The Influence of Fermentation Vessels on Yeast Microbiota and Main Parameters of Sauerkraut
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation of Sauerkraut
2.2. Determination the Number of Yeasts and Their Isolation
2.3. Determining the Number of Lactic Acid Bacteria
2.4. DNA Isolation
2.5. RAPD-PCR
2.6. PCR-RFLP
2.7. 5.8S-ITS rRNA Gene Region Sequencing
2.8. Determination of Total and Reducing Sugars Using 3,5-Dinitrosalicylic Acid (DNS)
2.9. Determination of Sugars and Acids by HPLC
2.10. Free Amine Nitrogen (FAN)
2.11. Sensory Analysis (QDA)
2.12. Statistical Analysis
3. Results and Discussion
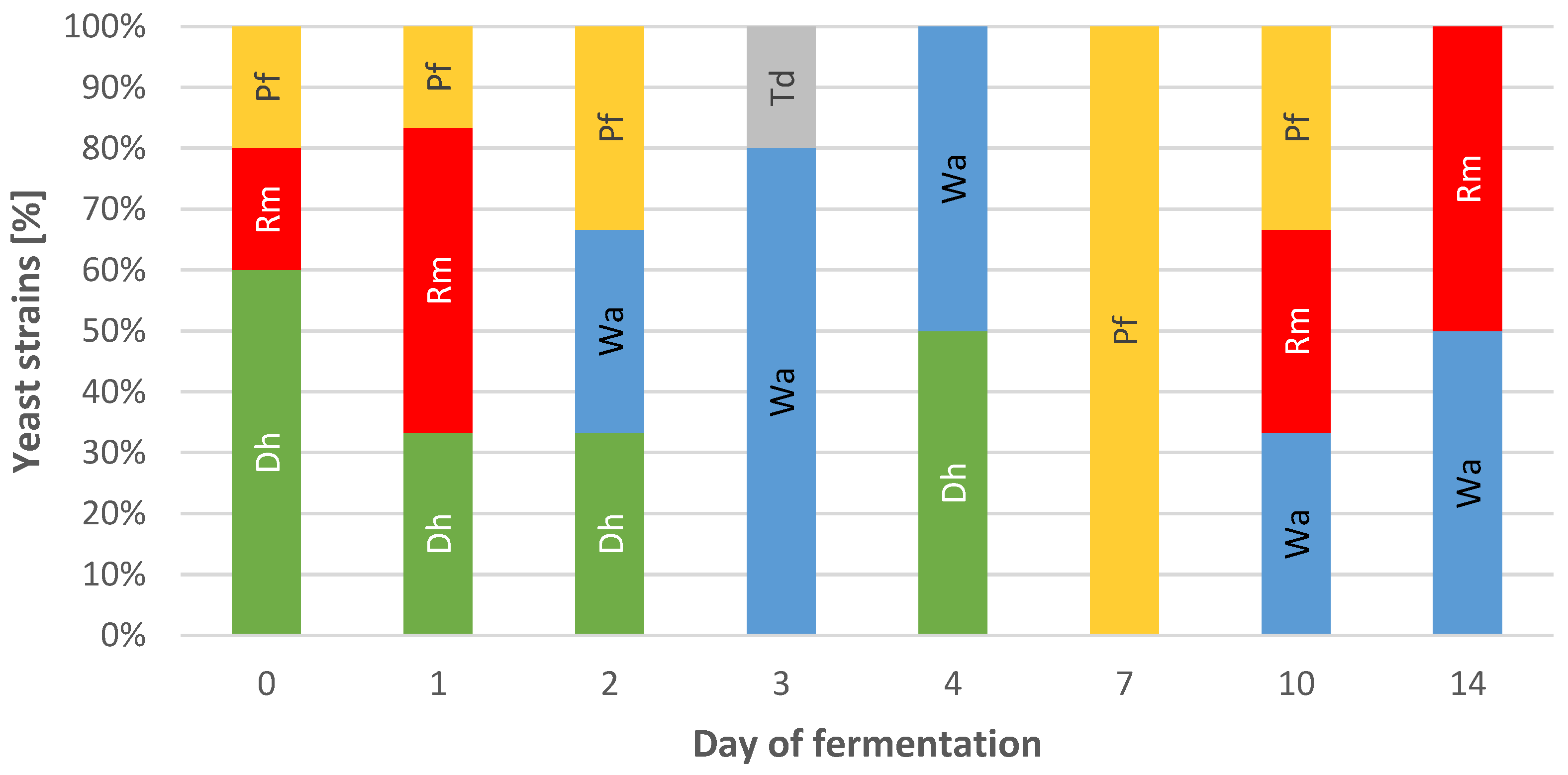
3.1. The Yeast Microbiota during Fermentation in Stoneware Vessels of Cabbages of Different Varieties—Preliminary Studies
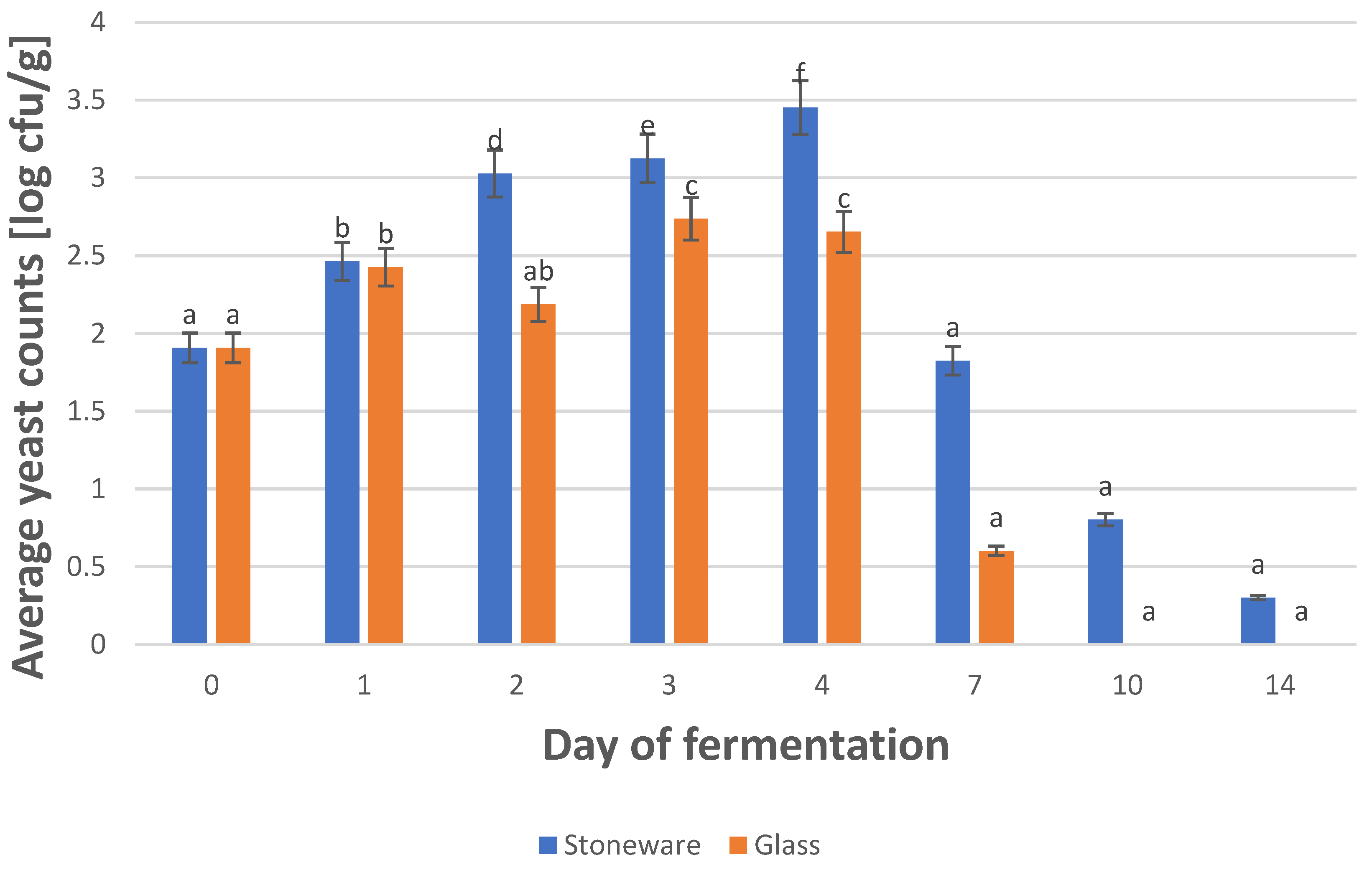
3.2. The Influence of the Vessel Used for Fermentation on the Yeast Microbiota of Sauerkraut
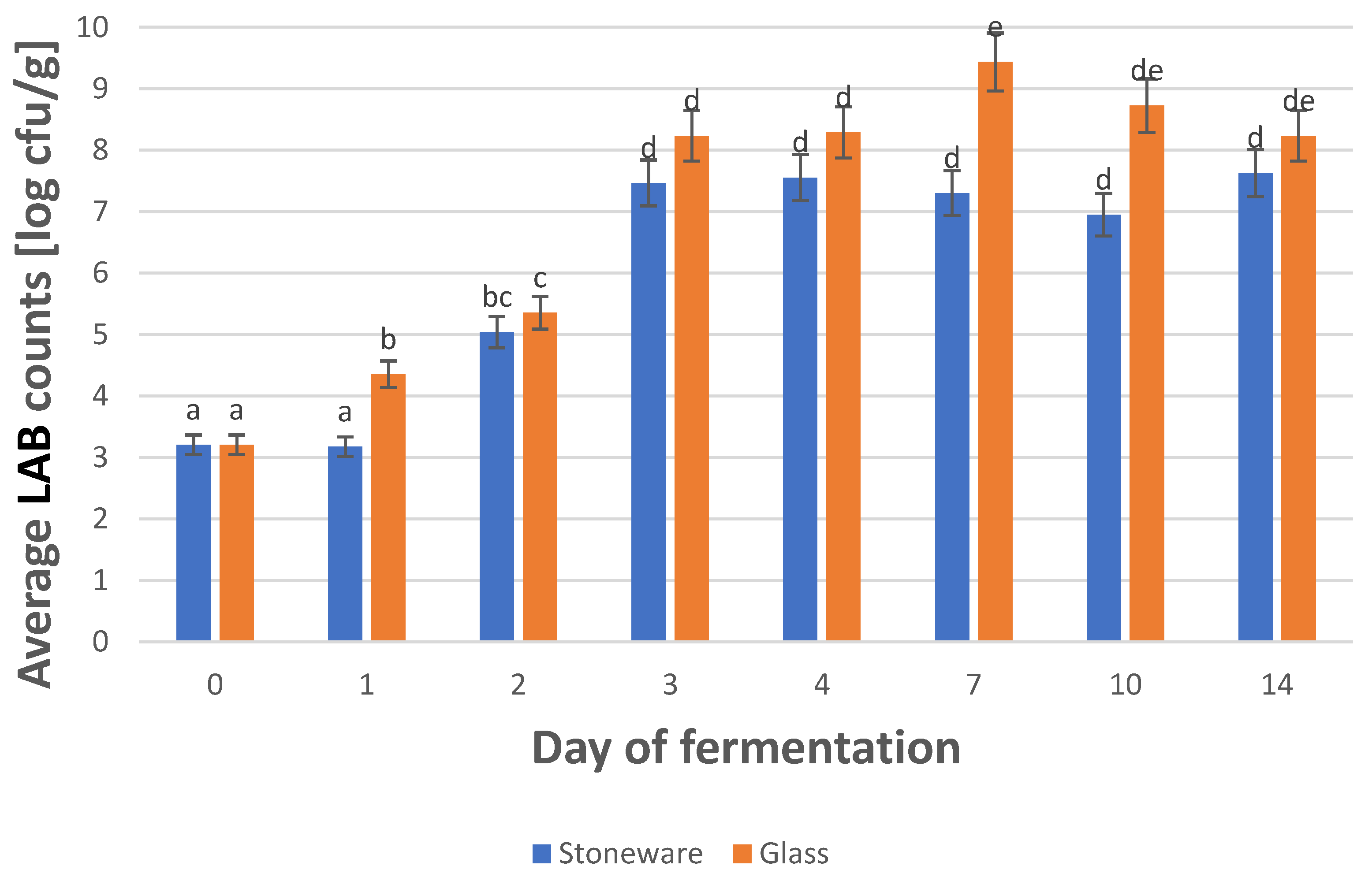
3.3. The Influence of the Vessel Used for Fermentation on the LAB Content, pH, and Selected Organic Acids Level of Sauerkraut
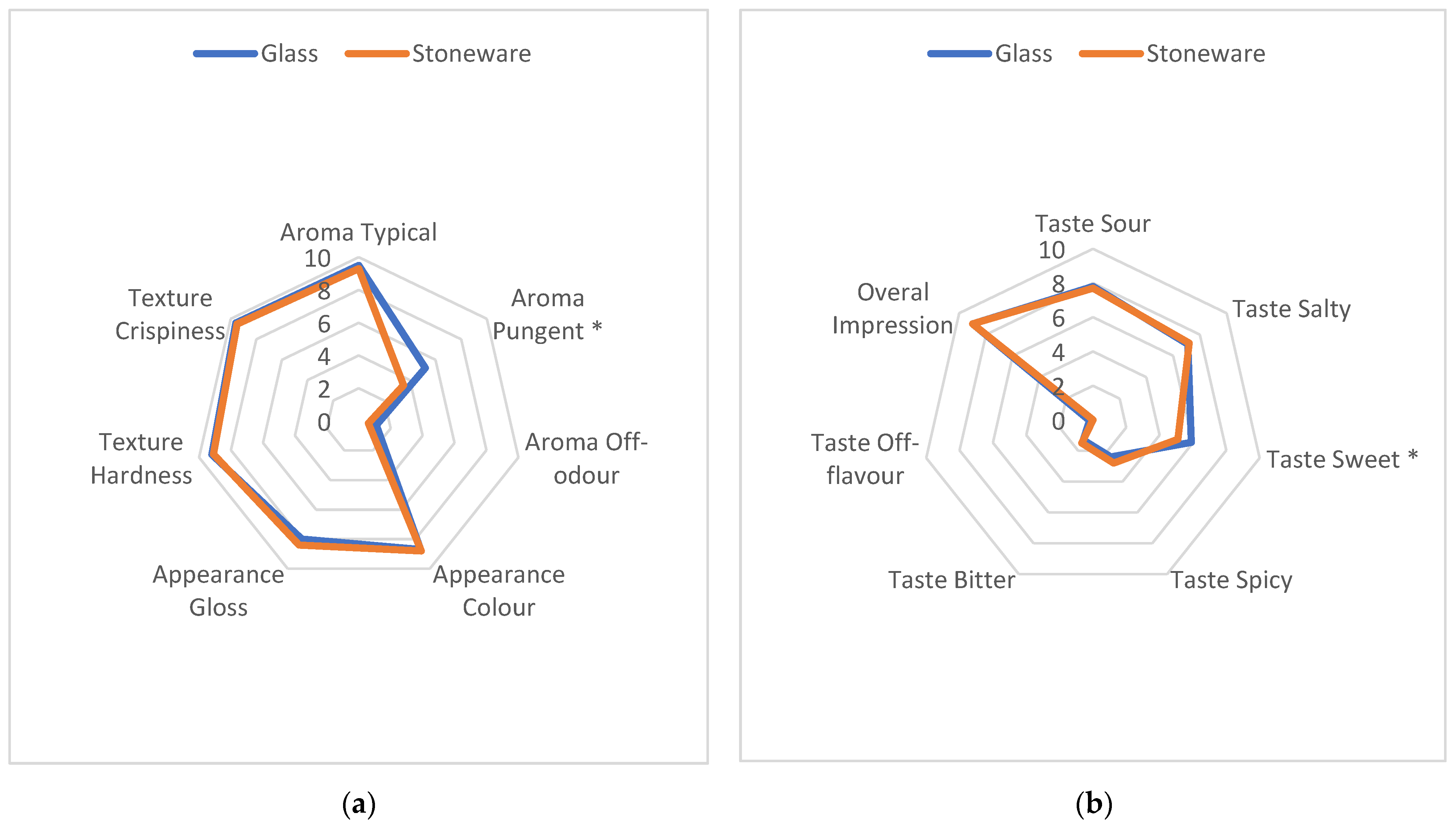
3.4. The Influence of the Vessel Used for Fermentation on the Selected Sugars, Polyols, FAN Level, and Sensory Profile of Sauerkraut
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinkraus, K.H. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Control 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Motarjemi, Y. Impact of small-scale fermentation technology on food safety in developing countries. Int. J. Food Microbiol. 2002, 75, 213–229. [Google Scholar] [CrossRef]
- Satora, P.; Celej, D.; Skotniczny, M.; Trojan, N. Identyfikacja drożdży obecnych w kiszonej kapuście komercyjnej i otrzymywanej w gospodarstwach rolnych. Żywność Nauka Technol. Jakość 2017, 24, 27–36. [Google Scholar] [CrossRef]
- Li, K.-Y. Fermentation. In Handbook of Food and Beverage Fermentation Technology; Hui, Y.H., Meunier-Goddik, L., Hansen, Å.S., Josephsen, J., Nip, W.K., Stanfield, P.S., Toldrá, F., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 595–610. [Google Scholar]
- Satora, P.; Skotniczny, M.; Strnad, S.; Ženišová, K. Yeast Microbiota during Sauerkraut Fermentation and Its Characteristics. Int. J. Mol. Sci. 2020, 21, 9699. [Google Scholar] [CrossRef]
- Marsden, L.M.; Gray, P.P.; Nippard, G.J.; Quinlan, M.R. Evaluation of the DNS Method for Analysing Lignocellulosic Hydrolysates. J. Chem. Technol. Biotechnol. 1982, 32, 1016–1022. [Google Scholar] [CrossRef]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Gajewski, M.; Radzanowska, J. Skład chemiczny i jako sensoryczna kapusty głowiastej w zaleznosci od jej odmiany i dawki azotu stosowanej w nawozeniu mineralnym. Żywność Nauka Technol. Jakość 2004, 2, 108–120. [Google Scholar]
- Quereda Vázquez, F.; Lorente-Ayza, M.; Ignacio, N.; Maria, D.A.; Escrig Marin, P.; Ojeda Domenech, M. Red firing clays for the manufacture of vessels for wine fermentation and maturation by means of technological processes. Open Ceram. 2022, 9, 100232. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2008, 4, 68. [Google Scholar] [CrossRef]
- Passoth, V.; Fredlund, E.; Druvefors, U.Ã.; Schnorer, J. Biotechnology, physiology and genetics of the yeast Pichia anomala. FEMS Yeast Res. 2006, 6, 3–13. [Google Scholar] [CrossRef]
- Liu, K.-F.; Li, X.-H.; Hui, F.-L. Yarrowia brassicae f.a., sp. nov., a new yeast species from traditional Chinese sauerkraut. Int. J. Sys. Evol. Microbiol. 2018, 68, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.S.; Verón, H.; Contreras, L.; Isla, M.I. An overview of plant-autochthonous microorganisms and fermented vegetable foods. Food Sci. Hum. Wellness 2020, 9, 112–123. [Google Scholar] [CrossRef]
- Coda, R.; Cassone, A.; Rizzello, C.; Nionelli, l.; Cardinali, G.; Gobetti, M. Antifungal activity of Wickerhamomyces anomalus and Lactobacillus plantarum during sourdough fermentation: Identification of novel compounds and longterm effect during storage of wheat bread. Appl. Environ. Microbiol. 2011, 77, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Tarko, T.; Sroka, P.; Blaszczyk, U. The influence of Wickerhamomyces anomalus killer yeast on the fermentation and chemical composition of apple wines. FEMS Yeast Res. 2014, 14, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Q.; Luan, Y.; Duan, C.-Q.; Yan, G.-L. Use of Torulaspora delbrueckii Co-fermentation With Two Saccharomyces cerevisiae Strains With Different Aromatic Characteristic to Improve the Diversity of Red Wine Aroma Profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, K.; Urso, R.; Dolci, P.; Comi, G.; Cocolin, L. Microflora of Feta cheese from four Greek manufacturers. Int. J. Food Microbiol. 2008, 126, 36–42. [Google Scholar] [CrossRef]
- Alan, Y.; Yildiz, N. Effects of Lactobacillus used as the starter culture on naturally fermented pickled cabbage. Food Sci. Technol. 2022, 42, e45020. [Google Scholar] [CrossRef]
- Geeson, J.D. The Fungal and Bacterial Flora of Stored White Cabbage. J. Appl. Bacteriol. 1979, 46, 189–193. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Saren, G.; Feng, K. Effect of salt concentration on microbial communities, physicochemical properties, and metabolite profile during spontaneous fermentation of Chinese northeast sauerkraut. J. Appl. Microbiol. 2020, 129, 1458–1471. [Google Scholar] [CrossRef]
- Shih, C.T.; Hang, Y.D. Production of carotenoids by Rhodotorula rubra from sauerkraut brine. LTW-Food Sci. Technol. 1996, 29, 570–572. [Google Scholar]
- Lücke, F.-K.; Zangerl, P. Food safety challenges associated with traditional foods in German-speaking regions. Food Control 2004, 43, 217–230. [Google Scholar] [CrossRef]
- Mendoza, L.M.; de Nadra, M.C.M.; Farías, M.E. Antagonistic interaction between yeasts and lactic acid bacteria of oenological relevance. Food Res. Int. 2010, 43, 1990–1998. [Google Scholar] [CrossRef]
- Du Toit, M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons from nature’s own arsenal—A review. S. Afr. J. Enol. Vitic. 2000, 21, 74–92. [Google Scholar] [CrossRef]
- Legan, J.D.; Voysey, P.A. Yeast spoilage of bakery products and ingredients. J. Appl. Bacteriol. 1991, 70, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.-D.; Kyung, K.H. Inhibition of yeast film formation in fermented vegetables by materials derived from garlic using cucumber pickle fermentation as model system. Food Sci. Biotechnol. 2006, 15, 469–473. [Google Scholar]
- Shah, N.N.; Singhal, R.S. Fermented Fruits and Vegetables–Fermented Cabbage. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3.3.1, pp. 59–62. [Google Scholar]
- Tkáčová, J.; Furdíková, K.; Klempová, T.; Ďurčanská, K.; Čertík, M. Screening of carotenoid-producing Rhodotorula strains isolated from natural sources. Acta Chim. Slov. 2015, 8, 34–38. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT-Food Sci. Technol. 2021, 136, 110325. [Google Scholar] [CrossRef]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Che, Z.; Li, H.; Liu, L. Influence of oxygen exposure on fermentation process and sensory qualities of Sichuan pickle (paocai). RSC Adv. 2019, 9, 38520–38530. [Google Scholar] [CrossRef]
- Viander, B.; Maki, M.; Palva, A. Impact of low salt concentration, salt, quality on natural large-scale sauerkraut fermentation. Food Microbiol. 2003, 20, 391–395. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, F.; Shi, H.; He, M.; Ge, J.; Ling, H.; Cheng, K. Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods 2023, 12, 1164. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.; Schillinger, U.; Buckenhüskes, H.; Sauerkraut. Handbook of Fermented Functional Foods; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 395–412. [Google Scholar]
- Andress, E.; Ingham, B.; Snyder, A.; Breidt, F. Manufacture of Traditionally Fermented Vegetable Products: Best Practice for Small Businesses and Retail Food Establishments. Food Prot. Trends 2021, 40, 251–263. [Google Scholar]
- Daeschel, M.A.; Andersson, R.E.; Fleming, H.P. Microbial ecology of fermenting plant materials. FEMS Microbiol. Lett. 1987, 46, 357–367. [Google Scholar] [CrossRef]
- Gomes, A.L.M.; Bueno, A.V.I.; Osmari, M.P.; Machado, J.; Nussio, L.G.; Jobim, C.C. Effects of Obligate Heterofermentative Lactic Acid Bacteria Alone or in Combination on the Conservation of Sugarcane Silage. Front. Microbiol. 2021, 12, 643879. [Google Scholar] [CrossRef] [PubMed]
- Saeki, A.; Taniguchi, M.; Matsushita, K.; Toyama, H.; Theeragool, G.; Lotong, N.; Adachi, O. Microbiological Aspects of Acetate Oxidation by Acetic Acid Bacteria, Unfavorable Phenomena in Vinegar Fermentation. Biosci. Biotechnol. Biochem. 1997, 61, 317–323. [Google Scholar] [CrossRef]
- Drašković, M.; Horecki, A.Y.; Šumić, Z.; Malbaša, R.; Vitas, J.; Pavlić, B.; Vakula, A. Variation of bioactive compounds content in fermented cabbage: Influence of temperature. J. Process. Energy Agric. 2017, 21, 136–141. [Google Scholar] [CrossRef]
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of processing on oxalate contents in plant foods: A review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Jeong, E.J.; Moon, D.W.; Oh, J.S.; Moon, J.S.; Seong, H.; Kim, K.Y.; Han, N.S. Development of Cabbage Juice Medium for Industrial Production of Leuconostoc mesenteroides Starter. J. Microbiol. Biotechnol. 2017, 27, 2112–2118. [Google Scholar] [CrossRef]
- Wennberg, M.; Ekvall, J.; Olsson, K.; Nyman, M. Changes in carbohydrate and glucosinolate composition in white cabbage (Brassica oleracea var. capitata) during blanching and treatment with acetic acid. Food Chem. 2006, 95, 226–236. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Tyagi, P.; Sindhia, V.; Yadavilli, K.S. Invertase and its applications—A brief review. J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Johannigsmeier, S.D.; McFeeters, J.F.; Fleming, H.P.; Thompson, R.L. Effects of Leuconostoc mesenteroides Starter Culture on Fermentation of Cabbage with Reduced Salt Concentrations. J. Food Sci. 2007, 72, 166–172. [Google Scholar]
- Bhatt, S.M.; Mohan, A.; Srivastava, S.K. Challenges in Enzymatic Route of Mannitol Production. ISRN Biotechnol. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kostinek, M.; Specht, I.; Edward, V.A.; Pinto, C.; Egounlety, M.; Sossa, C.; Mbugua, S.; Dortu, C.; Thonart, P.; Taljaard, L.; et al. Characterisation and biochemical properties of predominant lactic acid bacteria from fermenting cassava for selection as starter cultures. Int. J. Food Microbiol. 2007, 114, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Shaigani, P.; Awad, D.; Redai, V. Oleaginous yeasts- substrate preference and lipid productivity: A view on the performance of microbial lipid producers. Microb. Cell Fact. 2021, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Kryachko, Y.; Batbayar, B.; Tanaka, T.; Nickerson, M.T.; Korber, D.R. Production of glycerol by Lactobacillus plantarum NRRL B-4496 and formation of hexamine during fermentation of pea protein enriched flour. J. Biotechnol. 2020, 323, 331–340. [Google Scholar] [CrossRef]
- Lekkas, C.; Stewart, G.G.; Hill, A.; Taidi, B.; Hodgson, J. The Importance of Free Amino Nitrogen in Wort and Beer. Tech. Q. Master Brew. Assoc. Am. 2005, 42, 113–116. [Google Scholar]
- Guadalupe-Daqui, M.; Chen, M.; Sarnoski, P.J.; Goodrich-Schneider, R.M.; MacIntosh, A.J. Impacts of Reduced (Vacuum) Pressure on Yeast Fermentation as Assessed Using Standard Methods and Automated Image Analysis. Fermentation 2023, 9, 155. [Google Scholar] [CrossRef]
- Bell, S.-J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Loureiro, V.; Querol, A. The prevalence and control of spoilage yeasts in foods and beverages. Trends Food Sci. Technol. 1999, 10, 356–365. [Google Scholar] [CrossRef]
- Li, H.; Guan, H.; Zhang, X.; Xing, S.; Liu, W.; Kim, I.-C.; Gong, H. The Impact of Different Cooking Methods on the Flavor Profile of Fermented Chinese Spicy Cabbage. Molecules 2023, 28, 6539. [Google Scholar] [CrossRef] [PubMed]

| Strain | 5.8S-ITS rRNA Gene (bp) | Restriction Fragments (bp) | Species Identification by 5.8S-ITS rRNA Gene Sequencing | ||||
|---|---|---|---|---|---|---|---|
| CfoI | HinfI | HaeIII | Closest Species | Homology % | |||
| Pf9 | 450 | 170 + 100 + 100 + 80 | 250 + 200 | 340 + 80 + 30 | Pichia fermentans | 100.0 | MT645416 |
| Wa1 | 650 | 575 | 310 + 310 | 600 + 50 | Wickerhamomyces anomalus | 99.0 | KY657575 |
| Wa7 | 650 | 575 | 310 + 310 | 600 + 50 | Wickerhamomyces anomalus | 99.6 | KY105860 |
| Wa22 | 650 | 575 | 310 + 310 | 600 + 50 | Wickerhamomyces anomalus | 98.4 | KT580795 |
| Rm8 | 640 | 320 + 240 + 80 | 340 + 225 + 75 | 425 + 215 | Rhodotorula mucilaginosa | 100.0 | OQ692821 |
| Rm26 | 640 | 320 + 240 + 80 | 340 + 225 + 75 | 425 + 215 | Rhodotorula mucilaginosa | 99.8 | JQ293997 |
| Td5 | 800 | 330 + 220 + 150 + 100 | 800 | 410 + 390 | Torulaspora delbrueckii | 98.2 | KY105612 |
| Dh17 | 650 | 300 + 300 + 50 | 325 + 325 | 420 + 150 + 90 | Debaryomyces hansenii | 99.0 | MT192508 |
| Dh19 | 650 | 300 + 300 + 50 | 325 + 325 | 420 + 150 + 90 | Debaryomyces hansenii | 99.2 | MK275230 |
| Dh23 | 650 | 300 + 300 + 50 | 325 + 325 | 420 + 150 + 90 | Debaryomyces hansenii | 98.1 | MH429890 |
| Dh31 | 650 | 300 + 300 + 50 | 325 + 325 | 420 + 150 + 90 | Debaryomyces hansenii | 98.3 | KM816678 |
| Cl6 | 370 | 210 + 180 | 180 + 160 | 370 | Clavispora lusitaniae | 99.0 | MK312615 |
| Vessel/Day | 0 | 1 | 2 | 3 | 4 | 7 | 10 | 14 | SEM 1 | Sig. 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Glass | 6.04 f | 5.78 ef | 5.73 ef | 4.64 c | 4.10 b | 3.82 ab | 3.81 ab | 3.73 a | 0.17 | *** |
| Stoneware | 6.04 f | 5.70 e | 5.57 e | 5.13 d | 4.53 c | 4.06 b | 3.91 ab | 3.86 ab | 0.19 | ||
| Lactic acid | Glass | 0.00 a | 0.87 ab | 1.05 ab | 3.74 abc | 4.42 bc | 12.42 efg | 15.78 g | 15.37 fg | 1.40 | *** |
| Stoneware | 0.00 a | 2.01 ab | 1.16 ab | 2.57 ab | 2.75 ab | 6.95 cd | 11.65 ef | 10.12 de | 0.86 | ||
| Oxalic acid | Glass | 0.31 abc | 0.36 abc | 0.54 c | 0.30 abc | 0.50 bc | 1.25 e | 1.00 d | 0.36 abc | 0.07 | *** |
| Stoneware | 0.31 abc | 0.53 c | 0.27 ab | 0.21 a | 0.39 abc | 0.82 d | 0.96 d | 0.15 a | 0.06 | ||
| Acetic acid | Glass | 0.00 a | 0.98 e | 1.60 g | 0.47 b | 1.52 fg | 3.75 k | 2.84 j | 0.89 de | 0.24 | *** |
| Stoneware | 0.00 a | 0.64 bc | 0.73 cd | 0.84 de | 0.99 e | 2.38 i | 1.39 f | 2.07 h | 0.15 | ||
| Succinic acid | Glass | 6.22 f | 5.85 f | 8.48 g | 3.53 cd | 3.74 cde | 2.86 abcd | 1.64 ab | 1.39 a | 0.51 | *** |
| Stoneware | 6.22 f | 5.92 f | 5.64 f | 4.68 def | 5.42 ef | 3.68 cde | 1.92 abc | 3.30 bcd | 0.34 |
 ).
).| Vessel/Day | 0 | 1 | 2 | 3 | 4 | 7 | 10 | 14 | SEM 1 | Sig. 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FAN | Glass | 69.6 ab | 59.4 a | 66.7 a | 58.5 a | 61.4 a | 111.2 bcd | 124.6 cd | 137.0 d | 8.4 | *** |
| Stoneware | 69.6 ab | 61.3 a | 61.6 a | 79.6 ab | 85.1 abc | 136.4 d | 138.6 d | 139.4 d | 7.0 | ||
| Total sugars | Glass | 2.98 ab | 4.52 cde | 4.20 bcd | 2.77 ab | 3.17 abc | 5.77 e | 5.65 e | 5.15 de | 0.18 | *** |
| Stoneware | 2.98 ab | 2.88 ab | 3.01 ab | 2.53 a | 2.89 ab | 3.23 abc | 2.98 ab | 3.31 abc | 0.24 | ||
| Reductive sugars | Glass | 1.91 cd | 3.57 h | 3.78 h | 1.26 a | 2.63 g | 5.18 j | 5.60 k | 4.27 i | 0.10 | *** |
| Stoneware | 1.91 cd | 1.02 a | 1.60 b | 1.78 bc | 2.45 fg | 2.65 g | 2.14 de | 2.21 ef | 0.30 | ||
| Glucose | Glass | 1.36 b | 0.84 a | 1.18 ab | 0.91 a | 0.92 a | 2.19 c | 2.06 c | 2.23 c | 0.12 | *** |
| Stoneware | 1.36 b | 1.39 b | 1.41 b | 0.90 a | 0.86 a | 6.60 e | 3.34 d | 0.89 a | 0.39 | ||
| Fructose | Glass | 1.39 e | 0.37 b | 0.03 a | 0.71 d | 0.07 a | 0.71 d | 0.62 cd | 0.51 bcd | 0.10 | *** |
| Stoneware | 1.39 e | 0.31 b | 0.46 bc | 0.71 d | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.09 | ||
| Glycerol | Glass | 0.00 a | 0.00 a | 0.00 a | 0.10 a | 0.67 c | 1.78 f | 1.51 e | 1.03 d | 0.15 | *** |
| Stoneware | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.24 b | 1.48 e | 1.58 e | 1.45 e | 0.14 | ||
| Mannitol | Glass | 1.70 b | 2.04 bc | 2.59 c | 1.74 b | 2.42 c | 6.80 f | 5.24 e | 4.42 d | 0.18 | *** |
| Stoneware | 1.70 b | 2.60 c | 2.52 c | 2.45 c | 1.70 b | 0.67 a | 0.52 a | 0.73 a | 0.37 |
 ).
).Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satora, P.; Strnad, S. The Influence of Fermentation Vessels on Yeast Microbiota and Main Parameters of Sauerkraut. Appl. Sci. 2024, 14, 236. https://doi.org/10.3390/app14010236
Satora P, Strnad S. The Influence of Fermentation Vessels on Yeast Microbiota and Main Parameters of Sauerkraut. Applied Sciences. 2024; 14(1):236. https://doi.org/10.3390/app14010236
Chicago/Turabian StyleSatora, Paweł, and Szymon Strnad. 2024. "The Influence of Fermentation Vessels on Yeast Microbiota and Main Parameters of Sauerkraut" Applied Sciences 14, no. 1: 236. https://doi.org/10.3390/app14010236
APA StyleSatora, P., & Strnad, S. (2024). The Influence of Fermentation Vessels on Yeast Microbiota and Main Parameters of Sauerkraut. Applied Sciences, 14(1), 236. https://doi.org/10.3390/app14010236






