1. Introduction
In the context of clinical diagnostics, there has been a continuous need to find non-invasive and efficient diagnostic tools. The ability to accurately assess the conditions affecting patients and proactively track symptoms, especially in cases of hand neuropathies and musculoskeletal disorders, can significantly improve treatment outcomes and enhance the overall quality of life. In recent years, infrared thermography (IRT) has emerged as a promising technology that has enormous potential to support the diagnosis and management of these conditions.
Hand neuropathies and musculoskeletal disorders are debilitating conditions that can manifest in various forms, causing pain, discomfort, and loss of function in the hands [
1,
2].
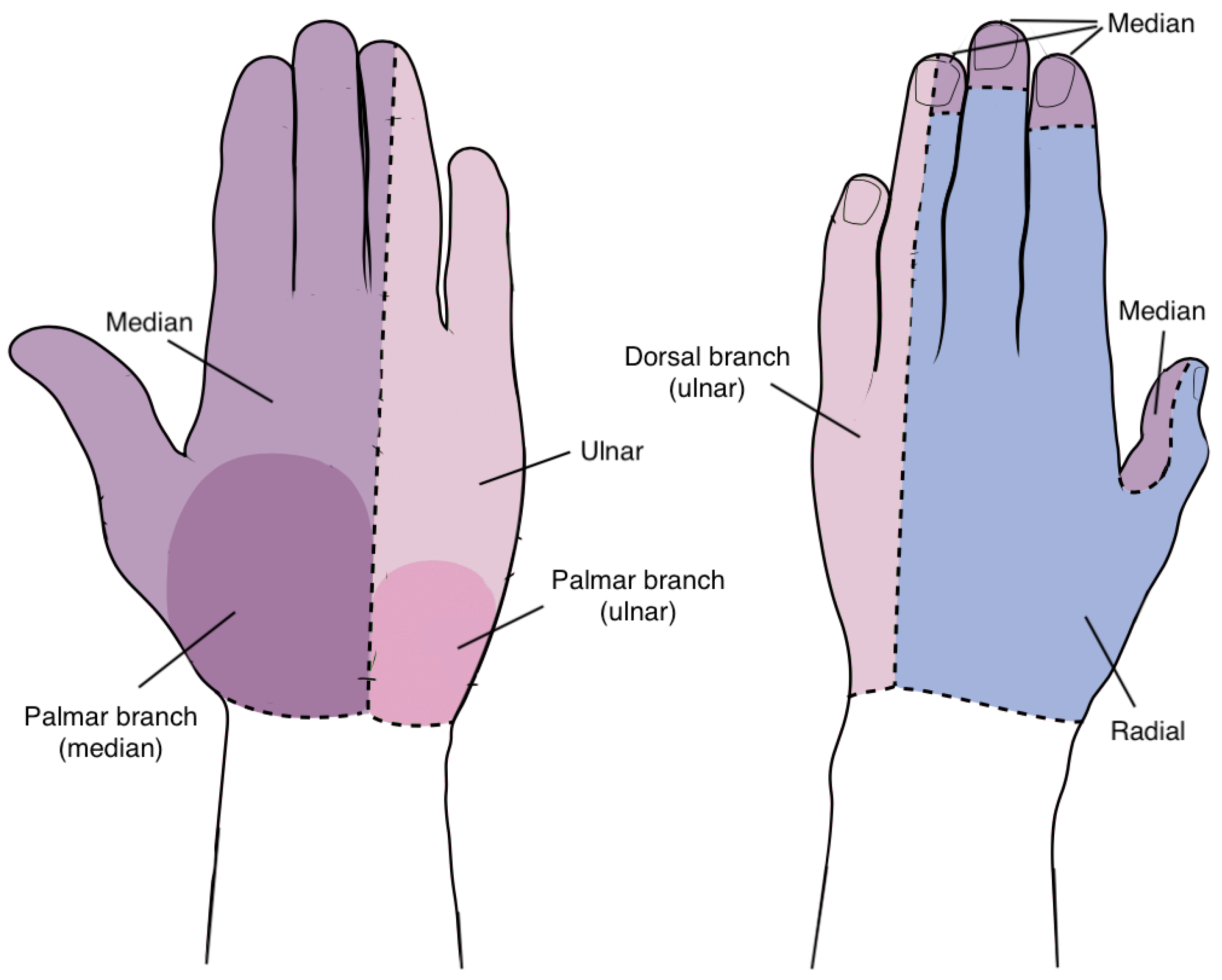
Upper extremity neuropathies refer to conditions where there is impairment or malfunction of the nerves in the upper limbs, often resulting from factors such as compression, inflammation, and trauma [
3]. The distinct sensory regions innervated by the hand’s nerves, namely the median, ulnar, and radial nerves, are displayed in
Figure 1.
Prolonged compression of the median nerve within the wrist leads to the development of Carpal Tunnel Syndrome (CTS), the most prevalent entrapment neuropathy [
4] affecting 7% to 19% of the population [
5]. CTS is more common in women than in men and its incidence tends to increase with age [
6]. In addition, conditions involving mechanical, traumatic, inflammatory, or hormonal factors, such as rheumatoid arthritis, diabetes mellitus, hypothyroidism, pregnancy, and morbid obesity, may contribute to heightened pressure within the carpal tunnel (higher than the normal pressure of 10 mmHg), thereby increasing the risk of CTS [
7,
8,
9,
10,
11,
12,
13]. Activities and professions characterised by repetitive hand and wrist movements of flexion and extension under significant force, such as forceful gripping, have also been linked to CTS [
14]. In the earliest stage of the disease, the typical symptoms reported by patients with CTS include sleep disturbances due to hand numbness and a sensation similar to swelling and nocturnal brachialgia paraesthetica, which is described by pain extending from the wrist to the shoulder and tingling in the hand and fingers, and can be relieved by repeatedly shaking the wrists [
5,
13,
15]. As the condition progresses, these symptoms also tend to occur during the day, and become more frequent when performing repetitive movements demanding prolonged wrist flexion, such as holding an object with the hands. In an advanced stage, weakness and potential hypotrophy or atrophy of the thenar muscles can be reported along with sensory loss in the first three fingers (thumb, index, and middle) and the radial side of the ring finger [
4].
On the other hand, when the ulnar nerve gets compressed while passing trough the cubital tunnel on the inner side of the elbow, the second most prevalent entrapment neuropathy arises, known as Cubital Tunnel Syndrome (CuTS) [
16]. Although the cubital tunnel is the most common area of compression, ulnar nerve damage can also occur from C8-T1 nerve root fibers to the wrist [
17]. In this condition, the compression of the ulnar nerve, responsible for controlling muscle movements in the forearm and ring and little fingers, can be caused by prolonged or repetitive elbow flexion, extension, pronation, or supination, gripping, lifting, traumatic injuries, and specific diseases [
17,
18]. While CuTS presents a risk four times greater for developing advanced disease compared to CTS [
3], its symptoms are quite similar, encompassing numbness, paresthesia, hand atrophy, pain in the affected hand and forearm, and reduced grip strength due to weakness in the muscles of the flexor carpi ulnaris (FCU) and the flexor digitorum profundi (FDP) [
1,
3,
17]. Nevertheless, CuTS mainly affects the medial hand and ring and pinky fingers. Patients with clinical conditions such as diabetes or advanced age complain of motor symptoms (loss of grip strength, weakness, difficulty with fine motor tasks) more often than sensory symptoms (numbness, tingling, paresthesia) usually reported by younger patients.
Furthermore, Radial Tunnel Syndrome (RTS) is a relatively uncommon neuropathic condition that occurs due to the compression of the posterior interosseous nerve, the terminal motor branch of the radial nerve, as it traverses the radial tunnel, a region within the proximal forearm [
3]. The prevalence of RTS is relatively low compared to Carpal Tunnel Syndrome or Cubital Tunnel Syndrome and it is estimated to impact a small percentage of the population, affecting 0.0035% of the population per year [
19,
20]. This rarity can often lead to misdiagnosis or delayed recognition, making accurate diagnosis and appropriate management crucial. The underlying causes of Radial Tunnel Syndrome are multifactorial and can include a combination of anatomical factors, repetitive overuse, and most commonly trauma, such as humerus fractures [
21]. The compression of the radial nerve can result from factors such as muscle hypertrophy or inflammation, as well as external pressure or repetitive motions that place stress on the nerve. Symptoms of RTS typically manifest as pain and discomfort in the forearm, particularly in the area around the radial tunnel [
22]. Individuals with RTS may experience aching, burning, or sharp pain, often exacerbated by activities that involve gripping, twisting, or extending the wrist and hand. Weakness in the affected arm and wrist, namely in the extensor carpi radialis longus and extensor carpi brevis, and in the metacarpophalangeal joints of the thumb and fingers and the interphalangeal joint of the thumb, may result, respectively, in disrupted extension of the fingers and radial deviation of the wrist during extension, impacting a person’s ability to perform everyday tasks and activities [
19].
Orthopaedic conditions of the hand encompass a diverse range of musculoskeletal disorders that affect its anatomy and functionality. Among the multitude of hand-related pathologies, conditions such as rhizarthrosis, tenosynovitis of Quervain, tendonitis of the flexors of the fingers, and osteoarthritis hold significant clinical relevance [
2]. These conditions share a common characteristic of causing discomfort, pain, and functional impairment within the hand and wrist, often necessitating medical attention and intervention.
Hand osteoarthritis is typically attributed to age-related joint wear and tear, although it can also be influenced by genetics. Joints in the hand, such as those in the fingers and the base of the thumb, are affected. Rhizarthrosis is primarily caused by wear and tear on the carpometacarpal (CMC) joint at the base of the thumb. Over time, the joint’s cartilage can deteriorate due to aging, repetitive use, or injury [
23]. Common symptoms include pain, stiffness, and reduced range of motion at the base of the affected hand joints [
24]. Swelling and the development of bony nodules (Heberden’s and Bouchard’s nodes) may also occur.
Tenosynovitis of Quervain arises from irritation or inflammation of the tendons (abductor pollicis longus and extensor pollicis brevis) on the thumb side of the wrist. Repetitive thumb motions or injury can trigger inflammation in the tendon sheath [
2]. Symptoms include pain and tenderness at the base of the thumb, especially when grasping or making a fist [
25]. Swelling and difficulty moving the thumb are also common.
Furthermore, flexor tendinopathy involves inflammation or irritation of the tendons in the fingers, often due to overuse, strain, or repetitive motions [
26]. It can be associated with conditions such as carpal tunnel syndrome. Affected individuals may experience pain, swelling, and stiffness in the fingers, particularly when bending or gripping objects [
27]. Decreased hand strength and difficulty with fine motor tasks can also occur.
Traditionally, clinicians have relied on a combination of clinical examination, electromyography (EMG), and imaging techniques such as X-rays and magnetic resonance imaging (MRI) to assess these conditions [
1,
3,
13,
18,
28]. While these methods have been instrumental in diagnosis, they are often limited in providing a comprehensive view of the underlying physiological changes [
29].
On the other hand, IRT offers a unique perspective by capturing the thermal patterns and temperature distribution in the affected area [
30]. This non-invasive technology is based on the principle that different tissues and structures in the human body emit varying levels of infrared radiation based on their metabolic activity and blood flow [
4]. Consequently, it can detect subtle changes in temperature distribution that may not be discernible through conventional means. However, the specific contextual relevance of IRT in medical applications, especially concerning hand pathologies, is not sufficiently explored in the existing literature. Furthermore, the potential for early detection of abnormalities, which can be instrumental in preventive healthcare, remains an understudied facet of IRT within the medical domain. Hence, understanding the distinct thermal patterns associated with different medical conditions, particularly those affecting the hands, is crucial for harnessing the full potential of IRT in clinical settings.
Numerous studies have sought to explore the clinical utility of infrared thermal imaging in the context of Carpal Tunnel Syndrome, but other hand neuropathies and musculoskeletal disorders are poorly explored. Park et al. [
4] studied the dynamic relationship between CTS duration, severity, and the diagnostic potential of IRT. The findings revealed a diminishing trend in temperature differences between areas innervated by the median and ulnar nerves as the disease progressed. Additionally, it was observed an increase in thermal anisometry correlated with prolonged duration and severity of CTS. However, a notable limitation of the study is the exclusive focus on the physiological changes in unmyelinated autonomic nerves, without drawing comparisons between temperature patterns in the injury and control groups. This suggests that while the study provides valuable insights into the physiological aspects of CTS, it lacks a direct comparison with control groups, potentially limiting the comprehensive understanding of temperature dynamics in the context of CTS pathology. Bargiel et al. [
29] studied the usefulness of the infrared thermography in the diagnosis of Carpal Tunnel Syndrome. They found a notable contrast in the thermographic patterns between the hands of participants with pathology and those of the control group. Even when a cold stimulus was applied to the affected hands, the control group exhibited a faster return to normal temperature, despite the fact that individuals who had undergone surgery were symptom-free. However, the thermographic readings during the postoperative period did not align with the symptomatic relief reported after surgery. Moreover, among individuals with unilateral CTS, there were no discernible variations in thermal images between the affected and healthy hands. While this study established the utility of IRT in diagnosing CTS, its ultimate conclusion was that IRT falls short in offering an objective assessment of patient complaints in the postoperative period.
This article intends to investigate the potential of IRT as a non-invasive complementary diagnostic tool for hand neuropathies and musculoskeletal disorders. This way, it explores case studies and clinical scenarios to determine IRT role and application in medical diagnostics, and the specific advantages it brings to the diagnosis and management of conditions affecting the upper limbs.
3. Results and Discussion
All thermal images of the hands, acquired for both the injury group and the control group, from dorsal and palmar perspectives, underwent processing to standardise the temperature scale and segmentation to eliminate the background. The selection of ROIs was based on the sensory territories controlled by the nerves on each surface of the hand, specifically the median, ulnar, and radial branches. The regions most commonly affected in orthopaedic lesions, which include the finger joints (metacarpophalangeal joint, proximal interphalangeal joint, and distal interphalangeal joint), as well as the region constituted by the radius (named as arm ROI), which is the larger bone situated on the thumb side of the arm, were also considered. After extracting the mean temperature values from all the hands of the injury and control groups, for each ROI, the data were subsequently separated into two variables, each represented in a column, one for the control and the other for injured, to be further used in the SPSS analysis. To evaluate the data distribution, the descriptive statistics by group was obtained by running the Compare Means test. This allowed to create a comparative box plot illustrating the mean temperature values in degrees Celsius (°C), for each hand perspective.
The graph relative to the dorsal view is presented in
Figure 5. The control group exhibits a distribution with a negative skew, indicating that the majority of data points are concentrated in the lower region of the distribution, corresponding to lower temperatures, as would be expected in healthy people. The injured group presents a normal distribution, with the data evenly distributed around the mean. None of the distributions display outliers. Considering the compact size of the boxes, it suggests that there is a high level of agreement between all the participants in both the control and the injured groups. The first group displays a maximum mean temperature of 34 °C, while the second group registers a notably higher temperature of 35.8 °C, reflecting a difference of 1.8 °C between the two. The control group demonstrates a significantly lower minimum mean temperature (27 °C) in comparison to the injured group (29 °C), signifying a substantial difference of 2 °C. The mean temperature in °C for the control group is 31.39, with a corresponding standard deviation of 1.65, which indicates a degree of consistency in the recorded temperatures. In the injured group, these values are 32.41 °C and 1.35 °C, respectively, which implies that the temperatures tend to be clustered around the mean temperature. This highlights a thermal difference of 1.02 °C, indicating the mean temperature of the control group is significantly higher than that of the injured group. Therefore, the maximum and mean values within the dataset are higher in the injured group, whereas the minimum is lower in the control. This conclusion is validated by the level of significance between the two groups **
p < 0.01, which indicates that the mean dorsum temperature were significantly different between the two groups.
The box plot correspondent to the palmar perspective is depicted in
Figure 6. Similarly to the previous plots, the control group shows a negatively skewed distribution, although more accentuated, with few data points on the upper side and more concentrated in the area of lower temperatures. The injured group shows a normal distribution, with even lower data dispersion than in the posterior part of the hand considering the smaller size of the box, although it presents four outliers below the lower quartile. The maximum mean temperature in the injured group, which reaches 36 °C, is greater than the 34.8 °C exhibited for the control group. However, the thermal difference between the two groups (1.2 °C) is less pronounced when compared to the back of the hand value of 1.8 °C, because both groups display higher maximum values. Regarding the minimum mean value, a value of 27.6 °C is observed in the group of healthy hands and 29 °C in the group of affected hands, showing a thermal difference of 1.4 °C, which is also smaller compared to the dorsal perspective. The mean temperature reveals a greater thermal difference of 1.21 °C between the two groups, with the first group exhibiting an average temperature of 33.34 °C and the second group of 32.13 °C. The standard deviation was, respectively, 1.62 °C and 1.46 °C, indicating low variability of values and suggesting that the temperature values in both datasets are relatively close to the mean. The observed level of significance, denoted as **
p < 0.01, signifies statistically significant differences in mean palm temperatures between the two groups. This box plot allows to take the same conclusions as in the previous, that the mean and maximum temperature values are significantly higher in the injury group and the minimum is notably lower in the control group.
After comparing means between the mean temperatures of the two groups (healthy and injured hands) to get a sense of the distribution and variation of the data, an independent samples T-test was implemented, for each ROI, in the back and palm of the hand, in order to determine if there is a statistically significant difference between the two independent groups. The null hypothesis (H0) states that there is no significant difference in the mean temperature between the two groups. In contrast, the alternative hypothesis (H1) suggests that there is a significant increase in mean temperature within the injury group, in comparison to the control group, which makes this a one-tailed test. In order to determine if the test result is significant, the significance level was set to = 0.05. The sample size consists of 15 control hands and 25 injured hands.
The Levene’s Test for Equality of Variances was conducted to evaluate if the variances of the two groups are significantly different. The results of the test are expressed in
Table 1 and
Table 2, respectively, for the back and palm of the hand. Given that all the
p-values resulting from Levene’s test (Sig.) for both dorsum and palm are greater than 0.05 (
p > 0.05), the null hypothesis (H0) is accepted, which assumes that the variances of the control and injured groups are approximately equal. This indicates that there is no statistically significant difference in variance between these groups.
The outcomes of the independent samples
t-test, used to compare the means of the control and injured groups, with respect to the back of the hand, are displayed in
Table 1. The mean thermal difference corresponds to the difference between the mean temperatures of the injured and the control groups. The calculated mean thermal differences across all the considered ROIs consistently indicate higher mean temperatures within the injured group. Since the
p-values for all considered ROIs are lower than the defined significance level
= 0.05, the null hypothesis can be rejected and the alternative hypothesis can be accepted. Therefore, it can be concluded that the mean temperature within the injury group is significantly higher than the control’s group mean temperature. This increment of temperature can result from inflammatory processes and increased blood flow caused by hand neuropathies and orthopaedic conditions. In addition, factors such as infection can also cause a temperature increase.
Table 2 summarises the results of the independent samples
t-test, employed to compare the mean thermal difference (in °C) between the injured and the control groups for various regions of the hand palm. Similar to the dorsal dataset, all the analysed ROIs exhibit positive mean thermal differences, which indicate that the mean temperatures within the injured group are higher than those in the control group. Given that the
p-values for all the examined ROIs are below the pre-defined significance level of
= 0.05, the null hypothesis is dismissed and the alternative hypothesis is embraced. Hence, it can be highlighted that the mean temperature in the injury group is substantially greater than the mean temperature in the control group.
The increment of temperature in the injury group is evident through the distinctions in thermal patterns between the control hand (left) and the hand with pathology (right), as can be seen in the example of
Figure 7. This illustration reflects a trend, showcasing comparatively cooler patterns in the control hand as opposed to the globally warmer thermal patterns observed in the hand with pathology. Hence, the temperature increase can result from inflammatory processes and increased blood flow associated with hand neuropathies and orthopaedic conditions. Additionally, factors such as infection can also contribute to an increase in temperature. In the context of medical diagnosis, monitoring temperature variations is crucial for identifying and understanding underlying health conditions. It serves as a valuable indicator in assessing the severity of inflammation and understanding the impact of neuropathies and orthopaedic disorders. A comprehensive understanding of these temperature increments aids healthcare professionals in formulating targeted treatment plans and providing more effective patient care.
In order to investigate the potential of infrared thermography as a complementary and validating tool for diagnosing hand neuropathies and musculoskeletal disorders, the results were segmented by pathology to establish associations between thermal differences in specific hand regions. Due to the volume of information, which was extensive for inclusion in a single chart, the data were categorised into three subgroups: CTS, Dupuytren’s contracture, and Osteoarthritis; CuTS, Tenosynovitis of Quervain, and Distal radius fracture; and Hemiparesis, Rhizarthrosis, and Rupture of ulnar collateral ligament. Thus, three radar graphs illustrating thermal asymmetry between pathological hands and their healthy contralaterals were produced for each considered ROI, as illustrated in
Figure 8. Participants with bilateral pathology were appropriately excluded from the dataset, since it would not be possible to compare the injured hand with the contralateral one.
According to
Figure 8, the ROIs exhibiting significant temperature asymmetry for patients with CTS include the median branch, forefinger proximal, middle distal, and thumb proximal with a thermal difference of up to 2.5 °C. Furthermore, the remaining ROIs also exhibited a positive thermal difference, between 0.2 °C and 1.3 °C, indicating warmer thermal patterns in the pathological hands. These outcomes consistently correspond to the expected areas affected by CTS, particularly in the sensory regions associated with the median nerve: median branch, thumb, forefinger, and middle finger. By contrast, patients presenting Dupuytren’s contracture in the fourth finger revealed negative thermal differences in the ring proximal and distal, while the other ROIs’ temperature asymmetry varied between 0 °C and 0.5 °C. Since this pathology is defined by a progressive stiffening of fibrous tissue bands within the palms, resulting in finger contraction, it would be expected that a reduction in blood flow could occur, which may consequently lead to a decrease in temperature in the affected fingers. Although there is less dispersion among the values, the results corresponding to osteoarthritis exhibit more pronounced thermal differences in the middle distal, little proximal, and thumb proximal areas. The observed findings align with expectations as hand osteoarthritis is characterised by inflammation in finger joints, notably at the base of the thumb and in proximal and distal joints.
In patients with CuTS, the most significant temperature asymmetries occurred in the ulnar branch, little metacarpophalangeal, and little distal. This pathology typically affects the areas controlled by the ulnar nerve, that is, the ulnar palmar branch, the little finger, and partially the ring finger. While the results generally align with expectations, there are some inconsistencies, such as the reduction in thermal difference at the proximal joint of the little finger compared to its metacarpophalangeal and distal joints. The temperature asymmetries observed in participants exhibiting Tenosynovitis of Quervain were more pronounced in the thumb metacarpophalangeal and proximal regions and the ulnar branch. This observation aligns with the pathology’s characteristic impact on the thumb’s short extensor and long abductor tendons. However, it is crucial to note that the differences between the ROIs are not substantial enough for diagnostic purposes. Instead, they serve to validate the diagnosis and confirm the patients’ reported complaints. In the case of distal radius fracture, while the thermal differences exhibited significant values (exceeding 0.3 °C), they appear relatively consistent across all ROIs. This uniformity hinders establishing a clear association between the pathology and specific affected areas.
Patients with hemiparesis presented warmer thermal patterns in the three joints of the ring, little, and middle fingers, as well as the median and ulnar branches, and forefinger metacarpophalangeal, with differences ranging from 1 °C to 2.2 °C. Given the nature of this pathology, which involves paralysis on one side of the body, the thermal asymmetries should be consistent across all ROIs. However, the results include exceptions in the thumb metacarpophalangeal and arm regions, where thermal differences fell below 0.3 °C. Regarding rhizarthrosis, the most notable thermal difference was encountered in the thumb metacarpophalangeal (2.4 °C) and proximal (3.1 °C) joints, aligning with expectations given that this condition is characterised by wear and degeneration of the thumb joints. Finally, the temperature asymmetries in cases involving the rupture of the ulnar collateral ligament were most prominent in the thumb metacarpophalangeal joint, along with the three joints of the ring and little fingers, registering a maximum difference of 3.2 °C. The tearing of the ulnar ligament is characterised by radial deviation in the thumb, impacting its metacarpophalangeal joint. Additionally, it may cause tingling in the ring and little fingers. Despite the overall alignment of results with expectations, it is noteworthy that the thermal difference in the thumb metacarpophalangeal joint should theoretically be greater than that in the ring and little joints, given that the rupture occurs precisely in the thumb joint.
The results strengthen the infrared thermography’s validity in highlighting temperature variations corresponding to the expected anatomical impact of specific pathologies, such as CTS, Dupuytren’s contracture, osteoarthritis, and rhizarthrosis.
Although infrared thermography holds promise as a non-invasive method to enhance clinical diagnostics, this study encountered certain limitations. The cross-sectional design of the research prevented the collection of follow-up data on disease progression. A longitudinal analysis, tracking the infrared thermal imaging results of the same patient over time, could further support our findings. Additionally, our careful inclusion criteria excluded over half of the initially considered hands from the dataset. Therefore, these findings might not be applicable to individuals with any complicating factors that could influence infrared thermography. Furthermore, the inherent susceptibility of measured temperatures to environmental conditions, equipment variations, and individual factors such as the menstrual cycle or daily temperature fluctuations requires caution in drawing conclusions solely based on thermal differences.
4. Conclusions
This study evaluated the potential of infrared thermography to provide valuable insights into the diagnosis and differentiation of hand neuropathies and musculoskeletal disorders. In this context, a series of thermal images were obtained from a group of participants with healthy hands (control) and from patients with hands affected by neuropathies and/or musculoskeletal disorders (injured). A statistical analysis was conducted to determine if the mean temperatures in the injury group were greater than in the control group.
The results revealed significant differences in mean temperature between the control and injury groups across all the analysed regions of interest, both on the dorsal and palmar aspects of the hand. In particular, the injury group consistently exhibited higher mean temperatures, implying distinct thermal patterns associated with hand pathologies.
Furthermore, Levene’s test confirmed the equality of variances between the control and injury groups, endorsing the validity of the statistical comparisons. The use of independent samples t-tests across multiple regions of interest highlighted consistent trends of increased mean temperatures in the injury group, further substantiating the potential of infrared thermography for differential diagnosis.
These findings underscore the utility of infrared thermography as a non-invasive and objective tool for assessing hand pathologies, offering a complementary approach to traditional diagnostic methods. The ability to detect thermal differences indicative of underlying conditions opens promising avenues for early detection, monitoring, and management of hand neuropathies and musculoskeletal disorders.
Overall, the integration of infrared thermography in clinical practice could significantly enhance diagnostic precision and improve patient care, facilitating timely interventions and personalised treatment strategies. Future research in this area may expand the scope of applications and refine diagnostic protocols, ultimately contributing to more effective healthcare practices for individuals with hand-related conditions.