Integration of Organic Waste for Soil Stabilization through MICP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Condition
2.1.1. Bacterial Strains
2.1.2. Extract from Brewer’s Spent Yeast
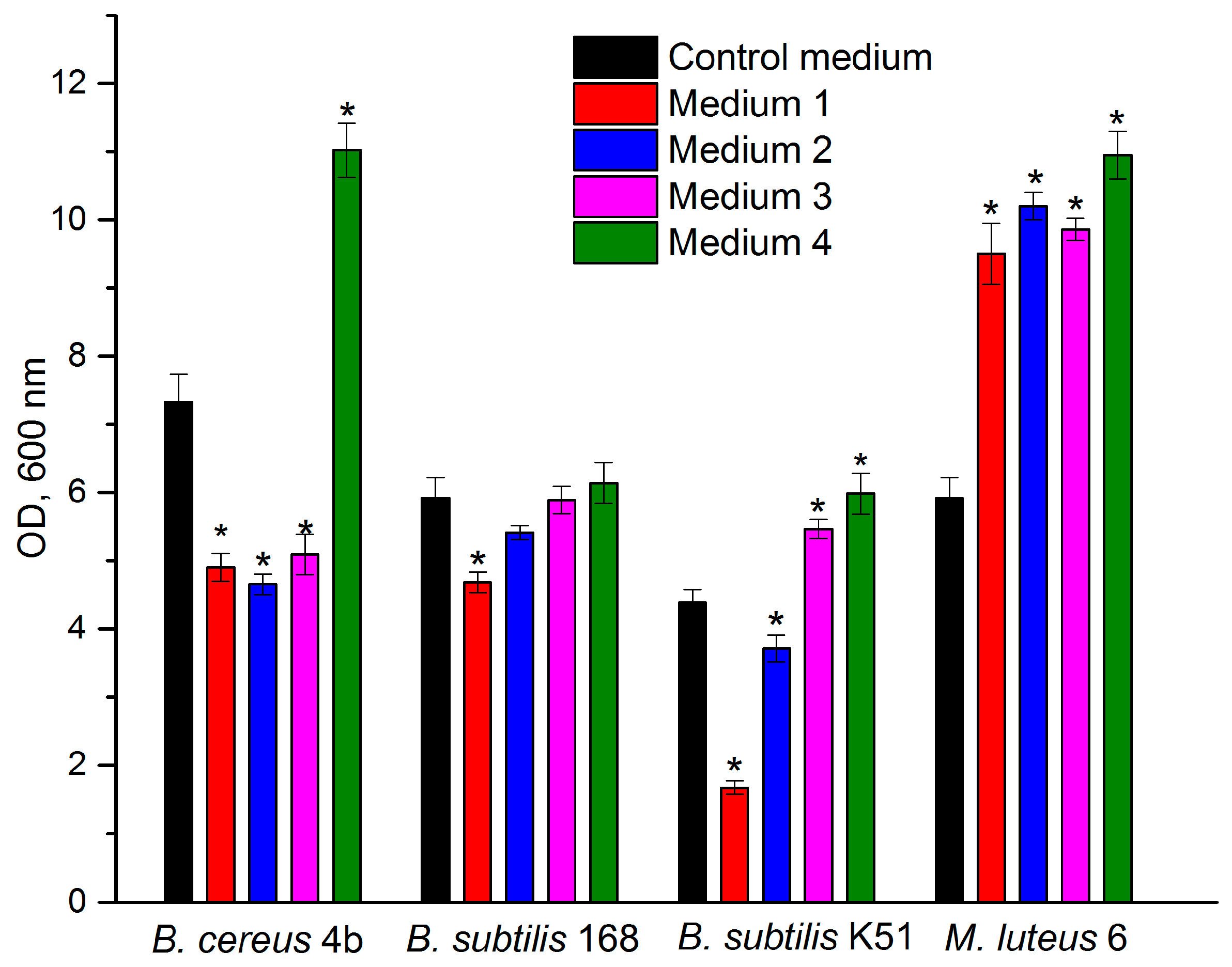
2.1.3. Growth Media
2.2. Urease Assay
2.3. Soil and Additives from Organic Waste
2.3.1. Soil Characteristics
2.3.2. Cellulose-Containing Organic Waste Additives
Shredded Wastepaper
Flax Shives
Sawdust
2.4. Stabilization of the Soil Samples through MICP
2.4.1. Sample Preparation
2.4.2. Assessment of Unconfined Compression Strength and the Calcium Carbonate Content
2.4.3. Microscopy Analysis
2.5. Statistical Processing
3. Results
3.1. Selection of a Growth Medium for Ureolytic Bacteria
3.2. Properties of MICP-Treated Soil Samples with Waste Additives
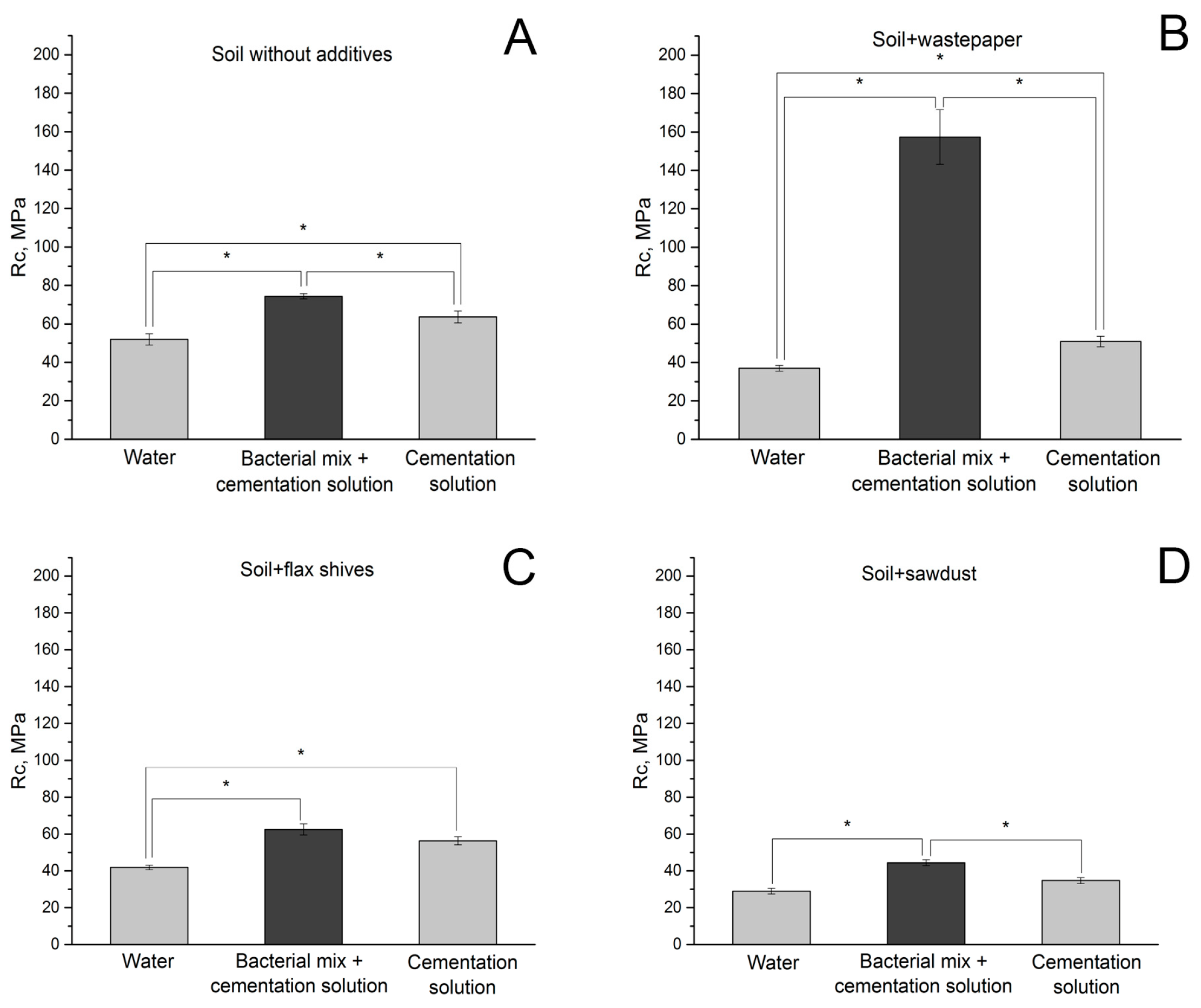
3.2.1. Effect of MICP Treatment and Additives on Compressive Strength of Soil Samples
3.2.2. Calcium Carbonate Formation during MICP Treatment of Soil Samples with Additives
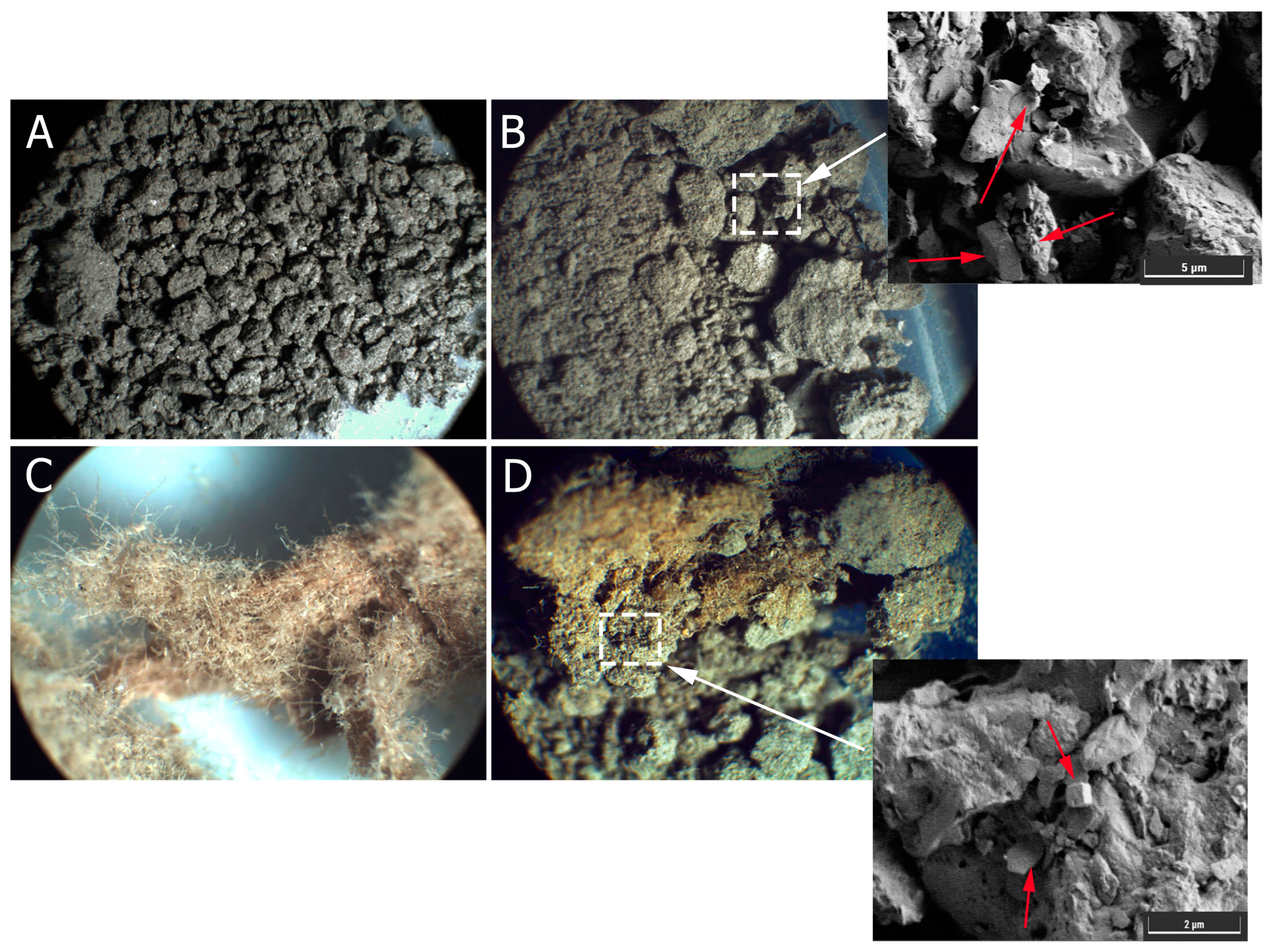
3.2.3. Microscopic Observations of MICP-Treated Soil Samples with Shredded Wastepaper Additive
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Xie, Y.-H.; Tang, C.-S.; Pan, X.-H.; Jiang, N.-J.; Singh, D.N.; Cheng, Y.-J.; Shi, B. Bio-Mediated Method for Improving Surface Erosion Resistance of Clayey Soils. Eng. Geol. 2021, 293, 106295. [Google Scholar] [CrossRef]
- Martinez, A.; Dejong, J.; Akin, I.; Aleali, A.; Arson, C.; Atkinson, J.; Bandini, P.; Baser, T.; Borela, R.; Boulanger, R.; et al. Bio-Inspired Geotechnical Engineering: Principles, Current Work, Opportunities and Challenges. Géotechnique 2022, 72, 687–705. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial Method for Construction of an Aquaculture Pond in Sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Tang, C.-S.; Yin, L.; Jiang, N.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors Affecting the Performance of Microbial-Induced Carbonate Precipitation (MICP) Treated Soil: A Review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Construction Biotechnology; Springer: Singapore, 2017; ISBN 978-981-10-1444-4. [Google Scholar]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Bacterial Roles in the Precipitation of Carbonate Minerals. In Microbial Sediments; Springer: Berlin/Heidelberg, Germany, 2000; pp. 32–39. [Google Scholar]
- Hammes, F.; Verstraete, W. Key Roles of PH and Calcium Metabolism in Microbial Carbonate Precipitation. Rev. Environ. Sci. Biotechnol. 2002, 1, 3–7. [Google Scholar] [CrossRef]
- Krajewska, B. Urease-Aided Calcium Carbonate Mineralization for Engineering Applications: A Review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, S.; Nawarathna, T.H.K.; Nayanthara, P.G.N.; Nakashima, K.; Kawasaki, S. The Amendments in Typical Microbial Induced Soil Stabilization by Low-Grade Chemicals, Biopolymers and Other Additives: A Review. In Building Materials for Sustainable and Ecological Environment; Springer: Singapore, 2021; pp. 49–72. [Google Scholar]
- Oualha, M.; Bibi, S.; Sulaiman, M.; Zouari, N. Microbially Induced Calcite Precipitation in Calcareous Soils by Endogenous Bacillus Cereus, at High PH and Harsh Weather. J. Environ. Manag. 2020, 257, 109965. [Google Scholar] [CrossRef] [PubMed]
- Al Imran, M.; Gowthaman, S.; Nakashima, K.; Kawasaki, S. The Influence of the Addition of Plant-Based Natural Fibers (Jute) on Biocemented Sand Using MICP Method. Materials 2020, 13, 4198. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-Mediated Soil Improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. A State-of-the-Art Review on Soil Reinforcement Technology Using Natural Plant Fiber Materials: Past Findings, Present Trends and Future Directions. Materials 2018, 11, 553. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Sustainable Energy from Waste Organic Matters via Efficient Microbial Processes. Sci. Total Environ. 2020, 722, 137927. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Yadav, B.; Atmakuri, A.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Bioconversion of Organic Wastes into Value-Added Products: A Review. Bioresour. Technol. 2022, 344, 126398. [Google Scholar] [CrossRef] [PubMed]
- Kuryntseva, P.; Galitskaya, P.; Selivanovskaya, S. Changes in the Ecological Properties of Organic Wastes during Their Biological Treatment. Waste Manag. 2016, 58, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gimeno, A.; Navarro-Pedreño, J.; Almendro-Candel, M.B.; Gómez, I.; Zorpas, A.A. The Use of Wastes (Organic and Inorganic) in Land Restoration in Relation to Their Characteristics and Cost. Waste Manag. Res. J. A Sustain. Circ. Econ. 2019, 37, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Masserov, D.; Kustov, M. Global Achievements in the Valorization of Organic Waste for Environmentally Sustainable Development of Territories. Russ. J. Resour. Conserv. Recycl. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.-S.; Jiang, N.-J.; Pan, X.-H.; Liu, B.; Wang, Y.-J.; Shi, B. Microbial-induced Carbonate Precipitation (MICP) Technology: A Review on the Fundamentals and Engineering Applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Lactose Mother Liquor as an Alternative Nutrient Source for Microbial Concrete Production by Sporosarcina Pasteurii. J. Ind. Microbiol. Biotechnol. 2009, 36, 433–438. [Google Scholar] [CrossRef]
- Saksinchai, S.; Suphantharika, M.; Verduyn, C. Application of Simple Yeast Extract from Brewer’s Yeast for Growth and Sporolation of Bacillus Thuringiensis Subsp. Kurstaki: A Physiological Study. World J. Microbiol. Biotechnol. 2001, 17, 307–316. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces Yeast Biomass: Characteristics and Potential Applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Golovkina, D.A.; Zhurishkina, E.V.; Ivanova, L.A.; Baranchikov, A.E.; Sokolov, A.Y.; Bobrov, K.S.; Masharsky, A.E.; Tsvigun, N.V.; Kopitsa, G.P.; Kulminskaya, A.A. Calcifying Bacteria Flexibility in Induction of CaCO3 Mineralization. Life 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Golovkina, D.A.; Zhurishkina, E.V.; Xu, J. Field Trials of Soil Improvement Technology with a Bacterial Mixture. In Biogenic—Abiogenic Interactions in Natural and Anthropogenic Systems 2022; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Ivanova, L.A.; Golovkina, D.A.; Zhurishkina, E.V.; Gorshkova, Y.E.; Yapryntsev, A.D.; Baranchikov, A.E.; Tsvigun, N.V.; Kopitsa, G.P.; Kulminskaya, A.A.; Lebedev, D.V. Structure Evolution of CaCO3 Precipitates Formed during the Bacillus Cereus Induced Biomineralization. Minerals 2023, 13, 740. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Egorov, V.V.; Zabrodskaya, Y.A.; Shaldzhyan, A.A.; Baranchikov, A.Y.; Tsvigun, N.V.; Lykholay, A.N.; Yapryntsev, A.D.; Lebedev, D.V.; Kulminskaya, A.A. Matrix Is Everywhere: Extracellular DNA Is a Link between Biofilm and Mineralization in Bacillus Cereus Planktonic Lifestyle. NPJ Biofilms Microbiomes 2023, 9, 9. [Google Scholar] [CrossRef]
- Babayan, T.L.; Bezrukov, M.G. Autolysis in Yeasts. Acta Biotechnol. 1985, 5, 129–136. [Google Scholar] [CrossRef]
- Microbial Enzymes: Roles and Applications in Industries; Arora, N.K.; Mishra, J.; Mishra, V. (Eds.) Springer: Singapore, 2020; Volume 11, ISBN 978-981-15-1709-9. [Google Scholar]
- Mukhutdinov, R.R.; Pilipenko, T.V.; Kruchina-Bogdanov, I.V. Identification of Powdered Products by Gas Chromatography Method with Preliminary Sample Derivatization. Bull. South. Ural. State Univ. Ser. Food Biotechnol. 2019, 7, 75–84. [Google Scholar]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, School of Biological Sciences & Biotechnology Murdoch University, Perth, WA, Australia, 2004. [Google Scholar]
- Shoba, S.A.; Rozhkov, V.A.; Alyabina, I.O.; Kolesnikova, V.M.; Urusevskaya, I.S.; Molchanov, E.N.; Stolbovoy, V.S.; Sheremet, B.V.; Konyushkov, D.E. Soil Geographic Database of Russia, 2nd ed.; Huang, P.M., Li, Y., Sumner, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System). ASTM: West Conshohocken, PA, USA, 2000.
- Nuez, L.; Beaugrand, J.; Shah, D.U.; Mayer-Laigle, C.; Bourmaud, A.; D’Arras, P.; Baley, C. The Potential of Flax Shives as Reinforcements for Injection Moulded Polypropylene Composites. Ind. Crops Prod. 2020, 148, 112324. [Google Scholar] [CrossRef]
- Ogah, A.O.; Afiukwa, J.N. Characterization and Comparison of Mechanical Behavior of Agro Fiber-Filled High-Density Polyethylene Bio-Composites. J. Reinf. Plast. Compos. 2014, 33, 37–46. [Google Scholar] [CrossRef]
- Krotov, V.S. Use of AAS Pulping for Flax and Hemp Shives. J. Int. Hemp Assoc. 1995, 3, 16–18. [Google Scholar]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–Carbohydrate Complexes: Properties, Applications, Analyses, and Methods of Extraction: A Review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- ASTM D7012-14e1; Standard Test Methods for Compressive Strength and Elastic Moduli of Intact Rock Core Specimens under Varying States of Stress and Temperatures. ASTM: West Conshohocken, PA, USA, 2014.
- ASTM D 4373; Standard Test 5 Method for Calcium Carbonate Content in Soil. ASTM: West Conshohocken, PA, USA, 2021.
- Babakhani, S.; Fahmi, A.; Katebi, H.; Ouria, A.; Majnouni-Toutakhane, A.; Ganbarov, K.; Kafil, H.S. Non-sterile Corn Steep Liquor a Novel, Cost Effective and Powerful Culture Media for Sporosarcina pasteurii Cultivation for Sand Improvement. J. Appl. Microbiol. 2021, 130, 1232–1244. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Surface Percolation for Soil Improvement by Biocementation Utilizing In Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiol. J. 2017, 34, 546–556. [Google Scholar] [CrossRef]
- Stabnikov, V.; Chu, J.; Myo, A.N.; Ivanov, V. Immobilization of Sand Dust and Associated Pollutants Using Bioaggregation. Water Air Soil. Pollut. 2013, 224, 1631. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Reddy, M.S. Original Research: Biocalcification by Sporosarcina pasteurii Using Corn Steep Liquor as the Nutrient Source. Ind. Biotechnol. 2010, 6, 170–174. [Google Scholar] [CrossRef]
- Cuzman, O.A.; Richter, K.; Wittig, L.; Tiano, P. Alternative Nutrient Sources for Biotechnological Use of Sporosarcina Pasteurii. World J. Microbiol. Biotechnol. 2015, 31, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gowthaman, S. Microbial induced slope surface stabilization using industrial-grade chemicals: A preliminary laboratory study. Int. J. Geomate 2019, 17, 110–116. [Google Scholar] [CrossRef]
- Qiu, R.; Tong, H.; Fang, X.; Liao, Y.; Li, Y. Analysis of Strength Characteristics of Carbon Fiber–Reinforced Microbial Solidified Sand. Adv. Mech. Eng. 2019, 11, 168781401988442. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of Fiber Addition on Mechanical Properties of MICP-Treated Sand. J. Mater. Civil. Eng. 2016, 28, 04015166. [Google Scholar] [CrossRef]
- Al Imran, M.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Durability Improvement of Biocemented Sand by Fiber-Reinforced MICP for Coastal Erosion Protection. Materials 2022, 15, 2389. [Google Scholar] [CrossRef]
- Chen, M.; Gowthaman, S.; Nakashima, K.; Komatsu, S.; Kawasaki, S. Experimental Study on Sand Stabilization Using Bio-Cementation with Wastepaper Fiber Integration. Materials 2021, 14, 5164. [Google Scholar] [CrossRef]
- Anderson, J.; Bang, S.; Bang, S.S.; Lee, S.J.; Choi, S.R.; Dho, N.Y. Reduction of Wind Erosion Potential Using Microbial Calcite and Soil Fibers. In Proceedings of the Geo-Congress 2014 Technical Papers; American Society of Civil Engineers, Reston, VA, USA, 24 February 2014; pp. 1664–1673. [Google Scholar]





| Medium | Ingredients, g/L |
|---|---|
| Medium 1 | BSYE, 5; NaCl, 10 |
| Medium 2 | BSYE, 10; NaCl, 10 |
| Medium 3 | BSYE, 5; Glucose, 5; NaCl, 10 |
| Medium 4 | BSYE, 5; Glucose, 10; NaCl, 10 |
| LB * | Yeast extract, 5; peptone, 10; NaCl, 10 |
| Sieve size, mm | >20 | 20–10 | 10–5 | 5–2 | 2–1 | 1–0.5 | 0.5–0.25 | 0.25–0.1 | 0.1–0.05 | 0.05–0.01 | 0.01–0.005 | <0.005 |
| Percentage passing | 10.5 | 3.5 | 6.0 | 3.7 | 4.0 | 5.7 | 4.4 | 15.0 | 3.8 | 3.2 | 5.5 | 45.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovkina, D.A.; Zhurishkina, E.V.; Filippova, A.D.; Baranchikov, A.E.; Lapina, I.M.; Kulminskaya, A.A. Integration of Organic Waste for Soil Stabilization through MICP. Appl. Sci. 2024, 14, 62. https://doi.org/10.3390/app14010062
Golovkina DA, Zhurishkina EV, Filippova AD, Baranchikov AE, Lapina IM, Kulminskaya AA. Integration of Organic Waste for Soil Stabilization through MICP. Applied Sciences. 2024; 14(1):62. https://doi.org/10.3390/app14010062
Chicago/Turabian StyleGolovkina, Darya A., Elena V. Zhurishkina, Arina D. Filippova, Alexander E. Baranchikov, Irina M. Lapina, and Anna A. Kulminskaya. 2024. "Integration of Organic Waste for Soil Stabilization through MICP" Applied Sciences 14, no. 1: 62. https://doi.org/10.3390/app14010062






