Centennial Lake Environmental Evolution Reflected by Diatoms in Yilong Lake, Yunnan Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
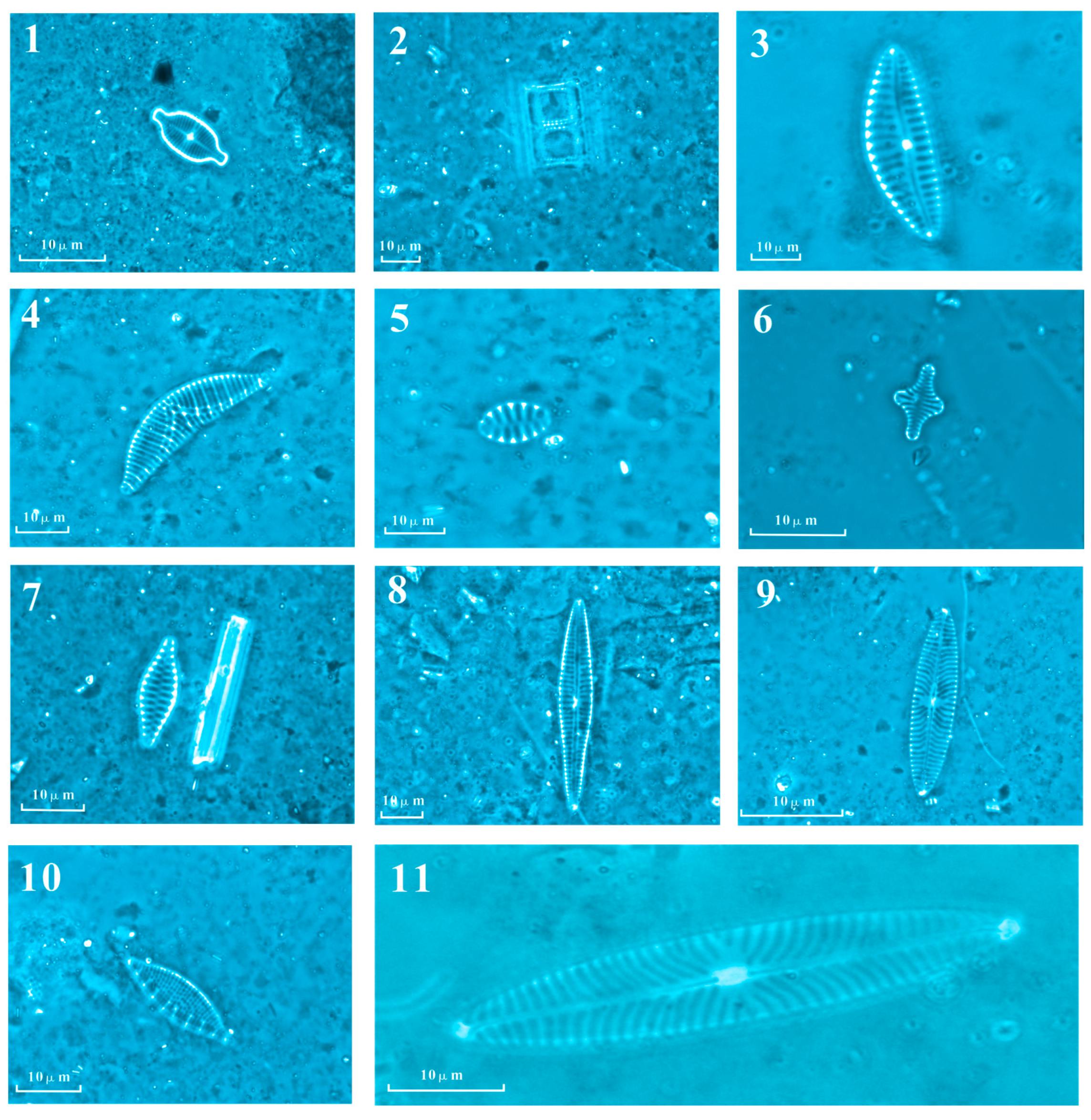
2.2. Diatom Analysis
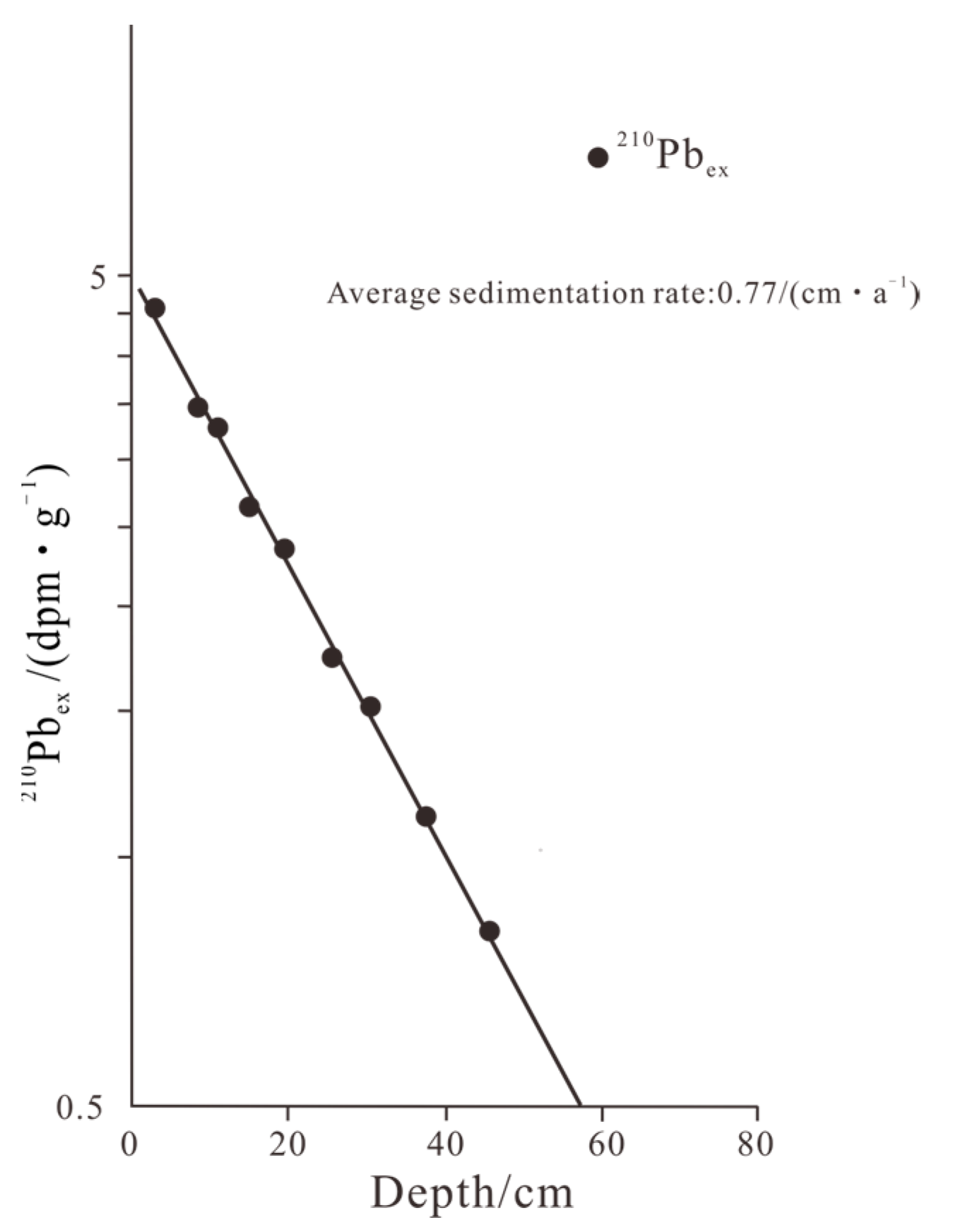
2.3. Age Model
2.4. Determination of Water Quality
2.5. Data Analysis
3. Results
3.1. Diatom Zones
3.1.1. Zone I (1938–1948 A.D.)
3.1.2. Zone II (1948–1962 A.D.)
3.1.3. Zone III (1962–1980 A.D.)
3.1.4. Zone IV (1980–1995 A.D.)
3.1.5. Zone V (1995–2008 A.D.)
3.1.6. Zone VI (2008–2020 A.D.)
3.2. PCA Results and Ecological Affinity of Diatoms
4. Evolution in pH Value and Eutrophication in Yilong Lake
4.1. 1938–1948 A.D.
4.2. 1948–1962 A.D.
4.3. 1962–1980 A.D.
4.4. 1980–1995 A.D.
4.5. 1995–2008 A.D.
4.6. 2008–2020 A.D.
5. Conclusions
- (1)
- The average deposition rate of Yilong Lake was 0.77 cm/a. The pH value and eutrophication were the two main environmental factors that affected the environmental change of Yilong Lake, which was in a high pH and eutrophication state for a long time.
- (2)
- In Yilong Lake, the main diatom species indicated a high pH value were A. exigua, N. menisculus, N. viridula, F. inflata, and N. denticule. Meanwhile, A. exigua, N. menisculus, and N. viridula could be used for the index of eutrophication. On the contrary, A.islandica and E. sorex could be considered indicators for lower eutrophication in the lake.
- (3)
- According to the pH value and eutrophication curves obtained from PCA, the evolution of Yilong Lake from 1938 to 2020 could be divided into six major stages. Though high pH value and eutrophication had been the main characteristics for a long time, the quality of Yilong Lake was gradually improved through planned treatment in the last decades.
- (4)
- Different stages of evolution in Yilong Lake corresponded to different natural changes and human activities. Due to the influence of lithology and sedimentation, the pH value of Yilong Lake was relatively low, and there were few affections by human activities in the preliminary stage. Since 1984, eutrophication has rapidly increased due to the increasing population and accompanying human activities such as agricultural development, deforestation, and land reclamation. After 1980, the water quality of Yilong Lake continued to improve due to planned treatment such as building sewage treatment plants, restoring forests, dredging, and planting lakeside wetlands.
- (5)
- The sediment diatom records provided effective information on the pH value and eutrophication of Yilong Lake, reflecting the history of the lake over the past century. In addition to the changes in nature, especially the drying up of the lake caused by drought, which might lead to the high pH value and eutrophication, human activities had also played an important role in the lake environment. The irrational human development in the lake basin caused abnormal changes in pH values and eutrophication, while the planned and targeted treatment could restore the natural state of the lake. This provided a scientific basis for research on the evolution of plateau lakes such as Yilong Lake, as well as for lake governance and protection.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nanjing Institute of Geography & Limnology Chinese Academy of Science. Report of Lakes in China; Science Press: Beijing, China, 2019. (In Chinese) [Google Scholar]
- Finger-Higgens, R. Diminishing Arctic lakes. Nat. Clim. Chang. 2022, 12, 782–783. [Google Scholar] [CrossRef]
- Rad, A.M.; Kreitler, J.; Abatzoglou, J.T.; Fallon, K.; Roche, K.R.; Sadegh, M. Anthropogenic stressors compound climate impacts on inland lake dynamics: The case of Hamun Lakes. Sci. Total. Environ. 2022, 829, 154419. [Google Scholar] [CrossRef]
- Kumar, R.; Parvaze, S.; Huda, M.B.; Allaie, S.P. The changing water quality of lakes–A case study of Dal Lake, Kashmir Valley. Environ. Monit. Assess. 2022, 194, 228. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, T.; Huang, Y.; Zan, C.; Pan, X.; Xu, Z. Response of water quality to climate warming and atmospheric deposition in an alpine lake of Tianshan Mountains, Central Asia. Ecol. Indic. 2023, 147, 109949. [Google Scholar] [CrossRef]
- Zeng, L.; Swann, G.E.; Leng, M.J.; Chen, X.; Ji, J.; Huang, X.; McGowan, S. Ecosystem deterioration in the middle Yangtze floodplain lakes over the last two centuries: Evidence from sedimentary pigments. Quat. Sci. Rev. 2023, 302, 107954. [Google Scholar] [CrossRef]
- Gunacti, M.C.; Gul, G.O.; Cetinkaya, C.P.; Gul, A.; Barbaros, F. Evaluating Impact of Land Use and Land Cover Change Under Climate Change on the Lake Marmara System. Water Resour. Manag. 2022. [Google Scholar] [CrossRef]
- Zhiqi, L.; Baozhu, P.; Xu, H.; Gang, L.; Taoyi, W. Water environmental characteristics and water quality assessment of lakes in Tibetan Plateau. Environ. Sci. 2022, 43, 5073–5083. (In Chinese) [Google Scholar]
- Xidong, G.; Hongchen, J.; Ying, J.; Xu, C. Lake environmental changes in response to acid deposition in Southwest China over the last century: Evidence from sedimentary diatoms in Lake Longtan of the Simian Mountains. J. Lake Sci. 2021, 33, 1940–1950. (In Chinese) [Google Scholar] [CrossRef]
- Xiaoli, S.; Boqiang, Q. Evolution and ecological environment of lakes in the middle and lower reaches of Yangtze River. J. Ningbo Univ. (Nat. Sci. Eng. Ed.) 2007, 20, 221–226. (In Chinese) [Google Scholar]
- Dayuan, Y.; Xusheng, L.; Zhenke, Z. Lake Evolution along Middle-Lower Reaches of the Yangtze River. J. Lake Sci. 2000, 12, 226–232. (In Chinese) [Google Scholar] [CrossRef]
- Fuming, Q.; Kai, Z.; Guangjie, C.; Yongxin, Y.; Jiaoyuan, W.; Kui, H. Sediment-inferred recent pattern and drivers of environmental and ecological changes at Lake Qilu, Yunnan Province. Lake Sci. 2018, 30, 1109–1122. (In Chinese) [Google Scholar] [CrossRef]
- Xiaolin, C.; Guangjie, C.; Yuanyuan, L.; Rui, L. Evaluation of the quantitative relationships between diatom communities and total phosphorus (TP) in 45 lakes and their applications for TP reconstruction in Yunnan, Southwest China. Lake Sci. 2023, 35, 88–104. (In Chinese) [Google Scholar]
- Yuanyuan, L.; Guangjie, C.; Haibin, S.; Xiaolin, C.; Huibin, L.; Lizeng, D.; Hucai, Z.; Wenxiang, Z. Responses of a diatom community to human activities and climate changes in Xingyun Lake. Acta Ecol. Sin. 2016, 36, 3063–3073. (In Chinese) [Google Scholar]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Paerl, H.W. Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecol. Eng. 2006, 26, 40–54. [Google Scholar] [CrossRef]
- Watson, S.B.; Miller, C.; Arhonditsis, G.; Boyer, G.L.; Carmichael, W.; Charlton, M.N.; Confesor, R.; Depew, D.; Höök, T.O.; Ludsin, S.A.; et al. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef]
- Morabito, G.; Rogora, M.; Austoni, M.; Ciampittiello, M. Could the extreme meteorological events in Lake Maggiore watershed determine a climate-driven eutrophication process? Hydrobiologia 2018, 824, 163–175. [Google Scholar] [CrossRef]
- Verschuren, D.; Johnson, T.C.; Kling, H.J.; Edgington, D.N.; Leavitt, P.R.; Brown, E.T.; Talbot, M.R.; Hecky, R.E. History and timing of human impact on Lake Victoria, East Africa. R. Soc. 2002, 269, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Shijie, L. An approach to accellerating innovative development of the lake science. Bull. Chin. Acad Sci. 2006, 21, 399–405. (In Chinese) [Google Scholar]
- Reddy, K.R.; DeBusk, T.A. State-of-the-Art Utilization of Aquatic Plants in Water Pollution Control. Water Sci. Technol. 1987, 19, 61–79. [Google Scholar] [CrossRef]
- Gumbricht, T. Nutrient removal processes in freshwater submersed macrophyte systems. Ecol. Eng. 1993, 2, 1–30. [Google Scholar] [CrossRef]
- Takemura, Y.; Syutsubo, K.; Kubota, K. Suppression of phosphorus release from eutrophic lake sediments by sediment microbial fuel cells. Environ. Technol. 2022, 43, 2581–2589. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, Y.; Chen, Y.; Shen, N.; Wang, G.; Yan, Y. Realignment of phosphorus in lake sediment induced by sediment microbial fuel cells (SMFC). Chemosphere 2021, 291, 132927. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xiao, E.-R.; Xu, D.; Zhou, Y.; He, F.; Liu, B.-Y.; Zeng, L.; Wu, Z.-B. Internal nitrogen removal from sediments by the hybrid system of microbial fuel cells and submerged aquatic plants. PLoS ONE 2017, 12, e0172757. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, J.; Lou, Y.; Fang, A.; Zhou, H.; Liu, B.; Xie, G.; Xing, D. MnO2/tourmaline composites as efficient cathodic catalysts enhance bioelectroremediation of contaminated river sediment and shape biofilm microbiomes in sediment microbial fuel cells. Appl. Catal. B Environ. 2020, 278, 119331. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, R. Restoration Study of Microorganisms in Lake Water Purification. Engineering 2013, 05, 459–462. [Google Scholar] [CrossRef]
- Dondajewska, R.; Kozak, A.; Rosińska, J.; Gołdyn, R. Water quality and phytoplankton structure changes under the influence of effective microorganisms (EM) and barley straw—Lake restoration case study. Sci. Total. Environ. 2019, 660, 1355–1366. [Google Scholar] [CrossRef]
- Angeler, D.G.; Allen, C.R.; Garmestani, A.; Gunderson, L.; Johnson, R.K. Panarchy and management of lake ecosystems. Ecol. Soc. 2021, 26, 1–7. [Google Scholar] [CrossRef]
- Abujraiban, A.; Assaf, G.J. Effect of Strategic Planning of Human Resources in Management Performance. Civ. Eng. J. 2022, 8, 1725–1738. [Google Scholar] [CrossRef]
- Carvalho, J.M.S. Modelling (Social) Intra/Entrepreneurship Process. Emerg. Sci. J. 2022, 6, 14–36. [Google Scholar] [CrossRef]
- Sumin, W.; Hongshen, D. Records of Lakes in China; Science Press: Beijing, China, 1998; pp. 379–381. (In Chinese) [Google Scholar]
- Editorial Committee of Book of Shiping County, Yunnan Province. Book of Shiping County; Yunnan people’s Publishing House: Kunming, China, 1990; pp. 1–870. (In Chinese) [Google Scholar]
- Yihui, C. Conservation and Management Plan for Yilong Lake (2018–2035); Kunming China International Research Center for Plateau-Lake: Kunming, China, 2019. (In Chinese) [Google Scholar]
- Xin, X.; Chongyuan, Z.; Ling, C.; Jianrong, M.; Jinxing, Z. Application of diatoms in water quality monitoring and reconstruction of palaeoenvironment. J. Anhui Agric. Sci. 2011, 39, 5216–5217 + 5386. (In Chinese) [Google Scholar]
- Hurlbert, S.H. The Nonconcept of Species Diversity: A Critique and Alternative Parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, S.; Charles, D.F.; Gerritsen, J.; Belton, T.J. A diatom-based biological condition gradient (BCG) approach for assessing impairment and developing nutrient criteria for streams. Sci. Total. Environ. 2016, 562, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Xiangdong, Y.; Ji, S.; Weilan, X.; Yuxin, Z. Diatom assemblages in sediment and dynamic process of lake trophic evolution from Longgan Lake, East China. Acta Palaeontol. Sin. 2002, 41, 455–460. (In Chinese) [Google Scholar]
- Fritz, S.C.; Juggins, S.; Battarbee, R.W.; Engstrom, D.R. Reconstruction of past changes in salinity and climate using a diatom-based transfer function. Nature 1991, 352, 706–708. [Google Scholar] [CrossRef]
- Fulford-Smith, S.P.; Sikes, E.L. The evolution of Ace Lake, Antarctica, determined from sedimentary diatom assemblages. Palaeogeogr. Palaeoclim. Palaeoecol. 1996, 124, 73–86. [Google Scholar] [CrossRef]
- Moser, K. Reconstructing Drought Using Diatoms Preserved in Alpine Lake Sediments from the Western United States. Quat. Int. 2012, 279–280, 339. [Google Scholar] [CrossRef]
- Brugam, R.B.; McKeever, K.; Kolesa, L. A diatom-inferred water depth reconstruction for an Upper Peninsula, Michigan, lake. J. Paleolimnol. 1998, 20, 267–276. [Google Scholar] [CrossRef]
- Narancic, B.; Saulnier-Talbot, É.; St-Onge, G.; Pienitz, R. Diatom sedimentary assemblages and Holocene pH reconstruction from the Canadian Arctic Archipelago’s largest lake. Écoscience 2021, 28, 347–360. [Google Scholar] [CrossRef]
- Ryves, D.B.; Clarke, A.L.; Appleby, P.G.; Amsinck, S.L.; Jeppesen, E.; Landkildehus, F.; Anderson, N.J. Reconstructing the salinity and environment of the Limfjord and Vejlerne Nature Reserve, Denmark, using a diatom model for brackish lakes and fjords. Can. J. Fish. Aquat. Sci. 2004, 61, 1988–2006. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Kamenik, C.; Schmidt, R.; Shen, J.; Zhu, L.; Li, S. Diatom assemblages and quantitative reconstruction for paleosalinity from a sediment core of Chencuo Lake, southern Tibet. Sci. China Ser. D Earth Sci. 2004, 47, 522–528. [Google Scholar] [CrossRef]
- Lan, B.; Zhang, D.; Yang, Y. Evolution of Lake Ailike (northern Xinjiang of China) during past 130 years inferred from diatom data. Quat. Int. 2018, 475, 70–79. [Google Scholar] [CrossRef]
- Song, B.; Wang, R.; Wang, Q.; Kong, L.; Hu, Z.; Zheng, W.; Yang, X. Pollen and diatom record long-term complex relationships between diversity and stability in a lake and nearby vegetation from Tingming Lake in Yunnan, SW China. Quat. Int. 2020, 580, 87–94. [Google Scholar] [CrossRef]
- Håkansson, H. The recent diatom succession of Lake Havgårdssjön, south Sweden. In Proceedings of the 7th International Diatom Symposium, Philadelphia, PA, USA, 22–27 August 1984; pp. 411–429. [Google Scholar]
- Hustedt, F. Die Kieselalgen Deutschlands Österreichs und der Schweiz unter Berücksichtigung der übrigen Länder Europas sowie angrenzender Meeresgebiete. In L. Rabenhorst’s Kryptogamen-flora von Deutschland Österreich und der Schweiz, Band VII; Otto Koeltz Science Publishers: Koenigstein, Germany, 1959; Volume 2, pp. 1–845. [Google Scholar]
- Foged, N. Diatoms from West Greenland; C.A. Reitzels Forlag: Copenhagen, Denmark, 1953; pp. 1–86. [Google Scholar]
- Foged, N. Diatoms from Rennell Island. In The Natural History of Rennell Island, British Solomon Islands; Danish Science Press: Copenhagen, Denmark, 1957; Volume 3, pp. 7–97. [Google Scholar]
- Foged, N. Diatoms from Afghanistan. In Biologiske Skrifter Udgivet af Det Kongelige Danske Videnskabernes Selskab; I kommission hos Ejnar Munksgaard: Copenhagen, Denmark, 1959; pp. 1–95. [Google Scholar]
- Foged, N. Freshwater Diatoms from Spitsbergen; Universitetsforlaget: Oslo, Norway, 1964; Volume 11, pp. 1–204. [Google Scholar]
- Foged, N. Diatoms in Eastern Australia. In Biblotheca Phycologica, Band 41; Cramer, J., Ed.; Vaduz: Berlin, Germany, 1978; pp. 1–242. [Google Scholar]
- Foged, N. Diatoms in New Zealand, the North Island. In Biblotheca Phycologica, Band 47; Cramer, J., Ed.; Vaduz: Berlin, Germany, 1979; pp. 1–224. [Google Scholar]
- Foged, N. Diatoms in Egypt. In Nova Hedwigia, Band 33; Cramer, J., Ed.; Gebruder Borntraeger: Berlin, Germany, 1980; pp. 629–707. [Google Scholar]
- Foged, N. Diatoms in Alaska. In Biblotheca Phycologica, Band 53; Cramer, J., Ed.; Vaduz: Berlin, Germany, 1981; pp. 1–316. [Google Scholar]
- Foged, N. Diatoms in Bornholm, Denmark. In Biblotheca Phycologica, Band 59; Cramer, J., Ed.; Vaduz: Berlin, Germany, 1982; pp. 1–174. [Google Scholar]
- Foged, N. Freshwater and littoral diatoms from Cuba. In Biblotheca Phycologica, Band 5; Cramer, J., Ed.; Vaduz: Berlin, Germany, 1984; pp. 1–242. [Google Scholar]
- Hartley, B. An Atlas of British Diatoms; Biopress Ltd.: Bristol, UK, 1996; pp. 1–601. [Google Scholar]
- Chengyan, H.; Shicheng, L.; Zhaodi, C.; Yuhua, M. Atlas of Chinese Fossil Diatoms in Lake Phase; China Ocean Press: Beijing, China, 1998; pp. 1–164. (In Chinese) [Google Scholar]
- Karbassi, A.R.; Amirnezhad, R. Geochemistry of heavy metals and sedimentation rate in a bay adjacent to the Caspian Sea. Int. J. Environ. Sci. Technol. 2004, 1, 191–198. [Google Scholar] [CrossRef]
- Sheli, C.; Lina, G.; Dianming, Q.; Yuan, C.; Jia, G.; Xuechun, X. 210Pb and 137Cs dating of the sediment core and its recent accumulation rates in Yueliang Lake in West Jilin Province. J. Jilin Univ. (Earth Sci. Ed.) 2013, 43, 134–141. (In Chinese) [Google Scholar]
- Regulations and Standards Department of the Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Chlorophyll a-Spectrophotometric Method: HJ 897-2017; China Environment Publishing Group: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Regulations and Standards Department of the Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Total Nitrogen-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method: HJ 636-2012; China Environment Publishing Group: Beijing, China, 2012. (In Chinese) [Google Scholar]
- Regulations and Standards Department of the Ministry of Ecology and Environment of the People’s Republic of China. Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method: GB 11893-89; China Environment Publishing Group: Beijing, China, 1990. (In Chinese) [Google Scholar]
- Ishizaka, A.; Lokman, B.; Tasiou, M. A Stochastic Multi-criteria divisive hierarchical clustering algorithm. Omega 2020, 103, 102370. [Google Scholar] [CrossRef]
- Jackson, J.E. A User’s Guide to Principal Components; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]
- Grimm, E.C. CONISS: A FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Juggins, S. C2 User Guide. Software for Ecological and Palaeoecological Data Analysis and Visualisation; University of Newcastle: Newcastle upon Tyne, UK, 2003; pp. 1–69. [Google Scholar]
- Yawen, F.; Zhengyu, H. Studied on Aulonoraphidinales from Xingkai Lake in Heilongjiang Province. Acta Hydrobiol. Sin. 2004, 28, 421–425. (In Chinese) [Google Scholar]
- Mingyue, L. Preliminary Study of Biraphidinales Diatom from Guangxi Zhuang Autonomous Region (Chongzuo Region). Master’s Thesis, Haerbin Normal University, Haerbin, China, 2017. (In Chinese). [Google Scholar]
- Kaku, K.; Kaifa, W.; Xumin, G. Diatom Analysis; Geological Publishing House: Beijing, China, 1984. (In Chinese) [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliterarurverzeichnis. In Süsswasserflora von Mitteleuropa, Band 2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1991; Volume 4, pp. 1–437. [Google Scholar]
- Hiromu, K. Pennatae. In Environmental Microbiology Illustrated; Sadao, K., Ryuichi, S., Mitsuo, C., Eds.; Kodansha: Tokyo, Japan, 1995; pp. 236–298. (In Japanese) [Google Scholar]
- Xiaomiao, Z.; Yuan, Z.; Jianing, L.; Shuping, W.; Xin, G.; Qian, Z.; Jingqi, W. Impact of talc ore mining on periphyton community structure and water environment. Environ. Sci. 2017, 38, 3721–3730. (In Chinese) [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Baeillariophyceae. In Süsswasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer: Stuttgart, Germany, 1986–1991; Volume 1–4. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato, Frustulia. In Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2001; Volume 2, pp. 1–526. [Google Scholar]
- Hongjun, H.; Yinxin, W. The Freshwater Algae of China Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006; pp. 300–416. (In Chinese) [Google Scholar]
- Stoermer, E.F.; Wolin, J.A.; Schelske, C.L.; Conley, D.J. Variations in Melosira islandica valve morphology in Lake Ontario sediments related to eutrophication and silica depletion1. Limnol. Oceanogr. 1985, 30, 414–418. [Google Scholar] [CrossRef]
- Genkal, S.I.; Popovskaya, G.I. New data on the frustule morphology of Aulacosira Islandica (Bacillariophyta). Diatom Res. 1991, 6, 255–266. [Google Scholar] [CrossRef]
- Serieyssol, C.A.; Edlund, M.B.; Kallemeyn, L.W. Impacts of settlement, damming, and hydromanagement in two boreal lakes: A comparative paleolimnological study. J. Paleolimnol. 2008, 42, 497–513. [Google Scholar] [CrossRef]
- Hitoharu, W.; Kazumi, A.; Taisuke, O.; Akihiro, T.; Akiko, H. Freshwater Diatom Ecology Illustrated; Rokakuho: Tokyo, Japan, 2005; pp. 1–784. (In Japanese) [Google Scholar]
- Krammer, K. The genus Cymbella. In Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2002; Volume 3, pp. 1–584. [Google Scholar]
- Yang, X.; Shen, J.; Dong, X.; Liu, E.; Wang, S. Historical trophic evolutions and their ecological responses from shallow lakes in the middle and lower reaches of the Yangtze River: Case studies on Longgan Lake and Taibai Lake. Sci. China Ser. D Earth Sci. 2006, 49, 51–61. [Google Scholar] [CrossRef]
- Yang, X.; Anderson, N.J.; Dong, X.; Shen, J. Surface sediment diatom assemblages and epilimnetic total phosphorus in large, shallow lakes of the Yangtze floodplain: Their relationships and implications for assessing long-term eutrophication. Freshw. Biol. 2008, 53, 1273–1290. [Google Scholar] [CrossRef]
- Xuhui, D.; Xiangdong, Y.; Enfeng, L. Diatom records and reconstruction of epilimnetic phosphorus concentration in Lake Taibai (Hubei Province) over the past 400 years. J. Lake Sci. 2006, 18, 597–604. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Kazumi, A.; Toshiharu, W. Statistic classification of epilithic diatom species into three ecological groups relating to organic water pollution. (2) Saprophilous and saproxenous taxa. Diatom 1995, 10, 35–47. [Google Scholar] [CrossRef]
- Yallop, M.; Hirst, H.; Kelly, M.; Juggins, S.; Jamieson, J.; Guthrie, R. Validation of ecological status concepts in UK rivers using historic diatom samples. Aquat. Bot. 2009, 90, 289–295. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Fan, Y. Diatom Taxonomic Composition as a Biological Indicator of the Ecological Health and Status of a River Basin under Agricultural Influence. Water 2020, 12, 2067. [Google Scholar] [CrossRef]
- Yu, W.; Gui, Z. On the desertification and genesis of Karst Stone Mountain area in East Yunnan. Adv. Earth Sci. 2003, 18, 933–938. (In Chinese) [Google Scholar]
- Sisi, C.; Hucai, Z.; Fengqin, C.; Han, W.; Huibin, L.; Dongsheng, L. Human activities indicated by the sediments at the Yilong Lake. Mountain Res. 2016, 34, 274–281. (In Chinese) [Google Scholar]
- Xiaohai, L.; Ping, N.; Junli, Z.; Jianguo, C.; Fengle, Y.; Xiumin, Z. Towards the effects of reclaiming the land from the lake and restoring the lake from the land in Yilong Lake. J. Kunming Univ. Sci. Technol. 2006, 31, 78–81 + 94. (In Chinese) [Google Scholar]
- Zhongwei, L.; Yunhui, W.; Miao, L.; Yanshun, C. Explore the uprising of pH value in lake water on dry season. Environ. Sci. Mgmt. 2007, 32, 80–81 + 84. (In Chinese) [Google Scholar]
- Editorial committee of Yunnan Yearbook. Yunnan Yearbook; Yunnan Yearbook Publisher: Kunming, China, 1986; pp. 1–556. (In Chinese) [Google Scholar]
- Qixin, G.; Shihao, B. Eutrophication of water in Yilong Lake. Environ. Sci. Surv. 1991, 16, 32–34. (In Chinese) [Google Scholar]
- Guoxin, Q. Research of environmental and economic development planning in Yilong Lake Area of Shiping Prefecture. Environ. Sci. Surv. 1997, 16, 11–16. (In Chinese) [Google Scholar]
- Honghe Prefecture Local Chronicle Office. Yearbook of Honghe Prefecture; Dehong Ethnic Press: Mangshi, China, 2005; pp. 1–476. (In Chinese) [Google Scholar]
- Aiying, Y. Environmental Benefit Assessment for Typical Water Pollution Treatment Project in Yilong Lake; Yunnan University Press: Kunming, China, 2018; pp. 1–170. (In Chinese) [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Ma, R.; Shi, H.; Li, J.; Tu, S. Centennial Lake Environmental Evolution Reflected by Diatoms in Yilong Lake, Yunnan Province, China. Appl. Sci. 2023, 13, 5288. https://doi.org/10.3390/app13095288
Huang Y, Ma R, Shi H, Li J, Tu S. Centennial Lake Environmental Evolution Reflected by Diatoms in Yilong Lake, Yunnan Province, China. Applied Sciences. 2023; 13(9):5288. https://doi.org/10.3390/app13095288
Chicago/Turabian StyleHuang, Yue, Ruiwen Ma, Hongbo Shi, Jie Li, and Shuyu Tu. 2023. "Centennial Lake Environmental Evolution Reflected by Diatoms in Yilong Lake, Yunnan Province, China" Applied Sciences 13, no. 9: 5288. https://doi.org/10.3390/app13095288



