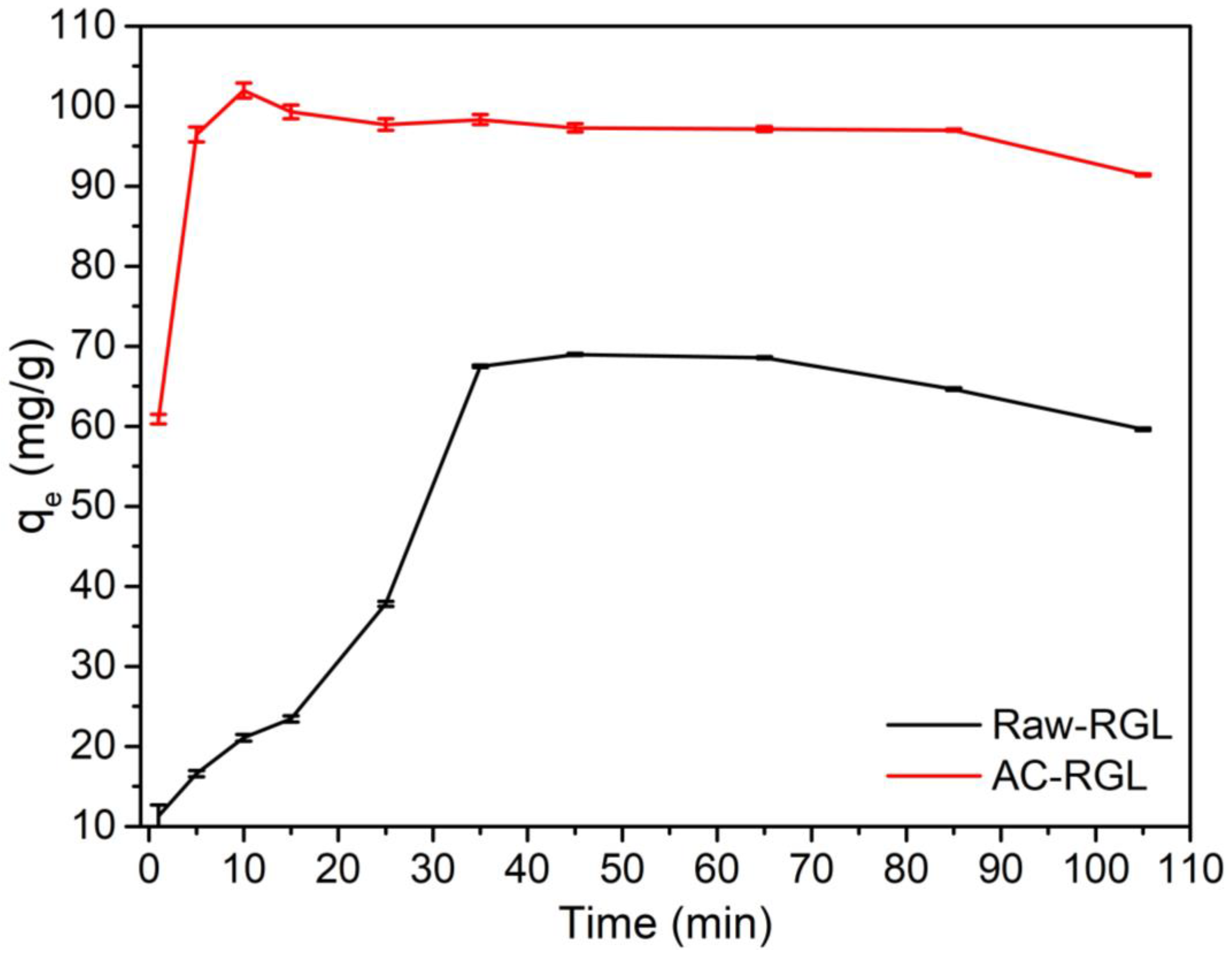
3.8. Time Effect and Rate Kinetics
3.9. Temperature and Thermodynamic Parameters
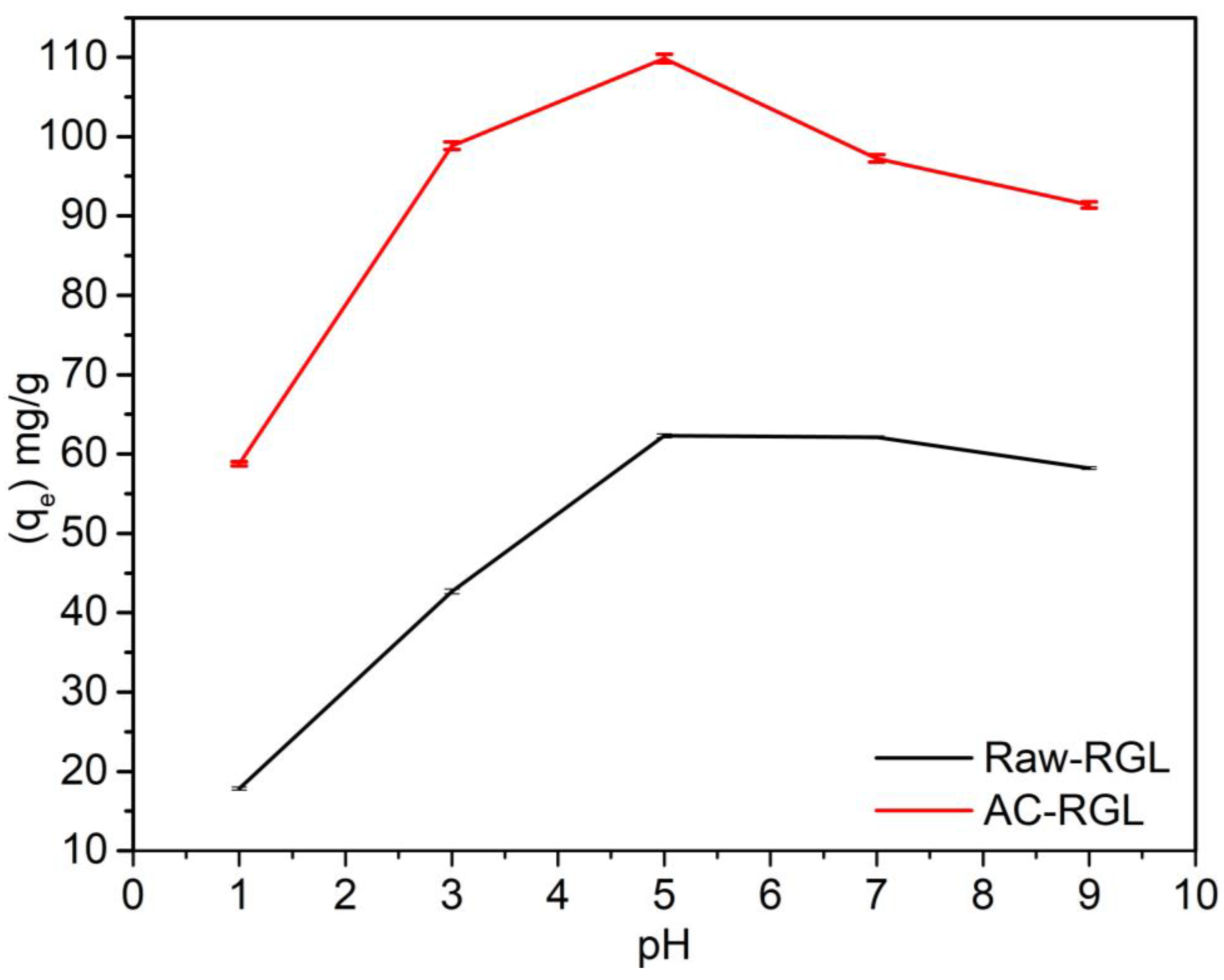
3.10. pH Effect
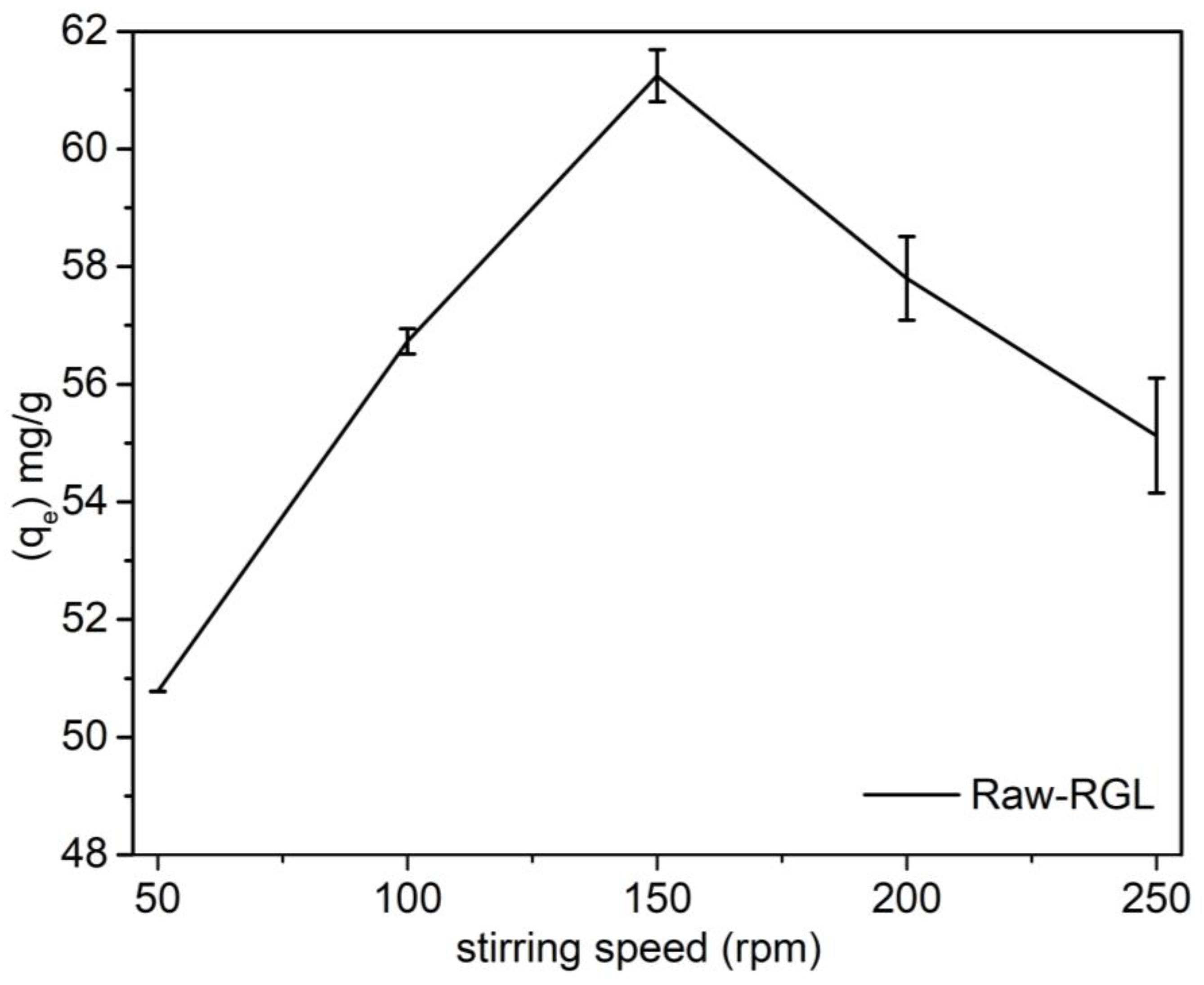
3.11. Stirring Speed Effect
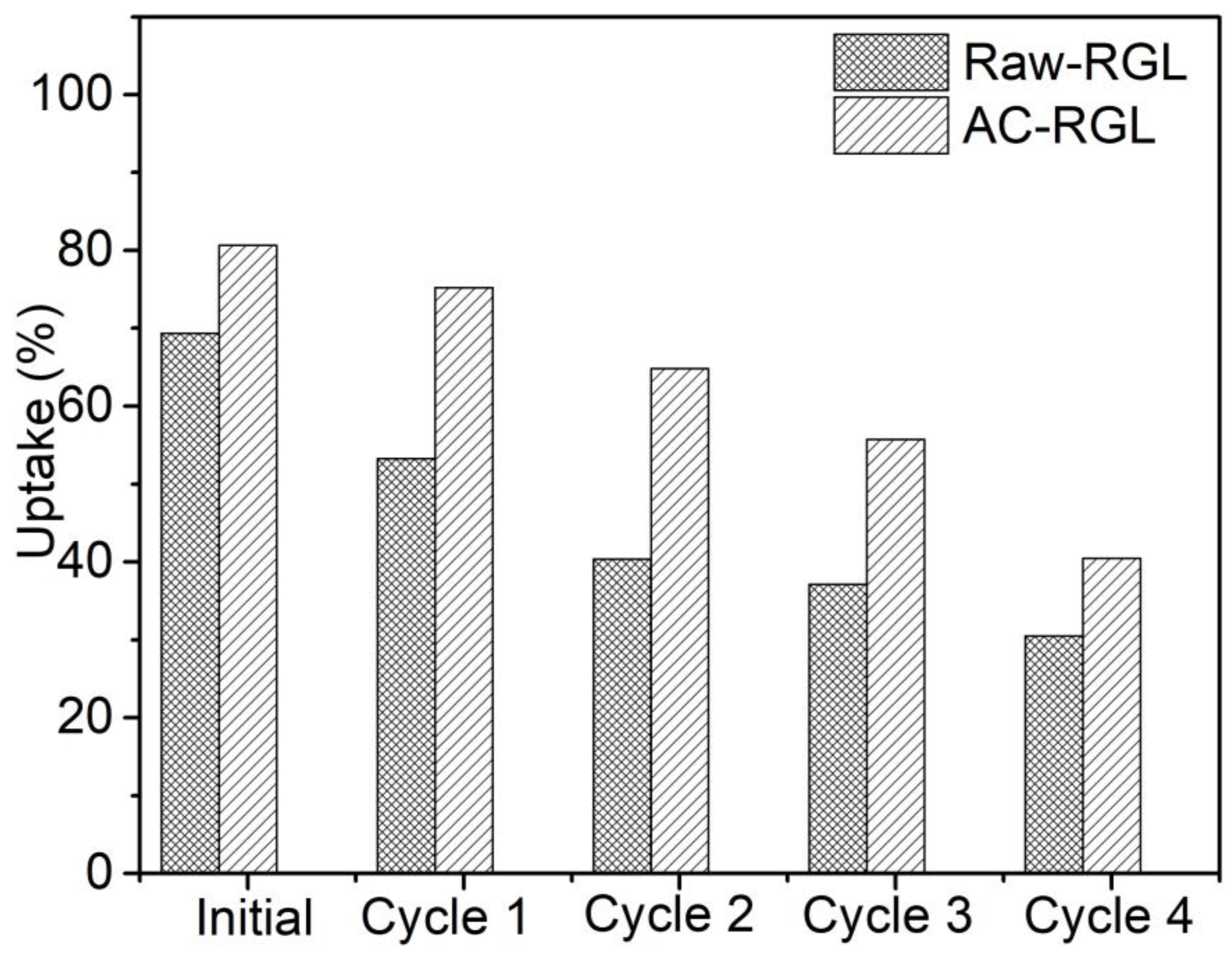
4. Regeneration Study
5. Post adsorption Characterization
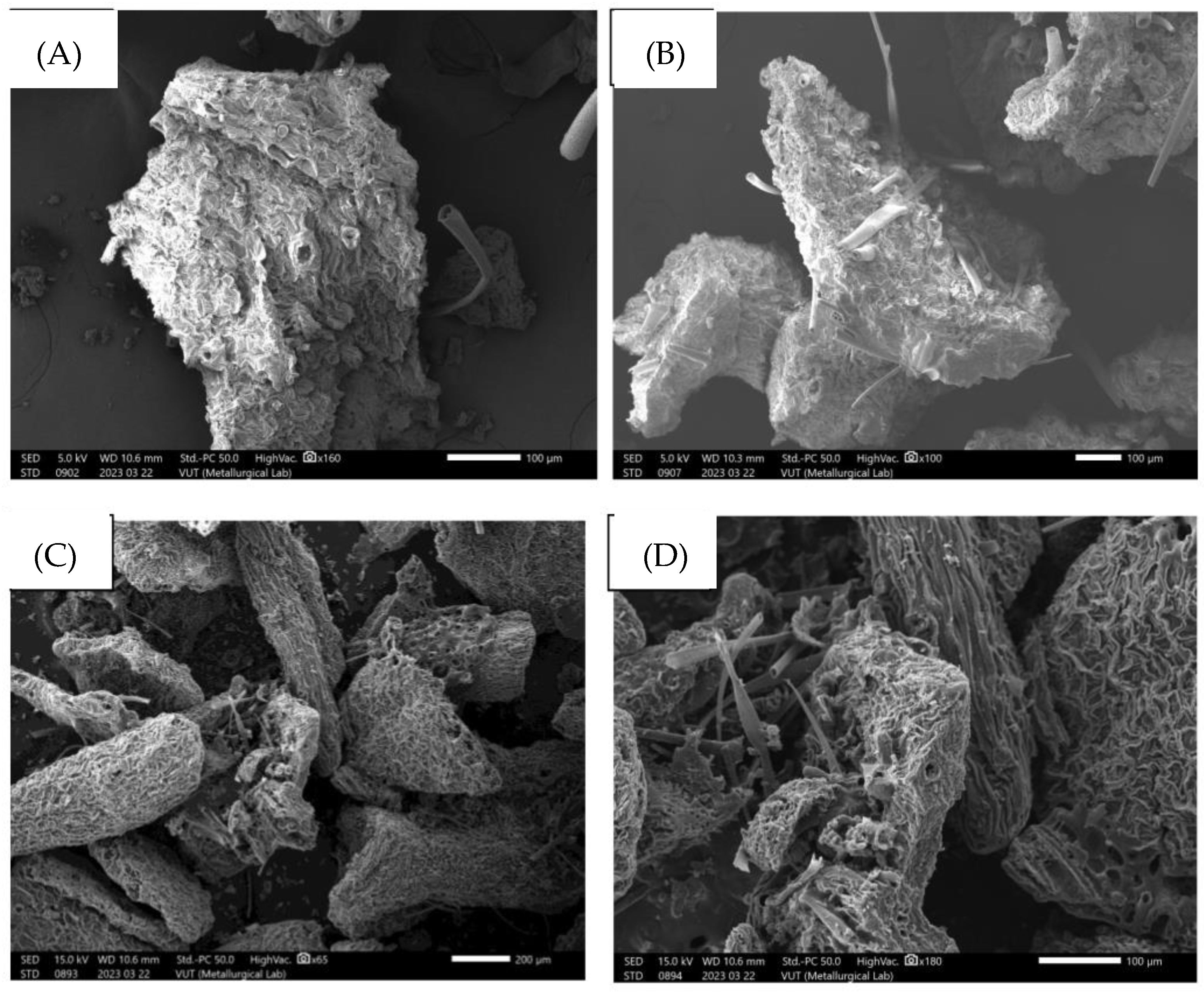
5.1. SEM Images
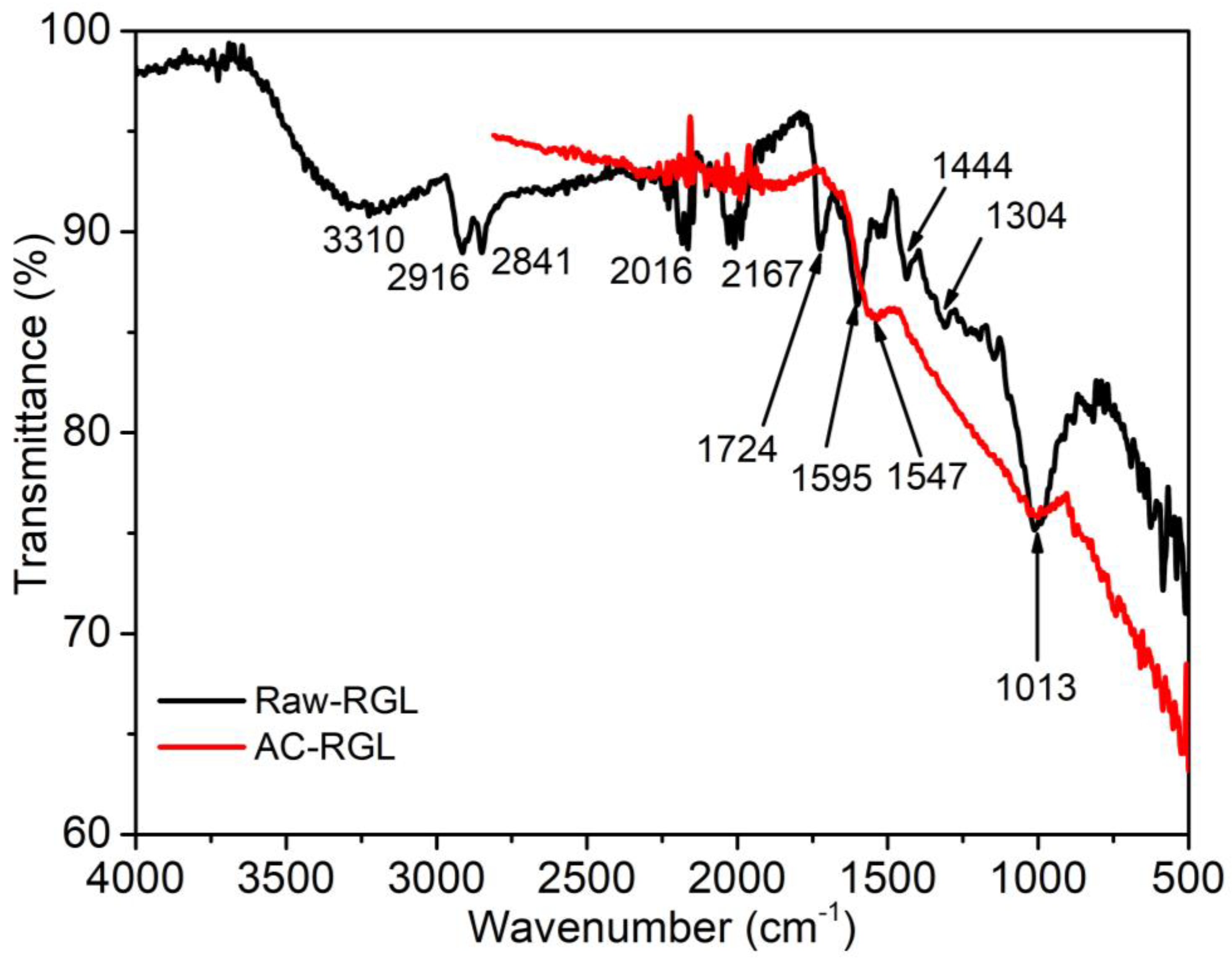
5.2. FTIR Analysis
6. Comparative Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Igwegbe, C.A. Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Wan, J.-C.; Kim, H.; Liang, C.-H.; Dai, Y.-D.; Chiang, P.-C. Adsorption of Selected Pharmaceutical Compounds onto Activated Carbon in Dilute Aqueous Solutions Exemplified by Acetaminophen, Diclofenac, and Sulfamethoxazole. Sci. World J. 2015, 2015, 186501. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Magalhães Filho, F.J. Ibuprofen and caffeine removal in vertical flow and free-floating macrophyte constructed wetlands with Heliconia rostrata and Eichornia crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Gaffar, J.; Gabrielli, S.; Lavine, E.; Pitt, T.; Abrams, E.; Atkinson, A.; Eiwegger, T.; Protudjer, J.; Wong, T.; O’Keefe, A. Diagnosis of ibuprofen allergy through oral challenge. Clin. Exp. Allergy 2020, 50, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Davarnejad, R.; Soofi, B.; Farghadani, F.; Behfar, R. Ibuprofen removal from a medicinal effluent: A review on the various techniques for medicinal effluents treatment. Environ. Technol. Innov. 2018, 11, 308–320. [Google Scholar] [CrossRef]
- Caicedo, D.F.; dos Reis, G.S.; Lima, E.C.; De Brum, I.A.; Thue, P.S.; Cazacliu, B.G.; Lima, D.R.; dos Santos, A.H.; Dotto, G.L. Efficient adsorbent based on construction and demolition wastes functionalized with 3-aminopropyltriethoxysilane (APTES) for the removal ciprofloxacin from hospital synthetic effluents. J. Environ. Chem. Eng. 2020, 8, 103875. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced Ibuprofen Adsorption and Desorption on Synthesized Functionalized Magnetic Multiwall Carbon Nanotubes from Aqueous Solution. Materials 2020, 13, 3329. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Gan, Y.; Wei, Y.; Xiong, J.; Gang Cheng, G. Impact of post-processing modes of precursor on adsorption and photocatalytic capability of mesoporous TiO2 nanocrystallite aggregates towards ciprofloxacin removal. Chem. Eng. J. 2018, 349, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, J.; Yang, H.; Wen, Z.; Chen, R.; Cheng, G.; Li, J.; Zhang, K.; Zhang, H. Surface potential/wettability and interface charge transfer engineering of copper-oxide (Cu-MOx, M = W, Ti, and Ce) hybrids for efficient wastewater treatment through adsorption-photocatalysis synergy. Ind. Eng. Chem. Res. 2020, 59, 15454–15463. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Poll. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Zhang, M.; Xiong, J.; Zhu, J.; Li, W.; Zhang, C.; Cheng, G. Impact of Cu particles on adsorption and photocatalytic capability of mesoporous Cu@TiO2 hybrid towards ciprofloxacin antibiotic removal. J. Taiwan Inst. Chem. Eng. 2019, 96, 229–242. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Abasi, C.; Mtunzi, F.M.; Nyamukamba, P.; Dikio, E.D. Adsorption study of lead ions on nickel- metal organic framework. Asian J. Chem. 2019, 31, 1153–1157. [Google Scholar] [CrossRef]
- Fadzail, F.; Hasan, M.; Mokhtar, Z. Adsorption of ibuprofen using activated carbon derived from Dillenia indica peels. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012031. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Yan, W. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Optimization of microwave preparation conditions for activated carbon from Albizia lebbeck seed pods for methylene blue dye adsorption. J. Anal. Appl. Pyrol. 2014, 105, 199–208. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Naidoo, E.B. Sorption of chromium(VI), cadmium(II) ions and methylene blue dye by pristine, defatted and carbonized Nigella sativa L. seeds from aqueous solution. Asian J. Chem. 2022, 33, 471–483. [Google Scholar]
- Ofomaja, A.E. Kinetic study and sorption mechanism of methylene blue and methyl violet onto mansonia (Mansonia altissima) wood sawdust. Chem. Eng. J. 2008, 143, 85–95. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I.; Kumar, R. Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. Clean Soil Air Water 2010, 38, 437–445. [Google Scholar] [CrossRef]
- Villabona-Ortíz, A.; Figueroa-Lopez, K.J.; Ortega-Toro, R. Kinetics and Adsorption Equilibrium in the Removal of Azo-Anionic Dyes by Modified Cellulose. Sustainability 2022, 14, 3640. [Google Scholar] [CrossRef]
- DAFF (Department of Agriculture, Forestry and Fishery). Rose-Geranium Production. 2012. Available online: www.nda.agric.za/docs/Brochures/ProGuRosegeranium (accessed on 10 April 2022).
- Weiss, E.A. Essential Oil Crops; CAB International: Wallingford, UK, 1997; pp. 125–130. [Google Scholar]
- Motsa, N.M.; Soundy, P.; Steyn, J.M.; Learmonth, R.A.; Mojela, N.; Teubes, C. Plant shoot age and temperature effects on essential oil yield and oil composition of rose scented geranium (Pelargonium spp.) grown in South Africa. J. Essent. Oil Res. 2006, 1, 106–110. [Google Scholar] [CrossRef]
- Demarne, F.E. Geraniums and Pelargoniums: The Genera Geraniums and Pelargoniums; Taylor and Francis: London, UK, 2002; pp. 77–93. [Google Scholar]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Marshall, V.; Hamidpour, R. Pelargonium graveolens (Rose Geranium) A Novel Therapeutic Agent for Antibacterial, Antioxidant, Antifungal and Diabetics. Arch. Can. Res. 2017, 5, 1. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Abdel-Salam, M.A. Remediation of a Pb-Contaminated Soil Cultivated with Rose Geranium (Pelargonium graveolens) Using Nano-Zeolite. J. Soil Sci. Agric. Eng. Mansoura Univ. 2018, 9, 473–479. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zheng, M.; Jiang, X.; Jin, R.; Zhao, Y.; Zhan, J. Insights into the emission reductions of multiple unintentional persistent organic pollutants from industrial activities. Chemosphere 2016, 144, 420–424. [Google Scholar] [CrossRef]
- Acosta, R.; Fierro, V.; Martinez de Yuso, A.; Nabarlatz, D.; Celzard, A. Tetracycline adsorption onto activated carbons produced by KOH activation of tyre pyrolysis charcoal. Chemosphere 2016, 149, 168–176. [Google Scholar] [CrossRef]
- Islam, M.A.; Sabar, S.; Benhouria, A.; Khanday, W.A.; Asif, M.; Hameed, B.H. Nanoporous activated carbon prepared from karanj (Pongamia pinnata) fruit hulls for methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2017, 74, 96–104. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhu, T. Application of activated carbon derived from scrap tires for adsorption of Rhodamine B. J. Environ. Sci. 2010, 22, 1273–1280. [Google Scholar] [CrossRef]
- Njoku, V.O.; Foo, K.Y.; Asif, M.; Hameed, B.H. Preparation of activated carbons from rambutan (Nephelium lappaceum) peel by microwave-induced KOH activation for acid yellow 17 dye adsorption. Chem Eng. J. 2014, 250, 198–204. [Google Scholar] [CrossRef]
- Deng, H.; Li, G.; Yang, H.; Tang, J.; Tang, J. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation. Chem. Eng. J. 2010, 163, 373–381. [Google Scholar] [CrossRef]
- Yanga, D.; Song, S.; Li, J.; Tang, Z.; Ye, J.; Yang, J. Preparation and CO2 adsorption properties of porous carbon by hydrothermal carbonization of tree leaves. J. Mater. Sci. Technol. 2019, 35, 875–884. [Google Scholar] [CrossRef]
- Hassana, A.F.; Elhadidy, H. Production of activated carbons from waste carpets and its application in methylene blue adsorption: Kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2017, 5, 955–963. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Redondo, N.; Obeso, R.A.; Caracciolo, N.; Vázquez, C. Chromium and Lead adsorption by avocado seed biomass study through the use of Total Reflection X-Ray Fluorescence analysis. Appl. Radiat. Isot. 2019, 153, 108809. [Google Scholar] [CrossRef] [PubMed]
- Thabede, P.M.; Shooto, N.D.; Naidoo, E.B. Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. S. Afr. J. Chem. Eng. 2020, 33, 39–50. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Rafatullah, M.; Sulaiman, O.; Govind, A.A. Adsorption of Pb(II) ions from aqueous solutions by date bead carbon activated with ZnCl2. Clean Soil Air Water 2011, 39, 392–399. [Google Scholar] [CrossRef]
- Saucier, C.; Adebayo, M.A.; Lima, E.C.; Cataluna, R.; Thue, P.S.; Prola, L.D.T.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Dotto, G.L. Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 2015, 289, 18–27. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Xaba, T.; Naidoo, E.B. Sulfuric activated carbon of black cumin (Nigella sativa L.) seeds for the removal of cadmium(II) and methylene blue dye. Asian J. Chem. 2020, 32, 1361–1369. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Xaba, T.; Naidoo, E.B. Adsorption studies of toxic cadmium(II) and chromium(VI) ions from aqueous solution by activated black cumin (Nigella sativa) seeds. J. Environ. Chem. Eng. 2020, 8, 104045. [Google Scholar] [CrossRef]
- Chakraborty, P.; Show, S.; Banerjee, S.; Halder, G. Mechanistic insight into sorptive elimination of ibuprofen employing bidirectional activated biochar from sugarcane bagasse: Performance evaluation and cost estimation. J. Environ. Chem. Eng. 2018, 6, 5287–5300. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M. Binary Adsorption of chromium and cadmium metal ions by hemp (Cannabis sativa) based adsorents. Environmental Nanotechnology. Monit. Manag. 2022, 18, 100683. [Google Scholar]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Sillanpää, M.; Bhatnagar, A. A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae Effect of pre-treatment. Chem. Eng. J. 2017, 307, 435–446. [Google Scholar] [CrossRef]
- Matos, J.; Nahas, C.; Rojas, L.; Rosales, M. Synthesis and characterization of activated carbon from sawdust of Algarroba wood. 1.Physical activation and pyrolysis. J. Hazard. Mater. 2011, 196, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Obemah David Nartey, O.D. Characterization and evaluation of biochars derived from agricultural waste biomass from Gansu, China. In Proceedings of the World Congress on Advances in Civil, Environmental, and Materials Research, Busan, Republic of Korea, 24–28 August 2014. [Google Scholar]
- Wan, X.; Rong, Z.; Zhu, K.; Wu, Y. Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int. J. Biol. Macromol. 2022, 222, 725–735. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Anil, A.G.; Kapoor, D.; Khasnabis, S.; Shekar, S.; Pavithra, N.; Samuel, J.; Subramanian, S.; Singh, J.; et al. Adsorption and detoxification of pharmaceutical compounds from wastewater using nanomaterials: A review on mechanism, kinetics, valorization and circular economy. J. Environ. Manag. 2021, 300, 113569. [Google Scholar] [CrossRef]
- Mabungela, N.; Shooto, N.D.; Mtunzi, F.; Naidoo, E.B. The Adsorption of Copper, Lead Metal Ions, and Methylene Blue Dye from Aqueous Solution by Pure and Treated Fennel Seeds. Adsorp. Sci. Technol. 2022, 2022, 5787690. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisupar, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin materials for adsorption: Current trent, perspectives and opportunities. Bioresour. Technol. 2019, 272, 570–580. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. The nutritional composition of fennel (Foeniculum vulgare): Shoots, leaves, stems and inflorescences. LWT-Food Sci. Technol. 2010, 43, 814–818. [Google Scholar] [CrossRef]
- Shooto, N.D.; Naidoo, E.B.; Maubane, M. Sorption studies of toxic cations on ginger root adsorbent. J. Ind. Eng. Chem. 2019, 76, 133–140. [Google Scholar] [CrossRef]
- Sekulic, M.T.; Boskovic, N.; Slavkovic, A.; Garunovica, J.; Kolakovic, S.; Papa, S. Surface functionalised adsorbent for emerging pharmaceuticalremoval: Adsorption performance and mechanisms. Process Saf. Environ. Prot. 2019, 125, 50–63. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Bhila, B.; Harry Moloto, H.; Naidoo, E.B. Lead ions and methylene blue dye removal from aqueous solution by mucuna beans (velvet beans) adsorbents. J. Environ. Chem. Eng. 2020, 8, 103557. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Naidoo, E.B. Simultaneous adsorptive study of toxic metal ions in quaternary system from aqueous solution using low-cost black cumin seeds (Nigella sativa) adsorbents. S. Afr. J. Chem. Eng. 2019, 30, 15–27. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Dong, S.K. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar]
- Ghaedi, M.; Ansari, A.; Bahari, F.; Ghaedi, A.M.; Vafaei, A. A hybrid artificial neural network and particle swarm optimization for prediction of removal of hazardous dye brilliant green from aqueous solution using zinc sulfide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Sarkara, S.; Tiwari, N.; Basu, A.; Behera, M.; Das, B.; Chakrabortty, S.; Sanjay, K.; Suar, M.; Adhya, T.K.; Banerjee, S.; et al. Sorptive removal of malachite green from aqueous solution by magnetite/coir pith supported sodium alginate beads: Kinetics, isotherms, thermodynamics and parametric optimization. Environ. Technol. Innov. 2021, 24, 101818. [Google Scholar] [CrossRef]
- Basu, A.; Behera, S.S.; Dash, S.; Banerjee, S.; Sarkar, S.; Mohanty, C.K.; Dhal, N.K.; Parhi, P.K.; Tripathy, S.K. A study on removal of Cr(III) from aqueous solution using biomass of Cymbopogon flexuosus immobilized in sodium alginate beads and its use as hydrogenation catalyst. J. Taiwan Inst. Chem. Eng. 2019, 102, 118–132. [Google Scholar] [CrossRef]
- Raza, M.H.; Sadiq, A.; Farooq, U.; Athar, M.; Hussain, T.; Mujahid, A.; Salman, M. Phragmites karka as a Biosorbent for the removal of mercury metal ions from aqueous solution: Effect of modification. J. Chem. 2015, 2015, 293054. [Google Scholar] [CrossRef]
- Geng, J.; Lin, L.; Gu, F.; Chang, J. Adsorption of Cr(Ⅵ) and dyes by plant leaves: Effect of extraction by ethanol, relationship with element contents and adsorption mechanism. Ind. Crops Prod. 2022, 177, 114522. [Google Scholar] [CrossRef]
- Jedynak, K.; Szczepanik, B.; Rędzia, N.; Słomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered mesoporous carbons for adsorption of paracetamol and non-steroidal anti-inflammatory drugs: Ibuprofen and naproxen from aqueous solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef]
- Wong, K.T.; Yoon, Y.; Jang, M. Enhanced recyclable magnetized palm shell waste based powdered activated carbon for the removal of ibuprofen: Insights for kinetics and mechanisms. PLoS ONE 2015, 10, 0141013. [Google Scholar] [CrossRef]
- Frohlich, A.C.; dos Reis, G.S.; Pavan, F.A.; Lima, É.C.; Foletto, E.L.; Dotto, G.L. Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ. Sci. Pollut. Res. 2018, 25, 24713–24725. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.N.; El-Shafey, E.; Al-Busafi, S.; Al-Lawati, H.A. Adsorption of chlorpheniramine and ibuprofen on surface functionalized activated carbons from deionized water and spiked hospital wastewater. J. Environ. Chem. Eng. 2019, 7, 102860. [Google Scholar] [CrossRef]
- Labuto, G.; Carvalho, A.P.; Mestre, A.S.; dos Santos, M.S.; Modesto, H.R.; Martins, T.D.; Lemos, S.G.; da Silva, H.G.T.; Neide, E.; Carrilho, V.M.; et al. Individual and competitive adsorption of ibuprofen and caffeine from primary sewage effluent by yeast-based activated carbon and magnetic carbon nanocomposite. Sustain. Chem. Pharm. 2022, 28, 100703. [Google Scholar] [CrossRef]
- Dubeya, S.P.; Dwivedi, A.D.; Sillanpääa, M.; Gopal, K. Artemisia vulgaris-derived mesoporous honeycomb-shaped activated carbon for ibuprofen adsorption. Chem. Eng. J. 2010, 165, 537–544. [Google Scholar] [CrossRef]





| Element | Mass (%) | Atom (%) |
|---|---|---|
| C | 62.99 | 70.12 |
| O | 34.90 | 29.17 |
| K | 1.08 | 0.37 |
| Ca | 1.02 | 0.34 |
| Total | 100.00 | 100.00 |
| Element | Mass (%) | Atom (%) |
|---|---|---|
| C | 77.15 | 86.05 |
| O | 11.46 | 9.60 |
| Mg | 1.30 | 0.72 |
| Si | 1.33 | 0.64 |
| Cl | 1.42 | 0.54 |
| K | 1.63 | 0.56 |
| Ca | 5.70 | 1.96 |
| Total | 100.00 | 100.00 |
| Adsorbent | Surface Area (m2/g) | Micropore Volume (cm3/gh) | Mesopore Volume (cm3/g) | Total Pore Volume (cm3/g) | pH(pzc) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| Raw-RGL | 1.70 | - | - | - | 7.32 | 22.56 |
| AC-RGL | 17.69 | 0.500 | 0.103 | 0.606 | 6.61 | 41.13 |
| Isotherms | Raw-RGL | AC-RGL | |
|---|---|---|---|
| Langmuir | Qo | 45.12 | 35.68 |
| B | 2.13 | 3.82 | |
| r2 | 0.812 | 0.951 | |
| Freundlich | 1/n | 69.67 | 125.68 |
| kf | 3.67 | 1.67 | |
| r2 | 0.998 | 0.997 | |
| Experimental (qe) | 74.12 | 113.76 | |
| Models | Raw-RGL | AC-RGL | |
|---|---|---|---|
| PFO | K1 | 0.469 | 0.979 |
| r2 | 0.995 | 0.993 | |
| PSO | qe | 34.56 | 56.65 |
| K2 | 0.101 | 1.002 | |
| r2 | 0.861 | 0.757 | |
| IPD | C | 33.23 | 47.23 |
| Ki | 7.715 | 13.06 | |
| r2 | 0.867 | 0.823 | |
| EPA | % | 12.88 | 16.88 |
| ESA | % | 87.12 | 83.12 |
| Experimental (qe) | 68.97 | 101.96 | |
| Parameter | Raw-RGL | AC-RCL |
|---|---|---|
| ∆H° (KJ mol−1) | −2.39 | −2.34 |
| ∆S° (KJ mol−1K−1) | 3.23 | 4.78 |
| ∆G° (KJ mol−1) 298 K | −4.67 | −3.80 |
| 308 K | −5.65 | −4.89 |
| 318 K | −6.45 | −5.23 |
| 328 K | −7.55 | −6.01 |
| 338 K | −8.43 | −6.89 |
| Adsorbents | q(max) (mg/g) | References |
|---|---|---|
| Ibuprofen | ||
| Mesoporous carbon | 120.1 | [63] |
| Palm shell | 114.7 | [64] |
| Rose geranium leaves | 113.76 | This study |
| Standard activated carbon | 85.0 | [65] |
| Alkaline activated carbon | 68.0 | [66] |
| Yeast-based activated carbon | 51.0 | [67] |
| Leaves of mugwort weed | 16.95 | [68] |
| Sugarcane bagasse | 13.51 | [42] |
| Olive waste cake | 12.9 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thabede, P.M.; Mtunzi, F.; Nyamukamba, P. Sorption Behaviour of Ibuprofen Using Activated Carbon Derived from Rose Geranium (Pelargonium graveolens L.) Leaves. Appl. Sci. 2023, 13, 5133. https://doi.org/10.3390/app13085133
Thabede PM, Mtunzi F, Nyamukamba P. Sorption Behaviour of Ibuprofen Using Activated Carbon Derived from Rose Geranium (Pelargonium graveolens L.) Leaves. Applied Sciences. 2023; 13(8):5133. https://doi.org/10.3390/app13085133
Chicago/Turabian StyleThabede, Patience Mapule, Fanyana Mtunzi, and Pardon Nyamukamba. 2023. "Sorption Behaviour of Ibuprofen Using Activated Carbon Derived from Rose Geranium (Pelargonium graveolens L.) Leaves" Applied Sciences 13, no. 8: 5133. https://doi.org/10.3390/app13085133
APA StyleThabede, P. M., Mtunzi, F., & Nyamukamba, P. (2023). Sorption Behaviour of Ibuprofen Using Activated Carbon Derived from Rose Geranium (Pelargonium graveolens L.) Leaves. Applied Sciences, 13(8), 5133. https://doi.org/10.3390/app13085133






