Featured Application
A low-cost, easy-to-fabricate multi-electrode system used in electrical intervention of in-vitro cell culture experiments.
Abstract
Electrical intervention has been one of the prime approaches in therapeutics in recent times. Research studies have reported several instances of electrical intervention in in vitro, in vivo, ex vivo, and clinical experiments. In vitro research shows a direct relationship between applied modulation and changes in a biological entity and leads to proof of theory. Although the stimulus used in in vitro experiments is current, voltage, or electric field, the ionic current flowing through the biological samples is the key factor in biomodulation. The direction and density of ionic current through the biological sample depend heavily on the experimental setup and electrode configuration. Bio-interfacing electrodes within a biological system have been a matter of concern in in vitro experiments, leading to various expensive and commercially available electrode setups. However, most of the setups are tailored for a specific experiment and cannot be altered as required. This paper demonstrates a multi-electrode system designed for in vitro experiments in standard 24-well culture dishes. The electric field distribution and current density of the setup were analyzed using COMSOL Multiphysics. The system is designed using standard PCB building technology. It can be configured into bipolar or tetrapolar setups. The system was used to measure in vitro bio-impedance in a 24-well culture dish for both bipolar and tetrapolar configurations. Bio-compatibility was observed by keeping the system in contact with human dermal fibroblasts (HDFs) in an in vitro experiment environment. The results indicate no statistical difference in the proliferation of HDFs due to exposure to electrodes. Moreover, no corrosion on the electrodes was observed. In general, the system is a low-cost, easy-to-make alternative to commercially available in vitro electrical bio-interfacing studies.
1. Introduction
The electrical intervention of biological entities has been on the rise in recent times. These interventions include the modulation of cell and tissue behaviors through electrical stimulation [1], sensing cell and tissue properties through bioimpedance measurements [2], drug delivery, gene therapy [3], etc. Biomodulation is the cellular or biochemical response of a biological entity to any external modulation [4]. The external modulation can be due to biological causes, such as infection or cancerous invasion, or due to therapeutic approaches like electrical, optical, magnetic, pressure, and ultrasound stimulation [5,6,7,8,9]. In recent times, biomodulation has gained a noteworthy spot in the field of therapeutic research. Amongst all the modalities, electrical biomodulation or electro-biomodulation has been of keen interest. Several works depict the widespread use of electro-biomodulation in various therapeutics like pain management, nerve regeneration, muscle control, wound healing, and antibacterial effects [10,11,12,13,14].
As tissues consist of different types of cells, it is expected that the electro-biomodulation on a particular tissue will affect the constituent cells in different manners. Hence, to identify the specific physiological effect of any electro-biomodulation on any particular type of cell, researchers rely on in vitro experiments. In vitro research provides a more targeted approach, where a controlled environment is used to provide external intervention to a specific type of cell. A factor heavily affecting the outcome of in vitro experiments is the experimental setup. While environmental factors, such as temperature, humidity, and exposure to contaminants, can be controlled, deviations in electrode parameters, such as size, shape, inter-electrode distance, and electrode materials, can lead to outcomes that differ from expectations. Electrodes act as the interface between the electronic circuitry and biological tissue. The compatibility of the electrode material with the cells is a crucial factor for an experimental setup. Recent work proposed a set of protocols that involve parameters related to the setup, stimulation, and biological sample for in vitro experiments [15].
Repeatability is another factor of significance in in vitro experiments. Although experiments are conducted in similar setups and environments, researchers often opt to run experiments a number of times to make them statistically significant. A specific set of statistical considerations goes into planning in vitro research [16,17]. These considerations range from setting up experiments to acquiring the data for analysis. A common practice is to run the experiments in parallel, where each identical setup is kept in the same environment, receiving the same external effect.
A common trend in the in vitro experimental arena is using multi-well plates [18,19]. Each well is set up with its own cell lines and growth media, and the whole setup is kept in an incubator with controlled temperature, pressure, and humidity. For in vitro experiments with electrical intervention, the addition of electrodes to the existing protocol brings in compatibility issues. Some common choices for electrodes are agar salt bridges [20,21], inert noble materials such as gold, silver, platinum, stainless steel [22,23], conducting polymers like PEDOT:PSS, Ppy/HE/PLLA [24,25,26], and carbonized materials [27,28,29]. Even though the electrode choices are practical for electrical intervention, they have their drawbacks, such as being complex, hard to maintain, hard to replicate, or expensive to fabricate. Moreover, a single electrical source to modulate parallel wells together ensures the uniformity of the experiment.
Bio-interfacing electrodes within a biological system have been a matter of concern in in vitro experiments, leading to various expensive and commercially available electrode setups. An interesting setup for in vitro electro-biomodulation experiments is the C-Dish™, patented by IonOptix LLC [30]. The setup uses a simple approach of fabricating carbon electrodes to polycarbonate shells with additional support to hold electrical circuitry [31]. The setup is designed to fit standard multi-well culture dishes used in in vitro works. It has been successfully used in various research works for stimulating cell lines [32,33]. Although, the C-Dish™ only provides a bipolar configuration of electrodes. Experiments involving impedance tomography require tetrapolar configurations [34]. This limits the applicability of the C-Dish™ to only the in vitro stimulation of cells. Another recent setup used by Urabe et al. uses a PCB mounted with carbon electrodes (designed for a 24-well culture dish setup) to electrically stimulate human dermal fibroblasts [35].
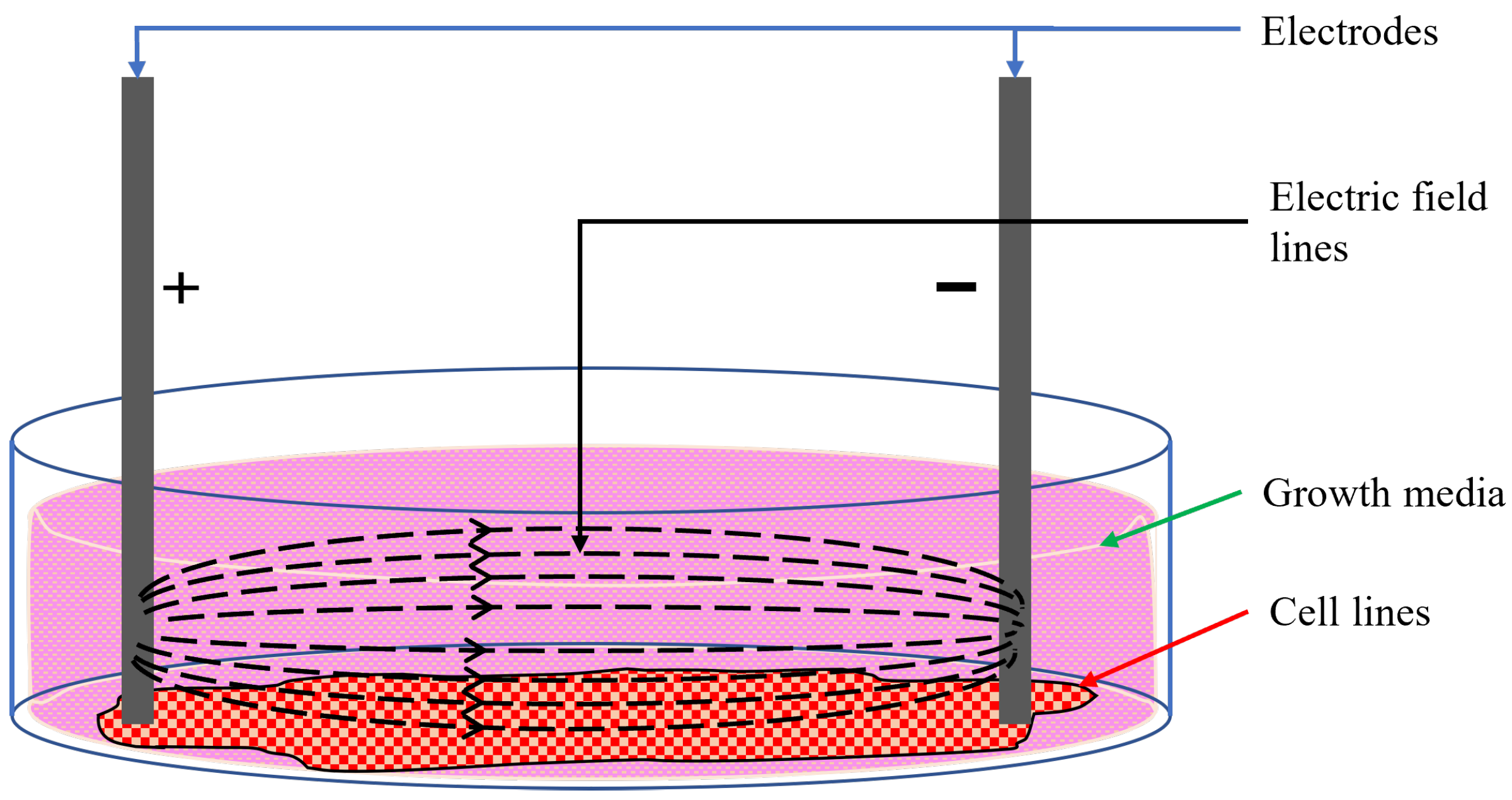
Moreover, in experiments involving cell lines, the cells are grown in the bottom plane of the wells, which are topped up with growth media. The growth media come in the form of liquids or gels that provide the necessary nutrients and conditions for cells to grow in in vitro setups [36]. The formation and type of growth media depend on the cells under consideration. However, most growth media contains glucose, amino acids, inorganic salts, vitamins, growth factors, hormones, and a pH buffer solution [36,37]. Fundamentally, growth media are highly conductive ionic solutions. Any electrode placed in a culture well containing cells and growth media will conduct electricity through the entirety of the interfacing surface; a part of the stimulus will flow through the cells at the bottom, and the rest will conduct through the growth media, as shown in Figure 1. As only a fraction of the stimulus flows through the targeted cells at the bottom of the well, it is difficult to correlate the external current or voltage to the effective modulating stimulus through the cells.
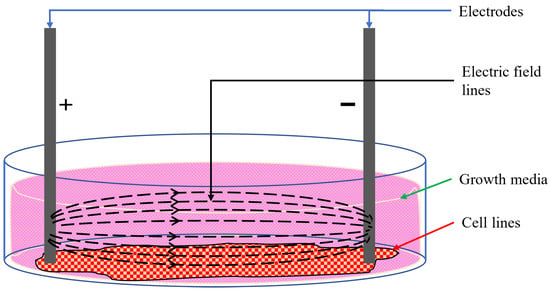
Figure 1.
Electric field lines in cell culture [15].
The setup of an in vitro experiment plays a decisive part in the outcomes of the experiment. It is evident that the setups used in research works encompass a wide variety of designs and materials. The commercially available setups are designed for specific experiment protocols. Hence, a versatile, easy-to-fabricate, and configurable electrode setup is highly needed. This work proposes an inexpensive method of building standard biocompatible electrodes for multi-well in vitro experiments. The setup is made of inexpensive, printed circuit board (PCB) technology and is designed for a standard 24-well culture dish. The electrodes are designed to provide effective stimulation at the bottom of the wells. The electric field distribution of the electrodes in the culture medium is analyzed in COMSOL Multiphysics. The electrodes are plated with gold to provide an interface that is compatible with the target biological cells. It also provides an option for setting the electrodes in bipolar or tetrapolar configurations, as required by the experiment protocol. Moreover, there is the flexibility of connecting any number of wells in a series to provide the same modulation to different sets of experiments.
2. Multi-Electrode System Design
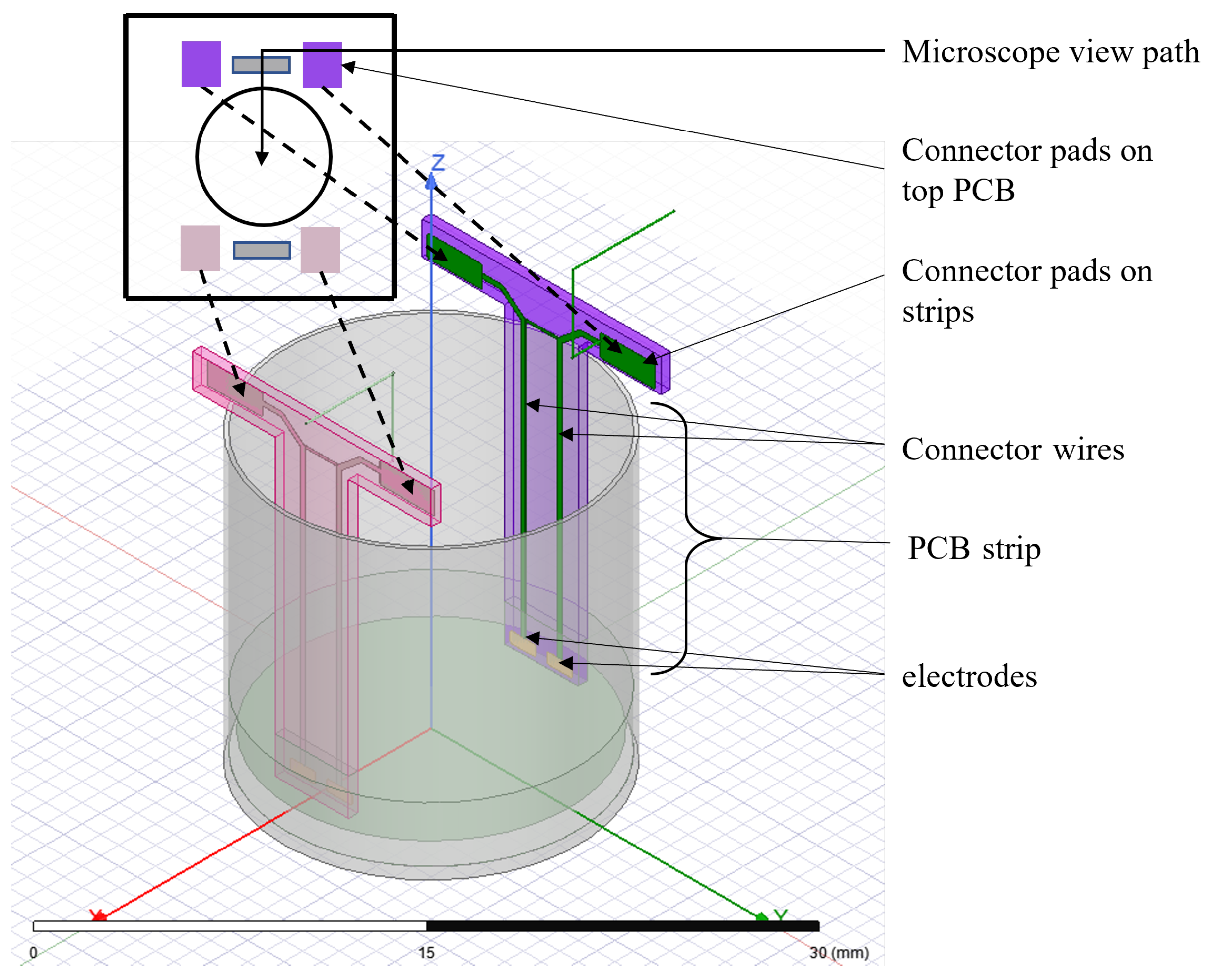
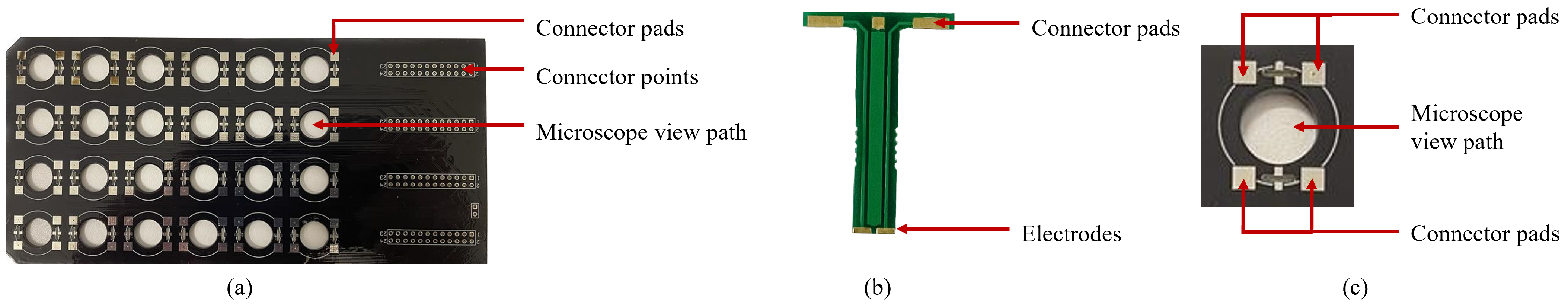
The multi-electrode system is designed for standard 24-well culture plates. Each well has a diameter of 16 mm and a depth of 16 mm. Each well is populated with four electrodes extended to the bottom of the wells via two strips of PCB board material (Figure 2). Each electrode has a dimension of 1.25 mm × 0.5 mm. The two electrodes on a strip have a separation of 0.5 mm, and the two strips are separated by 13 mm. The electrodes are plated with gold to ensure biocompatibility. Each electrode is connected to connector pads via copper wires embedded in the PCB. The connector pads are soldered with the corresponding connector pads on the main PCB board to create a continuous electric connection (Figure 2). Each of the connector pads on the main PCB board is wired to connection points at the end of the main board. These connection points can be used to interface external circuitry with the electrodes. Although each well has four electrodes, the two electrodes on one strip can be shortened by soldering the corresponding connector pads in the main PCB board to be used as a two-electrode system.
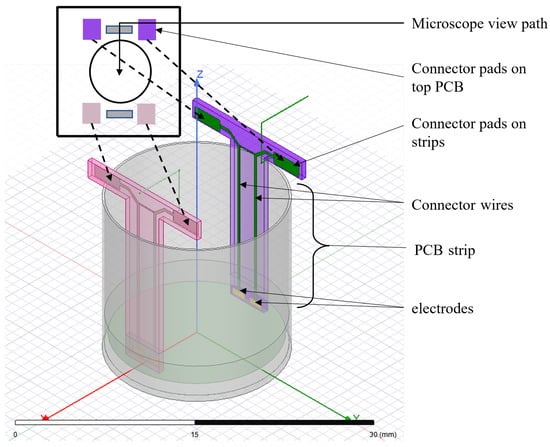
Figure 2.
Schematic diagram of proposed multi-electrode setup in culture well.
3. System Characterization Using FEM
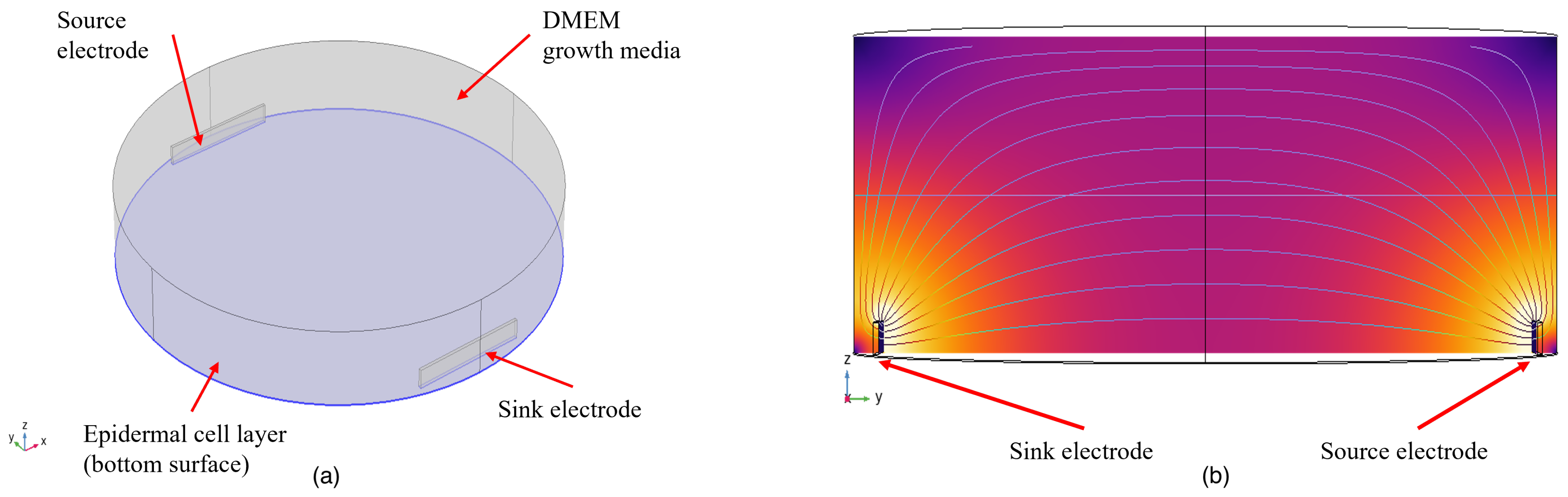
The analytical characterization of the multi-electrode setup was carried out using finite element modeling (FEM) in COMSOL Multiphysics. The model of the setup is shown in Figure 3. Skin epidermal cells are modeled as a 20 μm thick layer at the bottom of the culture well. The epidermal cell layers are modeled as a homogeneous layer at the bottom of the dish to reduce computational complexity. The conductivity of epidermal cells is taken as 0.4 S/m and the relative permittivity as [38]. The epidermal cell layer is topped by DMEM growth media, with a conductivity and relative permittivity of 1.68 S/m and 80, respectively [39]. The electrodes are designed for copper from the materials library in COMSOL Multiphysics ( S/m, = 1). Amongst the six surfaces of the electrode, the surface facing the center of the well is left intact, and the rest of the surfaces are defined with FR4 (circuit board) material from the materials library of COMSOL Multiphysics ( S/m, = 1) to achieve an insulating PCB layer.
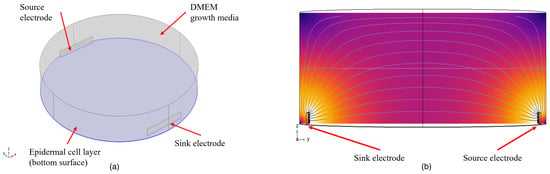
Figure 3.
Simulation model: (a) a 3D view of the setup (b); cross-sectional view of the setup.
Current density and electric field distribution at different regions of the culture well were evaluated to determine the efficacy of the design. The setup was simulated as per the design topology mentioned in Section 2. Copper electrodes of 3.5 mm × 0.5 mm were used as the source and sink electrodes. The electrodes were placed near the bottom of the well. The separation between the electrodes was 13 mm.
The analysis was carried out for different electrode surface areas. The width of the electrodes was kept fixed at 3.5 mm, and the height () varied from 0.5 mm to 2.00 mm to denote the changes in surface area. The electrodes were placed perpendicular to the bottom of the culture well. A comparative analysis of current density and electric field distribution was used to depict the effect of surface area and the position of the electrodes in providing electrical stimulation.
3.1. Current Density Analysis
The biological cells seeded at the bottom of the culture wells, along with the growth media on top, construct a bulk conductive media. Any current applied to the bulk will construct a 3D flow through the cells and media. The primary concern of electro-biomodulation is to ensure the flow of current through the targeted biological entity. Hence, current density analysis through the cells for varying electrode sizes gives a good view of the amount of current flowing through the targeted cells.
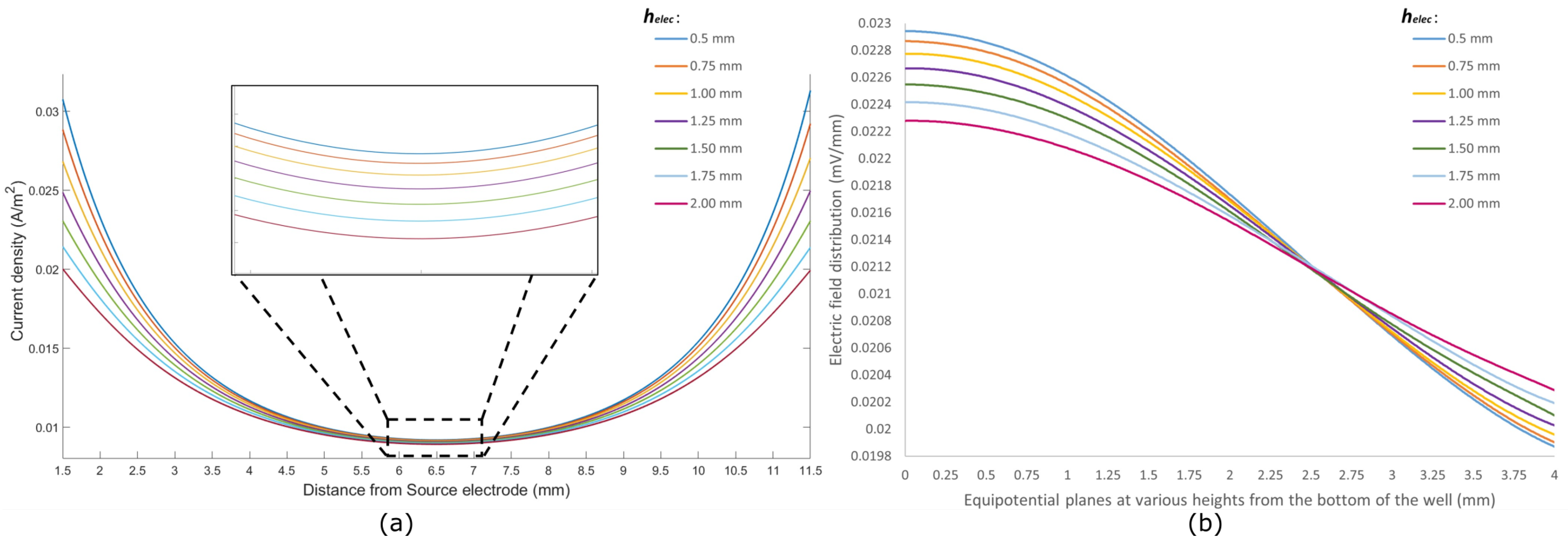
The current density through the cells for different sizes of electrodes is shown in Figure 4a. The density has been analyzed along a line passing through the cells from the source electrode towards the sink electrode and 1.5 mm away from both electrodes. This region is considered the region of interest where the cells are seeded. A constant current of 2 μA is reported to cause a proliferative effect on epidermal cells [40]. The same value was used as the injection current for simulation.
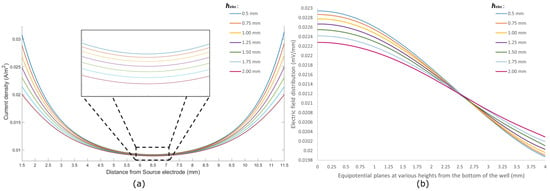
Figure 4.
(a) Current density (A/m2) along the axis from the source electrode to the sink electrode through the cell; (b) electric field distribution (mV/mm) at different equipotential planes along the height of the culture well.
From Figure 4a, it can be seen that the current density follows a similar pattern for all sizes of electrodes, being the highest at points close to the electrodes and reaching a lower value around the center of the dish. This outcome is expected as any cross-sectional plane perpendicular to the current flow path has a smaller area closer to the electrodes and a relatively larger area around the center of the dish. However, electrodes with a smaller surface area ( = 0.5 mm) pose a higher current density throughout the region of interest compared to electrodes with a larger surface area. Hence, placing smaller electrodes near the bottom of the culture well gives a better stimulus to the targeted cells in biomodulation.
The current from the source electrode to the sink electrode follows a bulk conduction pathway through the cells and growth media in the well. As such, the current density through the cells () and the current density through the media () depend heavily on the size and positioning of the electrodes. By placing electrodes close to the bottom surface of the well and changing the size of the electrodes, a difference in current densities can be observed. Table 1 shows the ratio between the current densities in the cell and in the media. It can be seen that electrodes with a height of 0.50 mm have a of around 0.27174. This signifies that the current flowing through the cells is around 27% of the current flowing through the media. As the size of the electrodes increases, this ratio falls off and reaches a value of 0.25856 for electrodes with a height of 2.00 mm. Hence, it can be concluded that smaller electrodes placed near the bottom of the wells provide better electrical bio-interfacing for in vitro studies.

Table 1.
The ratio between the current density in cells () and the current density in growth media () for different sizes of electrodes.
3.2. Electric Field Analysis
The electric field distribution was analyzed for different equipotential planes from the bottom of the culture well, as shown in Figure 4b. The bottom of the well, where the epidermal cells are modeled, is depicted as a plane at 0 mm. The electric field is observed at equipotential planes from 0 mm to 4.00 mm at intervals of 0.25 mm. The field distribution depends on the current injected through the source electrode. By keeping the injecting current and width of electrodes unchanged, the height of the electrodes () was increased from 0.5 mm to 2.00 mm.
It can be seen from Figure 4b that the electric field distribution is highest at the bottom of the well for all electrode sizes, and this gradually falls off at subsequent equipotential planes away from the bottom. This depicts that the electrodes placed close to the bottom of the culture wells give the highest electric field along the surface. Electrodes with smaller surface areas show higher electric fields towards the bottom of the well compared to their large surface area counterparts.
Furthermore, it can be seen that electrodes with different construct different field distribution trends for the same amount of injected current. Electrodes with a = 0.5 mm show a rather constant field distribution in planes up to 0.5 mm from the bottom of the well, and they show a notable drop in planes toward the top of the well. As the value of increases, the field distribution starts to flatten out at subsequent equipotential planes from the bottom to the top of the well. Table 2 summarizes the difference between the maximum and minimum values of field distribution for different electrode sizes. Electrodes with a small surface area, such as the one with = 0.50 mm, show a difference of mV/mm between the equipotential plane at the bottom of the well and a plane at 4.00 mm from the bottom. On the contrary, electrodes with = 2.00 mm exhibit a much lower difference of mV/mm between the same equipotential planes. For smaller electrodes, the field is much more concentrated around the vicinity of the bottom of the well, where the target cells are seeded. Thus, it can be considered that smaller electrodes placed near the bottom of the culture wells give better electrical bio-interfacing in terms of electric fields.

Table 2.
Difference in the maximum and minimum electric field along the height of the culture well for different sizes of electrodes.
4. Multi-Electrode System Fabrication
The multi-electrode system is designed to sit on top of standard, 24-well culture plates with dimensions of 127.6 mm × 85.3 mm × 23.1 mm. It has 24 groups of electrodes, with each group corresponding to one well. The connector pads on the main board are wired to external connector points at the end of the board. These connectors are used to apply stimulus from external circuitry. The main board is shown in Figure 5a.
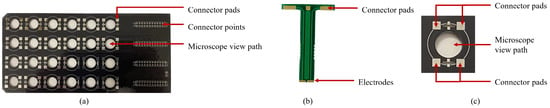
Figure 5.
Multi-electrode system; (a) main board (top view); (b) electrode strip; (c) microscopic view path.
Each standard well has a diameter of 16 mm and a depth of 16 mm. Two electrodes are fabricated at the end of a 16 mm strip, enabling the electrodes to reach the bottom of the well. Each electrode has a gold-plated surface area of 1.25 mm × 0.5 mm that is in contact with the bottom of the well. The two electrodes on one strip are separated by 1 mm of insulation. The total width of each strip is 3.5 mm. A circular view path 10 mm in diameter was made between the four electrodes. This allows for viewing the contents of the well under a microscope while the electrode setup is in place. The electrode strips are shown in Figure 5b, and the microscope view path is shown in Figure 5c. The complete setup is shown in Figure 6.

Figure 6.
Complete electrode setup in a 24-well culture dish.
5. Hardware Analysis
The designed multi-electrode system is a suitable choice for the electrical bio-interfacing of in vitro cell culture studies. Amongst the prominent studies, impedance measurement holds a strong place. The artifacts introduced due to the hardware configuration of the designed multi-electrode system have been examined by implementing the system for measuring impedance.
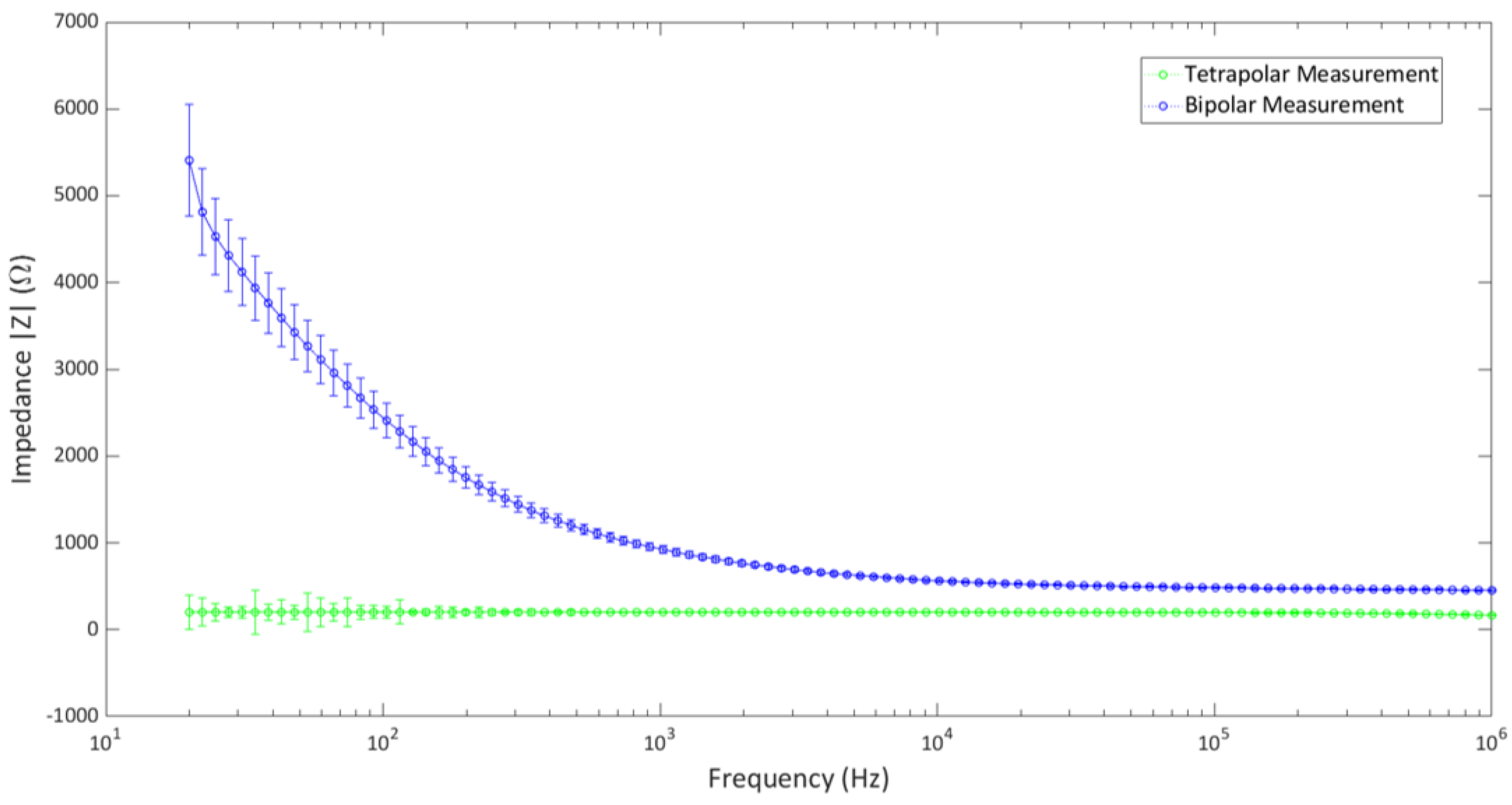
The setup was used to determine the impedance measurements of a popular cell growth media, Dulbecco’s Modified Eagle Medium (DMEM). The impedance measurement was carried out in both tetrapolar and bipolar configurations. As each well contains four electrodes, the tetrapolar configuration was made by using one electrode from each strip as the source and sink electrodes for current injection, and the remaining two were used as voltage-recording electrodes. The electrodes were connected with a precision LCR meter (Keysight Technologies, model E4980A), and the impedance of the media was determined over the range of 20 Hz–1 MHz. In order to achieve the bipolar configuration, the two electrodes on one strip were shorted together on the main board and connected to the LCR meter for impedance measurements. The experiment was carried out in a controlled laboratory environment of 37 °C and 5% CO2.
The impedance measurements of the two configurations are shown in Figure 7. The measurements were averaged over the values taken from five culture wells (separately) in the 24-well setup. It shows that the impedance values in the tetrapolar configuration are lower than those of the bipolar configuration. This is in agreement with the known theory, as tetrapolar measurements determine the transfer impedance rather than the actual impedance. The bipolar configuration shows deviations in the low-frequency end, although it poses a rather constant value at higher frequencies. Both configurations show little to no deviation at higher frequencies. It can be concluded that the multi-electrode system does not show notable artifacts due to the hardware configuration over a wide range of frequencies in the controlled laboratory environment, and it can be successfully implemented in bioimpedance measurement applications.
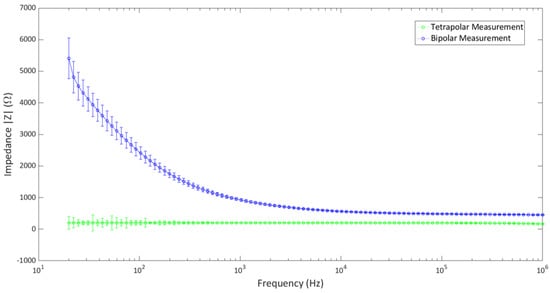
Figure 7.
Bipolar and tetrapolar impedance measurement of DMEM using the multi−electrode system.
6. Biocompatibility Analysis
Biocompatibility is a key factor for PCB systems intended for biological applications. A few research groups have reported the biocompatibility of several PCB systems for in vitro cell cultures [41,42]. The proposed system has been implemented in a 24-well cell culture plate with Human Dermal Fibroblast (HDF) cell culture to explore biocompatibility. Two 24-well culture plates were taken, where four wells from each plate were seeded with HDF and supplementary growth media. One culture plate was exposed to the electrode setup, referred to as experimental setup, whereas the other one was kept as the control setup. The electrodes were placed in the experimental setup such that they were submerged in the wells, reaching near the bottom of each well. Research by Sugimoto et al. suggests that fibroblasts were stimulated for 4 h to achieve targeted biomodulation [43]; hence, the electrodes were placed in the HDF cell culture for 4 h. Both culture plates were placed in an incubator with the following conditions: 37 °C, 5% CO2, and 95% humidity.
Live cell count was performed on the culture plates at 48 h, and those wells in which the electrodes had been placed for 4 h (experimental setup) were compared against those that were not exposed to the electrodes (control setup). The proliferation rate of HDF after 48 h was (−3.9%) in the experimental setup when compared to the control setup. There was a standard deviation of 4.9%, indicating overlapping data points between the two setups. There were no statistically significant differences between the two groups (p = 0.91), as determined by a Mann−Whitney U test (n = four per group).
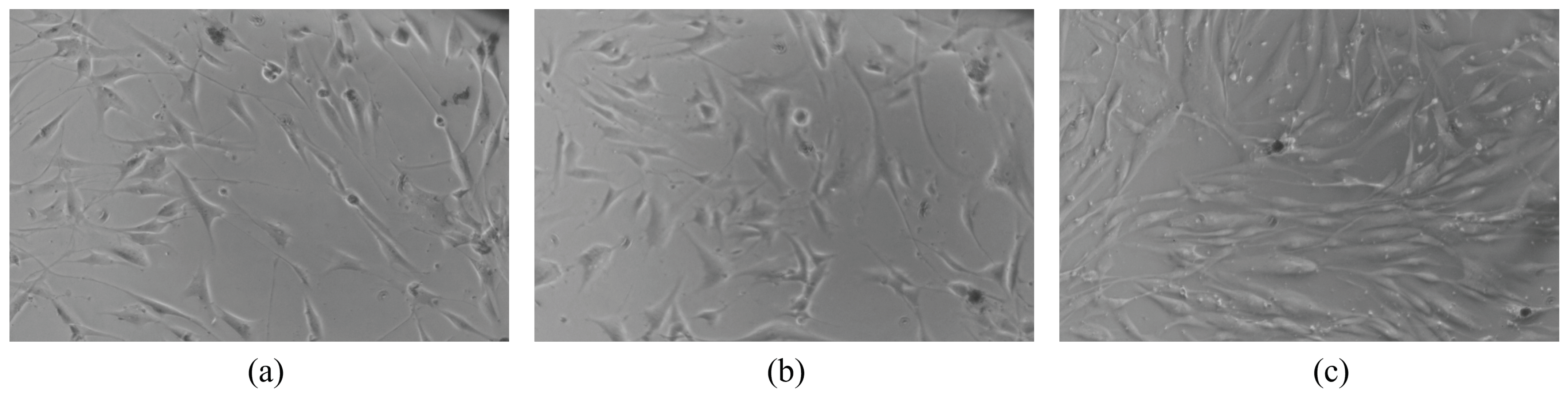
The microscope images of the HDF cell cultures are shown in Figure 8. Figure 8a shows the cell culture at the time of placement, noted as 0 h. Subsequent images of the culture plates were taken 24 h after placement (Figure 8b) and 48 h after placement (Figure 8c). A natural proliferation of HDF over a period of 48 h was observed. No evidence of any corrosion or adverse effect on the cells was observed due to the presence of the electrodes. It can be concluded that the proposed electrodes provide good biocompatibility for in vitro cell cultures.

Figure 8.
Human dermal fibroblasts: (a) 0 h; (b) 24 h; (c) 48 h.
7. Discussion
In vitro experiments provide a fundamental approach to designing protocols for clinical studies. Statistical significance plays a key part in proposing any protocol for which researchers tend to adopt multiple attempts at the same setup. A way to deal with multiple attempts of the same biological experiment is to run parallel setups under the same controlled conditions. Hence, most of the research works undergo experiments in multiple culture well plates. Multiple electrically bio-interfacing samples in a parallel setup pose a challenge that is addressed in many ways, as reported in the literature. Commercially available setups, like C-Dish™, provide a standard bipolar platform. However, the issue of the configurability, scalability, and multiplexing of the setup remains unanswered.
This work implemented an easy-to-design multi-electrode system for biological samples in multiple culture well dishes. The efficacy of the system has been determined by analyzing the distribution of the electric field generated due to the injection of a constant current through a biological sample. A finite element model of the cell culture well and electrode was designed in COMSOL Multiphysics. The current density and electric field distribution at various regions in the culture well were analyzed for different surface areas of electrodes to find the optimum electrode size and to maximize stimulus through the cell layer. The results show that a higher electric field is generated through the biological sample located at the bottom of the culture well if the electrodes are placed close to the bottom surface of the well with a small surface area. An increasing electrode surface area of results in a decrease in the electric field at the region of interest (through the cells). When analyzing the current density for different surface areas of electrodes, it can be seen that a higher current density is achieved with electrodes having a smaller surface area are placed at the bottom of the culture well. It can also be concluded that in order to maintain the optimal current density with an electrode of a larger surface area, the injection current should be increased accordingly. Most of this current would tend to flow through the ionic growth medium and only a portion would take part in the biomodulation process. Hence, by decreasing the size of the electrodes and by placing them near the bottom of the culture wells, it is possible to maximize the biomodulation effect in in vitro experiments.
The system was designed with basic PCB fabrication technology. It has four independent electrodes per well, giving a tetrapolar arrangement. However, the connectors of each electrode can be configured to provide bipolar setups. When using the setup, the impedance measurement of a uniform medium showed a constant impedance profile over a range of frequencies, suggesting good interfacing between the electrodes and media. For biocompatibility, the electrode surfaces were coated with gold. Prolonged contact with live cells in a culture medium shows regular cell proliferation with no corrosion from the electrodes. The proposed setup was designed for a 24-well culture dish, although it can easily be replicated for any set of standard culture dishes. Moreover, as each electrode has its own connector, multiple wells can be connected together to provide a singular stimulation from one source. The setup is designed to be used in a controlled laboratory environment with the following conditions: 37 °C, 5% CO2, and 95% humidity, with very small deviations. The PCB structure is made from FR4 PCB board, which is made from low-moisture-absorbing polymers that perform well in humid conditions. Within the controlled environment, the setup showed data consistency in consecutive trials, asserting no effect from the external environment.
Apart from in vitro stimulation cells, the setup can also be implemented in the in vitro measurement of bioimpedance. The electrodes have been applied in both bipolar and tetrapolar configurations to determine the impedance of DMEM growth media. It showed a constant impedance profile through a wide range of frequencies, although there were some deviations in the low-frequency region. This shows that the system does not introduce any disturbances due to frequency variation and can be used reliably for bioimpedance measurements.
A wide variety of electrode systems are used for the electrical intervention of cell cultures. Systems involving direct stimulation mechanisms use electrodes made of noble materials like gold, silver, carbon, stainless steel, etc., in the form of plates or wires. The plates or wires are submerged in the biological culture setup, where the entire surface in contact with the culture provides stimulation. As the surface in contact depends heavily upon the volume of culture media in the wells, the current density and electric field distribution through the media may vary for various volumes, although the injecting current remains the same. This work demonstrates that electrodes with smaller surface areas provide higher current density and targeted electric field distribution (when compared to larger ones) while keeping the injection current constant. A commonly used reliable electrode setup is the Ag/AgCl electrode. However, when comparing the costs of Ag/AgCl electrodes for multi-well multi-electrode stimulation, the proposed setup fabricated in PCB technology gives a low-cost alternative for such traditional setups. In comparison to the commercially available design for multi-well electro-biomodulation (C-Dish™), which provides only the bipolar configuration, the proposed setup can be altered between bipolar and tetrapolar configurations, adding versatility. Moreover, as the proposed system is designed using computer-aided software for designing PCBs, the electrode separations and dimensions can be replicated with high accuracy, giving a good grasp of experimental repeatability. Hence, when using comparative analysis, the proposed system provides a low-cost, easy-to-fabricate, configurable, and scalable electrical bio-interfacing system for multi-well in vitro cell culture experiments.
8. Conclusions
Electrical intervention has been widely explored in therapeutics for quite some time. Although many of the approaches have been relatively causative, quite a few have outlined the relationship between electrical intervention and biological modulation. In order to establish a proof of theory, in vitro experiments on specific cell lines have proven to be effective. Various research groups have successfully shown the multiple outcomes of in vitro electrical intervention on different types of cell lines. They have also reported diverse setups for electrical bio-interfacing, ranging from custom-made to commercially available versions.
This work reports the possibility of a low-cost, easy-to-build multi-electrode system with a view of making scalable and configurable electrodes. The setup is designed using standard PCB fabrication methods, which gives the benefit of designing electrodes for any standard culture well configuration. The electrodes in the proposed system are also easily configurable among bipolar and tetrapolar setups and according to the necessity of the experiment. The electrodes are plated with gold to ensure biocompatibility. A coat of gelatin or hydrogel could be applied to provide more biocompatibility between the electrodes and the biological entities. Overall, the proposed setup could provide an easy-to-build, low-cost, preliminary platform to provide electrical bio-interfacing for in vitro cell culture experiments and would have a positive impact on in vitro cell culture experiments worldwide.
Author Contributions
Conceptualization, I.F.T. and M.B.P.; methodology, I.F.T. and M.B.P.; software, M.R. and E.R.; validation, I.F.T., E.R. and M.B.P.; formal analysis, M.R.; investigation, M.R. and A.A.A.; resources, M.R. and A.A.A.; data curation, M.R; writing—original draft preparation, M.R.; writing—review and editing, I.F.T., E.R. and M.B.P.; visualization, M.R.; supervision, I.F.T., E.R. and M.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding in the form of doctoral studentship from The School of Science and Technology, City, University of London, UK.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to acknowledge The Research Centre for Biomedical Engineering, School of Science and Technology, and The Centre for Applied Vision Research, School of Health and Psychological Sciences at City, University of London, UK, for the support in conducting this research.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DMEM | Dulbecco’s Modified Eagle Medium |
| FEM | Finite Element Modelling |
References
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 1–12. [Google Scholar] [CrossRef]
- Amini, M.; Hisdal, J.; Kalvøy, H. Applications of bioimpedance measurement techniques in tissue engineering. J. Electr. Bioimpedance 2018, 9, 142. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Furth, P.A. Cancer prevention as biomodulation: Targeting the initiating stimulus and secondary adaptations. Ann. N. Y. Acad. Sci. 2012, 1271, 1–9. [Google Scholar] [CrossRef]
- Kloth, L.C. Electrical stimulation technologies for wound healing. Adv. Wound Care 2014, 3, 81–90. [Google Scholar] [CrossRef]
- Alborova, A.; Lademann, J.; Kramer, A.; Richter, H.; Patzelt, A.; Sterry, W.; Koch, S. In vivo analysis of wound healing by optical methods. GMS Krankenhaushygiene Interdiszip. 2008, 3, 1. [Google Scholar]
- Henry, S.L.; Concannon, M.J.; Yee, G.J. The effect of magnetic fields on wound healing: Experimental study and review of the literature. Eplasty 2008, 8, e40. [Google Scholar]
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef]
- Bailey, M.; Khokhlova, V.; Sapozhnikov, O.; Kargl, S.; Crum, L. Physical mechanisms of the therapeutic effect of ultrasound (a review). Acoust. Phys. 2003, 49, 369–388. [Google Scholar] [CrossRef]
- Du, J.; Shi, P.; Liu, J.; Yu, H.; Fang, F. Analgesic electrical stimulation combined with wrist-ankle acupuncture reduces the cortical response to pain in patients with myofasciitis: A randomized clinical trial. Pain Med. 2023, 24, 351–361. [Google Scholar] [CrossRef]
- Lu, S.; Chen, W.; Wang, J.; Guo, Z.; Xiao, L.; Wei, L.; Yu, J.; Yuan, Y.; Chen, W.; Bian, M.; et al. Polydopamine-decorated PLCL conduit to induce synergetic effect of electrical stimulation and topological morphology for peripheral nerve regeneration. Small Methods 2023, 7, 2200883. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.R.; Csont, T.; Csonka, C. The effect of electrical stimulation of skeletal muscle on cardioprotection and on muscle-derived myokine levels in rats: A pilot study. Physiol. Int. 2023, 110, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Szołtys-Brzezowska, B.; Bańkowska, A.; Piejko, L.; Zarzeczny, R.; Nawrat-Szołtysik, A.; Kloth, L.C.; Polak, A. Electrical Stimulation in the Treatment of Pressure Injuries: A Systematic Review of Clinical Trials. Adv. Ski. Wound Care 2023, 36, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Imani, I.M.; Kim, B.; Xiao, X.; Rubab, N.; Park, B.J.; Kim, Y.J.; Zhao, P.; Kang, M.; Kim, S.W. Ultrasound-Driven On-Demand Transient Triboelectric Nanogenerator for Subcutaneous Antibacterial Activity. Adv. Sci. 2023, 10, 2204801. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Rahman, E.; Powner, M.B.; Triantis, I.F. Making Sense of Electrical Stimulation: A Meta-analysis for Wound Healing. Ann. Biomed Eng. 2023, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Compton, M.E.; Mize, C.W. Statistical considerations for in vitro research: I—Birth of an idea to collecting data. In Vitro Cell. Dev.-Biol.-Plant 1999, 35, 115–121. [Google Scholar] [CrossRef]
- Mize, C.W.; Koehler, K.J.; Compton, M.E. Statistical considerations for in vitro research: II—Data to presentation. In Vitro Cell. Dev.-Biol.-Plant 1999, 35, 122–126. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef]
- Drury, K.C.; Konicek, J.R.; Kipersztok, S.; Williams, R.S. Twelve-well culture plate for the efficient collection and culture of human oocytes and embryos. J. Assist. Reprod. Genet. 1996, 13, 557–561. [Google Scholar] [CrossRef]
- Snyder, S.; DeJulius, C.; Willits, R.K. Electrical stimulation increases random migration of human dermal fibroblasts. Ann. Biomed. Eng. 2017, 45, 2049–2060. [Google Scholar] [CrossRef]
- Hoare, J.I.; Rajnicek, A.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Electric fields are novel determinants of human macrophage functions. J. Leucoc. Biol. 2016, 99, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, J.; Berger, T.; Barranco, S.; Chapin, S.; Becker, R. Antibacterial effects of silver electrodes with weak direct current. Antimicrob. Agents Chemother. 1974, 6, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.W.; Tooms, R.E.; Mendius, R.A.; Clifft, J.K.; Vander Zwaag, R.; El-Zeky, F. Efficacy of high voltage pulsed current for healing of pressure ulcers in patients with spinal cord injury. Phys. Ther. 1991, 71, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.; Meng, S.; Derbali, H.; Zhang, Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS ONE 2013, 8, e71660. [Google Scholar] [CrossRef]
- Reich, J.D.; Cazzaniga, A.L.; Mertz, P.M.; Kerdel, F.A.; Eaglstein, W.H. The effect of electrical stimulation on the number of mast cells in healing wounds. J. Am. Acad. Dermatol. 1991, 25, 40–46. [Google Scholar] [CrossRef]
- Ko, U.H.; Choi, J.; Choung, J.; Moon, S.; Shin, J.H. Physicochemically tuned myofibroblasts for wound healing strategy. Sci. Rep. 2019, 9, 16070. [Google Scholar] [CrossRef]
- Demir, H.; Balay, H.; Kirnap, M. A comparative study of the effects of electrical stimulation and laser treatment on experimental wound healing in rats. J. Rehabil. Res. Dev. 2004, 41, 147–153. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G.; Hedayati, M.; Mofid, M. Role of sensory and motor intensity of electrical stimulation on fibroblastic growth factor-2 expression, inflammation, vascularization, and mechanical strength of full-thickness wounds. J. Rehabil. Res. Dev. 2013, 50, 489–498. [Google Scholar] [CrossRef]
- Mehmandoust, F.G.; Torkaman, G.; Firoozabadi, M.; Talebi, G. Anodal and cathodal pulsed electrical stimulation on skin wound healing in guinea pigs. J. Rehabil. Res. Dev. 2007, 44, 611. [Google Scholar] [CrossRef]
- Tillotson, D.L.; Udale, R.T.; Haer, F.A.; Worden, K.L. Myocyte Culture Pacing Apparatus. US7148059B1, 21 February 2003. [Google Scholar]
- Xiong, G.M.; Do, A.T.; Wang, J.K.; Yeoh, C.L.; Yeo, K.S.; Choong, C. Development of a miniaturized stimulation device for electrical stimulation of cells. J. Biol. Eng. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Genovese, J.A.; Spadaccio, C.; Langer, J.; Habe, J.; Jackson, J.; Patel, A.N. Electrostimulation induces cardiomyocyte predifferentiation of fibroblasts. Biochem. Biophys. Res. Commun. 2008, 370, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Nedachi, T.; Kanzaki, M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp. Cell Res. 2007, 313, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, E.; Bortolani, B.; Corazza, I.; Cercenelli, L. A novel sensorized heart valve prosthesis: Preliminary in vitro evaluation. Sensors 2018, 18, 3905. [Google Scholar] [CrossRef] [PubMed]
- Urabe, H.; Akimoto, R.; Kamiya, S.; Hosoki, K.; Ichikawa, H.; Nishiyama, T. Pulsed electrical stimulation and amino acid derivatives promote collagen gene expression in human dermal fibroblasts. Cytotechnology 2023, 1–13. [Google Scholar] [CrossRef]
- Arora, M. Cell culture media: A review. Mater. Methods 2013, 3, 24. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Stinstra, G.; Hendriks, M. Estimation of the Electrical Conductivity of Human Tissue. Electromagnetics 2001, 21, 545–557. [Google Scholar] [CrossRef]
- Lang, Q.; Wu, Y.; Ren, Y.; Tao, Y.; Lei, L.; Jiang, H. AC Electrothermal Circulatory Pumping Chip for Cell Culture. ACS Appl. Mater. Interfaces 2015, 7, 26792–26801. [Google Scholar] [CrossRef]
- Konstantinou, E.; Zagoriti, Z.; Pyriochou, A.; Poulas, K. Microcurrent Stimulation Triggers MAPK Signaling and TGF-β1 Release in Fibroblast and Osteoblast-Like Cell Lines. Cells 2020, 9, 1924. [Google Scholar] [CrossRef]
- Urbano-Gámez, J.D.; Valdés-Sánchez, L.; Aracil, C.; De la Cerda, B.; Perdigones, F.; Plaza Reyes, Á.; Díaz-Corrales, F.J.; Relimpio López, I.; Quero, J.M. Biocompatibility Study of a Commercial Printed Circuit Board for Biomedical Applications: Lab-on-PCB for Organotypic Retina Cultures. Micromachines 2021, 12, 1469. [Google Scholar] [CrossRef]
- Mazzuferi, M.; Bovolenta, R.; Bocchi, M.; Braun, T.; Bauer, J.; Jung, E.; Iafelice, B.; Guerrieri, R.; Destro, F.; Borgatti, M.; et al. The biocompatibility of materials used in printed circuit board technologies with respect to primary neuronal and K562 cells. Biomaterials 2010, 31, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Maeshige, N.; Honda, H.; Yoshikawa, Y.; Uemura, M.; Yamamoto, M.; Terashi, H. Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J. Wound Care 2012, 21, 5–10. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).