Predictive Assessment of Mycological State of Bulk-Stored Barley Using B-Splines in Conjunction with Genetic Algorithms
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Data Collection
2.2. Data Preprocessing
2.3. Development of the Model to Assess the Mycological State of Grain
2.3.1. Methodology of B-Spline
2.3.2. Methodology of Genetic Algorithms
- If a new organism exhibited characteristics superior to the best one in the population, then it unconditionally replaced the worst organism/solution in the population.
- If a new organism exhibited characteristics superior to the worst one in the population and meets the requirement to maintain population diversity that the smallest of the distances between the new organism and any of the organisms existing in the population was greater than the limit value, defined as 1/3 of the current average distance in the population.
2.4. Statistical Evaluation of Model Performance
3. Results and Discussion
3.1. B-Spline Curve Structure Determiantion
3.2. Genetic Algorithms Design
3.3. Evaluation of Model Performance
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henry, R.J. Barley: Harvesting, Storage, and Transport, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Piltz, J.W.; Rodham, C.A.; Wilkins, J.F.; Hackney, B.F. A comparison of cereal and cereal/vetch crops for fodder conservation. Agriculture 2021, 11, 459. [Google Scholar] [CrossRef]
- Martínez-Subirà, M.; Moralejo, M.; Puig, E.; Romero, M.P.; Savin, R.; Romagosa, I. Impact of rising temperature in the deposition patterns of bioactive compounds in field grown food barley grains. Plants 2021, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trębicki, P. Yield losses caused by barley yellow dwarf virus-pav infection in wheat and barley: A three-year field study in south-eastern Australia. Microorganisms 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia (Bologna) 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Mankevičienė, A.; Semaškienė, R.; Dabkevičius, Z.; Kochiieru, Y.; Janavičienė, S.; Jonavičienė, A. Do black dots on wheat grains have an impact on deoxynivalenol accumulation? Zemdirbyste 2019, 106, 249–256. [Google Scholar] [CrossRef]
- Bretträger, M.; Becker, T.; Gast, M. Screening of Mycotoxigenic Fungi in Barley and Barley Malt (Hordeum vulgare L.) Using Real-Time PCR—A Comparison between Molecular Diagnostic and Culture Technique. Foods 2022, 11, 1149. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.; Currie, V.; Duncan, G.; Filipe, J.A.N.; Yoshinari, T.; Stoddart, G.; Roberts, D.; Gratz, S.W. Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland. Toxins 2023, 15, 247. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Tadla, M.; Rusinek, R. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Meas. J. Int. Meas. Confed. 2017, 103, 227–234. [Google Scholar] [CrossRef]
- Kochiieru, Y.; Mankeviciene, A.; Janaviciene, S.; Jonaviciene, A.; Ceseviciene, J. the Influence of Milling and Sifting Processes on Deoxynivalenol Distribution in Whole-Wheat Flour and Its Products. World Mycotoxin J. 2019, 12, 133–140. [Google Scholar] [CrossRef]
- Bhat, R.; Reddy, K.R.N. Challenges and issues concerning mycotoxins contamination in oil seeds and their edible oils: Updates from last decade. Food Chem. 2017, 215, 425–437. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Rudzińska, M.; Siger, A.; Bartkowiak-Broda, I. A high drying temperature causes degradation of sterols and tocopherols in yellow-seeded Brassica napus oils. Eur. J. Lipid Sci. Technol. 2015, 117, 483–490. [Google Scholar] [CrossRef]
- Ryniecki, A.; Gawrysiak-Witulska, M.; Wawrzyniak, J. Correlation for the automatic identification of drying endpoint in near-ambient dryers: Application to malting barley. Biosyst. Eng. 2007, 98, 437–445. [Google Scholar] [CrossRef]
- Wontner-Smith, T.J.; Bruce, D.M.; Cardwell, S.K.; Armitage, D.M.; Jennings, P. Ochratoxin A production during ambient-air drying. J. Stored Prod. Res. 2014, 56, 1–7. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Wawrzyniak, J.; Ryniecki, A.; Perkowski, J. Relationship of ergosterol content and fungal contamination and assessment of technological quality of malting barley preserved in a metal silo using the near-ambient method. J. Stored Prod. Res. 2008, 44, 360–365. [Google Scholar] [CrossRef]
- Orina, I.; Manley, M.; Williams, P.J. Non-destructive techniques for the detection of fungal infection in cereal grains. Food Res. Int. 2017, 100, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Rusinek, R.; Gancarz, M.; Krekora, M.; Nawrocka, A. A Novel Method for Generation of a Fingerprint Using Electronic Nose on the Example of Rapeseed Spoilage. J. Food Sci. 2019, 84, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Santos, J.L.P.; Chaves, R.D.; Sant’Ana, A.S. Estimation of growth parameters of six different fungal species for selection of strains to be used in challenge tests of bakery products. Food Biosci. 2017, 20, 62–66. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Vermeulen, A.; Jacxsens, L.; Mavromichali, E.; De Saeger, S.; De Meulenaer, B.; Devlieghere, F. Mycotoxin production and predictive modelling kinetics on the growth of Aspergillus flavus and Aspergillus parasiticus isolates in whole black peppercorns (Piper nigrum L). Int. J. Food Microbiol. 2016, 228, 44–57. [Google Scholar] [CrossRef]
- Jacxsens, L.; Yogendrarajaha, P.; De Meulenaer, B. Risk assessment of mycotoxins and predictive mycology in Sri Lankan spices: Chilli and pepper. Procedia Food Sci. 2016, 6, 326–330. [Google Scholar] [CrossRef]
- Aldars-García, L.; Sanchis, V.; Ramos, A.J.; Marín, S. Single vs multiple-spore inoculum effect on growth kinetic parameters and modeled probabilities of growth and aflatoxin B1 production of Aspergillus flavus on pistachio extract agar. Int. J. Food Microbiol. 2017, 243, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J. Model of Fungal Development in Stored Barley Ecosystems as a Prognostic Auxiliary Tool for Postharvest Preservation Systems. Food Bioprocess Technol. 2021, 14, 298–309. [Google Scholar] [CrossRef]
- Wawrzyniak, J. A Predictive Model for Assessment of the Risk of Mold Growth in Rapeseeds Stored in a bulk as a Decision Support Tool for Postharvest Management Systems. J. Am. Oil Chem. Soc. 2020, 97, 915–927. [Google Scholar] [CrossRef]
- Gauthier, J.; Wu, Q.V.; Gooley, T.A. Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant. 2020, 55, 675–680. [Google Scholar] [CrossRef]
- Majeed, A.; Abbas, M.; Qayyum, F.; Miura, K.T.; Misro, M.Y.; Nazir, T. Geometric modeling using new cubic trigonometric B-spline functions with shape parameter. Mathematics 2020, 8, 2102. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Medina, E.; Salinas, F.; Bugueño, W.; Fuentes, J.L.; Vílchez, C.; Garbayo, I.; Cerezal-Mezquita, P. Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis. Antioxidants 2022, 11, 1248. [Google Scholar] [CrossRef]
- Córdoba, A.T.; Gata, P.M.; Reina, D.G. Optimizing the Layout of Run-of-River Powerplants Using Cubic Hermite Splines and Genetic Algorithms. Appl. Sci. 2022, 12, 8133. [Google Scholar] [CrossRef]
- Bernstein, J.T.; Lou, W.; L’abbe, M.R. Examining the relationship between free sugars and calorie contents in Canadian prepacked foods and beverages. Foods 2017, 6, 75. [Google Scholar] [CrossRef]
- Gackowski, M.; Szewczyk-Golec, K.; Pluskota, R.; Koba, M.; Madra-Gackowska, K.; Woźniak, A. Application of Multivariate Adaptive Regression Splines (MARSplines) for Predicting Antitumor Activity of Anthrapyrazole Derivatives. Int. J. Mol. Sci. 2022, 23, 5132. [Google Scholar] [CrossRef]
- Bahar, A.; Cavusoglu, S.; Yilmaz, N.; Tekin, O.; Ercisli, S. The Effect of Different Doses of 1-Methylcyclopropene on Postharvest Physiology and Predicting Ethylene Production through Multivariate Adaptive Regression Splines in Cocktail Tomato. Horticulturae 2022, 8, 567. [Google Scholar] [CrossRef]
- Najjar, Y.M.; Basheer, I.A.; Hajmeer, M.N. Computational neural networks for predictive microbiology: I. methodology. Int. J. Food Microbiol. 1997, 34, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Panagou, E.Z.; Kodogiannis, V.; Nychas, G.J.E. Modelling fungal growth using radial basis function neural networks: The case of the ascomycetous fungus Monascus ruber van Tieghem. Int. J. Food Microbiol. 2007, 117, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Panagou, E.Z.; Kodogiannis, V.S. Application of neural networks as a non-linear modelling technique in food mycology. Expert Syst. Appl. 2009, 36, 121–131. [Google Scholar] [CrossRef]
- Mateo, F.; Gadea, R.; Medina, Á.; Mateo, R.; Jiménez, M. Predictive assessment of ochratoxin A accumulation in grape juice based-medium by Aspergillus carbonarius using neural networks. J. Appl. Microbiol. 2009, 107, 915–927. [Google Scholar] [CrossRef]
- Mateo, F.; Gadea, R.; Mateo, E.M.; Jiménez, M. Multilayer perceptron neural networks and radial-basis function networks as tools to forecast accumulation of deoxynivalenol in barley seeds contaminated with Fusarium culmorum. Food Control 2011, 22, 88–95. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Application of Artificial Neural Networks to Assess the Mycological State of Bulk Stored Rapeseeds. Agriculture 2020, 10, 567. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Prediction of fungal infestation in stored barley ecosystems using artificial neural networks. LWT 2021, 137, 110367. [Google Scholar] [CrossRef]
- Esfahanian, M.; Shokuhi Rad, A.; Khoshhal, S.; Najafpour, G.; Asghari, B. Mathematical modeling of continuous ethanol fermentation in a membrane bioreactor by pervaporation compared to conventional system: Genetic algorithm. Bioresour. Technol. 2016, 212, 62–71. [Google Scholar] [CrossRef]
- Du, X.L.; Li, X.Y.; Liu, Y.; Zhou, W.H.; Li, J.L. Genetic algorithm optimized non-destructive prediction on property of mechanically injured peaches during postharvest storage by portable visible/shortwave near-infrared spectroscopy. Sci. Hortic. 2019, 249, 240–249. [Google Scholar] [CrossRef]
- Wang, C.; Wang, P.; Han, S.; Wang, L.; Zhao, Y.; Juan, L. FunEffector-Pred: Identification of Fungi Effector by Activate Learning and Genetic Algorithm Sampling of Imbalanced Data. IEEE Access 2020, 8, 57674–57683. [Google Scholar] [CrossRef]
- Holland, J.H. Genetic Algorithms. Computer programs that "evolve" in ways that resemble natural selection can solve complex problems even their creators do not fully understand. Sci. Am. 1992, 267, 66–73. [Google Scholar] [CrossRef]
- Mondal, D.; Chakrabarti, A.; Sengupta, A. Chapter 8—Optimal and Robust Control. In Power System Small Signal Stability Analysis and Control, 2nd ed.; Mondal, D., Chakrabarti, A., Sengupta, A., Eds.; Academic Press: Boston, MA, USA, 2020; pp. 243–286. ISBN 978-0-12-817768-6. [Google Scholar]
- Gómez-Sanchis, J.; Gómez-Chova, L.; Aleixos, N.; Camps-Valls, G.; Montesinos-Herrero, C.; Moltó, E.; Blasco, J. Hyperspectral system for early detection of rottenness caused by Penicillium digitatum in mandarins. J. Food Eng. 2008, 89, 80–86. [Google Scholar] [CrossRef]
- Jahedi, A.; Salehi, M.; Goltapeh, E.M.; Safaie, N. Multilayer perceptron-genetic algorithm as a promising tool for modeling cultivation substrate of Auricularia cornea Native to Iran. PLoS ONE 2023, 18, e0281982. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, J.; Waśkiewicz, A.; Ryniecki, A. Evaluation of critical points of mould growth and mycotoxin production in the stored barley ecosystem with a hazardous initial microbiological state of grain. J. Stored Prod. Res. 2018, 77, 166–176. [Google Scholar] [CrossRef]
- Wawrzyniak, J. Methodology for Quantifying Volatile Compounds in a Liquid Mixture Using an Algorithm Combining B-Splines and Artificial Neural Networks to Process Responses of a Thermally Modulated Metal-Oxide Semiconductor Gas Sensor. Sensors 2022, 22, 8959. [Google Scholar] [CrossRef]
- Menaga, D.; Saravanan, S. 7—Application of artificial intelligence in the perspective of data mining. In Artificial Intelligence in Data Mining; Binu, D., Rajakumar, B.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 133–154. ISBN 978-0-12-820601-0. [Google Scholar]
- ElAshmawy, W.R.; Okello, E.; Williams, D.R.; Anderson, R.J.; Karle, B.; Lehenbauer, T.W.; Aly, S.S. Effectiveness of Intramammary Antibiotics, Internal Teat Sealants, or Both at Dry-Off in Dairy Cows: Milk Production and Somatic Cell Count Outcomes. Vet. Sci. 2022, 9, 559. [Google Scholar] [CrossRef]
- Wang, A.; Luo, J.; Zhang, T.; Zhang, D. Dietary vitamin C and vitamin C derived from vegetables are inversely associated with the risk of depressive symptoms among the general population. Antioxidants 2021, 10, 1984. [Google Scholar] [CrossRef]
- Hensel, M.; Nonno, S.D.; Mayer, Y.; Scheiermann, M.; Fahrer, J.; Durner, D.; Ulber, R. Specification and Simplification of Analytical Methods to Determine Wine Color. Processes 2022, 10, 2707. [Google Scholar] [CrossRef]
- Perperoglou, A.; Sauerbrei, W.; Abrahamowicz, M.; Schmid, M. A review of spline function procedures in R. BMC Med. Res. Methodol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Moscetti, R.; Monarca, D.; Cecchini, M.; Haff, R.P.; Contini, M.; Massantini, R. Detection of mold-damaged chestnuts by near-infrared spectroscopy. Postharvest Biol. Technol. 2014, 93, 83–90. [Google Scholar] [CrossRef]
- Salehi, M.; Farhadi, S.; Moieni, A.; Safaie, N.; Ahmadi, H. Mathematical Modeling of Growth and Paclitaxel Biosynthesis in Corylus avellana Cell Culture Responding to Fungal Elicitors Using Multilayer Perceptron-Genetic Algorithm. Front. Plant Sci. 2020, 11, 11481. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Alizadeh, M.; Naderi, R.; Tohidfar, M. Forecasting and optimizing Agrobacterium-mediated genetic transformation via ensemble model-fruit fly optimization algorithm: A data mining approach using chrysanthemum databases. PLoS ONE 2020, 15, e0239901. [Google Scholar] [CrossRef] [PubMed]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef]
- Tremarin, A.; Aragão, G.M.F.; Salomão, B.C.M.; Brandão, T.R.S.; Silva, C.L.M. Modeling the Soluble Solids and Storage Temperature Effects on Byssochlamys fulva Growth in Apple Juices. Food Bioprocess Technol. 2017, 10, 720–729. [Google Scholar] [CrossRef]
 experimental data points,
experimental data points,  B-spline curve,
B-spline curve,  knots,
knots,  control points, R–correlation coefficients between estimations of B-spline curves and experimental points adopted from Wawrzyniak et al. [47].
control points, R–correlation coefficients between estimations of B-spline curves and experimental points adopted from Wawrzyniak et al. [47].
 experimental data points,
experimental data points,  B-spline curve,
B-spline curve,  knots,
knots,  control points, R–correlation coefficients between estimations of B-spline curves and experimental points adopted from Wawrzyniak et al. [47].
control points, R–correlation coefficients between estimations of B-spline curves and experimental points adopted from Wawrzyniak et al. [47].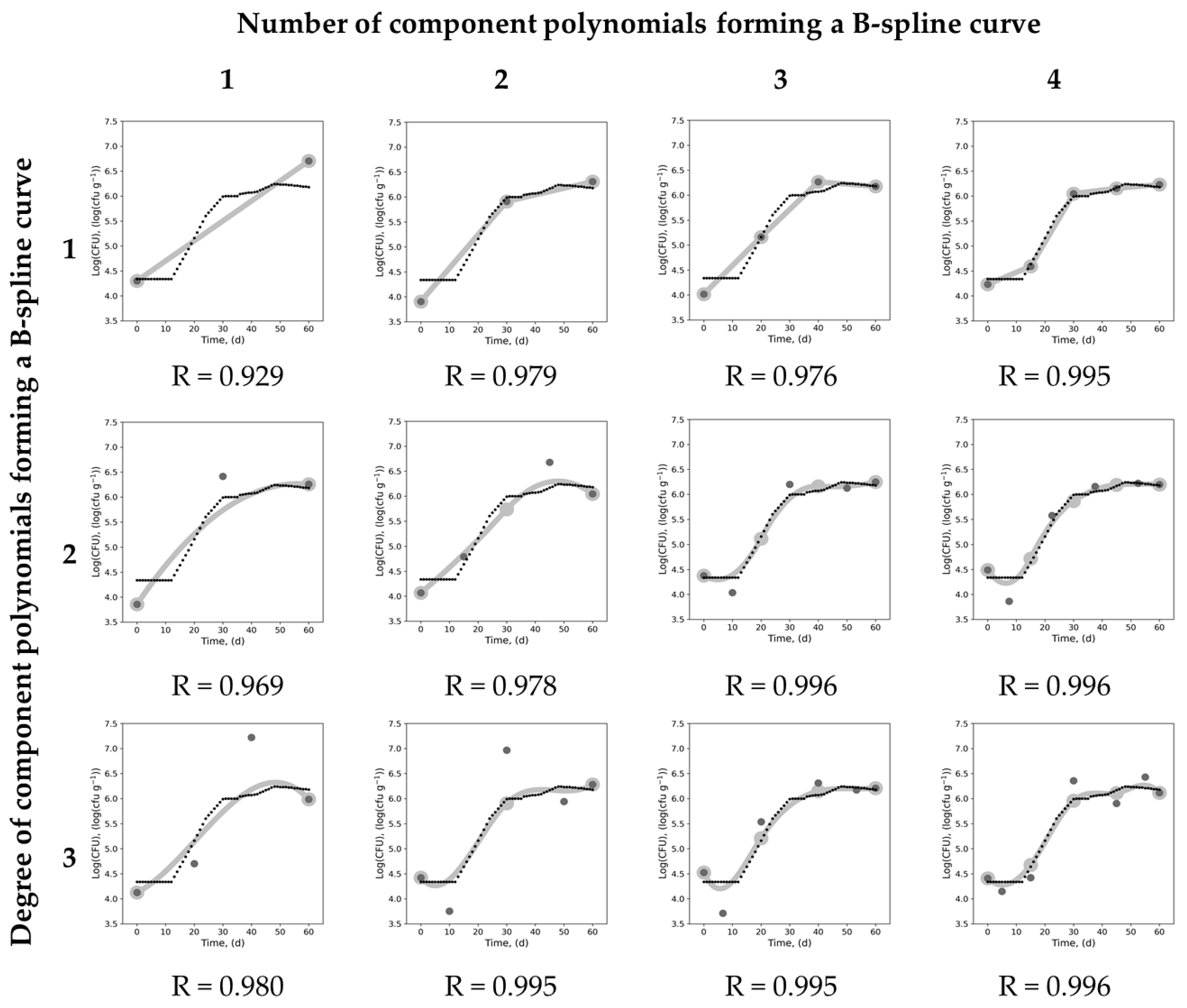




| Temperature (°C) | Water Activity | Role of Experimental Data in Model Development |
|---|---|---|
| 12 | 0.80 | Learning |
| 0.86 | Validation | |
| 0.89 | Learning | |
| 0.96 | Learning | |
| 18 | 0.80 | Learning |
| 0.85 | Learning | |
| 0.91 | Validation | |
| 0.95 | Learning | |
| 24 | 0.81 | Learning |
| 0.85 | Validation | |
| 0.91 | Learning | |
| 0.93 | Learning | |
| 30 | 0.78 | Learning |
| 0.80 | Learning | |
| 0.84 | Validation | |
| 0.92 | Learning |
| CP No.: j | 1 | 2 | 3 | 4 | 5 | 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP value: yi | y1 | y2 | y3 | y4 | y5 | y6 | ||||||||||||
| Factor | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T |
| Gene no.: i | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Gene value: xi | x1 | x2 | x3 | x4 | x5 | x6 | x13 | x8 | x9 | x10 | x11 | x12 | x13 | x14 | x15 | x16 | x17 | x18 |
| CP No.: j | 1 | 2 | 3 | 4 | 5 | 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP value: yi | y1 | y2 | y3 | y4 | y5 | y6 | ||||||||||||
| Factor | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T |
| Gene no.: i | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Gene value of input organism 1: 1-xi | 1-x1 | 1-x2 | 1-x3 | 1-x4 | 1-x5 | 1-x6 | 1-x7 | 1-x8 | 1-x9 | 1-x10 | 1-x11 | 1-x12 | 1-x13 | 1-x14 | 1-x15 | 1-x16 | 1-x17 | 1-x18 |
| Gene value of input organism 2: 2-xi | 2-x1 | 2-x2 | 2-x3 | 2-x4 | 2-x5 | 2-x6 | 2-x7 | 2-x8 | 2-x9 | 2-x10 | 2-x11 | 2-x12 | 2-x13 | 2-x14 | 2-x15 | 2-x16 | 2-x17 | 2-x18 |
| Gene value of output organism: 1/2-xi | 1-x1 | 2-x2 | 1-x3 | 1-x4 | 2-x5 | 2-x6 | 1-x7 | 2-x8 | 1-x9 | 2-x10 | 1-x11 | 1-x12 | 2-x13 | 1-x14 | 2-x15 | 2-x16 | 1-x17 | 2-x18 |
| CP no.: j | 1 | 2 | 3 | 4 | 5 | 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP value: yi | y1 | y2 | y3 | y4 | y5 | y6 | ||||||||||||
| Factor | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T | aw | T | aw × T |
| Gene no.: i | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Gene value of input organism 1: 1-xi | 1-x1 | 1-x2 | 1-x3 | 1-x4 | 1-x5 | 1-x6 | 1-x7 | 1-x8 | 1-x9 | 1-x10 | 1-x11 | 1-x12 | 1-x13 | 1-x14 | 1-x15 | 1-x16 | 1-x17 | 1-x18 |
| Gene value of output organism: 1/N-xi | 1-x1 | N-x2 | N-x3 | N-x4 | 1-x5 | N-x6 | 1-x7 | N-x8 | 1-x9 | N-x10 | 1-x11 | 1-x12 | N-x13 | 1-x14 | N-x15 | N-x16 | 1-x17 | 1-x18 |
| S no. | L_B | V_B | A(L_B + V_B)/2 | L_W |
|---|---|---|---|---|
| 1 | 0.0773 | 0.0717 | 0.0745 | 0.0803 |
| 2 | 0.0774 | 0.0760 | 0.0767 | 0.0808 |
| 3 | 0.0778 | 0.0733 | 0.0756 | 0.0816 |
| 4 | 0.0778 | 0.0740 | 0.0759 | 0.0865 |
| 5 | 0.0781 | 0.0762 | 0.0772 | 0.0817 |
| 6 | 0.0782 | 0.0718 | 0.0750 | 0.0835 |
| 7 | 0.0783 | 0.0790 | 0.0786 | 0.0840 |
| 8 | 0.0783 | 0.0770 | 0.0777 | 0.0826 |
| 9 | 0.0784 | 0.0823 | 0.0804 | 0.0820 |
| 10 | 0.0784 | 0.0776 | 0.0780 | 0.0832 |
| 11 | 0.0786 | 0.0780 | 0.0783 | 0.0828 |
| 12 | 0.0786 | 0.0778 | 0.0782 | 0.0847 |
| 13 | 0.0789 | 0.0778 | 0.0784 | 0.0825 |
| 14 | 0.0789 | 0.0806 | 0.0797 | 0.0866 |
| 15 | 0.0794 | 0.0771 | 0.0783 | 0.0850 |
| 16 | 0.0795 | 0.0764 | 0.0779 | 0.0917 |
| 17 | 0.0798 | 0.0731 | 0.0764 | 0.0855 |
| 18 | 0.0798 | 0.0966 | 0.0882 | 0.0858 |
| 19 | 0.0801 | 0.0819 | 0.0810 | 0.0830 |
| 20 | 0.0803 | 0.0824 | 0.0813 | 0.0840 |
| 21 | 0.0804 | 0.0757 | 0.0781 | 0.0884 |
| 22 | 0.0805 | 0.0709 | 0.0757 | 0.0857 |
| 23 | 0.0809 | 0.0699 | 0.0754 | 0.0922 |
| 24 | 0.0813 | 0.0730 | 0.0771 | 0.0862 |
| 25 | 0.0838 | 0.0785 | 0.0811 | 0.0912 |
| Statistical Indices | DATASET | ||
|---|---|---|---|
| Learning | Validation | Full | |
| Number of experimental points (N) | 201 | 63 | 264 |
| Coefficient determination (R2) | 0.944 | 0.916 | 0.938 |
| Mean absolute error (MAE) | 0.208 | 0.210 | 0.209 |
| Root mean square error (RMSE) | 0.278 | 0.247 | 0.276 |
| Bias factor (Bf) | 0.996 | 1.009 | 0.999 |
| Mean relative percentage error (MRPE), (%) | −0.162 | −1.058 | −0.048 |
| Accuracy factor (Af) | 1.041 | 1.040 | 1.041 |
| Mean absolute percentage error (MAPE), (%) | 4.000 | 4.000 | 4.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, J. Predictive Assessment of Mycological State of Bulk-Stored Barley Using B-Splines in Conjunction with Genetic Algorithms. Appl. Sci. 2023, 13, 5264. https://doi.org/10.3390/app13095264
Wawrzyniak J. Predictive Assessment of Mycological State of Bulk-Stored Barley Using B-Splines in Conjunction with Genetic Algorithms. Applied Sciences. 2023; 13(9):5264. https://doi.org/10.3390/app13095264
Chicago/Turabian StyleWawrzyniak, Jolanta. 2023. "Predictive Assessment of Mycological State of Bulk-Stored Barley Using B-Splines in Conjunction with Genetic Algorithms" Applied Sciences 13, no. 9: 5264. https://doi.org/10.3390/app13095264
APA StyleWawrzyniak, J. (2023). Predictive Assessment of Mycological State of Bulk-Stored Barley Using B-Splines in Conjunction with Genetic Algorithms. Applied Sciences, 13(9), 5264. https://doi.org/10.3390/app13095264





