Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DSM
2.2. Preparation of PFE
2.2.1. Preliminary Investigations of Initial Mixture for Extrusion
2.2.2. Sample Preparations for Adsorption and Desorption
2.3. Determination of EMC
2.4. Data Analysis
2.5. Determination of Net Isosteric Heat of Sorption
2.6. Statistical Analyses
3. Results and Discussion
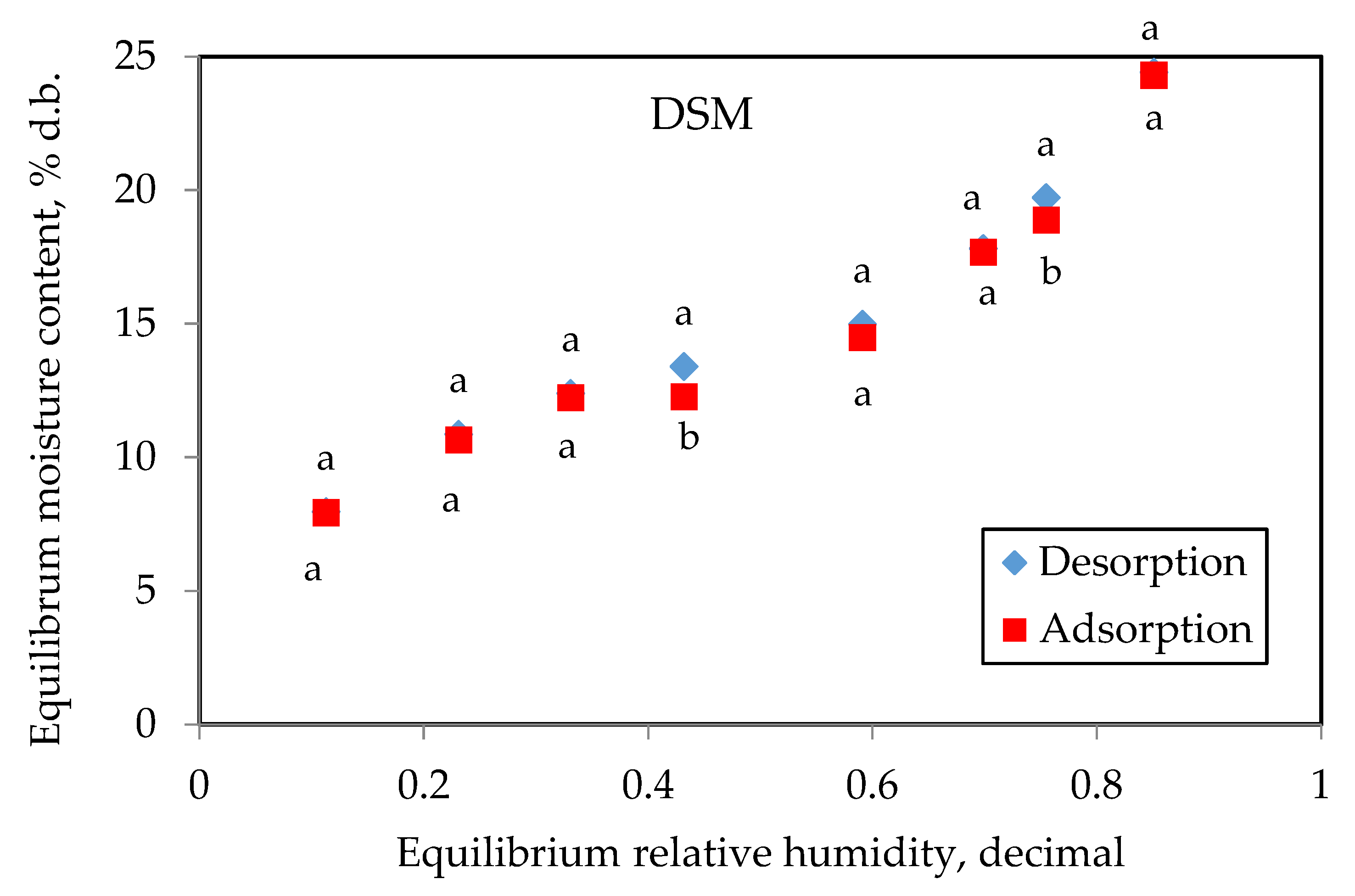
3.1. EMC for DSM and PFE
3.2. Hysteresis Effect
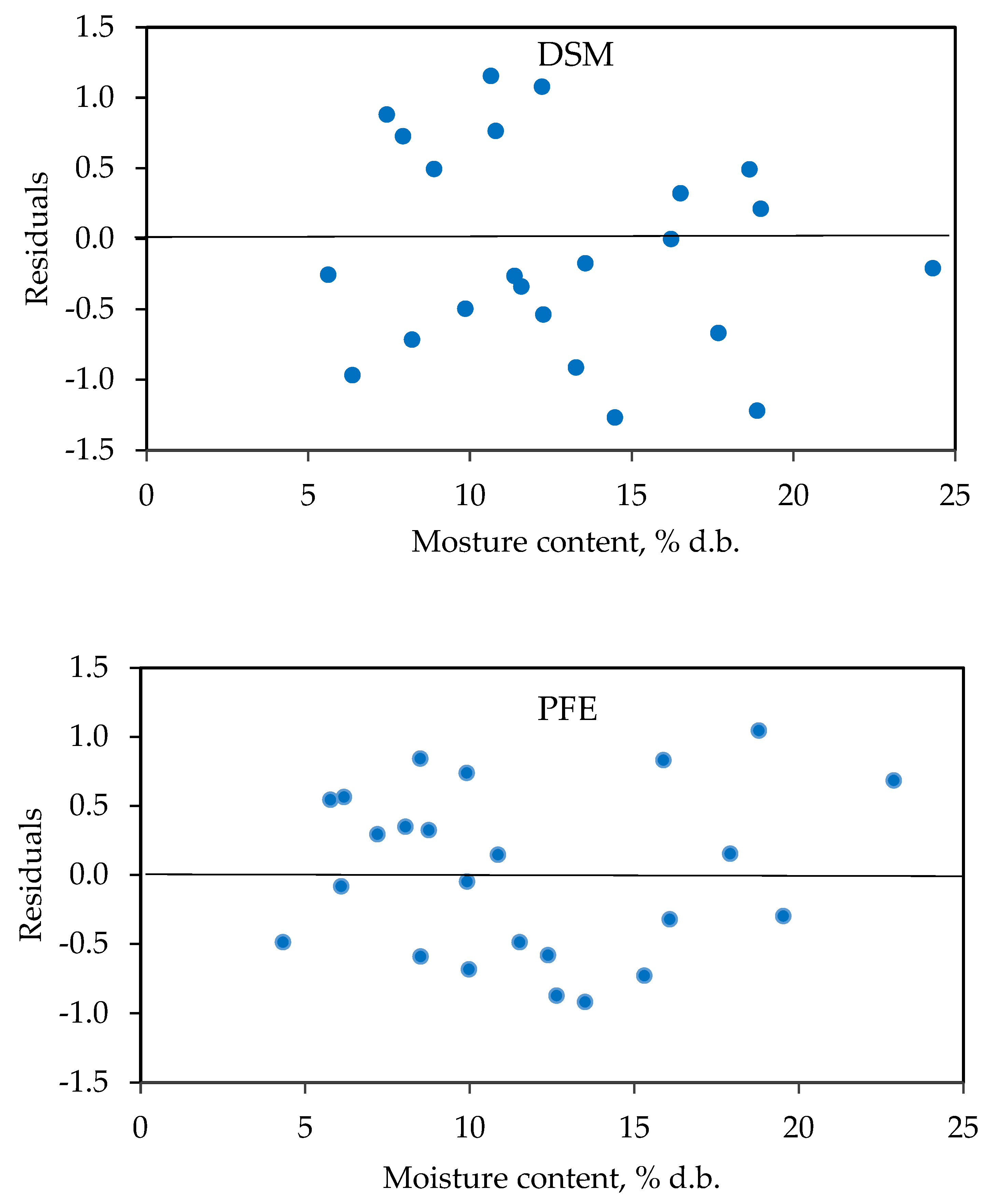
3.3. Models Evaluation
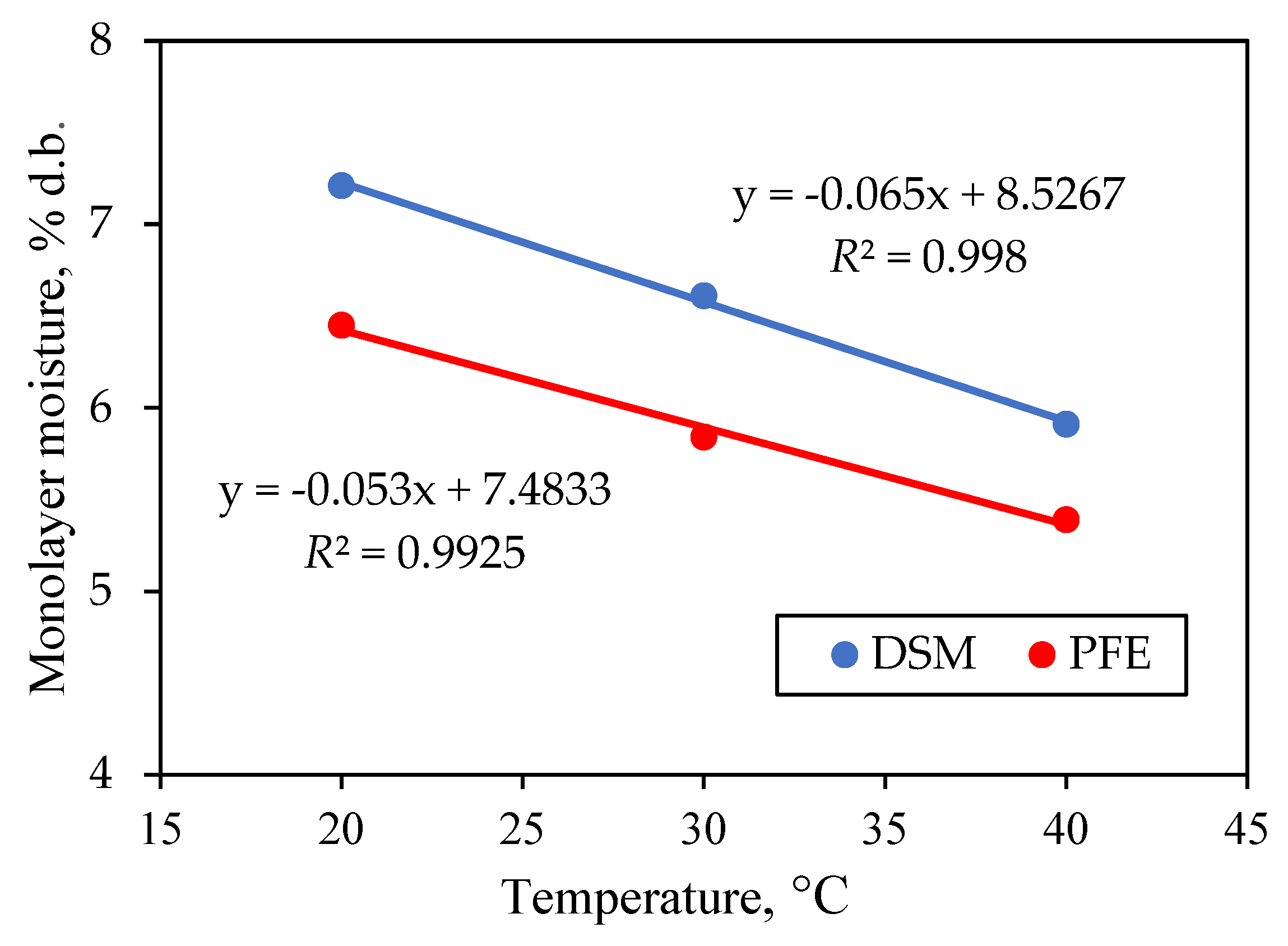
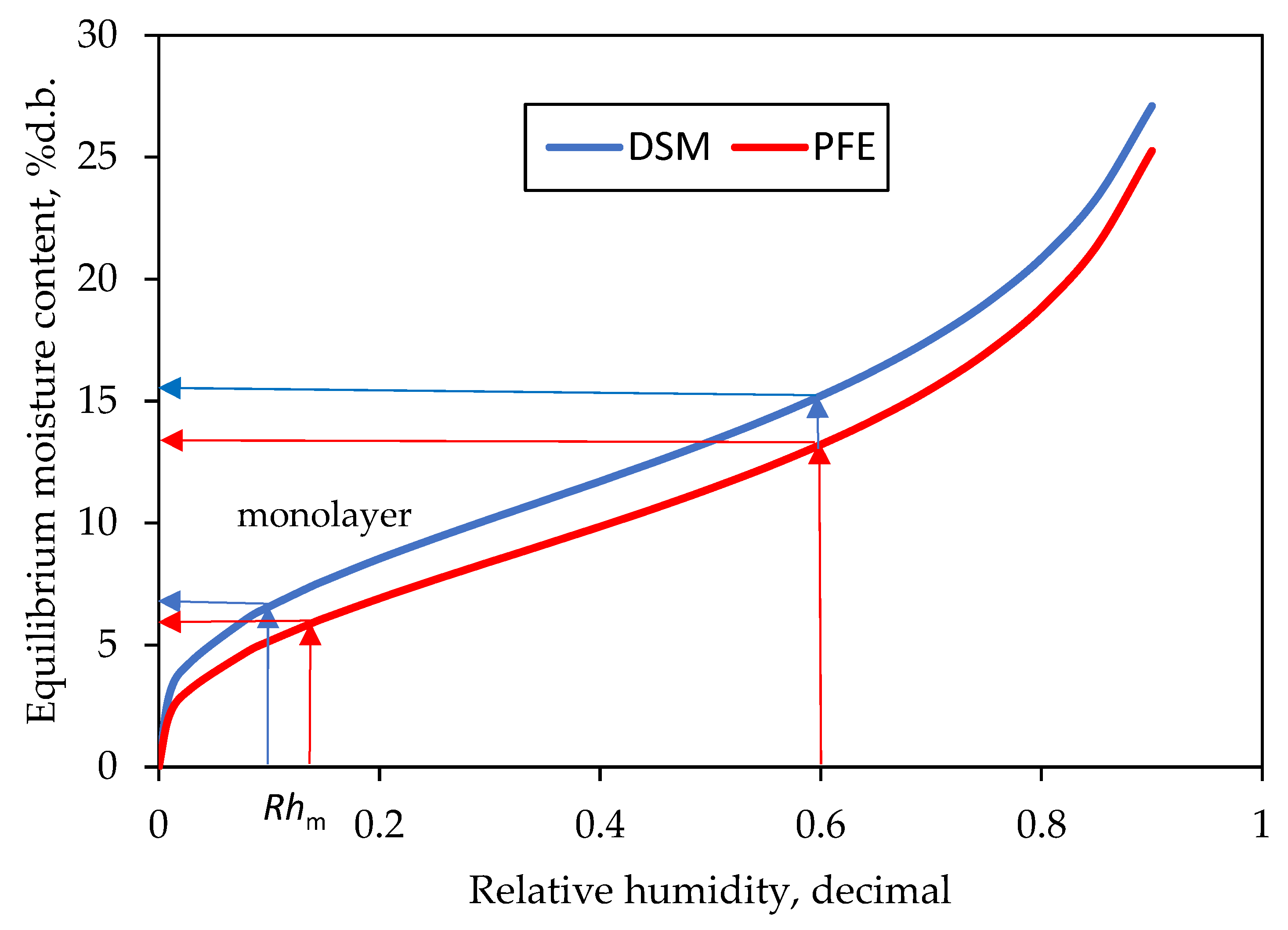
3.4. Monolayer Moisture Content
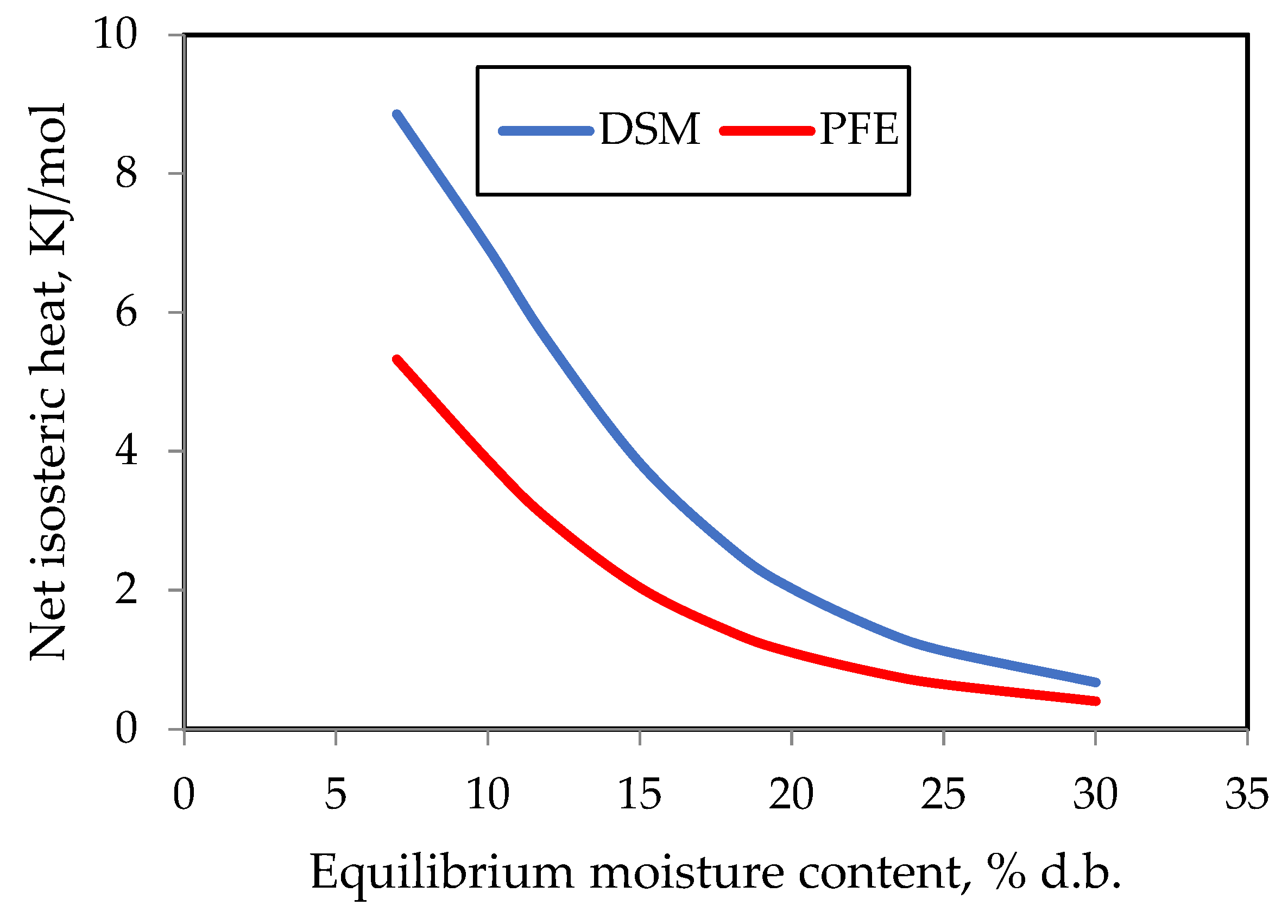
3.5. Net Isosteric Heat of Sorption
3.6. Long-Term Storage Ranges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, R.; Agarwal, R.; Bajada, C.; Arshinder, K. Redesigning a food supply chain for environmental sustainability—An analysis of resource use and recovery. J. Clean. Prod. 2020, 242, 118374. [Google Scholar] [CrossRef]
- Dey, D.; Richter, J.K.; Ek, P.; Bon-Jae, G.; Ganjyal, G.M. Utilization of food processing by-products in extrusion processing: A review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Masli, M.D.P.; Gu, B.J.; Rasco, B.A.; Ganjyal, G.M. Fiber-rich food processing by-products enhance the expansion of cornstarch extrudates. J. Food Sci. 2018, 83, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Rivera-González, G.; Amaya-Guerra, C.A.; De la Rosa-Millán, J. Physicochemical characterisation and in vitro starch digestion of avocado seed flour (Persea americana V. Hass) and its starch and fibrous fractions. Int. J. Food Sci. Technol. 2019, 54, 2447–2457. [Google Scholar] [CrossRef]
- Wang, S.; Kowalski, R.J.; Kang, Y.; Kiszonas, A.M.; Zhu, M.J.; Ganjyal, G.M. Impacts of the particle sizes and levels of inclusions of cherry pomace on the physical and structural properties of direct expanded corn starch. Food Bioprocess Technol. 2017, 10, 394–406. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Wanjari, N.; Waghmare, J. Phenolic and antioxidant potential of sunflower meal. Adv. Appl. Sci. Res. 2015, 6, 221–229. [Google Scholar]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Ivanova, P.; Kalaydzhiev, H.; Slavov, A.; Chalova, V. Value-added dietary fiber concentrate obtained as waste after protein isolation from ethanol-treated sunflower meal. Int. J. Food Sci. 2022, 2022, 4289059. [Google Scholar] [CrossRef]
- Bhise, S.; Kaur, A.; Manikantan, M.R.; Singh, B. Optimization of extrusion process for production of texturized flaxseed defatted meal by response surface methodology. Int. J. Res. Eng. Technol. 2013, 2, 302–308. [Google Scholar] [CrossRef]
- Reyes-Jáquez, D.; Casillas, F.; Flores, N.; Andrade-González, I.; Solís-Soto, A.; Medrano-Roldán, H.; Carrete, F.; Delgado, E. The effect of glandless cottonseed meal content and process parameters on the functional properties of snacks during extrusion cooking. Food Nutr. Sci. 2012, 3, 1716–1725. [Google Scholar] [CrossRef]
- Vidal, N.P.; Roman, L.; Shiva Swaraj, V.J.; Ragavan, K.V.; Simsek, S.; Rahimi, J.; Kroetsch, B.; Martinez, M.M. Enhancing the nutritional value of cold-pressed oilseed cakes through extrusion cooking. Innov. Food Sci. Emerg. Technol. 2022, 77, 102956. [Google Scholar] [CrossRef]
- Sisay, M.T.; Emire, S.A.; Ramaswamy, H.S.; Workneh, T.S. Effect of feed components on quality parameters of wheat–tef–sesame–tomato based extruded products. J. Food Sci. Technol. 2018, 55, 2649–2660. [Google Scholar] [CrossRef] [PubMed]
- Teangpook, C.; Paosangtong, U.; Temtrakool, K. Sunflower meal snack production using a village texturizer and its shelf life. Witthayasan Kasetsat 2011, 45, 900–908. [Google Scholar]
- Bhise, S.; Kaur, A. The effect of extrusion conditions on the functional properties of defatted cake of sunflower-maize based expanded snacks. Int. J. Res. Eng. Technol. 2015, 5, 247–252. [Google Scholar] [CrossRef]
- Grasso, S. Extruded snacks from industrial by-products: A review. Trends Food Sci. Technol. 2020, 99, 284–294. [Google Scholar] [CrossRef]
- Huffman, V.L.; Lee, C.K.; Burns, E.E. Selected functional properties of sunflower meal (Helianthus annuus). J. Food Sci. 1975, 40, 70–74. [Google Scholar] [CrossRef]
- White, N.D.G.; Jayas, D.S. Physical properties of canola and sunflower meal pellets. Can. Biosyst. Eng. 2001, 43, 49–53. [Google Scholar]
- Anjappa, R.H.; Oghbaei, M.; Nagarajaiah, S.B.; Prakash, J. Influence of water activity on physico-chemical properties of proteins from selected oilseed flours. Food Sci. Technol. Res. 2011, 17, 257–266. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Influence of extrusion cooking on phytochemical, physical and sorption isotherm properties of rice extrudate infused with microencapsulated anthocyanin. Food Sci. Biotechnol. 2021, 30, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rosentrater, K.A.; Verbeek, C.J.R. Water adsorption characteristics of extruded blends of corn gluten meal and distillers dried grains with solubles. Food Bioprod. Process. 2017, 101, 110–117. [Google Scholar] [CrossRef]
- Gandova, V.; Ivanova, P.; Kalaydzhiev, H.; Perifanova-Nemska, M.; Chalova, V.I. Dissolution and surface tension properties of ethanol-wash solute obtained from industrial sunflower meal. Biointerface Res. Appl. Chem. 2021, 11, 11284–11292. [Google Scholar] [CrossRef]
- Ivanova, P.; Chalova, V.; Koleva, L.; Pishtiyski, I.; Perifanova-Nemska, M. Optimization of protein extraction from sunflower meal produced in Bulgaria. Bulg. J. Agric. Sci. 2012, 18, 153–160. [Google Scholar]
- Khushbu, K.; Mridula, D.; Kishore, A.; Chitranayak. Extrusion processing of agri-horti and dairy products: A review. Int. J. Chem. Stud. 2020, 8, 1424–1433. [Google Scholar] [CrossRef][Green Version]
- ISO 712:2009; Cereals and Cereal Product—Determination of Moisture Content—Reference Method. ISO: Geneva, Switzerland, 2009.
- Adekola, K.A. Engineering review food extrusion technology and its applications. J. Food Sci. Eng. 2016, 6, 149–168. [Google Scholar] [CrossRef][Green Version]
- Dushkova, M.A.; Simitchiev, A.T.; Kalaydzhiev, H.R.; Ivanova, P.; Menkov, N.D.; Chalova, V.I. Comparison and modeling of moisture sorption isotherms of deproteinized rapeseed meal and model extrudate. J. Food Process. Preserv. 2022, 46, e16978. [Google Scholar] [CrossRef]
- Wolf, W.; Spiess, W.E.L.; Jung, G. Standardization of Isotherm Measurements (Cost-project 90 and 90 BIS). In Properties of Water in Foods, 1st ed.; Simatos, D., Multon, J.L., Eds.; Springer: London, UK, 1985; Volume 90, pp. 661–679. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Bell, L.N.; Labuza, T.P. Moisture Sorption: Practical Aspects of Isotherm Measurement and Use, 2nd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000; pp. 33–36. [Google Scholar]
- Fontana, A.J. Appendix A: Water Activity of Saturated Salt Solutions. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.I., Schmidt, S.J., Labuza, T.P., Eds.; Blackwell Publishing and the Institute of Food Technologists: Boston, MA, USA, 2007; pp. 391–393. [Google Scholar]
- Deshmukh, G.P.; Ravindra, M.R.; Jose, N.; Wasnik, P.G.; Dhotre, A.V. Moisture sorption behavior and thermodynamic properties of dry-crystallized Palada payasam (rice flakes milk pudding) mix determined using the dynamic vapor sorption method. J. Food Process. Preserv. 2020, 44, e14819. [Google Scholar] [CrossRef]
- D245.6; Moisture Relationships of Plant-Based Agricultural Products. American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2007.
- Jayas, D.S.; Mazza, G. Comparison of five three-parameter equations for the description of adsorption data of oats. Trans. ASAE 1993, 36, 119–125. [Google Scholar] [CrossRef]
- Menkov, N.D.; Paskalev, H.M.; Galyazkov, D.I.; Kerezieva-Rakova, M. Applying the linear equation of correlation of Brunauer-Emmet-Teller (BET)-monolayer moisture content with temperature. Food/Nahrung 1999, 43, 118–121. [Google Scholar] [CrossRef]
- Sahu, C.; Patel, S.; Khokhar, D. Sorption behavior and isosteric heat of maize-millet based protein enriched extruded product. Heliyon 2021, 7, e06742. [Google Scholar] [CrossRef] [PubMed]
- Jose, N.; Ravindra, M.R.; Deshmukh, G.P.; Rao, K.J. Sorption and thermodynamic properties of dry crystallized Palada payasam mix prepared using manual and mechanical stirring. J. Food Sci. Technol. 2022, 59, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Basha, R.K.; Leong, K.Y.; Othman, S.H.; Talib, R.A.; Naim, M.N.; Hasnan, N.Z.N.; Azmi, N.S. Sorption characteristic of starch-based film. Food Res. 2021, 5, 193–200. [Google Scholar] [CrossRef]
- Al-Muhtaseb, A.H.; McMinn, W.A.M.; Magee, T.R.A. Moisture sorption isotherm characteristics of food products: A review. Food Bioprod. Process 2002, 80, 118–128. [Google Scholar] [CrossRef]
- Tanska, M.; Konopka, I.; Ruszkowska, M. Sensory, physico-chemical and water sorption properties of corn extrudates enriched with spirulina. Plant Foods Hum. Nutr. 2017, 72, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Stasiak, M.; Jamroz, J. Analysis of sorption properties of starch–protein extrudates with the use of water vapour. J. Food Eng. 2008, 85, 580–589. [Google Scholar] [CrossRef]
- Witczak, T.; Stępień, A.; Zięba, T.; Gumul, D.; Witczak, M. The influence of extrusion process with a minimal addition of corn meal on selected properties of fruit pomaces. J. Food Process Eng. 2020, 43, e13382. [Google Scholar] [CrossRef]
- Manchuliantsau, A.; Tkacheva, A. Upcycling Solid Food Wastes and By-Products into Human Consumption Products. U.S. Patent 0223475, 25 July 2019. Available online: https://patents.google.com/patent/US20190223475A1/en (accessed on 25 July 2019).
- Aviara, N.A. Moisture sorption isotherms and isotherm model performance evaluation for food and agricultural products. In Sorption in 2020s, 1st ed.; Kyzas, G., Lazaridis, N., Eds.; IntechOpen: London, UK, 2020; pp. 14–18. [Google Scholar] [CrossRef]
- Ajala, A.S.; Ngoddy, P.O.; Olajide, J.O. Sorption isotherms and their fitted equations for dried chips of cassava roots (Manihot Esculenta Crantz; Tme-7 variety) and the resulting isosteric heats of sorption. Food Res. 2020, 4, 703–711. [Google Scholar] [CrossRef]
- Kapsalis, J.G. Influences of Hysteresis and Temperature on Moisture Sorption Isotherms. In Water Activity: Theory and Applications to Food, 1st ed.; Rockland, L.B., Beuchat, L.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1987; pp. 173–213. [Google Scholar]
- Yeasmin, F.; Hira, N.N.; Rahman, H.; Islam, M.N. Development of powder based ginger drink: Analysis of dehydration kinetics and moisture sorption isotherm. Food Res. 2021, 5, 322–329. [Google Scholar] [CrossRef]
- Bryan, W.P. Thermodynamic models for water–protein sorption hysteresis. Biopolymers 1987, 26, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Jamroz, J.; Sokołowska; Hajnos, M. Moisture sorption hysteresis in potato starch extrudates. Int. Agrophys. 1999, 13, 451–455. [Google Scholar]
- Iglesias, H.; Chirife, J. Correlation of BET monolayer moisture content in foods with temperature. J. Food Technol. 1984, 19, 503–506. [Google Scholar] [CrossRef]
- Echavarria, J.A.C.; Torres, A.M.R.; Montoya, J.E.Z. Sorption isotherms and thermodynamic properties of the dry silage of red tilapia viscera (Oreochromis spp.) obtained in a direct solar dryer. Heliyon 2021, 7, e06742. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.C.; Corrêa, P.C.; Zaidan, U.R.; Zaidan, I.R.; Leite, R.A. Moisture sorption isotherms of sunflower seeds: Thermodynamic analysis. Cienc. Agrotec. 2019, 43, e011619. [Google Scholar] [CrossRef]
- Barden, L.; Decker, E.A. Lipid oxidation in low-moisture food: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R. Influence of water activity on growth, metabolic activities and survival of yeasts and molds. J. Food Prot. 1983, 46, 135–141. [Google Scholar] [CrossRef]
- Abdullaha, N.; Nawawia, A.; Othman, I. Fungal spoilage of starch-based foods in relation to its water activity (aw). J. Stored Prod. Res. 2000, 36, 47–54. [Google Scholar] [CrossRef]


| 20 °C | 30 °C | 40 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| Rh | DSM | PFE | Rh | DSM | PFE | Rh | DSM | PFE |
| 0.113 | 7.93 ± 0.06 a | 6.18 ± 0.18 b | 0.113 | 7.43 ± 0.28 a | 5.77 ± 0.26 b | 0.112 | 5.62 ± 0.08 a | 4.33 ± 0.24 b |
| 0.231 | 10.65 ± 0.13 a | 8.50 ± 0.10 b | 0.216 | 8.89 ± 0.21 a | 7.20 ± 0.09 b | 0.201 | 6.37 ± 0.25 a | 6.10 ± 0.24 a |
| 0.331 | 12.23 ± 0.06 a | 9.91 ± 0.16 b | 0.324 | 10.8 ± 0.10 a | 8.76 ± 0.09 b | 0.316 | 8.21 ± 0.21 a | 8.05 ± 0.18 a |
| 0.432 | 12.27 ± 0.07 a | 10.86 ± 0.12 b | 0.432 | 11.38 ± 0.24 a | 9.92 ± 0.15 b | 0.423 | 9.86 ± 0.15 a | 8.51 ± 0.17 b |
| 0.591 | 14.48 ± 0.32 a | 12.64 ± 0.18 b | 0.560 | 13.57 ± 0.27 a | 11.52 ± 0.18 b | 0.532 | 11.59 ± 0.13 a | 9.98 ± 0.11 b |
| 0.699 | 17.68 ± 0.27 a | 15.31 ± 0.08 b | 0.679 | 16.51 ± 0.26 a | 13.51 ± 0.31 b | 0.661 | 13.28 ± 0.12 a | 12.38 ± 0.24 b |
| 0.755 | 18.88 ± 0.16 a | 17.92 ± 0.18 b | 0.751 | 18.64 ± 0.27 a | 16.08 ± 0.21 b | 0.747 | 16.22 ± 0.21 a | 15.89 ± 0.31 b |
| 0.851 | 24.31 ± 0.25 a | 22.89 ± 0.32 b | 0.836 | 23.7 ± 0.30 a | 19.53 ± 0.22 b | 0.823 | 18.99 ± 0.14 a | 18.79 ± 0.25 a |
| Model | Coefficients | R | SEE | ARE, % | Plot of Residuals |
|---|---|---|---|---|---|
| Chung–Pfost | a = 32.76313 b = 5.88479 c = 12.07121 | 0.98 | 1.03 | 6.18 | Random |
| Halsey | a = 5.475196 b = −0.033334 c = 1.916773 | 0.98 | 2.30 | 8.03 | Patterned |
| Oswin | a = 16.52953 b = −0.12708 c = 3.10444 | 0.99 | 0.89 | 5.81 | Random |
| Henderson | a = 0.00013 b = 34.86715 c = 1.74088 | 0.97 | 1.60 | 10.85 | Patterned |
| GAB | a = 8.5282 b = 0.7385 c = 682.19 | 0.97 | 1.31 | 8.18 | Random |
| BET | a = 10.03405 b = −0.10825 c = 83.45321 | 0.96 | 0.71 | 5.81 | - |
| Model | Coefficients | R | SEE | ARE, % | Plot of Residuals |
|---|---|---|---|---|---|
| Chung–Pfost | a = 349.2721 b = 24.3482 c = 0.2017 | 0.99 | 1.16 | 4.92 | Patterned |
| Halsey | a = 4.231838 b = −0.023758 c = 1.617364 | 0.98 | 2.89 | 11.18 | Patterned |
| Oswin | a = 13.47429 b = −0.08232 c = 2.76633 | 0.99 | 0.63 | 5.08 | Random |
| Henderson | a = 0.00016 b = 56.2774 c = 71.80561 | 0.98 | 1.22 | 11.08 | Random |
| GAB | a = 6.9442 b = 0.7921 c = 672.57 | 0.99 | 0.844 | 5.99 | Random |
| BET | a = 8.07627 b = −0.07179 c = 39.37878 | 0.97 | 0.49 | 4.83 | - |
| M | DSM | PFE | |||||
|---|---|---|---|---|---|---|---|
| T | 293 | 303 | 313 | 293 | 303 | 313 | |
| 7 | aw | 0.105 | 0.136 | 0.179 | 0.190 | 0.222 | 0.262 |
| 10 | 0.261 | 0.322 | 0.397 | 0.386 | 0.434 | 0.488 | |
| 12 | 0.383 | 0.455 | 0.536 | 0.510 | 0.560 | 0.612 | |
| 15 | 0.554 | 0.625 | 0.698 | 0.659 | 0.702 | 0.745 | |
| 18 | 0.686 | 0.746 | 0.803 | 0.762 | 0.796 | 0.829 | |
| 20 | 0.752 | 0.803 | 0.849 | 0.810 | 0.839 | 0.866 | |
| 23 | 0.8245 | 0.863 | 0.897 | 0.863 | 0.885 | 0.905 | |
| 25 | 0.8589 | 0.890 | 0.918 | 0.888 | 0.906 | 0.9231 | |
| 30 | 0.914 | 0.935 | 0.952 | 0.929 | 0.941 | 0.952 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dushkova, M.A.; Simitchiev, A.T.; Kalaydzhiev, H.R.; Ivanova, P.; Menkov, N.D.; Chalova, V.I. Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate. Appl. Sci. 2024, 14, 65. https://doi.org/10.3390/app14010065
Dushkova MA, Simitchiev AT, Kalaydzhiev HR, Ivanova P, Menkov ND, Chalova VI. Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate. Applied Sciences. 2024; 14(1):65. https://doi.org/10.3390/app14010065
Chicago/Turabian StyleDushkova, Mariya A., Apostol T. Simitchiev, Hristo R. Kalaydzhiev, Petya Ivanova, Nikolay D. Menkov, and Vesela I. Chalova. 2024. "Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate" Applied Sciences 14, no. 1: 65. https://doi.org/10.3390/app14010065
APA StyleDushkova, M. A., Simitchiev, A. T., Kalaydzhiev, H. R., Ivanova, P., Menkov, N. D., & Chalova, V. I. (2024). Moisture Sorption Behavior of Deproteinized Sunflower Meal and Patterned Food Extrudate. Applied Sciences, 14(1), 65. https://doi.org/10.3390/app14010065









