Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation
Abstract
Featured Application
Abstract
1. Introduction
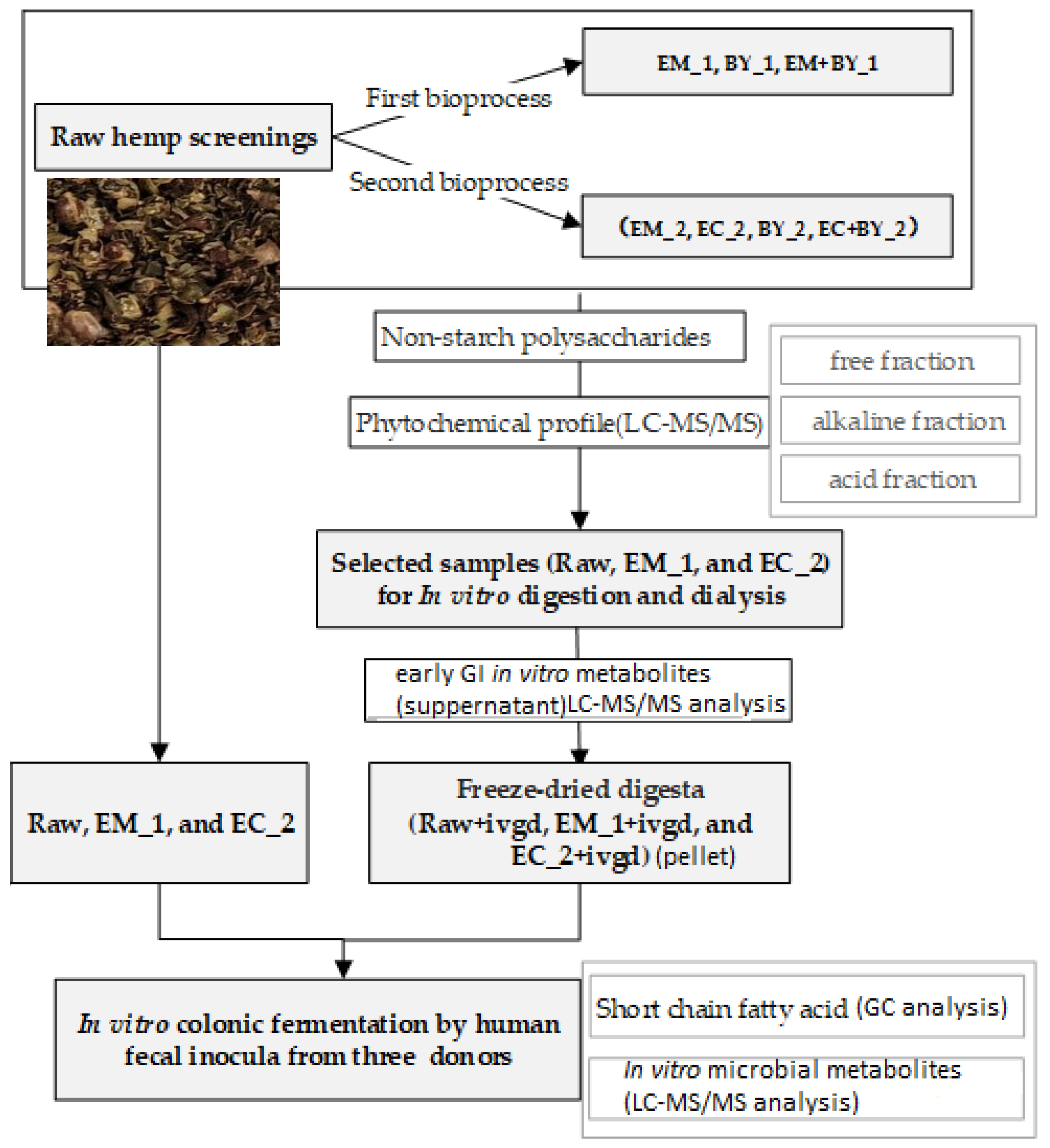
2. Materials and Methods
2.1. Standards and Reagents
2.2. Preparation of Hempseed Screenings for Analysis
2.3. Macronutrient Analysis
2.4. Bioprocessing of Hempseed Screenings
2.5. Non-Starch Polysaccharide (NSP) Analysis
2.6. Extraction of Phytochemicals
2.7. LC-MS Analysis
2.8. In Vitro Gastrointestinal Digestion (IVDG) Model with Dialysis Process
2.9. In Vitro Incubation of Hemp Samples Using Human Mixed Microbiota (Faecal Samples)
2.9.1. Human Faecal Incubation
2.9.2. Short Chain Fatty Acid (SCFA) Analysis
2.9.3. LC-MS/MS Microbial Metabolites Analysis
2.10. Statistical Analysis
3. Results
3.1. Hempseed Screening Composition
Hempseed Screenings Are a Rich Source of Nutrients
3.2. Hempseed Screenings Bioprocessing
3.2.1. Hempseed Screenings Bioprocessing Treatments Increased the Solubility of Dietary Fibre (NSP)
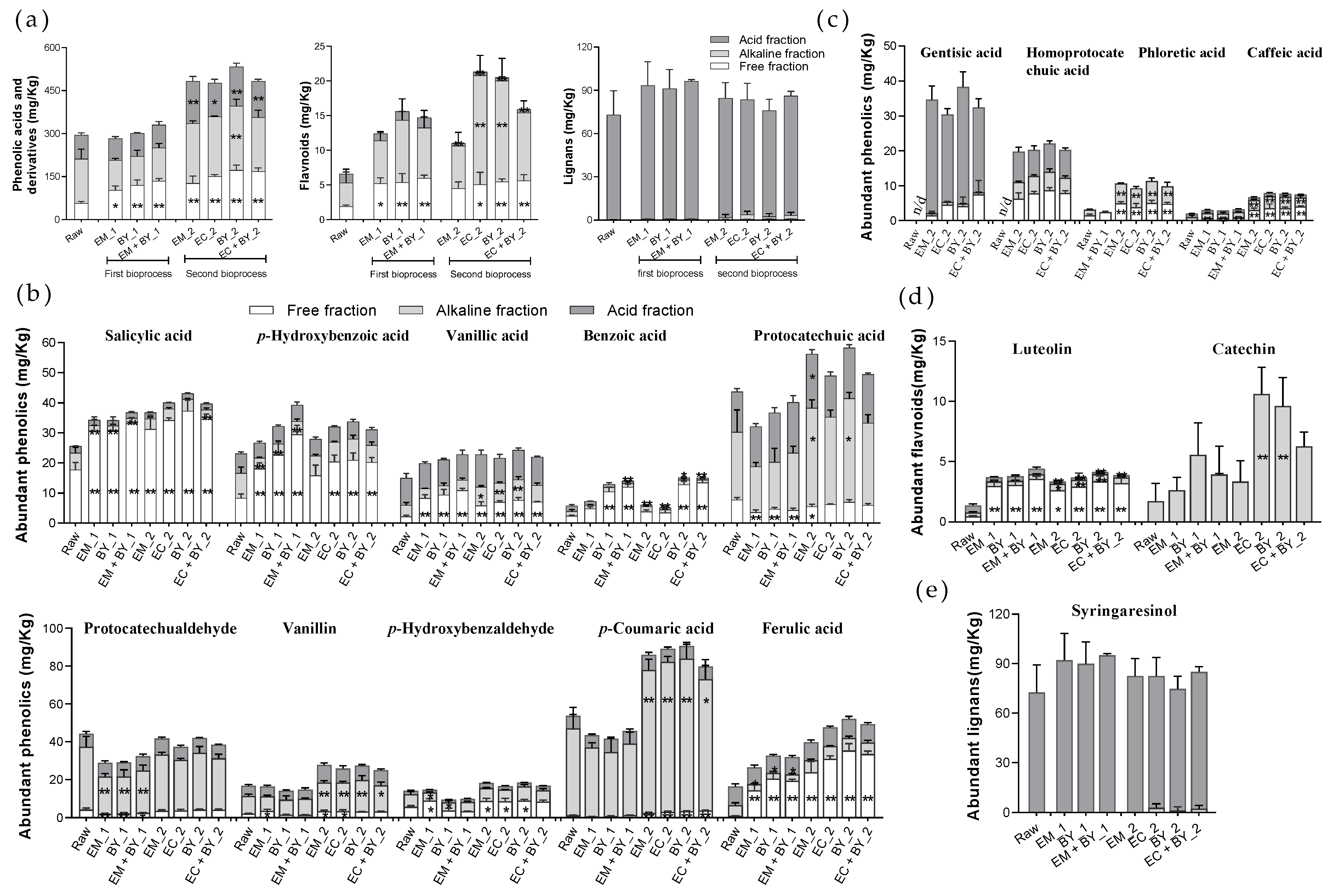
3.2.2. Hempseed Screenings Bioprocessing Treatments Increased the Extractability of Several Plant Metabolites
3.2.3. Hemp Screening Bioprocessing Treatments Improved the Upper Gastrointestinal Release of Several Plant Metabolites in the In Vitro Digestion Model
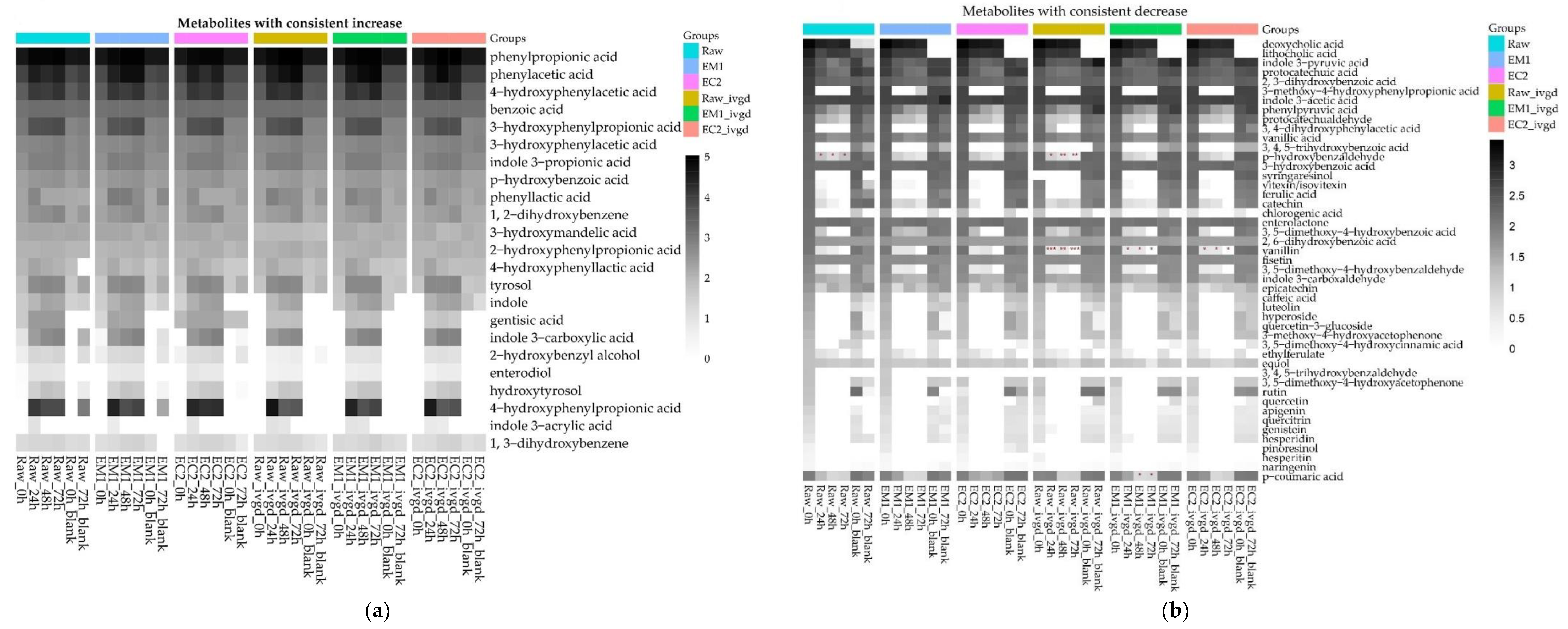
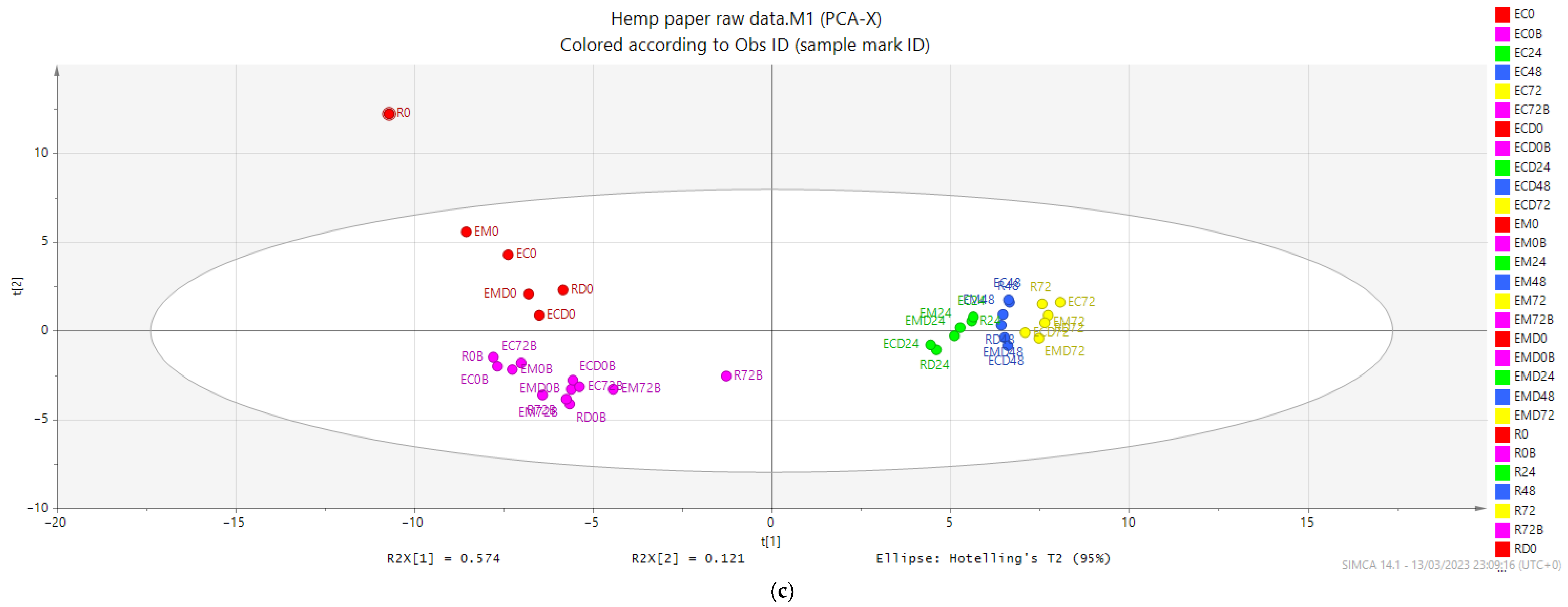
3.2.4. The Bioprocessing Treatments and In Vitro Digestion Treatments Showed no Impact on the Microbial Metabolism, and Both Raw and Bioprocessed Screenings were Rapidly Metabolised within 24 Hours with Little Further Transformation up to 72 Hours
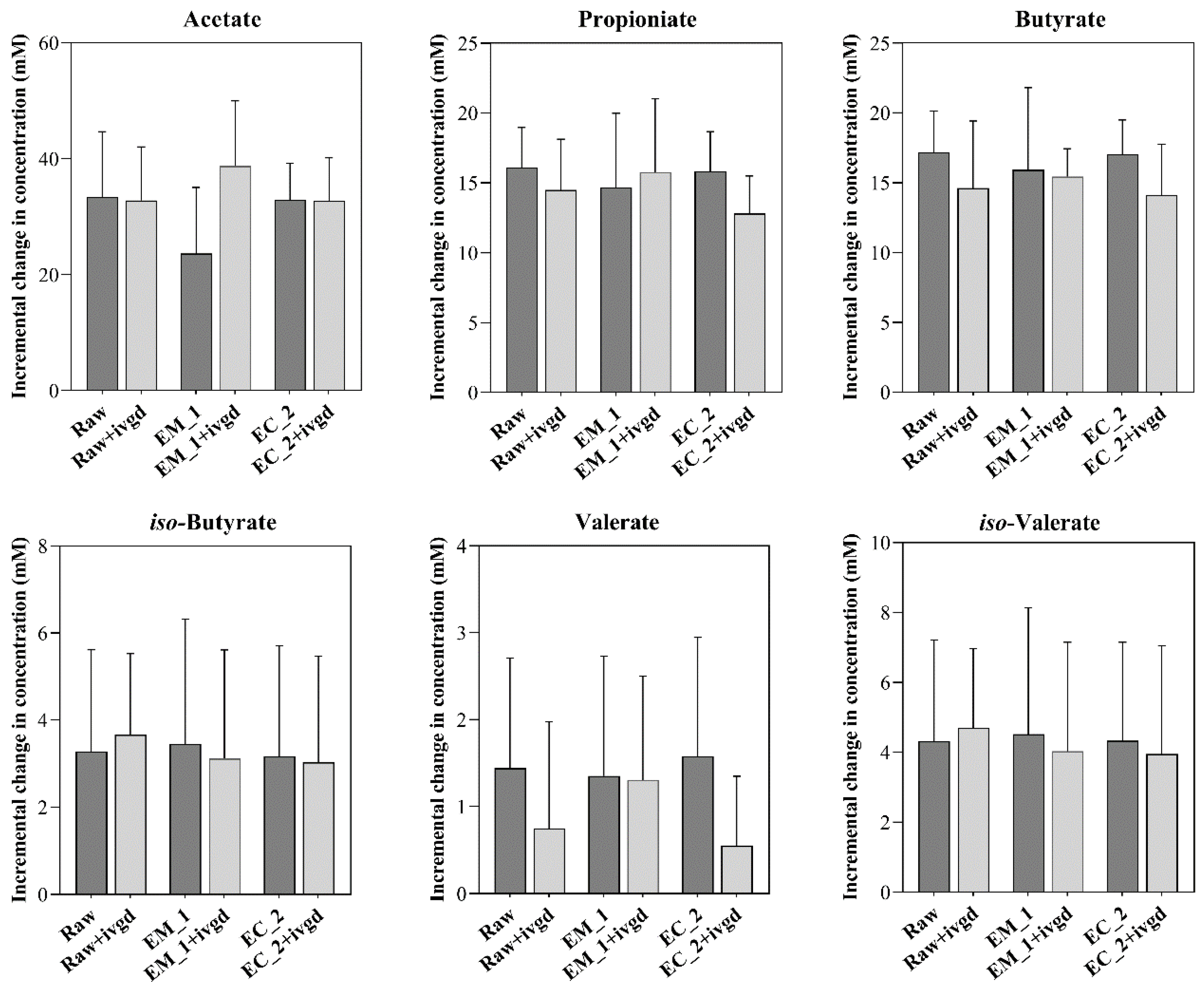
3.2.5. Hemp Screenings Are Highly Fermentable Sources of Dietary Fibre, but the Bioprocessing Treatments and In Vitro Digestion Treatment Showed Limited Impact on the Short Fatty Acids Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Madden, S.M.; Ryan, A.; Walsh, P.J.S. A Systems Thinking Approach Investigating the Estimated Environmental and Economic Benefits and Limitations of Industrial Hemp Cultivation in Ireland from 2017–2021. Sustainability 2022, 14, 4159. [Google Scholar] [CrossRef]
- Callaway, J.C.J.E. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and phytochemical content of high-protein crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef] [PubMed]
- British Nutrition Foundation. Available online: https://www.nutrition.org.uk (accessed on 29 December 2022).
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P.J.F.R.I. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Analyst 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Motilva, M.-J.; Ludwig, I.A. Beta-glucan and phenolic compounds: Their concentration and behavior during in vitro gastrointestinal digestion and colonic fermentation of different barley-based food products. J. Agric. Food Chem. 2018, 66, 8966–8975. [Google Scholar] [CrossRef]
- Osborn, L.J.; Schultz, K.; Massey, W.; DeLucia, B.; Choucair, I.; Varadharajan, V.; Banerjee, R.; Fung, K.; Horak, A.J., III; Orabi, D.; et al. A gut microbial metabolite of dietary polyphenols reverses obesity-driven hepatic steatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202934119. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.J.; Multari, S.; Haljas, E.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; Fyfe, C.; Anderson, S.; Horgan, G.; et al. Hemp and buckwheat are valuable sources of dietary amino acids, beneficially modulating gastrointestinal hormones and promoting satiety in healthy volunteers. Eur. J. Nutr. 2022, 61, 1057–1072. [Google Scholar] [CrossRef]
- United Nations Conference on Trade and Development. Circular Economy. UNCTAD. 2022. Available online: https://unctad.org/topic/trade-and-environment/circular-economy (accessed on 29 December 2022).
- Coda, R.; Rizzello, C.G.; Curiel, J.A.; Poutanen, K.; Katina, K.J.I.F.S.; Technologies, E. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innov. Food Sci. Emerg. Technol. 2014, 25, 19–27. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Nordlund, E.; Katina, K.; Mattila, I.; Poutanen, K.; Hanhineva, K.; Aura, A.-M. Effect of bioprocessing on the in vitro colonic microbial metabolism of phenolic acids from rye bran fortified breads. J. Agric. Food Chem. 2017, 65, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, E.; Katina, K.; Aura, A.-M.; Poutanen, K. Changes in bran structure by bioprocessing with enzymes and yeast modifies the in vitro digestibility and fermentability of bran protein and dietary fibre complex. J. Cereal Sci. 2013, 58, 200–208. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Prebiotic potential and bioactive volatiles of hemp byproduct fermented by lactobacilli. LWT 2021, 151, 112201. [Google Scholar]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Beneficial metabolic transformations and prebiotic potential of hemp bran and its alcalase hydrolysate, after colonic fermentation in a gut model. Sci. Rep. 2023, 13, 1552. [Google Scholar] [CrossRef]
- Russell, W.R.; Forrester, A.R.; Chesson, A.; Burkitt, M.J. Oxidative coupling during lignin polymerization is determined by unpaired electron delocalization within parent phenylpropanoid radicals. Arch. Biochem. Biophys. 1996, 332, 357–366. [Google Scholar] [CrossRef]
- Russell, W.R.; Burkitt, M.J.; Scobbie, L.; Chesson, A. Radical formation and coupling of hydroxycinnamic acids containing 1, 2-dihydroxy substituents. Bioorganic Chem. 2003, 31, 206–215. [Google Scholar] [CrossRef]
- AOAC. Determination of Moisture, Ash, Protein and Fat. Official Method of Analysis of the Association of Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Anderson, S. Soxtec: Its principles and applications. In Oil Extraction and Analysis; AOCS Publishing: New York, NY, USA, 2019; pp. 11–24. [Google Scholar]
- Englyst, H.N.; Quigley, M.E.; Hudson, G.; Cummings, J.H. Determination of dietary fibre as non-starch polysaccharides by gas–liquid chromatography. Analyst 1992, 117, 1707–1714. [Google Scholar] [CrossRef]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.-M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G.R.M.M. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Improved method for measurement of dietary fiber as non-starch 6olysaccharides in plant foods. J. Assoc. Off. Anal. Chem. 1988, 71, 808–814. [Google Scholar]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.; Wallace Duthie, G.G.; et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011, 5, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Duncan, S.H. Advanced analytical methodologies to study the microbial metabolome of the human gut. TrAC Trends Anal. Chem. 2013, 52, 54–60. [Google Scholar] [CrossRef]
- Neacsu, M.; McMonagle, J.; Fletcher, R.; Scobbie, L.; Duncan, G.; Cantlay, L.; De Roos, B.; Duthie, G.; Russell, W. Bound phytophenols from ready-to-eat cereals: Comparison with other plant-based foods. Food Chem. 2013, 141, 2880–2886. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.; Duthie, G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.J.; Perri, V.; Duncan, G.J.; Walker, R.; Coleman, M.; Russell, W.R. Analysis Nutritional and chemical profiling of UK-grown potato bean (Apios americana Medik) reveal its potential for diet biodiversification and revalorisation. J. Food Compos. Anal. 2021, 98, 103821. [Google Scholar] [CrossRef]
- Barik, S.K.; Dehury, B.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Hoggard, N.J.B.P. Analysis of polyphenolic metabolites from in vitro gastrointestinal digested soft fruit extracts identify malvidin-3-glucoside as an inhibitor of PTP1B. Biochem. Pharmacol. 2020, 178, 114109. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.J.F.; et al. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Richardson, A.; Calder, A.; Stewart, C.; Smith, A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 1989, 9, 5–8. [Google Scholar] [CrossRef]
- USDA National Nutrient Database. 2021. Available online: http://www.nal.usda.gov/fnic/foodcomp/search/https://fdc.nal.usda.gov/fdc-app.html#/food-details/1032521/nutrients (accessed on 29 November 2022).
- SACN. Scientific Advisory Committee on Nutrition (SACN)—GOV.UK. In Dietary Reference Values for Energy; The Stationery Office: London, UK, 2015. [Google Scholar]
- Vonapartis, E.; Aubin, M.-P.; Seguin, P.; Mustafa, A.F.; Charron, J.-B. Seed composition of ten industrial hemp cultivars approved for production in Canada. J. Food Compos. Anal. 2015, 39, 8–12. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Nie, S.; Liu, S.; Fang, Z.J.F.H. Post-extrusion physical properties, techno-functionality and microbiota-modulating potential of hempseed (Cannabis sativa L.) hull fiber. Food Hydrocoll. 2022, 131, 107836. [Google Scholar] [CrossRef]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.; Baxter, G.; Thies, F.; Kyle, J.; Duthie, G. A systematic review of salicylates in foods: Estimated daily intake of a Scottish population. Mol. Nutr. Food Res. 2011, 55, S7–S14. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N.J.L. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- mMusial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943–952952. [Google Scholar] [CrossRef]
- Karbownik, M.; Stasiak, M.; Zygmunt, A.; Zasada, K.; Lewiński, A. Protective effects of melatonin and indole-3-propionic acid against lipid peroxidation, caused by potassium bromate in the rat kidney. Cell Biochem. Funct. 2006, 24, 483–489. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.Y.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Jeong, Y.G.; Lee, Y.L.; Kim, Y.M.; Kwon, Y.G. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J. Neurosci. Res. 2009, 87, 2126–2137. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
| Proximate Composition | Content (%) | Amino Acids Composition | |||
|---|---|---|---|---|---|
| EAA2 | Content (%) | Non-EAA3 | Content (%) | ||
| dry matter | 94.32 ± 0.13 | tryptophan | 3.83 | glutamic acid | 3.02 |
| ash | 4.47 ± 0.01 | leucine | 1.21 | aspartic acid | 2.03 |
| protein | 20.15 ± 0.11 | valine | 0.88 | arginine | 1.91 |
| total fat | 25.06 ± 0.22 | phenylalanine | 0.85 | serine | 0.95 |
| total carbohydrate | 8.27 ± 0.14 | isoleucine | 0.71 | glycine | 0.79 |
| total starch | 0.03 ± 0 | threonine | 0.71 | proline | 0.77 |
| resistant starch | n.d.4 | histidine | 0.67 | alanine | 0.76 |
| total NSP1 | lysine | 0.67 | cysteic acid1 | 0.52 | |
| -Insoluble | 16.46 ± 0.86 | methionine | 0.27 | tyrosine | 0.42 |
| -Soluble | 0.32 ±0.05 | cysteine | 0.20 | ||
| NSP | Raw | First Bioprocess | Second Bioprocess | |||||
|---|---|---|---|---|---|---|---|---|
| EM_1 | BY_1 | EM+BY_1 | EM_2 | EC_2 | BY_2 | EC+BY_2 | ||
| Insoluble (%) | ||||||||
| Xylose | 7.01 ± 0.40 | 7.06 ± 0.04 | 7.05 ± 0.16 | 7.50 ± 0.06 | 6.48 ± 0.61 | 7.07 ± 0.19 | 6.95 ± 0.38 | 6.83 ± 0.14 |
| Glucose | 4.46 ± 0.52 | 3.95 ± 0.03 | 4.32 ± 0.11 | 3.81 ± 0.14 | 3.17 ± 0.15 * | 3.25 ± 0.14 * | 2.73 ± 0.39 | 3.05 ± 0.29 |
| Arabinose | 0.86 ± 0.01 | 0.39 ± 0.02 ** | 0.48 ± 0.00 | 0.34 ± 0.00 | 0.32 ± 0.01 ** | 0.34 ± 0.01 ** | 0.52 ± 0.01 | 0.33 ± 0.01 |
| Galactose | 0.48 ± 0.01 | 0.31 ± 0.01 ** | 0.41 ± 0.00 | 0.34 ± 0.03 | 0.29 ± 0.03 ** | 0.28 ± 0.00 ** | 0.43 ± 0.04 | 0.26 ± 0.01 |
| Rhamnose | 0.27 ± 0.01 | 0.20 ± 0.01 ** | 0.25 ± 0.00 | 0.19 ± 0.00 | 0.17 ± 0.02 ** | 0.20 ± 0.01 ** | 0.22 ± 0.00 | 0.18 ± 0.01 |
| Mannose | 0.22 ± 0.01 | 0.18 ± 0.01 ** | 0.32 ± 0.00 | 0.30 ± 0.01 | 0.12 ± 0.04 * | 0.13 ± 0.01 ** | 0.27 ± 0.01 | 0.19 ± 0.01 |
| Fucose | 0.08 ± 0.01 | 0.05 ± 0.00 ** | 0.06 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.00 ** | 0.05 ± 0.01 * | 0.08 ± 0.02 | 0.04 ± 0.00 |
| Uronic Acid | 3.10 ± 0.03 | 2.18 ± 0.03 ** | 2.80 ± 0.05 | 2.06 ± 0.06 | 1.37 ± 0.14 ** | 1.55 ± 0.17 ** | 2.57 ± 0.20 | 1.62 ± 0.18 |
| Total | 16.46 ± 0.86 | 14.32 ± 0.05 | 15.70 ± 0.31 | 14.58 ± 0.16 | 11.96 ± 0.84 ** | 12.87 ± 0.46 ** | 13.77 ± 0.86 | 12.50 ± 0.18 |
| Soluble (%) | ||||||||
| Xylose | 0.03 ± 0.01 | 0.06 ± 0.00 ** | 0.02 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.02 | 0.04 ± 0.03 | 0.08 ± 0.07 |
| Glucose | 0.04 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.00 |
| Arabinose | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.07 ± 0.00 | 0.08 ± 0.01 | 0.09 ± 0.02 | 0.08 ± 0.04 |
| Galactose | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.05 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.02 |
| Rhamnose | 0.02 ± 0.01 | 0.06 ± 0.01 ** | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.02 ± 0.02 |
| Mannose | n.d. | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.00 | n.d. | 0.02 ± 0.03 | 0.04 ± 0.02 | 0.03 ± 0.02 |
| Fucose | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.03 | 0.04 ± 0.00 |
| Uronic Acid | 0.06 ± 0.01 | 0.17 ± 0.00 ** | 0.05 ± 0.00 | 0.13 ± 0.00 | 0.17 ± 0.00 ** | 0.16 ± 0.01 ** | 0.05 ± 0.00 | 0.15 ± 0.01 |
| Total | 0.32 ± 0.05 | 0.58 ± 0.01 ** | 0.39 ± 0.04 | 0.47 ± 0.01 | 0.46 ± 0.01 ** | 0.52 ± 0.12 | 0.40 ± 0.17 | 0.48 ± 0.05 |
| Plant Metabolites (mg/Kg) | Raw | EM_1 | EC_2 | |||
|---|---|---|---|---|---|---|
| Total Content (Initial) | Content after In Vitro Digestion | Total Content (Initial) | Content after In Vitro Digestion | Total Content (Initial) | Content after In Vitro Digestion | |
| p-coumaric acid | 53.71 ± 10.72 | 3.26 ± 0.44 | 43.56 ± 2.39 | 1.23 ± 0.11 * | 89.09 ± 4.22 | 1.53 ± 0.05 * |
| protocatechualdehyde | 44.24 ± 5.40 | 1.58 ± 0.05 | 28.81 ± 1.90 | 1.05 ± 0.09 * | 37.38 ± 1.06 | 1.52 ± 0.12 |
| protocatechuic acid | 43.66 ± 7.13 | 6.52 ± 0.20 | 32.09 ± 1.75 | 2.95 ± 0.02 ** | 49.01 ± 1.85 | 5.47 ± 0.44 |
| salicylic acid | 25.59 ± 4.11 | 36.32 ± 1.48 | 34.24 ± 4.65 | 45.30 ± 3.25 | 40.04 ± 1.11 | 48.77 ± 1.55 ** |
| p-hydroxybenzoic acid | 23.12 ± 2.71 | 20.92 ± 0.93 | 26.73 ± 2.01 | 28.29 ± 1.49 * | 32.00 ± 2.01 | 31.35 ± 0.58 ** |
| vanillin | 16.67 ± 0.65 | 1.61 ± 0.21 | 16.34 ± 0.73 | 2.05 ± 0.37 | 25.77 ± 2.58 | 1.98 ± 0.22 |
| ferulic acid | 16.30 ± 0.47 | 13.91 ± 0.57 | 26.51 ± 3.45 | 21.06 ± 1.02 ** | 47.59 ± 2.27 | 36.97 ± 1.03 ** |
| vanillic acid | 14.97 ± 0.93 | 4.48 ± 0.36 | 19.91 ± 1.33 | 10.35 ± 0.33 ** | 21.67 ± 0.61 | 10.66 ± 0.54 ** |
| p-hydroxybenzaldehyde | 14.22 ± 1.64 | 8.05 ± 0.22 | 14.77 ± 0.68 | 8.24 ± 0.52 | 16.58 ± 1.84 | 8.65 ± 0.22 |
| syringaldehyde | 10.81 ± 0.56 | 1.86 ± 0.07 | 10.27 ± 0.51 | 1.94 ± 0.21 | 19.65 ± 2.58 | 1.99 ± 0.04 |
| benzoic acid | 5.77 ± 0.27 | 7.04 ± 0.71 | 7.26 ± 0.60 | 7.74 ± 1.06 | 6.11 ± 0.85 | 7.36 ± 1.14 |
| 3, 5-dimethoxy-4-hydroxybenzoic acid | 5.22 ± 0.49 | 0.92 ± 0.09 | 5.55 ± 0.75 | 1.32 ± 0.05 * | 7.91 ± 1.34 | 1.49 ± 0.02 * |
| phloretic acid | 3.14 ± 0.32 | 5.41 ± 0.27 | n.d. | 4.51 ± 0.18 * | 10.63 ± 0.45 | 4.52 ± 0.31 * |
| caffeic acid | 2.03 ± 0.49 | 3.48 ± 0.13 | 3.02 ± 0.38 | 2.07 ± 0.04 ** | 7.93 ± 1.32 | 3.01 ± 0.07 * |
| gentisic acid | n.d. | 7.15 ± 0.44 | n.d. | 4.41 ± 0.34 ** | 30.41 ± 2.62 | 26.45 ± 1.15 ** |
| homoprotocatechuic acid | n.d. | 4.61 ± 0.54 | n.d. | 8.38 ± 0.48 ** | 20.28 ± 1.07 | 8.54 ± 0.67 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Zhang, Z.; Duncan, G.J.; Morris, A.; Scobbie, L.; Henderson, D.; Morrice, P.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation. Appl. Sci. 2023, 13, 5781. https://doi.org/10.3390/app13095781
Fan S, Zhang Z, Duncan GJ, Morris A, Scobbie L, Henderson D, Morrice P, Russell WR, Duncan SH, Neacsu M. Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation. Applied Sciences. 2023; 13(9):5781. https://doi.org/10.3390/app13095781
Chicago/Turabian StyleFan, Songtao, Zhihong Zhang, Gary J. Duncan, Amanda Morris, Lorraine Scobbie, Donna Henderson, Philip Morrice, Wendy R. Russell, Sylvia H. Duncan, and Madalina Neacsu. 2023. "Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation" Applied Sciences 13, no. 9: 5781. https://doi.org/10.3390/app13095781
APA StyleFan, S., Zhang, Z., Duncan, G. J., Morris, A., Scobbie, L., Henderson, D., Morrice, P., Russell, W. R., Duncan, S. H., & Neacsu, M. (2023). Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation. Applied Sciences, 13(9), 5781. https://doi.org/10.3390/app13095781






