Abstract
To achieve diesel engine ultra-low nitrogen oxide emission, light-off selective catalyst reduction (LO-SCR) has been suggested for better performance with lower exhaust temperature. An electric heater upstream of the exhaust aftertreatment system was applied to significantly decrease the NOx emission at a low exhaust temperature. With a 7.2 kW electric heater coupled with LO-SCR, the NOx emission during 200~500 s of the world harmonized transient cycle (WHTC) decreased from 282.6 ppm to 61.5 ppm, which is a decrease of 45%. Application of an upstream diesel oxidation catalyst (DOC) decreased the NOx emission by 63% at the same interval at the cost of worse cold-start performance. The urea input was also adjusted to avoid NOx emission during the latter part of the WHTC.
1. Introduction
Diesel engines have been widely used on various occasions because of their excellent thermal efficiency and reliability. However, nitrogen oxide (NOx) emissions from diesel engines have an adverse effect on human beings and the environment [1], and regulations for diesel engine NOx emission have been put forward by governing bodies all over the world [2]. According to Euro VI legislation, NOx are restricted under 0.40 g/kW·h (steady-state testing) and 0.46 g/kW·h (transient-state testing), respectively [3]. Even so, the super ultra-low NOx (ULN) emission standards for medium-duty vehicles, established by the California Air Resources Board, requires that the total emissions of nonmethane organic gases and NOx are no more than 0.02 g/mile under the Heavy-Duty Federal Test Procedure (HD-FTP) [4]. This requires further alteration and improvement in NOx emission control technologies.
Current legislation limitations already strictly require combining aftertreatment technologies. As the mainstream exhaust aftertreatment (EAT) technology route, the combination of a diesel oxidation catalyst (DOC) + a catalytic diesel particulate filter (CDPF) + selective catalytic reduction (SCR) + an ammonia slip catalyst (ASC) presents excellent performance on emissions control at the cost of limited decrease in thermal efficiency [5]. Even though the current method has already achieved above 95% NOx conversion, the EAT system scheme still requires further adjustment and optimization in schemes and strategies for ULN emission regulation. Zavala et al. [4] proposed different aftertreatment configurations for ULN emission control, with light-off selective catalyst reduction (LO-SCR) applied close to the engine to take advantage of the higher exhaust temperatures.
SCR is the critical device in the EAT De-NOx progress. The Cu-zeolite catalyst is a common choice to meet current regulations. Metkar et al. [6] studied the effects of the Fe-ZSM-5 and Cu-CHA catalysts on the NOx reduction performance of NH3, gave the kinetic parameter values for Cu-CHA catalysts, and established a global kinetic model, which can better predict the conversion rate of NOx and NH3. Mohan et al. [7] reviewed the performance of NH3-SCR using Cu-based catalysts in reducing NOx at low temperatures and compared the ability of several Cu-based catalysts to reduce NOx (standard SCR reaction) in different temperature ranges. Among them, Cu/SSZ is currently the most suitable catalyst for low-temperature reduction of NOx, and the NOx removal rate can reach 100% when the temperature is between 150 and 350 °C. When the temperature is lower than 150 °C, the NOx reduction activity of Cu/SSZ drops sharply. Lei et al. [8] studied the effect of exhaust temperature on the efficiency of selective catalytic reduction (SCR) denitrification. Lei summarizes that the change in SCR denitrification efficiency generally includes three states based on exhaust gas temperature. In state I, the SCR carrier temperature is high and urea is injected, resulting in high NOx conversion efficiency (>90%); state III is a cold-start condition with the lowest NOx conversion efficiency (<50%). State II is a transitional stage involving ammonia storage. The NOx conversion efficiency is linearly related to exhaust temperature and increases with increasing temperature; the slope of the NOx conversion efficiency decreases with increasing exhaust temperature. Gholami et al. [9] reviewed different NOx emission-reduction technologies and introduced the chemical reaction mechanism of NH3-SCR (standard SCR) under aerobic and anaerobic conditions. Xie et al. [10] conducted experimental research on the NOx conversion efficiency and NH3 leakage of three aftertreatment schemes by introducing SCR size strategies. Xie used postprocessing systems with different SCR lengths of the same SCR diameter and found that the nitrogen oxide emissions of the three SCR systems were similar, but the average NH3 leakage varied greatly. As the SCR length decreased, the average NH3 leakage increased. Ciardelli et al. [11] proposed a novel catalytic mechanism for the fast SCR reaction of NH3, NO, and NO2 at low temperatures and supported a theoretical basis for fast SCR-related research. Yang et al. [12] studied the effect of fast SCR reaction on commercial SCR catalysts and found that fast SCR can apparently promote catalytic activity and decrease catalyst consumption and replacement frequency.
However, Cu-based catalysts may generate N2O and have little capability to clarify it. Yao et al. [13] suggested reducing the amount of Cu in Cu-zeolite SCR to reduce N2O generation without increasing NOx emission. Kim et al. [14] examined the Fe-zeolite catalyst and achieved significant suppression of N2O formation. Zhang and Yang analyzed the formation pathways of N2O in a combined zeolite-supported SCR with both Fe and Cu catalysts. The later research of Sharp et al. also selected this method for better N2O reduction [15].
The DOC is applied to oxidize CO, HC, and other combustible emissions of diesel engines [16]. A DOC coupled with DPF or CDPF has been widely applied for particulate matter clarification [17]. The oxidization process within DOC also affects NO, which can benefit the passive regeneration in downstream CDPF [18] and SCR. As the EAT thermal management largely focused on the DOC, the exhaust temperature and constituents of NOx, mainly NO and NOx, will also affect the NH3–NOx reactions of downstream SCR [19]. Nova et al. [20] suggested that, compared to other NO2/NOx ratios, the efficiency of the SCR reaction can be promoted to the greatest extent when the NO2/NOx ratio reaches 50%. Based on the inherent characteristics of high NO emission (more than 90%) of diesel engines, a feasible solution to improve De-NOx efficiency and decrease urea water solution consumption is increasing the SCR inlet NO/NO2 ratio to 1:1 [21]. Li et al. [22] proposed that the optimal ratio of NO2 to NOx in SCR may be 1:2, at which the nitrogen removal efficiency and the amount of N2 generated are maximized, with relatively less N2O produced. By increasing the ratio of NO2 to NOx, the NO content at the outlet gradually decreases, while the N2 content first increases and then decreases and the N2O content increases.
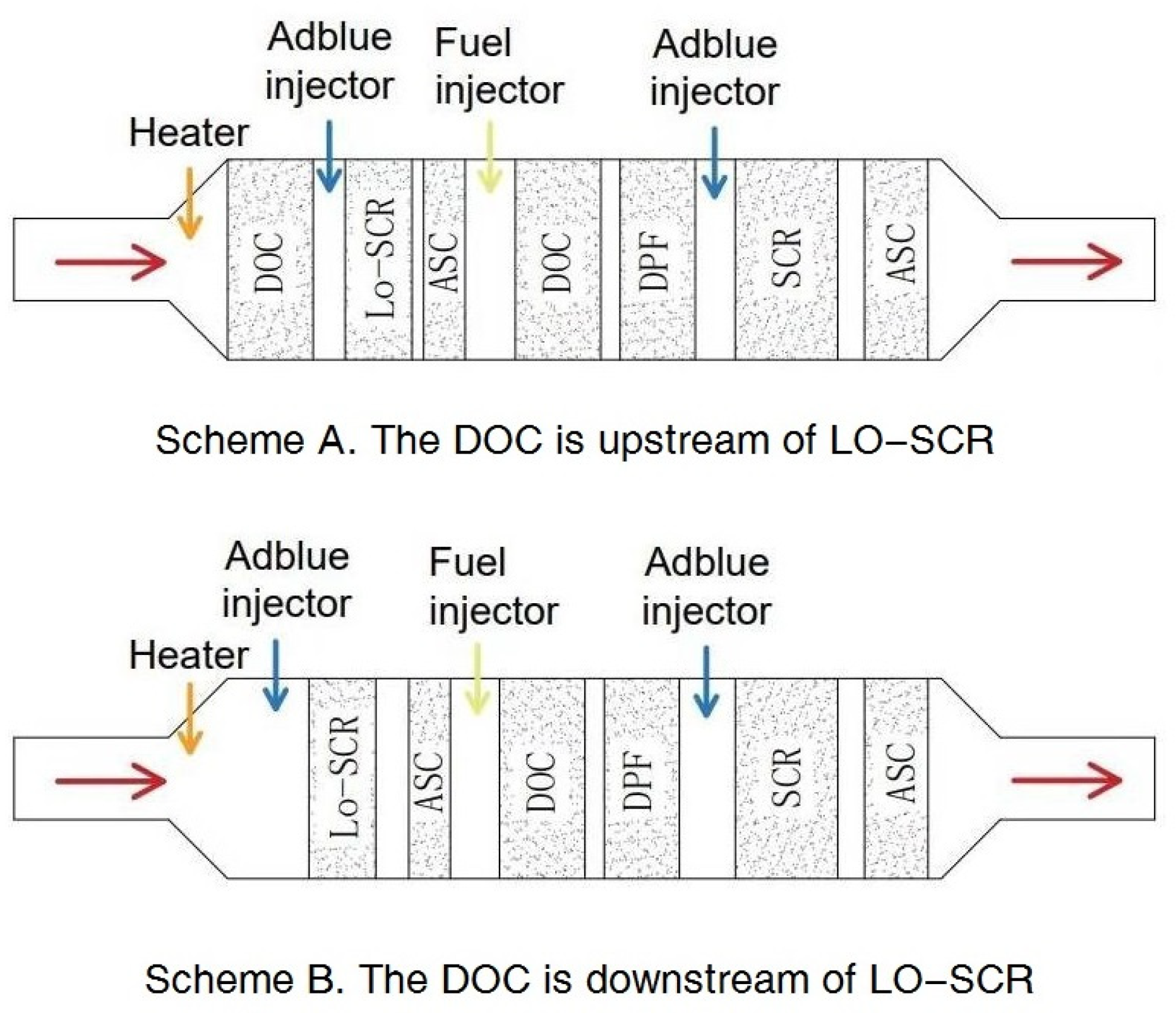
The relative location of the DOC, whether upstream (scheme A in Figure 1) or downstream (scheme B in Figure 1) of LO-SCR, also requires consideration. A DOC deployed upstream provides the possibility to partially oxidize NO into NO2 and increase the proportion of fast reactions. However, at lower exhaust temperatures, a higher NO2 concentration will increase N2O generation, [23], while, at higher exhaust temperatures, possible NO2 surplus caused by an increased generation rate in the DOC will further hinder the NH3–NOx reactions in SCR. Another problem for the upstream DOC scheme is its effect on exhaust temperature; at cold start conditions, the cold DOC upstream will delay the catalyst temperature increase of LO-SCR [4].
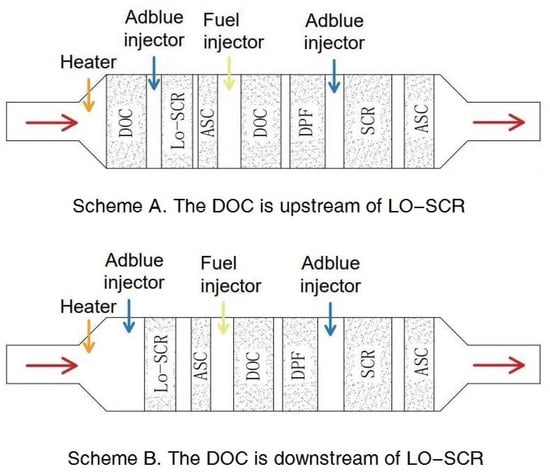
Figure 1.
Related exhaust aftertreatment system schemes.
Therefore, to achieve ULN emission control at mid to low load, the EAT thermal management strategy requires detailed analysis. This work focused on the NH3–NOx reactions in LO-SCR and related thermal management strategy optimization. Two major aspects were evaluated in this work: the effect of thermal management strategy on exhaust parameters before SCR and the effect of thermal management strategy on LO-SCR performance at ULN emission control. The former was analyzed through a diesel engine EAT bench test, focused on exhaust temperature increase with an electric heater and NO oxidization in a DOC. The latter was evaluated through simulation, mainly for the comparison of different EAT system schemes, and the final Lo-SCR performance with the optimized thermal management strategy. This paper can be a reference for ULN emission control at mid- to low-load working conditions.
2. Method
2.1. EAT System Experiments
2.1.1. Test Bench Structure
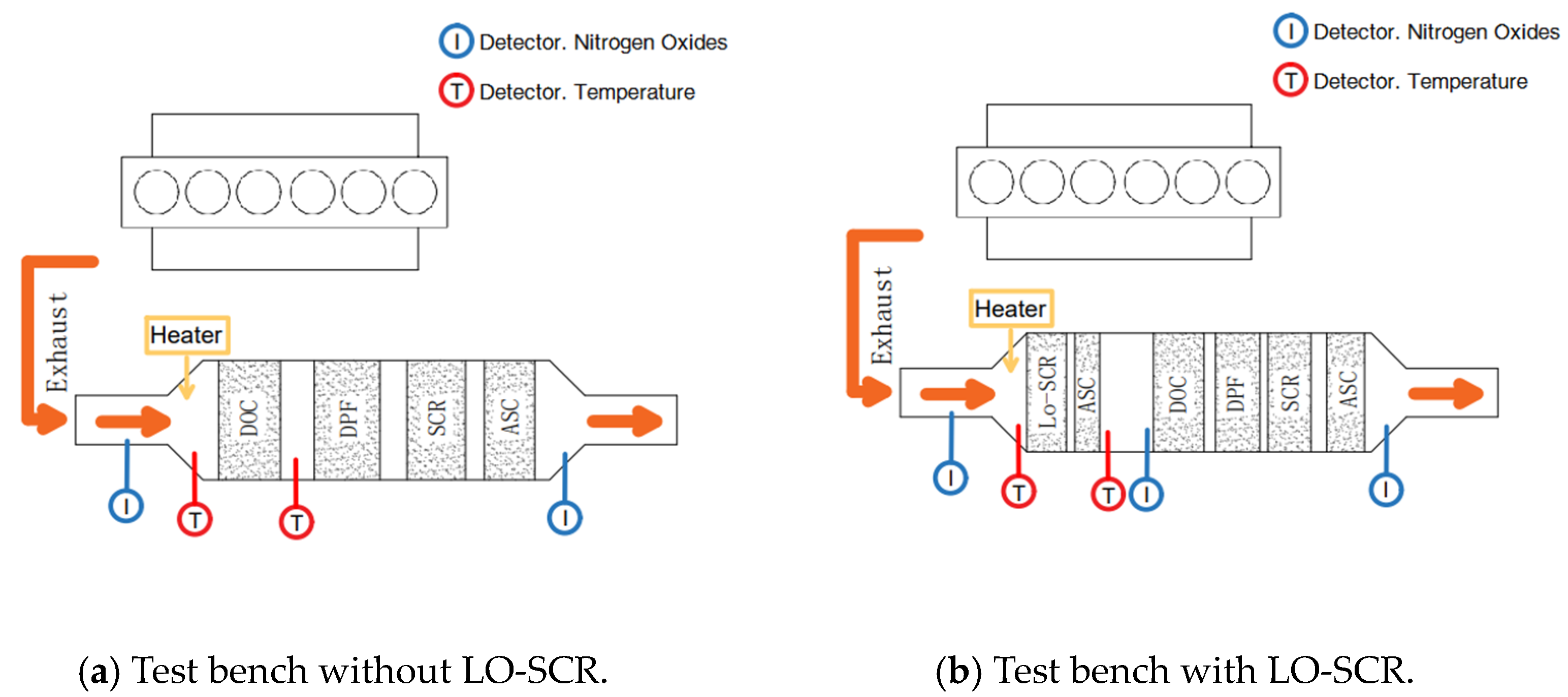
The experiments were performed on a certain six-cylinder in-line China VI engine that was equipped with a high-pressure common rail system and a VGT with an intercooler. The EAT system test bench is given in Figure 2, and Figure 3 is the scheme of the test bench. The parameters of the test engine are listed in Table 1. The EAT system, focused on DOC and LO-SCR, was arranged for this research. The details of the measurement equipment are listed in Table 2. NOx and O2 were measured by chemiluminescence detection (CLD) and paramagnetic detection (PMD), respectively.

Figure 2.
EAT system test bench.
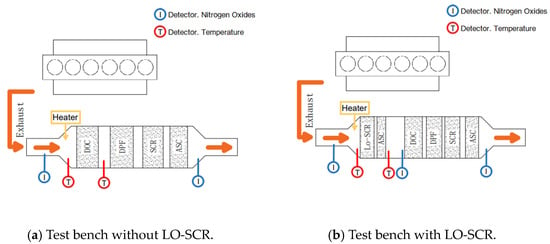
Figure 3.
Test bench structure.

Table 1.
Engine parameters.

Table 2.
Specification of measurement equipment.
2.1.2. Cycle Experiments
To investigate the NOx emission and other exhaust parameters during cold-start and mid- to low-load working conditions, a diesel engine emission experiment was carried out using the World Harmonized Transient Cycle (WHTC). The exhaust parameter before and after LO-SCR during cold-start WHTC was recorded for simulation. The NO oxidization capability of DOC was also investigated during the cycle experiment.
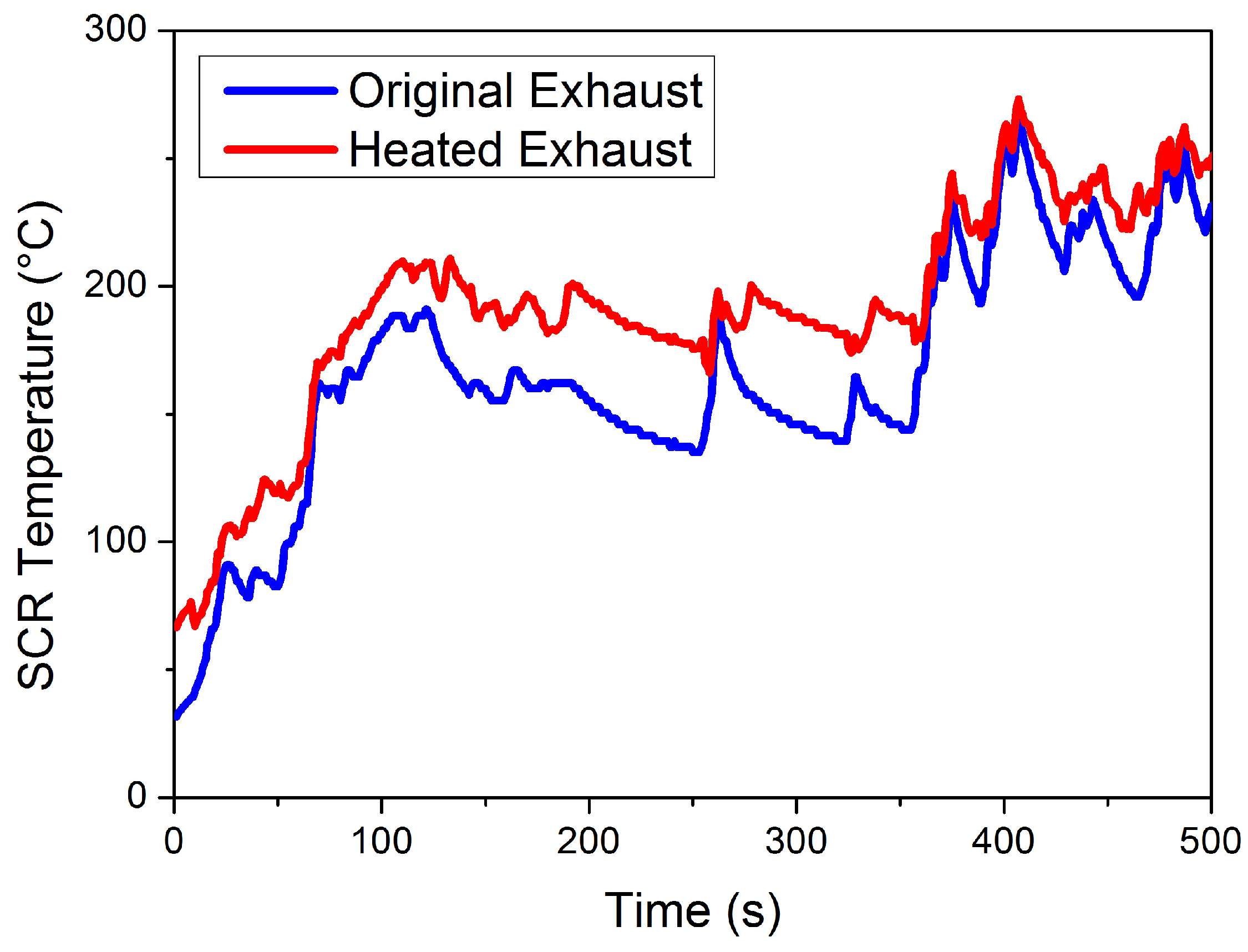
This paper was focused on reducing NOx emission at mid- to low-load working points, where exhaust temperature is relatively low. Therefore, the traditional thermal management of throttle valve opening adjustment and postinjection is insufficient in this case. A separate heater is placed before DOC, heating the exhaust before entering the EAT system, to effectively increase the catalyst temperature at a lower load. Temperature variations of the LO-SCR catalyst (at the inlet) before and after thermal management are given in Figure 4. After the application of an electrical heater, the SCR catalyst temperature increased by 25.75 °C on average during 0~500 s of WHTC.
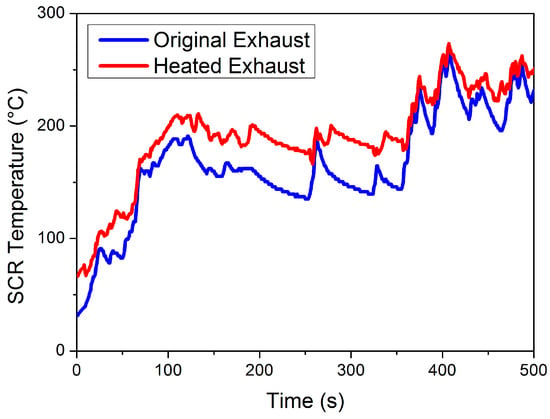
Figure 4.
Heated SCR catalyst temperature (at the inlet) during WHTC.
The altered catalyst temperature will be taken into the simulation model to fully simulate the effect of electrical heating on the EAT system with ULN emission. It is possible to place the DOC before LO-SCR to partially oxidize NO into NO2 and increase the proportion of fast reaction in SCR. For scheme A in Figure 1, the temperature input of the upstream DOC is taken from the LO-SCR inlet during the thermal management bench test, while the LO-SCR inlet parameter will be determined by the upstream DOC.
2.2. Modeling
2.2.1. Chemical Model
- De-NOx Reactions inside SCR
The NOx conversion of diesel engine exhaust mostly occurred in SCR, chemical reactions inside SCR are listed in Table 3 [5], and the three main reactions in SCR are R4 (standard reaction), R5 (fast reaction), and R6 (slow reaction). The reaction rate of a fast reaction can be 17 times higher than a standard reaction [13]; optimizing the ratio between NO and NO2 towards a larger proportion of fast reactions can thus effectively improve the NH3–NOx reaction rate. The slow reaction of R6 is neglectable in practical simulation models. Still, R6 is taken into consideration during model construction. It should be mentioned that NH3 may be oxidized by oxygen, as represented in R2. The NH3 consumption caused by the NH3–O2 reaction, or ammonia chemical loss, increases at higher exhaust temperatures, which requires further consideration after the application of electrical heating.

Table 3.
Related SCR reactions.
- 2.
- Reactions inside DOC
Reactions inside DOC are summarized as R9~R16 in Table 4, referred to by researchers of Hsieh et al. [24].

Table 4.
DOC reactions.
Reactions between NO and CO, H2, and unburned HC consume limited NO, while NO2 generated through R9 has a significantly greater effect on the SCR downstream. Increased NO2 at SCR inlet has both pros and cons on the NH3–NOx reaction rate. Limited NO2 in SCR will mostly be consumed in a fast reaction; the high reaction rate can improve the conversion rate, especially at lower temperatures with low reaction rates. However, if NO is completely clarified before NO2, the remaining NO2 will be difficult to clarify with only a slow reaction. In addition, R7 and R8 together will generate N2O, which is extremely difficult to clarify with Cu-zeolite SCR [20], and excess NO2 may increase the possibility of R7 happening, especially at lower temperatures [11]. The NO oxidation in DOC shall be taken into consideration.
2.2.2. EAT System Model
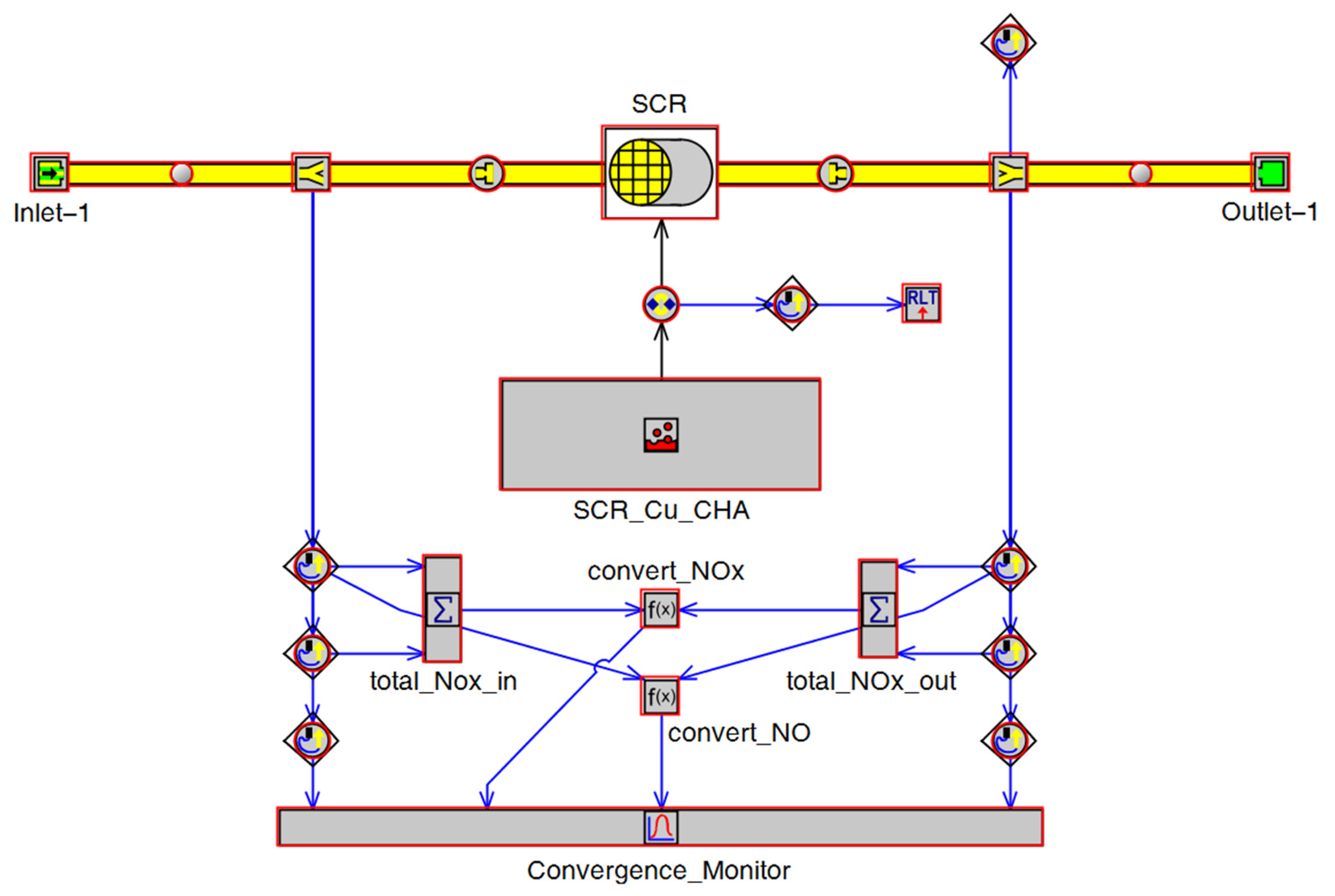
Modeling of the EAT system is focused on LO-SCR. The LO-SCR model was constructed in GT-Power(GT-SUITE version 2021), as shown in Figure 5. Related SCR parameters are given in Table 5.
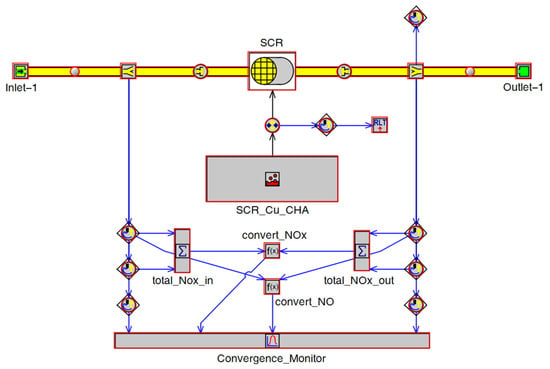
Figure 5.
SCR simulation model.

Table 5.
EAT system parameters.
3. Result and Analysis
3.1. SCR Reaction at Steady Working Point
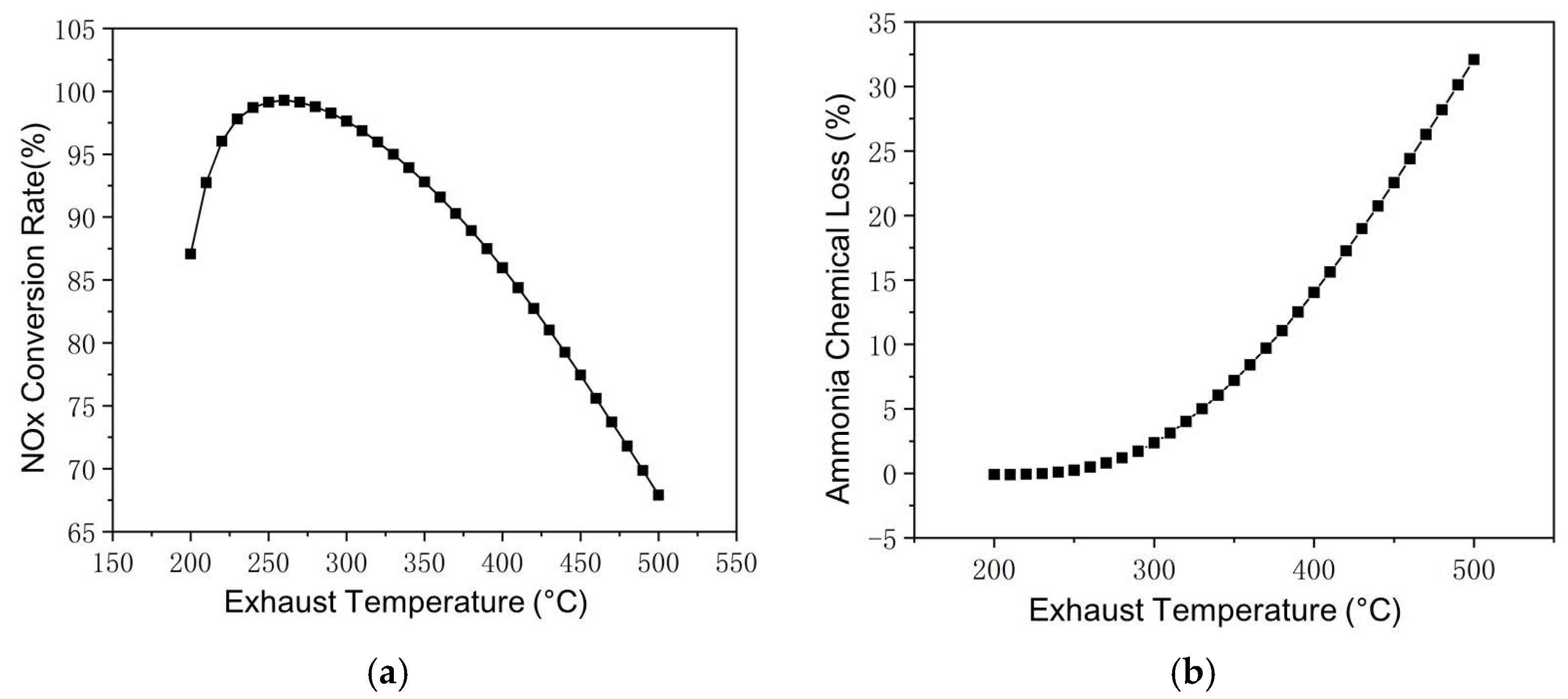
At a certain working point taken from the world harmonized steady-state cycle (WHSC), the reaction within SCR at exhaust temperatures between 200 °C and 500 °C is shown in Figure 6a, where exhaust mass flow and inlet NOx remain constant. At lower exhaust temperatures, the NH3–NOx reaction rate is hindered by the low temperature, as exhaust temperature increases, the NOx conversion rate gradually increases from 87.07% at 200 °C to 97.81% at 260 °C. After peaking at 260 °C, the NOx conversion rate decreases with a further increase in the exhaust temperature. With the ammonia–nitrogen ratio fixed at 1:1, a higher proportion of ammonia reacting with oxygen will hinder the reaction of ammonia with nitrogen oxides; the ratio of unclarified nitrogen oxides is almost equal to ammonia consumed by the NH3–O2 reaction. It should be pointed out that this is not the case in practical Cu-zeolite SCR reactions, as the ammonia storage generally provides sufficient ammonia.
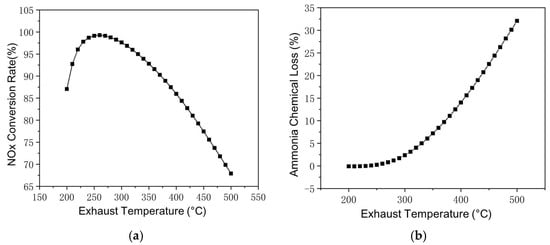
Figure 6.
Reactions in SCR at different exhaust temperatures. (a) Changes of NOx Conversion Rate with Exhaust Temperature. (b) Changes of Ammonia Chemical Loss with Exhaust Temperature.
Within the exhaust temperature range selected in Figure 6, the reaction between ammonia and oxygen also increases with increasing temperature, as shown in Figure 6b. At an exhaust temperature below 250 °C, the ammonia–oxygen reaction consumes less than 0.1% NH3 input and the NH3–O2 reaction is neglectable. As the exhaust temperature increases to over 300 °C, the NH3–O2 reaction rate begins to increase significantly, from 2.37% at 300 °C to 7.2% at 350 °C, 14.03% at 400 °C, and 32.09% at 500 °C. For road vehicles, the diesel engine is less likely to operate constantly at a high exhaust temperature range. Even so, with SCR catalyst temperature above 250 °C, the loss still requires consideration.
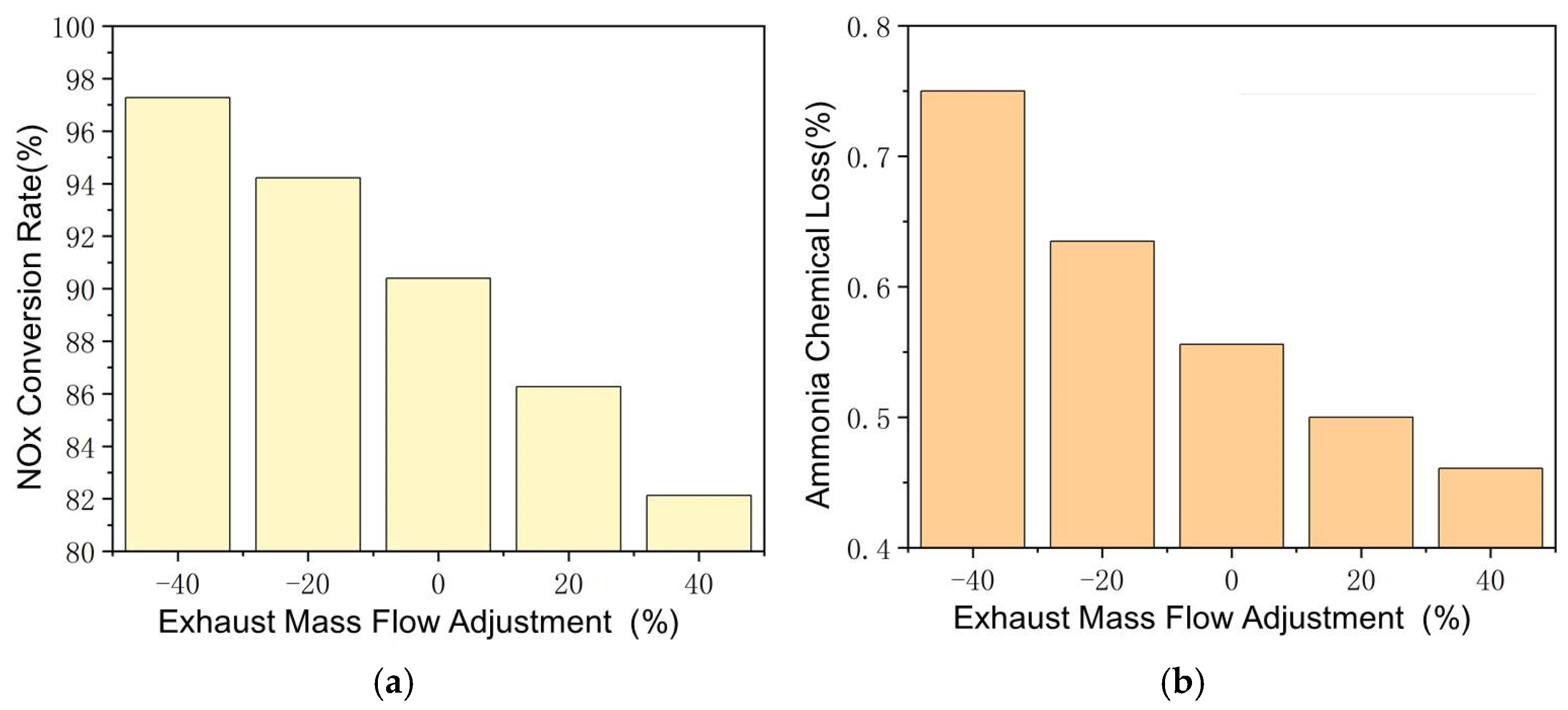
The effect of exhaust mass flow is shown in Figure 7, with other parameters remaining constant. It should be noted that the NOx inlet in PPM is fixed, thus increasing exhaust mass flow, which increases the NOx quantity in SCR. This is the main factor that affects the NOx conversion rate at different mass flow rates. Increasing the mass flow by 40% reduces the NOx conversion rate to 82.1%, while decreasing it by 40% improves the conversion rate to 97.6%. The effect of exhaust flow change on the ammonia–oxygen reaction is also significant and the NH3–O2 reaction rate decreases with the increase in exhaust mass flow and the ammonia chemical loss decreases from 0.75% to 0.46% between −40% and +40%. The effect of mass flow itself is less significant than exhaust temperature and SCR carrier length.
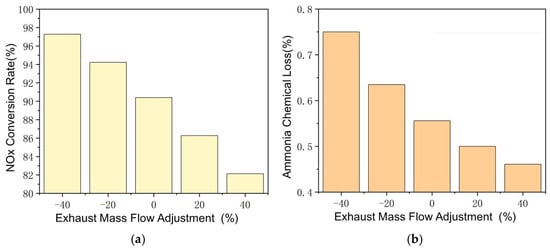
Figure 7.
Reactions in SCR at different exhaust mass flows. (a) Changes of NOx Conversion Rate with Exhaust Mass Flow Adjustment. (b) Changes of Ammonia Chemical Loss with Exhaust Mass Flow Adjustment.
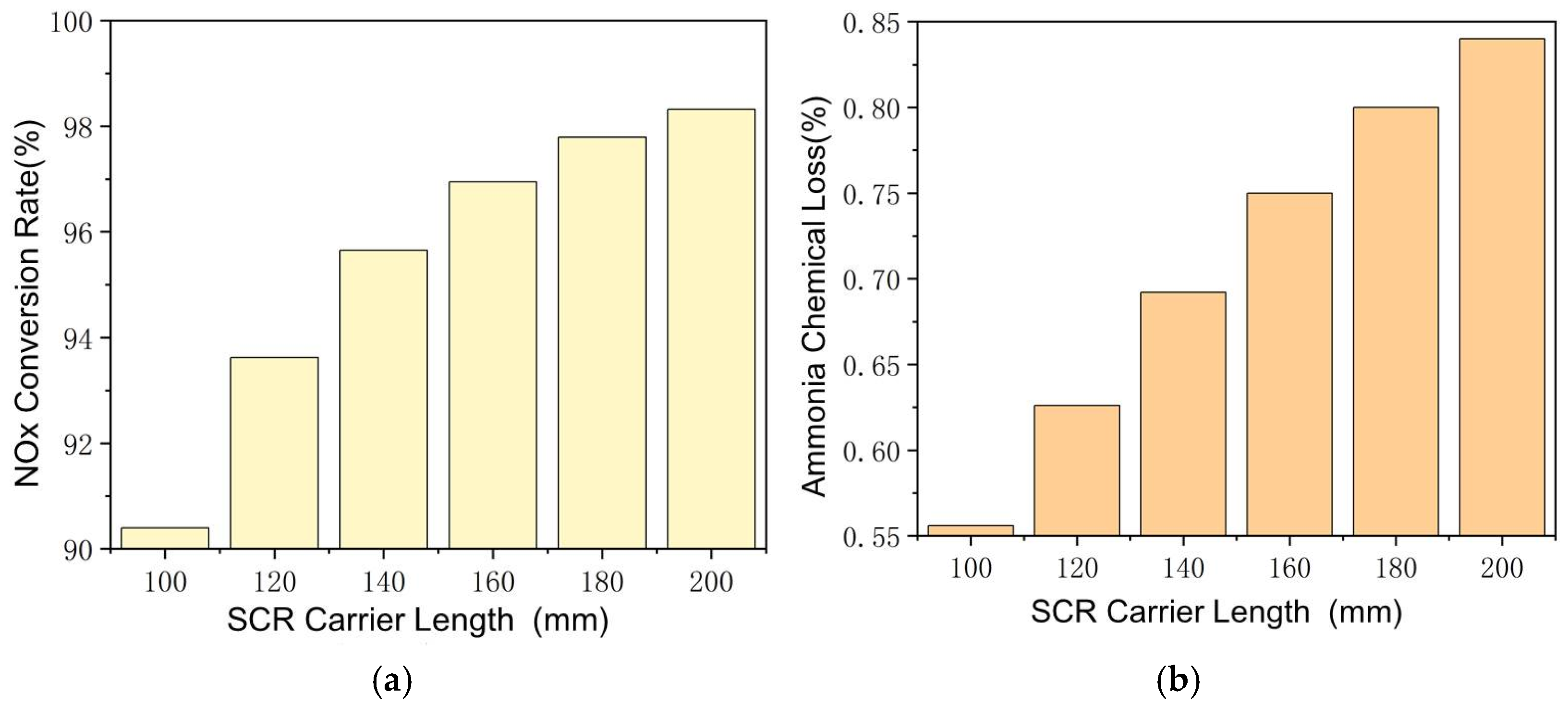
The effect of SCR carrier length is shown in Figure 8, with other parameters also remaining constant. Longer carrier length means a larger area and longer time for catalyzed reactions, which improves the NH3–NOx reaction rate and NOx conversion rate, even at lower exhaust temperatures. The conversion rate of NOx increases from 92.1% to 98.2% as the SCR carrier length increases from 100 mm to 200 mm. The NH3–O2 reaction rate is also significantly affected by the change in carrier length. At lower exhaust temperatures, as the carrier length increases from 100 mm to 200 mm, the ammonia chemical loss increases from 0.55% to 0.83%. Since the ammonia–nitrogen ratio is fixed at 1:1, the increase in the NH3–O2 reaction rate hinders the NH3–NOx reaction at longer carrier length. It is necessary to further improve the NH3–NOx reaction rate, and the ammonia–nitrogen ratio in steady-state working points should be greater than 1. It should be noted that a longer SCR carrier can store more ammonia, which may cause larger ammonia leakage at transient working points.
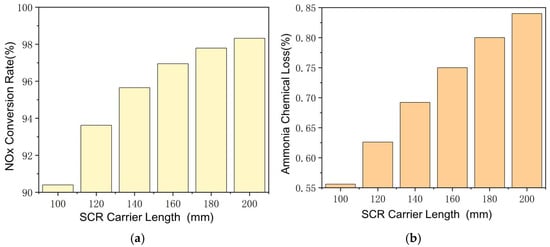
Figure 8.
Reactions in SCR at different carrier lengths. (a) Changes of NOx Conversion Rate with SCR Carrier Length. (b) Changes of Ammonia Chemical Loss with SCR Carrier Length.
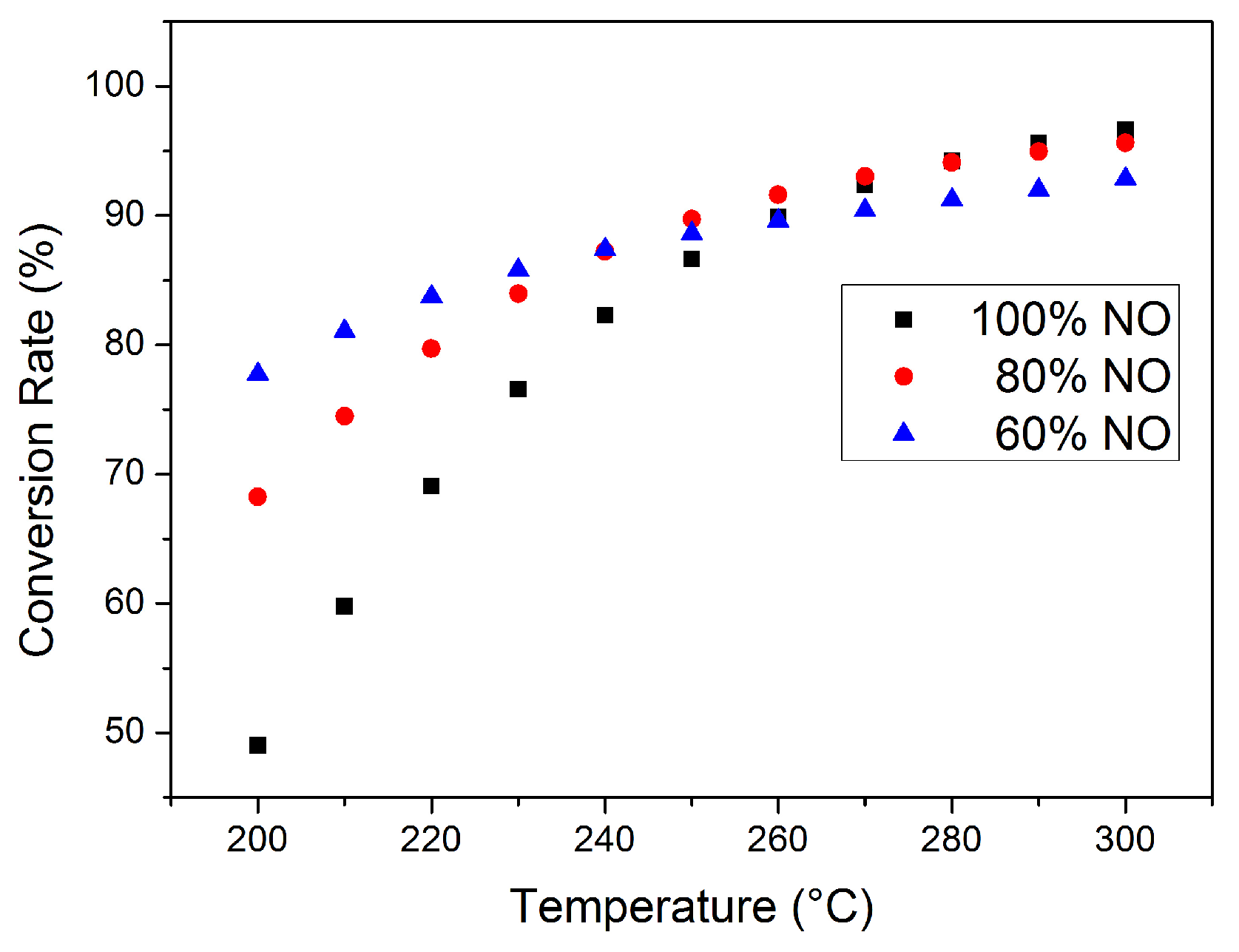
The effect of DOC on the NOx conversion rate after thermal management should also be emphasized. Nitrogen oxides generated during combustion are mostly NO, and the DOC at SCR upstream can partially oxidize NO into NO2. The NOx conversion rate at different SCR inlet NOx compositions (NO and NO2 only; other possible nitrogen oxides were neglected) is given in Figure 9, with the exhaust parameter and ammonia input remaining constant. The NOx concentration at the SCR inlet is higher than in Figure 6, Figure 7 and Figure 8 for better comparison. The effect of exhaust temperature at this working point is still generally positive for NOx conversion, while the effect of NO oxidization depends on actual SCR temperature. At lower temperatures with lower catalyst activity, a higher NO2 proportion can increase the proportion of fast reactions. With exhaust temperature at 200 °C, the NOx conversion rate of 40% NO2 (60% NO) is 77.57%, significantly higher than the 49.01% of 100% NO; at 250 °C, the difference caused by NO2 is limited. However, the consumption rate of NO is higher than NO2; thus, NO may be completely clarified before complete NO2 conversion, especially at higher temperatures, and NH3–NO2 reaction rate is significantly low and N2O is likely to be generated during this process. With 40% NO2 at the inlet and exhaust temperature at 300 °C, the NOx emission at the SCR outlet increased to 7.15%, while, with 100% NO, the emission is only 3.38%. It should be noted that the NOx at the SCR inlet in Figure 6, Figure 7 and Figure 8 is completely NO. The effect of DOC after thermal management requires further consideration.
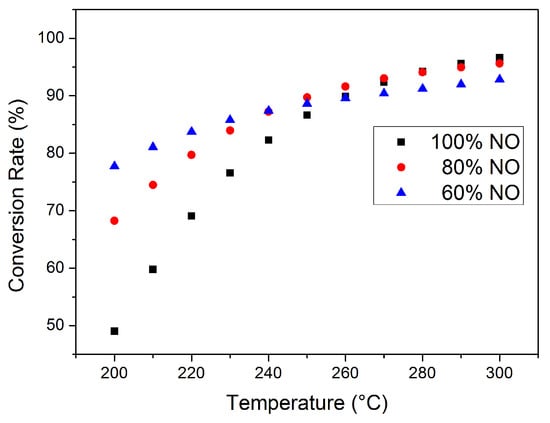
Figure 9.
Total NOx conversion rate at different inlet conditions.
In summary, the low exhaust temperature at lower load working points will inevitably decrease the SCR catalyst activity. To improve the NH3–NOx reaction rate, a higher exhaust temperature is required and a proper NO/NO2 ratio may also improve the conversion [19]. Methods that improve the reaction between ammonia and nitrogen oxides will also improve the ammonia–oxygen reaction. Thermal management is required to further decrease NOx emission, and the increase in ammonia chemical loss should also be taken into consideration [4].
3.2. SCR Reaction at Transient Cycle
The steady-state condition analysis in the previous section investigated the effect of various factors. Under transient working conditions, the exhaust temperature and mass flow rate change frequently and the temperature of the SCR carrier will also be affected, thereby affecting the NH3–NOx reaction. Therefore, before analyzing thermal management methods to further decrease NOx emission, it is necessary to analyze the SCR NOx conversion capacity at a transient cycle.
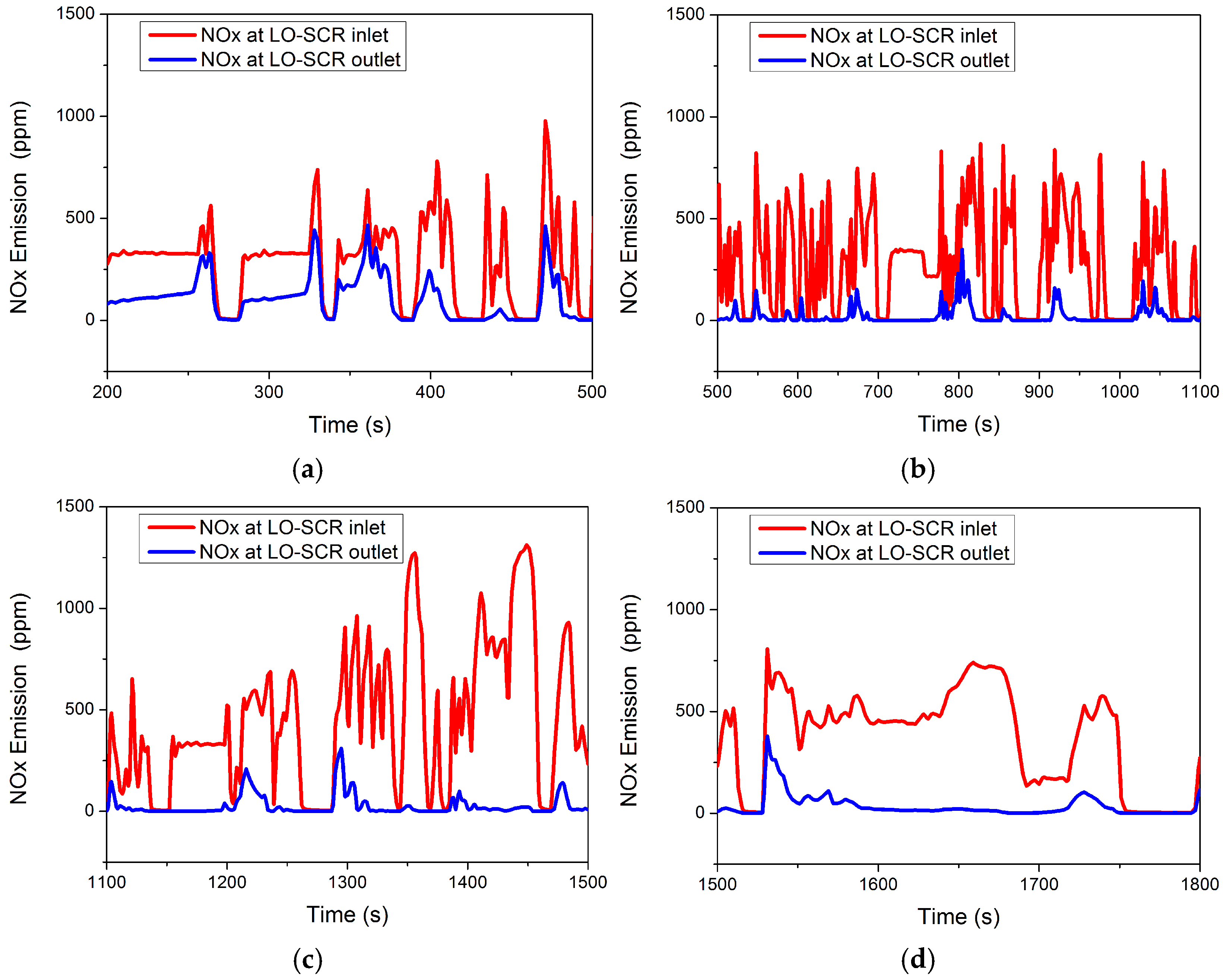
The WHTC cycle test is relatively close to the actual driving cycle and the engine operates under frequent varying conditions. The NOx emission of a conventional EAT system under the WHTC cycle without thermal management intervention is shown in Figure 10. High NOx emission levels mainly occur in the first third of the entire cycle, while the emission at the latter part of the cycle remains low. The results of NOx emission before 200 s are not taken into consideration in Figure 10, as thermal management is inevitably required to reduce NOx emission under cold starting conditions. In Figure 10a, compared to the 500~1800 s range (Figure 10b–d), the NOx emission is higher in the 200~500 s range (indicated by the blue line in Figure 10a). The peak NOx emission occurs at 361 s, with a value of 465.4 ppm. The average conversion rate of NOx at 200~500 s is only 62.58%, which may be due to the SCR carrier not having been raised to the appropriate temperature. Even with the NOx emissions of the starting condition, the NOx emissions of 200~500 s account for 39.21% of the total emissions.
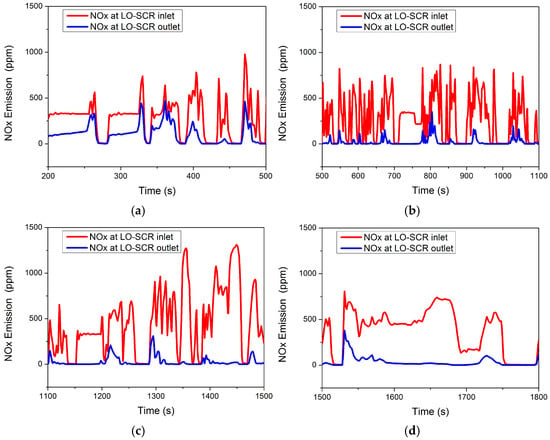
Figure 10.
NOx at LO-SCR inlet and outlet during WHTC cycle at (a) 200~500 s, (b) 500~1200 s, (c) 1200~1500 s, and (d) 1500~1800 s.
In Figure 10b, the NOx content in the engine exhaust gas does not change significantly compared to before (indicated by the red broken line in Figure 10b). The average NOx content is 282.6 ppm in the interval of 500~1100 s, while it is 280.2 ppm in the interval of 200~500 s. However, due to the increase in SCR carrier temperature, the NOx emission decreases significantly. In the interval of 500~1100 s, the peak NOx emission decreases to 349.2 ppm, occurring at 801 s, and the average conversion efficiency of NOx increases to 92.48%.
In the interval of 1100~1500 s, as shown in Figure 10c, the NOx content in the engine exhaust increases compared to the previous period, and the average NOx content rises to 457.8 ppm. At the same time, due to the high temperature of the SCR carrier, the NOx emission does not change significantly compared to the interval of 500~1100 s, which further increases the average NOx conversion rate to 93.96%.
In the interval of 1500~1800 s, as shown in Figure 10d, the average content of NOx in the engine exhaust gas decreases to 361.6 ppm; except for a few operating points where the NOx emission changes significantly, the overall trend is smooth and the average conversion rate of NOx is 90.01%. The average NOx emission level in the first 500 s is 260 ppm, while the average NOx emission from 500–1800 s is 10.5 ppm.
Under the WHTC cycle, there are more low-temperature conditions and more nitrogen oxide emissions. For the SCR system, the high-efficiency window of the catalyst is mainly 250~400 °C, so the catalytic conversion efficiency of the catalyst is affected under the condition of low exhaust temperature, resulting in the NOx not being efficiently clarified. The high NOx emissions in the first 500 s are mainly due to the low SCR carrier temperature, which only improved after higher exhaust temperature at the latter part of the cycle increased catalyst temperature to higher NOx conversion capability. Therefore, the key to further improving the purification capacity under SCR variable working conditions and achieving ultra-low nitrogen emissions lies in mid- to low-load working conditions, and thermal management methods should also focus on this period, as suggested by the experimental investigation of Zavala et al. [4] and Sharp et al. [13].
3.3. Effect of DOC on SCR
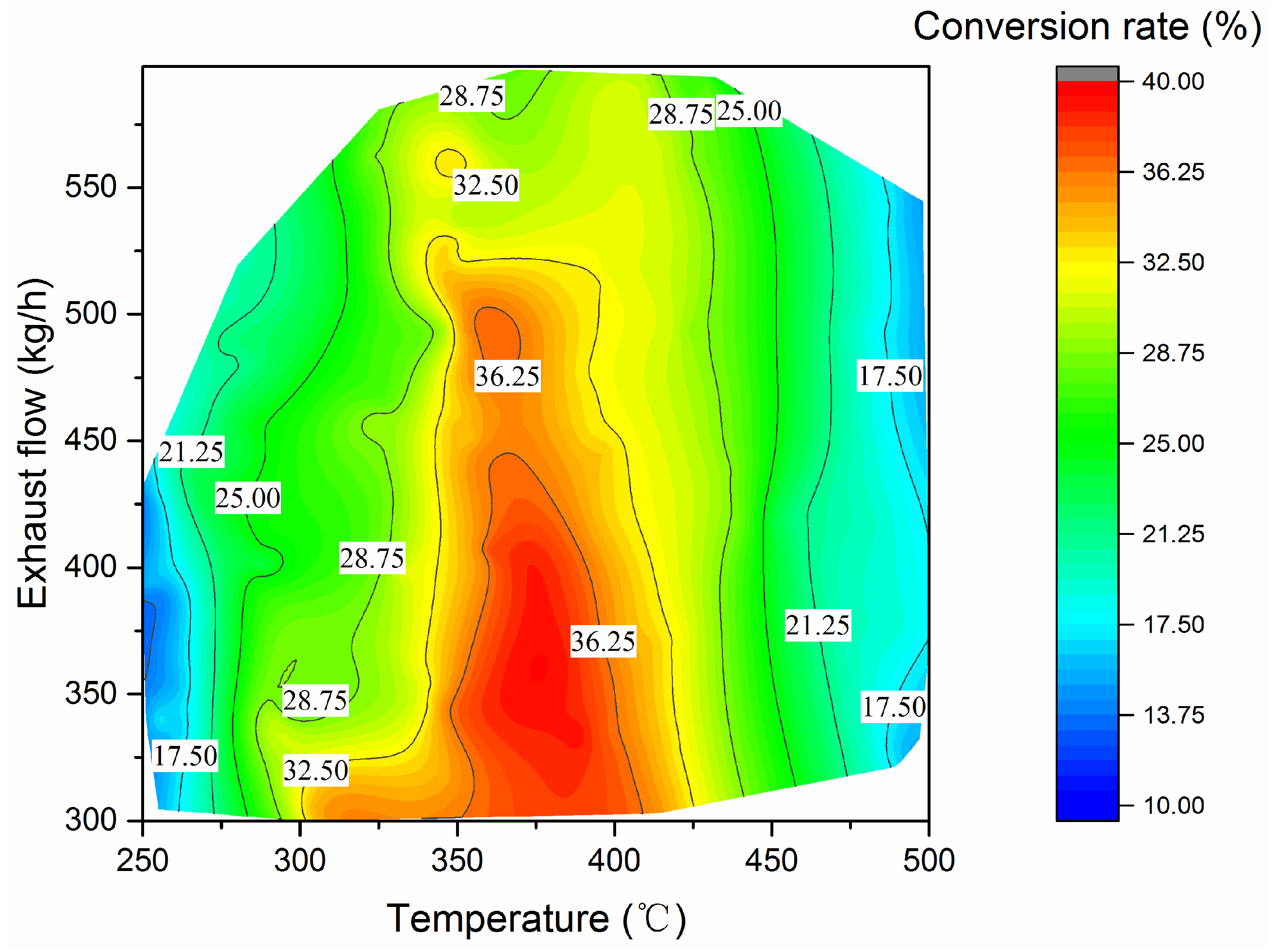
The NO–NO2 conversion rate within DOC at different exhaust temperatures and mass flows is given in Figure 11. When the exhaust temperature exceeds 425 °C, the conversion rate does not change significantly with the exhaust flow, while gradually decreasing with the increase in the exhaust temperature. Under medium load working points (in the range of 325 °C~425 °C), the exhaust mass flow has a significant effect on the NOx conversion rate in DOC, and the variety of conversion rates is limited at higher exhaust mass flow. The effect of exhaust temperature on NO–NO2 conversion rate is simple and significant. The high conversion rate mainly occurred between 350 °C and 400 °C, with medium to low exhaust flow rate from 300 kg/h to 525 kg/h. The highest conversion rate among all selected operating conditions is about 39.5%; the corresponding exhaust temperature and exhaust mass flow rate are 386 °C and 336.6 kg/h, respectively. It should be noted that the temperature is also within the optimistic temperature range of the SCR [17].
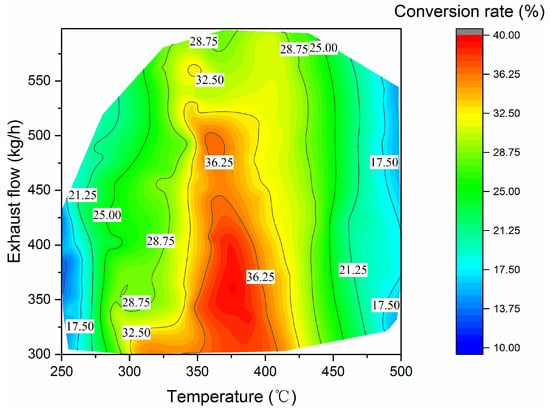
Figure 11.
DOC NO2 conversion rate.
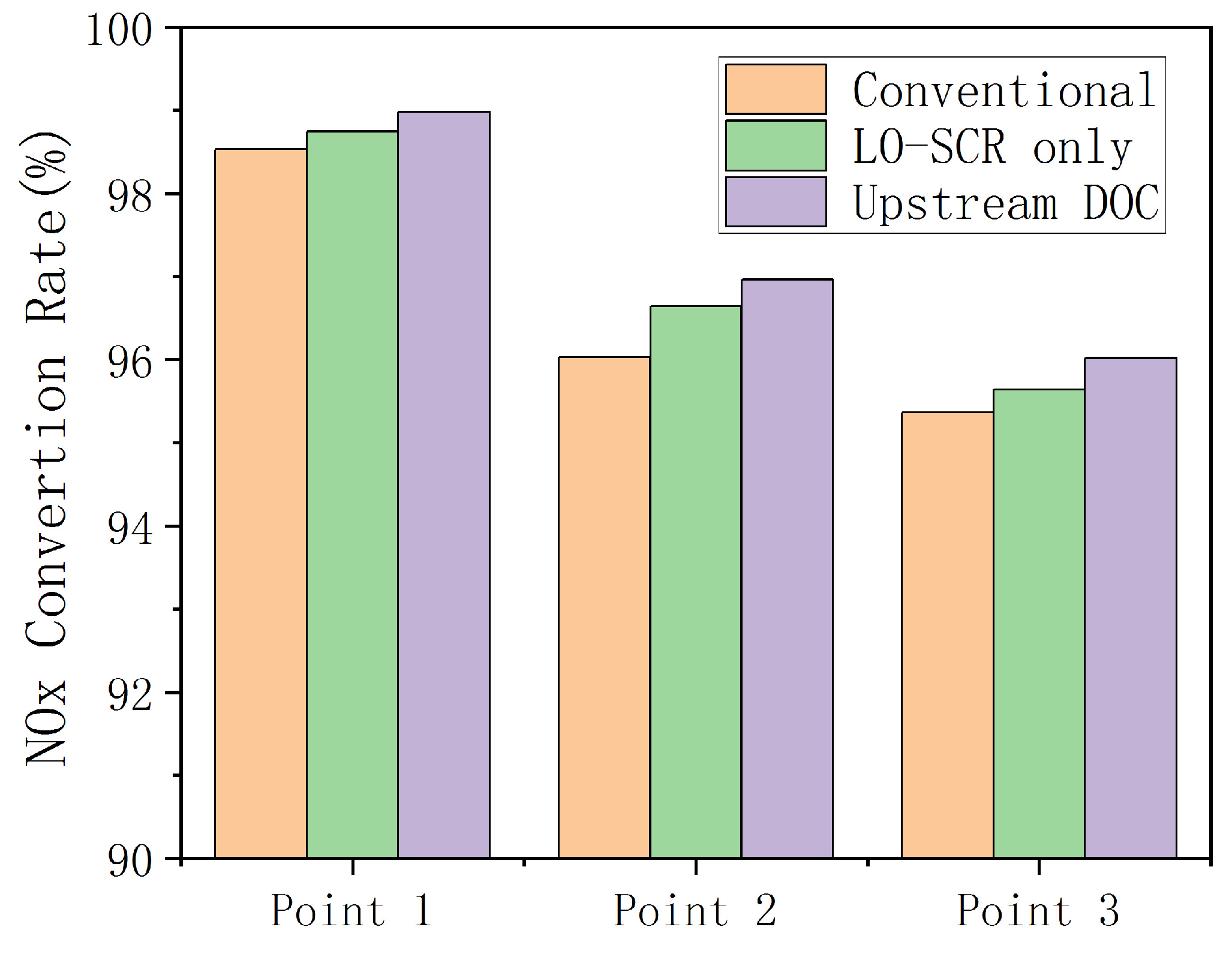
Figure 12 shows the NOx conversion rates at three different EAT system schemes of the working points under low- to medium-load conditions of the engine. Exhaust temperature is increased through exhaust electric heating, with or without upstream DOC, which is reflected in the figure as the NOx conversion efficiency at all three operating points being at a high level. At steady state and higher carrier temperature, the NO2 generated in DOC still increases the proportion of fast reaction in SCR and improves the conversion rate of NOx, which makes the NOx conversion efficiency of the scheme involving upstream DOC slightly higher than the other two schemes at the three operating points. Under three working conditions, the NOx emissions of the EAT system with upstream DOC decreases by 18.69%, 9.61%, and 14.15% compared to the conventional arrangement, although the NOx conversion efficiency increases to a limited extent compared to the other two EAT systems without upstream DOC. It should be also noted that, at cold start conditions, an upstream DOC will consume the heat of the exhaust and delay the catalyst temperature increase of LO-SCR and the effect on the cycle NOx emission may be negative.
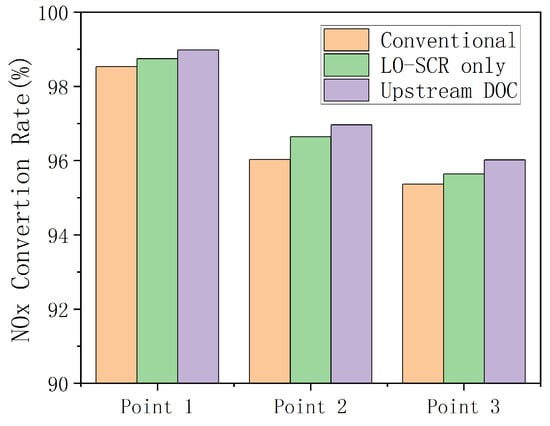
Figure 12.
NOx Conversion rate with different EAT system schemes.
4. Discussion
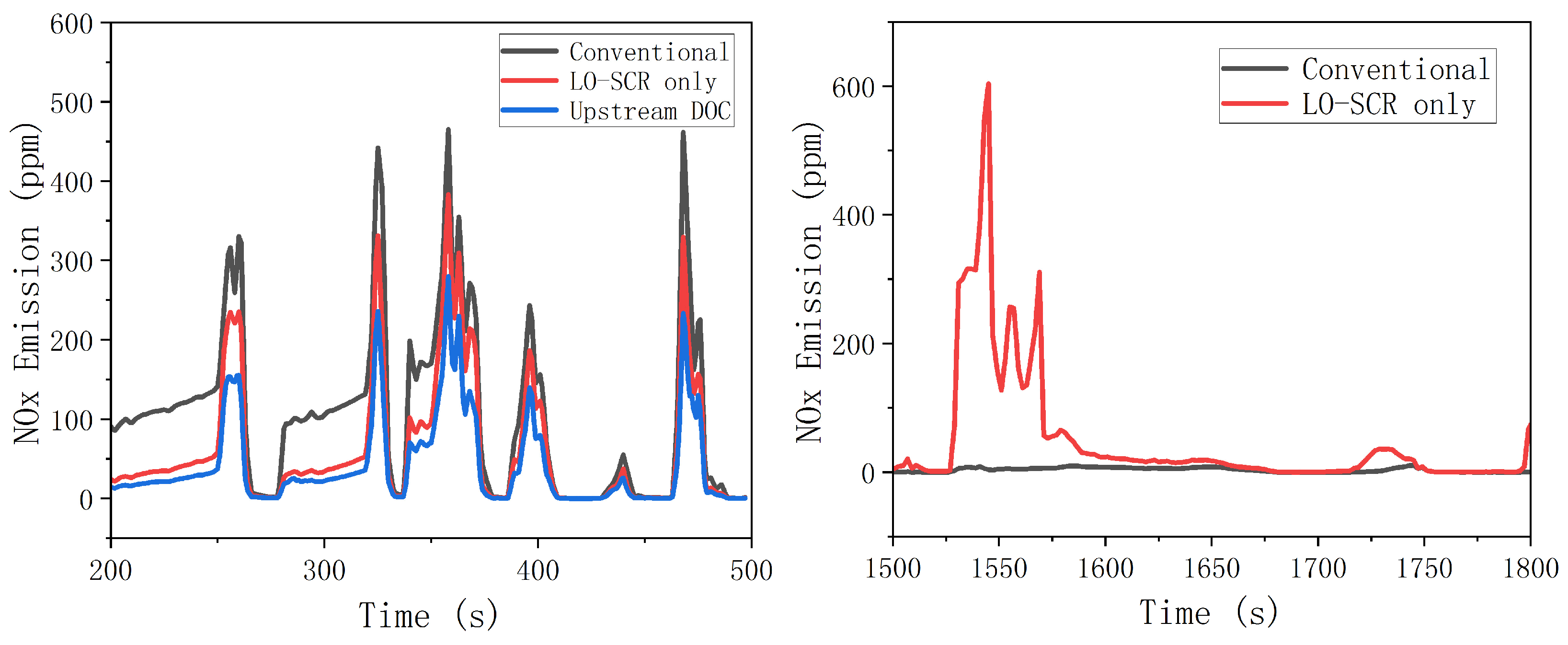
To effectively reduce NOx emission at mid- to low-load working points, an electric heater was applied at SCR upstream. During the entire WHTC bench test, the exhaust was heated before entering the EAT system to improve the activity of the SCR catalyst at a lower exhaust temperature. The simulation result is shown in Figure 13. Increased temperature has improved the activity of cold-start SCR catalyst, and the effect of thermal management on NOx emission is significant. During 200~500 s of WHTC test, after electrical heating, the average NOx emission of the LO-SCR system is 61.5 ppm, a reduction of 45% compared to the value without electrical heating before. The effect of upstream DOC is also moderately positive, as the NOx emission decreased by 63%, reaching 41.9 ppm. However, the effect of DOC will inevitably hinder the temperature increase of LO-SCR during the early stage of cold start. With higher exhaust temperature, the DOC before LO-SCR may generate NO2 in a larger proportion, which will increase the possibility of N2O generation. The moderate positive effect is insufficient to compromise these two concerns.
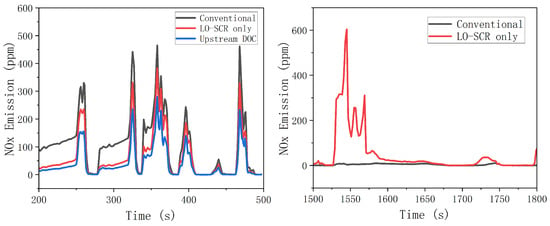
Figure 13.
Effect of thermal management on NOx conversion.
From 1500 to 1800 s, there was a significant difference in nitrogen oxide emissions between the EAT system with LO-SCR and the conventional arrangement system of 1500~1600 s. The increase in average exhaust temperature caused by thermal management increases the reaction rate between ammonia and oxygen, resulting in the increase in the ammonia chemical loss. If the urea input is not adjusted after thermal management, the ammonia storage is insufficient at 1500 s, which, in turn, leads to significant NOx emission. The problem can be largely avoided by proper urea input adjustment.
5. Conclusions
To further decrease the nitrogen oxide emission of diesel engines, electrical heating is applied for better EAT system performance at mid- to low working points. The effect of exhaust parameter and DOC on NOx conversion is investigated to further decrease the NOx emission during the cold-start WHTC cycle.
- At lower exhaust temperatures, the NH3–NOx reactions were hindered by the low temperature, which is consistent with the article mentioned in [8]. As exhaust temperature increases, the NOx conversion rate gradually increases from 87.07% at 200 °C to 97.81% at 260 °C. Longer SCR carriers can also increase the NH3–NOx reaction rate at steady working points at the cost of higher capital cost and ammonia chemical loss, as mentioned in [10].
- A separate LO-SCR coupled with an upstream electrical heater can effectively increase the NOx conversion rate at lower exhaust temperatures. With a 7.2 kW electrical heater, the NOx emission at the LO-SCR outlet decreased by 45% during the 200~500 s of the WHTC cycle.
- The effect of DOC on the NH3–NOx reactions is twofold. At lower working points, partially oxidized NO into NO2 increases the proportion of fast reaction in LO-SCR, while, at higher load working points, the high NO2 proportion may increase N2O generation and the proportion of slow reaction, similar to those mentioned in [21,22]. The upstream DOC decreased the LO-SCR outlet NOx emission by 63% during the 200~500 s of the WHTC cycle at the cost of worse cold-start performance and higher N2O emission.
- The application of thermal management increased the average exhaust temperature, which increased the NH3–O2 reaction rate in SCR. The urea input shall be adjusted properly to avoid NOx emission during the latter part of WHTC, as mentioned in [10].
Author Contributions
Conceptualization, K.S. and S.B.; methodology, K.S. and D.L.; software, Z.W.; validation, Z.W. and D.L.; formal analysis, D.L. and G.Z.; investigation, K.S., D.L. and G.Z.; resources, S.B. and C.L.; data curation, K.S. and G.Z.; writing—original draft preparation, K.S., Z.W. and D.L.; writing—review and editing, D.L., G.Z. and H.C.; visualization, G.Z. and Z.W.; supervision, G.L., S.B. and C.L.; project administration, K.S.; funding acquisition, G.L. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Construction Machinery Intelligent Equipment Innovation and Entrepreneurship Community of Shandong, China (grant numbers GTT2021105), Department of Science & Technology of Shandong Province, China (grant numbers 2021TSGC1334), Asset & Laboratory Management Department of Shandong University, China (grant numbers sy20232305), and the Undergraduate School of Shandong University, China (grant numbers 2022Y155).
Institutional Review Board Statement
Institutional review is not applicable as the paper did not involve humans or animals.
Informed Consent Statement
Informed consent is not applicable as the paper did not involve humans.
Data Availability Statement
All data and models used in the study appear in the submitted article.
Acknowledgments
We appreciate the editors and reviewers for their constructive and detailed critiques that contributed to the quality of this paper, and all individuals have confirmed this acknowledgment.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| A | Pre-exponential factor |
| b | Positive constants |
| E (kJ/mol) | Activation energy |
| LO-SCR | Light-Off Selective Catalyst Reduction |
| NOx | Nitrogen Oxides |
| HC | Hydrocarbon |
| WHTC | World Harmonized Transient Cycle |
| WHSC | World Harmonized Stationary Cycle |
| DOC | Diesel Oxidation Catalyst |
| EAT | Exhaust Aftertreatment |
| CDPF | Catalytic Diesel Particulate Filter |
| SCR | Selective Catalytic Reduction |
| ASC | Ammonia Slip Catalyst |
| VGT | Variable Geometry Turbocharger |
| ULN | Ultra-Low Nitrogen Oxides |
| CLD | Chemiluminescence Detector |
| PMD | Paramagnetic Detector |
References
- Lelieveld, J.; Evan, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Int. J. Engines 2018, 11, 1307–1330. [Google Scholar] [CrossRef]
- Hooftman, N.; Messagie, M.; Van Mierlo, J.; Coosemans, T. A review of the European passenger car regulations—Real driving emissions vs local air quality. Renew. Sustain. Energy Rev. 2018, 86, 1–21. [Google Scholar] [CrossRef]
- Zavala, B.; Sharp, C.; Neely, G.; Rao, S. CARB Low NOx Stage 3 Program—Aftertreatment Evaluation and Down Selection; No. 2020-01-1402; SAE Technical Paper: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Pan, Y.J.; Shen, B.X.; Liu, L.J.; Yao, Y.; Gao, H.P.; Liang, C.; Xu, H.J. Develop high efficient of NH3-SCR catalysts with wide temperature range by ball-milled method. Fuel 2020, 282, 118834. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, C.; Guo, D.; Yang, J.; Qiu, T.; Peng, G. Real-Time Evaluation Method of Heavy-Duty Diesel Vehicle SCR System Based on Ammonia Storage Characteristics in Real-Road Driving Emission Test. Appl. Sci. 2022, 12, 11197. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jiang, G.; Qian, F. Experimental Research on Aftertreatment SCR Sizing Strategy for a Nonroad Mid–Range Diesel Engine. Energies 2020, 13, 4462. [Google Scholar] [CrossRef]
- Ciardelli, C.; Nova, I.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. A “Nitrate Route” for the low temperature “Fast SCR” reaction over a V2O5–WO3/TiO2 commercial catalyst. Chem. Commun. 2004, 23, 2718–2719. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Ma, Q.; Niu, G.P.; Xu, X.T. Effect of fast SCR reaction on denitration characteristics of commercial V2O5-WO3/TiO2 catalysts. Chin. J. Environ. Eng. 2018, 12, 1968–1976. [Google Scholar]
- Yao, D.W.; Liu, B.A.; Wu, F.; Li, Y.X.; Hu, X.H.; Jin, W.Y.; Wang, X.L. N2O Formation Mechanism During Low-Temperature NH3-SCR over Cu-SSZ-13 Catalysts with Different Cu Loadings. Ind. Eng. Chem. Res. 2021, 60, 10083–10093. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.W. Selective reduction of NO by NH3 over Fe-zeolite-promoted V2O5-WO3/TiO2-based catalysts: Great suppression of N2O formation and origin of NO removal activity loss. Catal. Commun. 2016, 86, 82–85. [Google Scholar] [CrossRef]
- Sharp, C.; Zavala, B.; Neely, G.; Rao, S. An Update on Continuing Progress towards Heavy-Duty Low NOx and CO2 in 2027 and Beyond; No. 2023-01-0357; SAE Technical Paper: Warrendale, PA, USA, 2023. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Teoh, Y.H.; How, H.G. Higher alcohol–biodiesel–diesel blends: An approach for improving the performance, emission, and combustion of a light-duty diesel engine. Energy Convers. Manag. 2016, 111, 174–185. [Google Scholar] [CrossRef]
- Jung, Y.J.; Pyo, Y.; Jang, J.; Woo, Y.; Ko, A.; Kim, G.; Shin, Y.; Cho, C. Nitrous oxide in diesel aftertreatment systems including DOC, DPF and urea-SCR. Fuel 2021, 310, 122453. [Google Scholar] [CrossRef]
- Jiao, P.H.; Li, Z.J.; Shen, B.X.; Zhang, W.; Kong, X.J.; Jiang, R. Research of DPF regeneration with NOx-PM coupled chemical reaction. Appl. Therm. Eng. 2017, 110, 737–745. [Google Scholar] [CrossRef]
- Bai, S.Z.; Tang, J.; Wang, G.H.; Li, G.X. Soot loading estimation model and passive regeneration characteristics of DPF system for heavy-duty engine. Appl. Therm. Eng. 2016, 100, 1292–1298. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Weibel, M. Unifying Redox Kinetics for Standard and Fast NH3-SCR over a V2O5-WO3/TiO2 Catalyst. AIChE J. 2009, 55, 1514–1529. [Google Scholar] [CrossRef]
- Bai, S.Z.; Han, J.L.; Liu, M.; Qin, S.S.; Wang, G.H.; Li, G.X. Experimental investigation of exhaust thermal management on NOx emissions of heavy-duty diesel engine under the world Harmonized transient cycle (WHTC). Appl. Therm. Eng. 2018, 142, 421–432. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Zhang, N.; Liu, Z. Simulation of Denitrification of Vehicle Exhaust over Cu-CHA Bazite Catalyst for a Monolith Reactor. Catalysts 2021, 11, 930. [Google Scholar] [CrossRef]
- Liu, B.; Yao, D.W.; Wu, F.; Wei, L.; Li, X.W.; Wang, X.L. Experimental Investigation on N2O Formation during the Selective Catalytic Reduction of NOx with NH3 over Cu-SSZ-13. Ind. Eng. Chem. Res. 2019, 58, 20516–20527. [Google Scholar] [CrossRef]
- Hsieh, M.F.; Wang, J.M. NO and NO2 Concentration Modeling and Observer-Based Estimation Across a Diesel Engine Aftertreatment System. J. Dyn. Syst. Meas. Control Trans. ASME 2011, 133, 041005. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).