Abstract
Nowadays, the world is facing a general problem of resource overconsumption and waste overproduction: to address these two issues, the United Nations delivered the 12th Sustainable Development Goal (SDG), which has the objective of ensuring sustainable consumption and production patterns. Currently, polymers are present in every aspect of our lives and have the disadvantage of mostly coming from fossil sources and causing pollution when disposed of the wrong way. Agriculture plays a key role in the overall world environmental issues, being responsible for the creation of between 13 and 21% of global greenhouse gas (GHG) emissions. Moreover, it represents a continuously growing field, producing large amounts of waste. These residues can cause serious environmental concerns and high costs when disposed. However, agri-food waste (AFW) is a natural source of natural biopolymers, such as lignin, cellulose, pectin, and starch, but can also be used as a substrate to produce other non-toxic and biodegradable biopolymers, such as chitosan, polyhydroxyalkanoates (PHAs), and polylactic acid (PLA) through microbial fermentation. These polymers find applications in agricultural practices such as mulching films, soil stabilizers, hydrogels, nanocarriers, and coating for seeds, fruits, and vegetables. The employment of AFW in the production of non-toxic, sustainable, and biodegradable biopolymers for their agricultural utilization is an example of a virtuous circular economy approach that could help agriculture to be more sustainable.
Keywords:
agri-food waste; biopolymers; agriculture; biomass; lignin; cellulose; pectin; chitosan; PLA; PHA 1. Introduction
In 2023, EU Bioplastics reported that biopolymer global production capacity is expected to reach 7.43 million tons by 2028; however, the market demand corresponds to 400 million tons per year. The large difference between biopolymer supply and demand shows the need for raw material alternatives at sustainable costs to increase production [1]. This is also the reason why plastics of fossil origin are still widely used. Therefore, reducing the consumption of fossil fuels and using renewable and environmentally safe resources will probably be the main advantage of bioplastics, along with the possibility of biodegradability at the end of their life [2]. While biopolymers offer an alternative to fossil-based materials, their production still demands substantial energy input, largely sourced from fossil fuels. One approach involving renewable energy usage relies on the implementation of an integrated system, using AFW-derived energy to power the process (e.g., through AFW incineration to produce steam and electricity) [3].
Worldwide, around 1.3 million tons per year of food produced for human consumption is lost or wasted and this is equivalent to a third of all food produced [4]; for this reason, the United Nations has delivered the 12th sustainable development goal (SDG), which aims to ensure sustainable consumption and production patterns [5]. These losses occur during the processing of fruits and vegetables, as substantial amounts of pulp, stalks, peels, or seeds are generated and are destined for disposal [6]. These undervalued materials are rich sources of biopolymers, such as polysaccharides, proteins, and dietary fiber, as well as other valuable functional molecules such as tannins, flavonoids, polyphenols, and fatty acids [7]. Recently, the reuse of agri-food waste (AFW) as a raw material to obtain biopolymers for application in agriculture has gained increasing interest. Biopolymers with varying purity degrees and properties can be obtained through microbiological fermentation or biotechnological processes, as well as by treatment of biomass waste with enabling technologies to shorten the extraction/conversion times. The employment of sustainable, promising processes for sustainable agricultural operations may help to solve the dual challenges of waste management and natural resource conservation. Utilizing renewable resources, such as AFW, for biopolymer production offers the advantages of lower dependence on fossil fuels, resource recovery, and waste management [8]. The use of AFW mitigates the emission of greenhouse gases (GHGs) [9], for example, the GHG emissions from synthetic plastics production range between 1.8 and 3.55 (t CO2 eq./t plastic). Comparatively, for polylactic acid (PLA), the GHG emissions range between 0.4 and 1.3 (t CO2 eq./t plastic) [10]. Furthermore, the production of biopolymers from waste aligns with the principles of the circular economy, as this process transforms AFW into value-added products, minimizing the environmental impact and promoting efficient use of natural resources. Biopolymers deriving from AFW are indeed a renewable, often biodegradable, non-toxic, and environmentally friendly alternative to polymers obtained from non-renewable sources, such as petroleum [11,12]. However, the production cost of PLA is higher compared to conventional plastics: it costs between 1.31–2.02 € to manufacture petroleum-based plastics, meanwhile polyhydroxyalkanoates (PHA) and PLA cost between 4.00–12.02 € and 0–2.0 €, respectively [13].
In the agricultural sector, biopolymers derived from AFW have several applications, including mulching films, hydrogels (for water, biocides and/or biostimulants accumulation, and controlled release), soil stabilizers, and, lastly, coating for seeds, fruits, and vegetables [14,15]. The potential of using biopolymers to address the urgent challenges of both waste management and agricultural sustainability promises to promote a more resilient and environmentally conscious agricultural system. The sourcing of new, sustainable raw materials for biopolymer production and the examination of the practical implications of using them in agriculture are essential to advance sustainable agricultural practices in the 21st century [2].
The purpose of this review is to define and investigate the potential of AFW to produce biopolymers for agricultural applications; thus, for the sake of this review, only polymers directly deriving from agri-food waste or from microorganisms fed with agri-food waste will be explored. The proposed approach includes: (i) the identification of sustainable strategies to mitigate the environmental issues related to waste disposal, (ii) the process and technologies used to recover higher yields, and (iii) how the biopolymers can lead to higher crop yields and better preservation of fruits and vegetables, among other benefits.
2. Sources of Biopolymers
2.1. Lignin: Extraction Methods and Applications in Agriculture
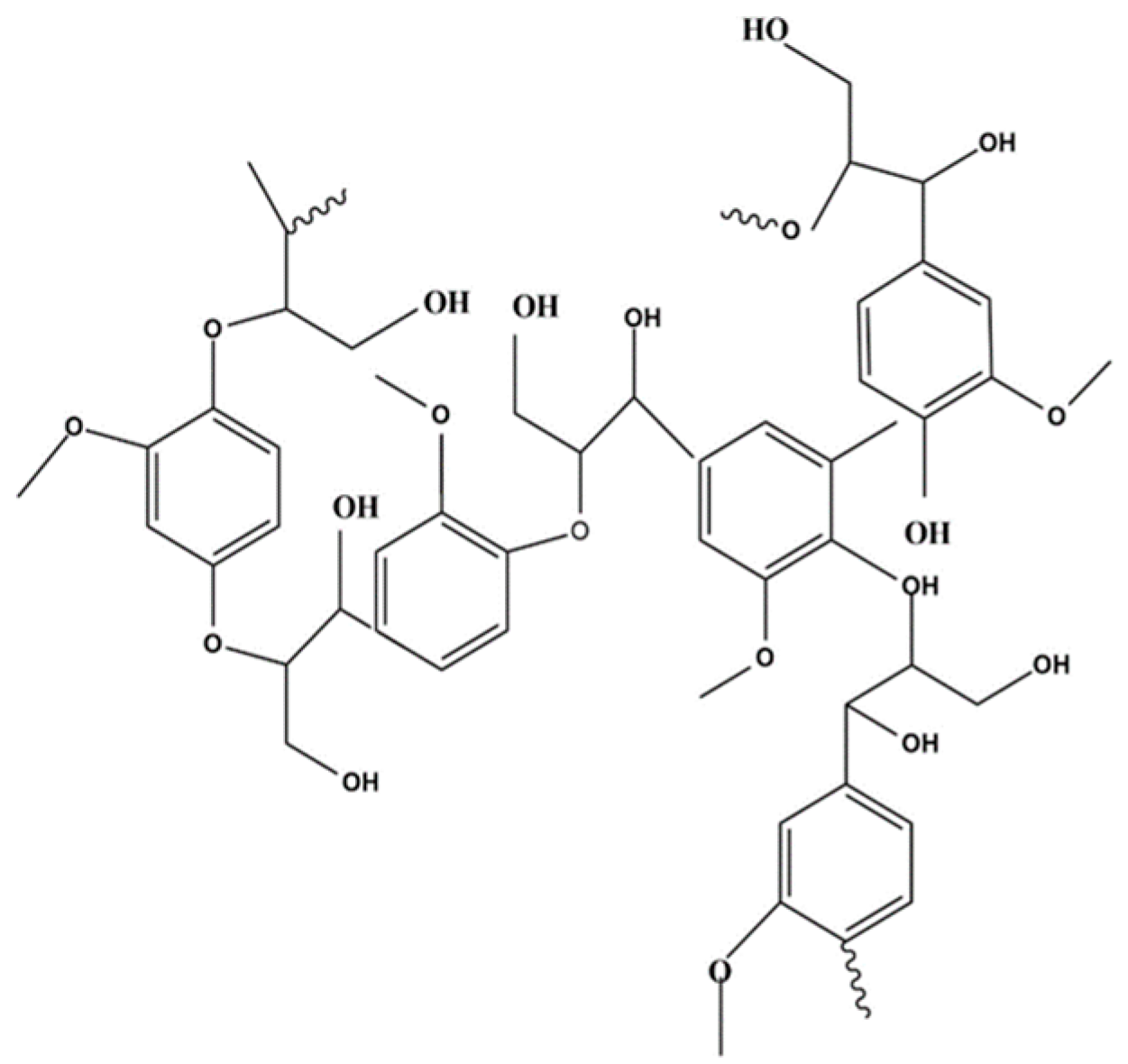
Lignin is a high molecular weight aromatic biopolymer (Figure 1) that can be found mainly in the woody stems of plants, but it is also a component of plant cell walls [16]; this characteristic makes lignin one of the most abundant polymers on earth. This polymer is mainly composed of the monomeric moieties of sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol.
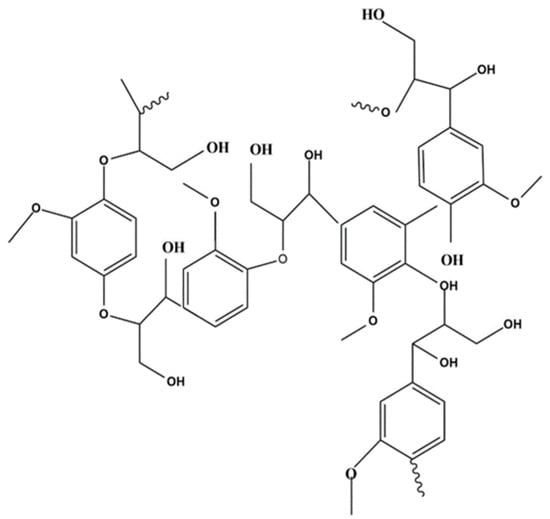
Figure 1.
Lignin chemical structure.
Valorization of lignin, a complex organic polymer found in the cell walls of plants, refers to the processes of converting it into higher-value products. Traditionally, lignin has been considered a by-product of the paper and pulp industry [17], often burnt for energy or used as a low-value filler in various applications. Nonetheless, with increasing interest in sustainable and renewable resources (pruning waste, straw, stalks, and bagasse), there has been growing attention towards finding more valuable uses for lignin.
Overall, lignin valorization holds great promise for reducing the environmental impact of industries relying on lignocellulosic biomass, creating new revenue streams and contributing to the development of a more sustainable bioeconomy. Biorefineries mainly using food industry waste are gaining importance in lignin production. Indeed, the only way to make a biorefinery economically viable encompasses the valorization of all the waste components, including lignin.
However, developing cost-effective and scalable processes aimed at the creation of a market for lignin-derived products is still challenging. Lignin has been explored for different applications in the medical, pharmaceutical, and electrochemical fields and as a plasticizer [18]. Moreover, depolymerized lignin is a platform chemical that can potentially substitute aromatic chemicals deriving from the fossil-fuel industry [18,19]. Delignification usually aims to remove lignin from a lignocellulosic biomass, obtaining a relatively pure holocellulose fraction for further purposes and lignin as a by-product. The literature displays a lot of different delignification methods, such as alkaline, acid delignification, organosolv process, autohydrolysis, or enzymatic methods [20]. The lignin pyrolysis method has also been explored to obtain lignin biochar. Although biochar may have agricultural applications, it is important to note that lignin biochar is usually exploited as a filler/additive for biocomposites, rather than biopolymer itself. Additionally, the roasting method is not effective in reducing GHG emissions [21]. The possibility to further valorize the lignin for different applications paved the way for research aiming to improve delignification processes through combination with green technologies such as microwave, ultrasound (US), or steam explosion, affording higher lignin yields with reduced extraction time. Recently, solvents such as ionic liquids, natural deep eutectic solvents (NaDESs), and dioxane have also been investigated for lignin extraction [20].
Delignification techniques employed in recent studies are shown in Table 1, where the best yields were found coupling alkaline delignification with other different pretreatments.

Table 1.
Delignification processes.
Indeed, Liu et al. (Entry 1) [22] observed that hydrothermal prehydrolysis promoted the depolymerization of lignin macromolecules, facilitating the delignification process. It was also observed that lignin obtained by alkaline delignification has a lower molecular weight and a higher content of hydroxyl groups, making it an interesting source of value-added functional bio-based materials. In addition, Fialho et al. (Entry 4) [25] successfully removed 93% of lignin from corncobs by coupling autohydrolysis with alkaline delignification, which was also more selective in lignin removal compared to dioxane delignification. Another advantage of a two-step delignification process is the fractionation of lignocellulosic components, as the first step of both the reported studies successfully removed and fractionated the hemicellulose fraction from the biomass. Conversely, corncobs treated solely with alkaline delignification afforded a lower delignification yield (Entry 3) [24].
The enzymatic treatment of pineapple leaf waste was reported in Entry 5 [26], presenting an effective removal of lignin from the biomass with high selectivity (78.5%); unfortunately, the lignin-rich fraction obtained was not characterized.
Ultrasound-assisted (UA) delignification using NaDES as green solvents is also an interesting route to explore; in Entry 2 [23], different NaDES were tested with different extraction times (60 to 120 min). When US technology was used in combination with NaDES, a higher delignification time was required; however, higher yields were achieved. This phenomenon probably occurred because NaDES are viscous and need more time for an optimal mass transfer. Overall, chlorine chloride-levulinic acid (ChLevA) afforded the highest yield of delignification and had a similar extraction yield at 60 and 120 min. Unfortunately, the recovery of lignin extracted with NaDES was very low (30 and 10 mg) for ChLA (choline chloride-lactic acid) and ChLevA, respectively, starting from 2.5 g of grape stalks, while for ChLAGly (choline chloride-lactic acid-glycerol), no lignin was recovered. This is probably caused by the depolymerization of lignin promoted by the treatment; moreover, NaDES could act as strong solubilizers towards lignin, preventing its precipitation, because deep eutectic solvents (DES) possess a very high polarity and can form H-bonds with the -OH moieties of lignin. This makes DES very efficient in lignin solubilization, however lignin isolation from the extract can be difficult, owing to the strong interaction with the solvent. However, the lignin precipitated from NaDES extraction was found to be less modified and presented a more cross-linked structure when compared to lignin obtained with alkaline treatment.
Lignin derived from food waste or agri-food by-products serves various purposes, categorizable into agricultural and food industry realms. In the agricultural field, lignin is a widely explored biopolymer, particularly for its application in soil enrichment.
This is because lignin’s intrinsic resistance to biodegradation makes it a durable contributor to soil organic matter [27]. Additionally, it integrates into a diverse range of polymers utilized in various materials, including those in outdoor applications like greenhouse construction and irrigation systems [28]. Moreover, lignin serves as a versatile agent, functioning as a dispersant, surfactant, insecticide [29], fungicide [30], antibacterial agent [31], or fertilizer [32]. Moreover, in the food industry, lignin finds utility as an additive in food packaging materials, enhancing their properties [20].
Poor mechanical properties of lignin make it unsuitable for direct plastic applications, however lignin added to other biopolymers can also improve the characteristics of the polymeric compound, since the chromophores naturally present in lignin absorb UV light and provide UV resistance to the composite [33,34,35,36]. Moreover, the addition of lignin has been proved to improve the thermal stability [37] and the tensile strength of other biopolymers, such as polylactic acid (PLA) [38]. The addition of lignin nanoparticles to a bioplastic derived from Kappaphycus alvarezii has been found capable of enhancing surface roughness, water barrier, hydrophobicity, and antimicrobial properties of the polymer, making it a perfect candidate for fruit packaging [39]. Overall, the incorporation of lignin in plastic biopolymers provides better performances, making them perform well as mulching films. Many works present in the literature investigated the functionalization of polymers with lignin, mainly for their strong antimicrobial characteristics [40,41]. All the characteristics mentioned above make lignin a perfect additive to polymers used for mulching films, food packaging, and fruit and vegetable coatings.
2.2. Pectin: Importance and Applicability as Biopolymer
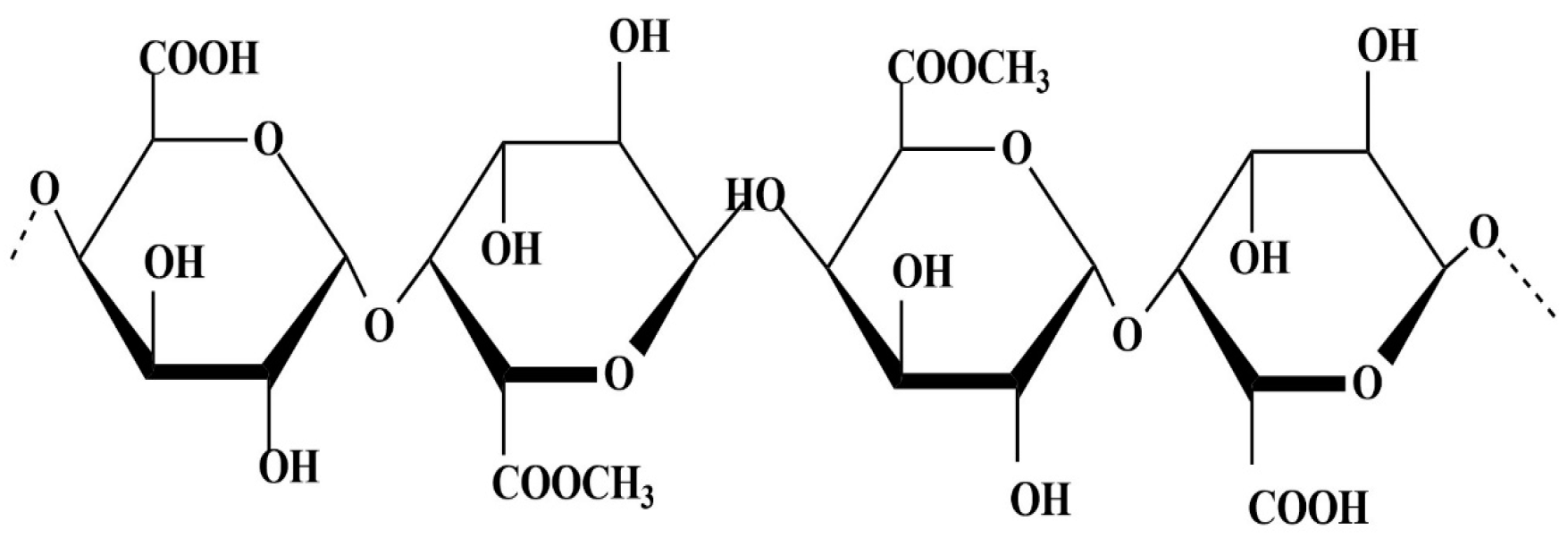
Pectin is a heteropolysaccharide mainly composed of methylated D-galacturonic acid units (Figure 2); this biopolymer can have a different composition, length, degree of esterification, and ramifications. The different characteristics that pectin can undertake are directly linked to the natural variability of the polymer. Pectin is present in all plant tissues, but it is particularly abundant in fruit peels and pomace; for this reason, peels are the part used more often for pectin extraction, with being citrus peel the most common AFW source for commercial manufacturing of pectin [42].
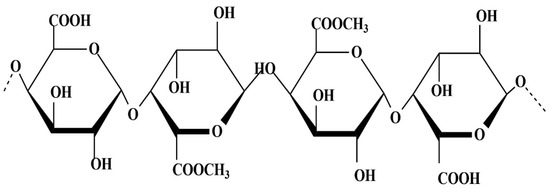
Figure 2.
Pectin chemical structure.
Pectin can be extracted using different methods: the most used one is the extraction under conventional heating (70–100 °C) and acidic conditions (pH 1.5–2.5) [43]. After the extraction step, pectin is usually isolated through ethanol precipitation. However, in conventional processes, pectin yield is lower compared to novel techniques [44].
In Table 2, some recent works on pectin extraction from biomass are reported. The yields and quality of pectin, measured by the degree of esterification, vary depending on the plant source, cultivar, growth conditions, and part of the plant being extracted.

Table 2.
Pectin extraction methods and yields.
For instance, subcritical water extraction (SWE) reveals different optimal extraction temperatures for apple pomace and citrus peel. The yield for apple pomace is 16.68% and for citrus peel is 21.95%, achieved through extractions at 150 °C and 120 °C, respectively. (Entry 1) [45]. In Entries 2 [46] and 3 [47] pectin was extracted from grape pomace and pomegranate peels using similar protocols, but the yield on pomegranate peel is 1.25 times higher in comparison to grape pomace. Furthermore, it can be noticed in Entry 4 [48] that the yield obtained from Citrus maxima albedo is significantly higher compared to the one of flavedo. The extraction method also influenced the amount of pectin recovered: for example, in Entry 5 [49] the yield of pectin on ananas peel was higher by 21.50% using microwave-assisted extraction (MAE) compared to conventional conditions (Table 2, Entry 1).
The usage of different methods and technologies to valorise AFW as a source of pectin represents a promising path, since pectin has different agricultural applications, such as an antimicrobial agent [52] that will help in the biotic control of the soil and will promote growth of crops; used as a hydrogel to retain water, thus helping to maintain soil moisture; and it is also used as a biopolymer to cover fruits, increasing its shelf-life. Therefore, AFW represents a low-priced source of this polysaccharide. For instance, pectin can be applied directly in the agricultural field as a hydrogel agent for drought stress management. A common virulence factor of plant pathogenic bacteria and fungi is the ability to produce pectinases, so plant pathogenic microbes can degrade pectins [53]. However, it has been found that the addition of agricultural waste polysaccharides improved plant growth and increased plant resistance against soil-borne pathogens [54]; additionally, pectin was found to be beneficial to Flavobacterium, a common plant holobiont [55].
Sayed et al. [56], for example, fabricated a guar gum-pectin/polyacrylamide/ZnO super absorbent hydrogel with satisfactory water retention and adequate biodegradation in soil, and Sharma et al. [57] created pectin nanoparticles which showed good performance in the retention of soil moisture. Applying a cascade treatment to orange peel, Figueira et al. (Table 2, Entry 6) [50] were able to obtain pectin with high anhydruyronic acid content and hesperidin, a high added-value compound. Şen et al. [51] (Table 2, Entry 7) observed that the combination of pulsed ultrasound assisted extraction (PUAE) and hot acid extraction (HAE) brought the highest yield on onion waste biomass compared to the two methods used separately.
Pectin is also a very important polysaccharide for fruit coating; Pholsin et al. [58] used cocoa shell powder as a raw material to recover the pectin, that was then used to coat tomatoes and was found to be more successful in maintaining the fruit characteristics compared to both the control and edible coating without pectin. Edible pectin films for fruit protection can also be functionalized with antimicrobial agents to enhance the protection against fruit pathogens, because the pectin does have bacteriostatic properties; in plants they serve as a barrier that protects them from biotic and abiotic stress [59] and pectin-derived saccharides have been found to exert antimicrobial activity [60]. Thus, pectin coating functionalized with trans-cinnamaldehyde was able to reduce weight loss, keep the firmness of the fruit, and reduce pericarp browning while maintaining the taste of rambutan [61].
2.3. Starch: A Promising Biopolymer for Agricultural Applications
Starch is a promising polysaccharide deriving from abundant renewable sources; for certain applications, it can be a valid replacement of traditional plastics. Starch is synthesized by most plants as an energy reservoir, stored in granular form within the cytosol of plant cells. This biopolymer is mainly composed of amylose and amylopectin; this latter forms the main crystalline parts, while the amorphous regions are formed by branches of both. The starch chemical structure can be seen in Figure 3 [62].
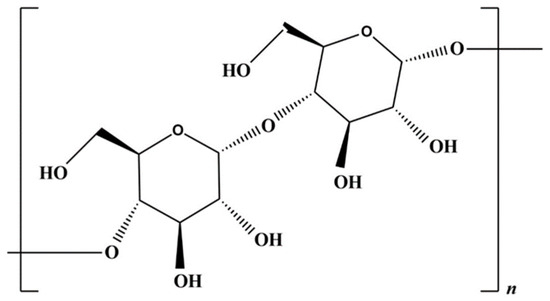
Figure 3.
Starch chemical structure.
Most AFW could pose as a valuable source of starch; indeed, there are several works in the literature that report its extraction from various residual biomasses (Table 3).

Table 3.
Starch extraction techniques and biomass sources.
Hernández-Carmona et al. [63], for example, observed that increasing the concentration of ascorbic acid from 1% to 5% w/v increased the extraction of starch from 23.8% to 32.5%. Kaur et al. (Table 3, Entry 2) [64] found that the highest starch recovery from biomass was obtained using a temperature of 30 °C and an extraction time of 11 h; moreover, the modification of extracted starch with citric acid caused a depletion of swelling power, solubility, and amylose content, coupled with an increase in water absorption capacity. Cassava peel yielded more starch compared to bagasse (Table 3, Entry 3) [65], and this starch was also more thermostable. Starch extracted from pineapple stem was compared to a commercial one, and was found to have a higher amylose content, gelatinization temperature, gelatinization enthalpy, and pasting temperature, making it thermoplastic (Table 3, Entry 4) [66].
Mieles-Gómez et al. (Table 3, Entry 5) [67] extracted starch rich in phenolic compounds with UAE; the employment of this technology enhanced the water-holding capacity, solubility, oil-holding capacity, and swelling power of starch, when compared to the polysaccharide obtained with conventional extraction.
Starch can be employed in agriculture for the creation of mulch films showing beneficial characteristics, such as maintaining soil moisture, preventing soil erosion, limiting the growth of weed species, and regulating solar radiation [62,63,64,65,66,67,68]. Additionally, it can be used for the encapsulation of biocontrol bacteria [69]; this encapsulation protects helpful microorganisms that can prevent the cultivated plant from biotic and abiotic stresses. Starch can also be employed in the construction of superabsorbent polymers for irrigation optimization and controlled release of biocides and biofertilizers [70].
2.4. Cellulose: A Resourceful Biopolymer in All Its Forms
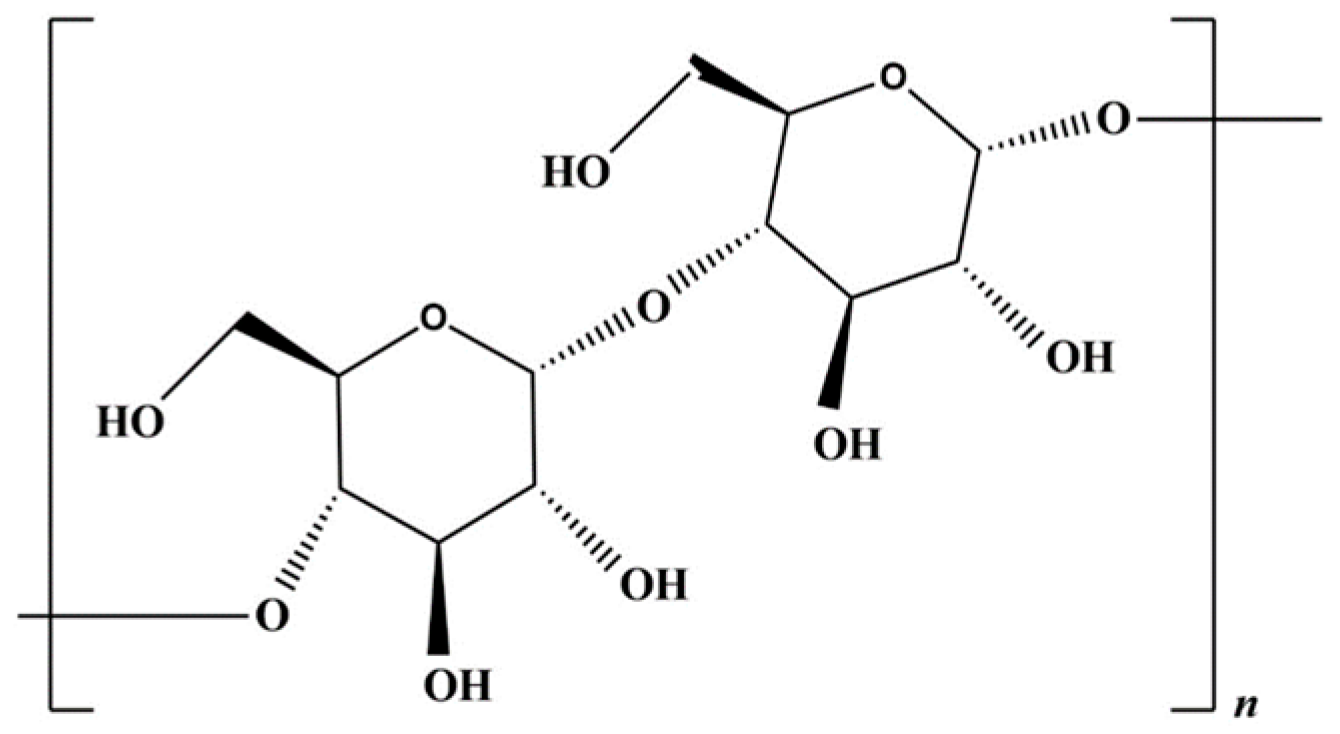
Cellulose is the most common polysaccharide in the world. The chemical structure can be seen in Figure 4: traditionally obtained from plants, it is formed by β-D-Glucopyranose molecules [71]. Nowadays, there is a growing interest in the use of bacterial cellulose (BC), which is characterized by the absence of lignin and hemicellulose, allowing for the avoidance of the downstream purification process. Another advantage is that BC is generally endowed with a high crystallinity and water holding capacity. Furthermore, bacteria are a more sustainable source compared to plants because they do not require extensive land use, which disrupts natural habitats. Additionally, bacteria can feed on waste, making them an environmentally friendly option [72]; however, its high production costs are a strong discouragement for its employment [73].
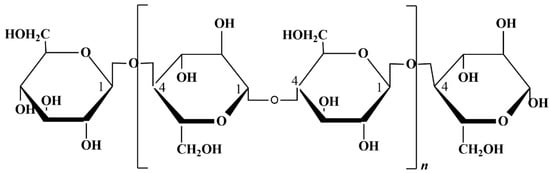
Figure 4.
Cellulose chemical structure.
Cellulose is present in the materials market in various derivative forms, such as methyl and ethyl cellulose, carboxymethyl cellulose (CMC), cellulose acetate, cellulose sulphates, amino celluloses, and hydroxypropyl methylcellulose. Cellulose can be present in crystalline, semicrystalline, or amorphous form, in nano or microparticles and in nanofibrils: each of these types of cellulose has its peculiar characteristics and can be used in the production of different materials [74].
As is evident in Table 4, cellulose purification is usually preceded by a delignification step and is often achieved by bleaching the sample or treating it under mild acid/alkaline conditions.

Table 4.
Cellulose purification from agri-food waste.
Palm leaves (23.5% of lignin) treated with alkaline hemicellulose removal followed by bleaching yielded a final cellulosic solid with a relatively high degree of purity (87.12%) and crystallinity (69.9%) (Table 4, Entry 1) [75]. By carrying out the cascade removal of hemicellulose and lignin, Fialho et al. (Table 4, Entry 2) [25] obtained a final solid with a glucan content of 77% after both organosolv and alkaline delignification.
The production of CMC from young and mature coconut coir is reported in Entry 3, reporting an improvement in the characteristics directly proportional to the number of bleaching times; it was also documented that mature coconut coir yields more cellulose and more CMC compared to the young ones [76].
Cellulose purification can also be done with biological methods involving, for example, the use of a consortium of two ligninolytic fungal strains with very low levels of cellulase activity (Table 4, Entry 4) [64], or with thermosolvent laccase enzymes combined with steam treatment and ethanol (Table 4, Entry 5) [78].
As mentioned before, cellulose can be also obtained in the form of BC, considering that BC-forming microorganisms can grow on AFW. Recent works focusing on BC production from waste are detailed in Table 5, revealing relatively modest yields and final titers. Future research efforts should concentrate on optimizing fermentation conditions to increase BC yield, as feeding waste biomass to bacteria is not enough to make the process economically feasible.

Table 5.
BC from waste biomass.
In this context, another cost-cutting strategy is the approach adopted by Karanicola et al. (Table 5, Entry 1) [79], as an alternative to fermentation optimization. This approach was aimed at the full utilization of the biomass, which entailed the extraction of valuable compounds such as essential oils and pectin before the fermentation; using this method, the cost of the entire process is supported by the obtainment of multiple value-added molecules from biomass.
Cellulose and its derivatives possess numerous applications in agriculture, as they have been employed for the controlled release of fertilizers [81] and essential micronutrients [82]. Another use of cellulose is as a fruit coating agent for ripening delay [83] or as a hydrogel for drought management [84,85]. Moreover, mulch films obtained with a CMC–PVA (polyvinyl alcohol) blend were able to degrade within two months [86], while those formed with CMC and soybean protein, compared with high density polyethylene (HDPE), led to an increased germination of cabbage seeds [87]. The production of mulch films was also found to be feasible in combination with antimicrobial elements such as silver nanowires for an enhanced crop protection [88].
2.5. Chitosan, an Important Adjuvant for Plant, Fruit, and Seed Protection
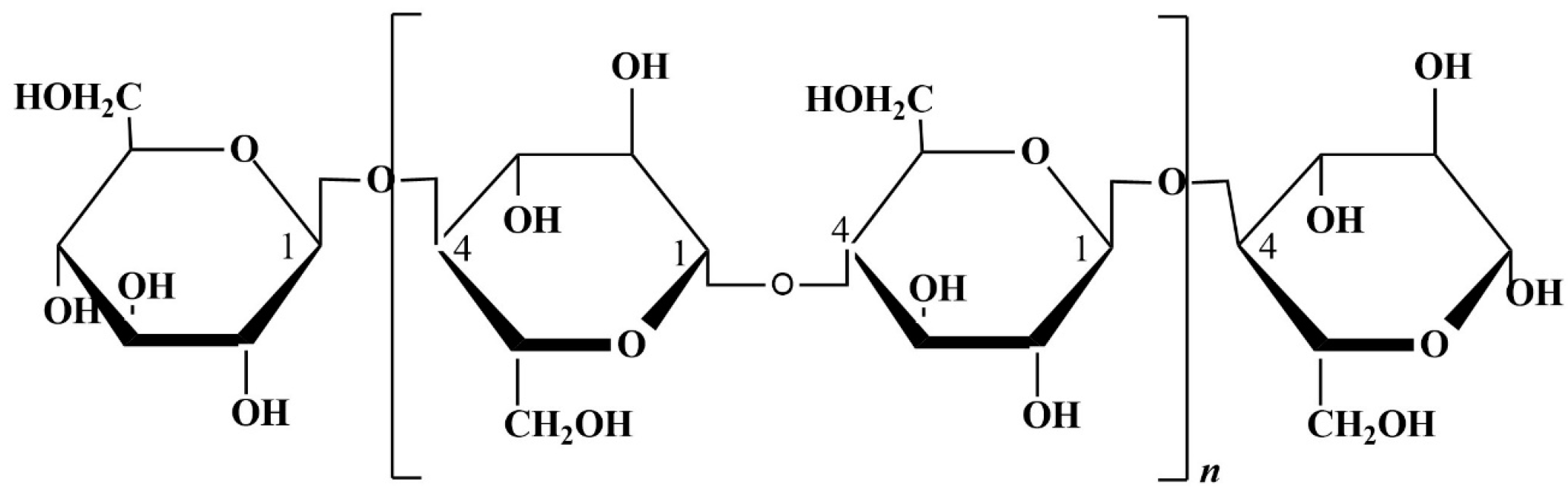
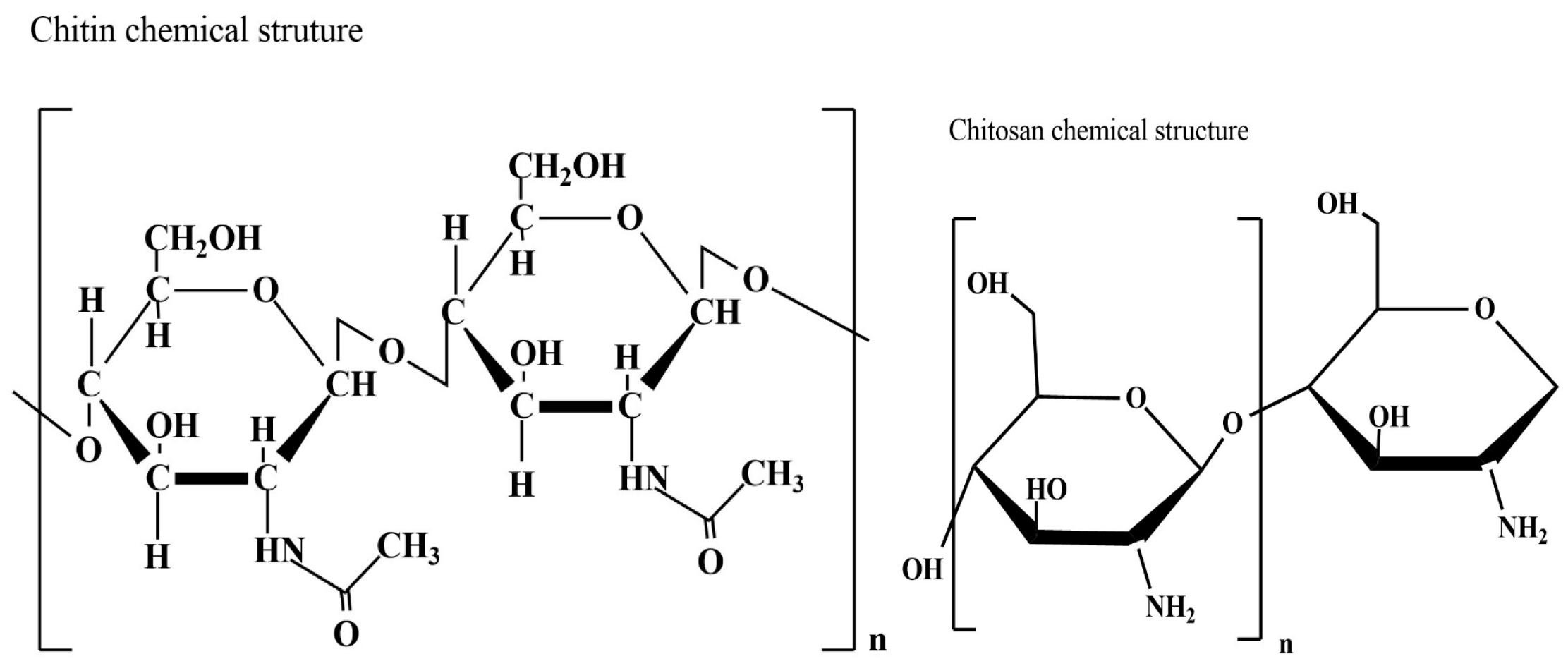
Chitin is the most abundant amino polysaccharide present in the world; it is formed by N-acetylglucosamine (GlcNAc) residues [89], and it is present in nature in crystalline forms of α, β, and γ-chitin. Traditionally, the main source of chitin is the crustacean waste deriving from the fishing industry [90], but it is not as sustainable as fungal chitin. Chitosan is a direct derivative of chitin obtained usually by alkaline deacetylation [91]; the result is a copolymer formed by β (1–4) linked D-glucosamine (GlcN) units. Chitosan (Figure 5) is characterized by a variable percentage of GlcNAc residues, as deacetylation is never complete. Chitin, and consequently chitosan, can be extracted from fungal waste biomass as a secondary product; in addition, fungi can also successfully grow on agri-food waste.
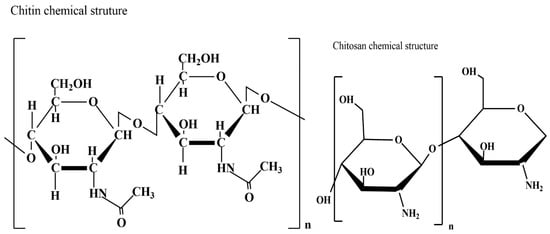
Figure 5.
Chitin and chitosan chemical structure.
Tayel et al. [92] successfully extracted chitosan from Aspergillus niger fungal waste following the production of citric acid, a common food additive. Mucor circinelloides was able to grow on sugar bagasse hydrolysate and corn steep solids, and a total of 1.36 g/L of chitosan was extracted from the fungal biomass [93]. Habibi et al. [94] obtained chitosan from Aspergillus terrerus grown using apple waste as the sole carbon source. Corn steep liquor and Cassava wastewater were used as a carbon and nitrogen source by Mucor subtilissimus UCP 1262 and Lichtheimia hyalospora UCP 1266; the chitosan extracted from the deriving fungal biomass exhibited a deacetylation degree >80%, comparable to commercial chitosan [95].
In each of the papers mentioned, the fungal biomass first underwent an alkaline treatment, then chitosan was extracted from the alkali-insoluble fraction using a mild acid treatment.
The antimicrobial properties of chitosan are useful in agriculture, as they can be employed to increase the shelf life of fresh fruit [93,96]. Moreover, chitosan can also be used in seed protection, since it facilitates the attachment of the biological priming agent Trichoderma asperellum to the seed [97], therefore significantly increasing seedling germination and inhibiting diseases [98]. Chitosan nanoparticles can also be used for the controlled release of herbicides, as they have been successfully used to encapsulate imazapic and imazapyr to reduce their toxicity and increase the efficacy of the treatment [99].
2.6. Polyhydroxyalkanoates (PHAs): A Versatile and Biodegradable Bioplastic
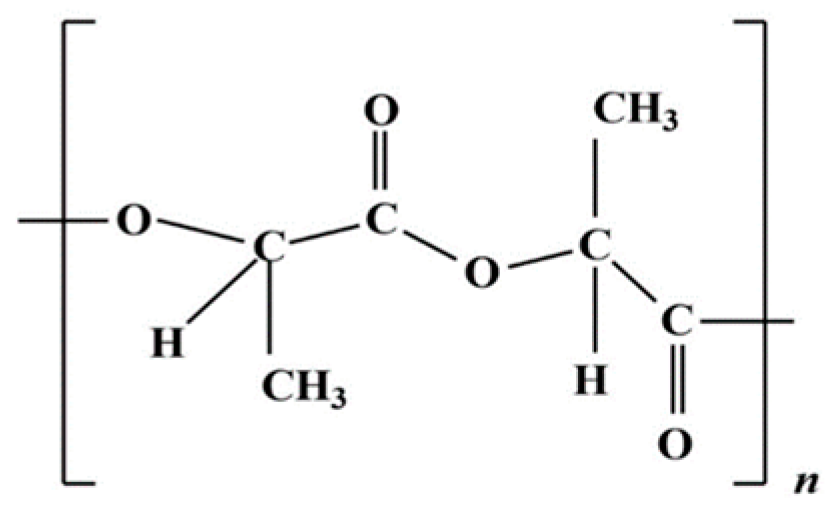
Chemically, PHAs are polyesters consisting of hydroxyalkanoate monomers linked by ester bonds. The general chemical structure of PHAs can be seen in Figure 6. PHAs are biopolymers naturally produced by several bacteria and archaea and stored under the form of granules inside the cell as a reserve carbon source. The specific chemical structure of PHAs can vary widely depending on the microbial species producing them, the carbon source provided during cultivation, and the environmental conditions. To synthetize PHAs, these microorganisms usually use glucose or fatty acids as a substrate: AFW can be a good and cheap source of these compounds, thus representing a good strategy for cutting costs, given that PHA is 15 times more expensive than petroleum-based plastics [100].
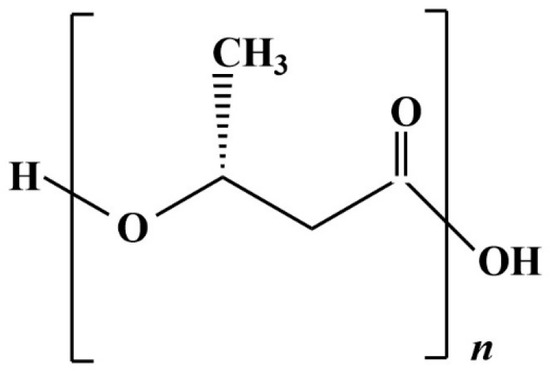
Figure 6.
Polyhydroxyalkanoates chemical structure.
Some recent works are reported in Table 6.

Table 6.
PHA production from agri-food waste.
Campanari et al. [101] (Table 6, Entry 1) utilized a multi-stage process in which they selected PHA-storing microorganisms under feast and famine (FF) conditions in a sequencing batch reactor (SBR) and found out that the PHA production is widely influenced by the selection of PHA producers, which is in turn influenced by the organic loading rate (ORL). Verdini et al. (Table 6, Entry 2) [102] used corn straw saccharified with the aid of microwave technology and obtained a greater yield during scaling up in a stirred tank 3L bioreactor, compared to a shake flask. Moreover, the authors were also able to determine the specific PHA that was being produced, which was poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). Similarly, Kag et al. (Table 6, Entry 6) [106] saccharified a waste biomass to accumulate PHA, however they obtained a lower final titer.
Razzaq et al. [103] (Table 6, Entry 3) determined that the type of PHA monomer is influenced by the selection of the substrate, as C.necator usually produces short-chain length PHAs, but with the mixture that they used as a substrate (without the addition of a nitrogen source), they were able to produce medium-chain length PHAs. In Entry 4 [104], P. monteilii was grown on sugarcane bagasse black liquor through a two-phase fermentation: (i) optimization of cell growth in batch configuration, (ii) pulse-feeding in fed-batch configuration, with the C:N ratio finely tuned to maximize the production of PHA. During the growth phase, the percentage of PHA in the total cell concentration was less than 1%; however, in the accumulation phase this percentage was increased to 12.8%.
Matos et al. [63] (Table 6, Entry 5) proposed a three-step valorization of fruit waste on a pilot scale: the first step aimed to convert the biomass into soluble fermentation products (SFP) through fermentation, then the SPF media, rich in butyrate, was fed to the second and third steps. The second step was aimed at microbial culture selection to improve PHA yield, and the third step involved PHA accumulation. Overall, the process was able to afford a remarkable maximum PHA content of 80.5% and a storage yield of 0.98 gCOD·gCOD−1; the overall process yielded 0.45 gCOD·gCOD−1. A more complex fermentation set-up, comprising the separation of SPF production step and the PHA creation, and the accumulation step can dramatically improve the PHA yield.
The usage of PHAs in agriculture has been explored mainly as mulch films [107,108]; for this application, PHA is usually blended with other biodegradable components to improve its processability. For example, the addition of medium-chain length PHA increased the processability of PLA [109] and slowed down its biodegradation rate [108]. It was found that the addition of a small percentage of UV stabilizer drastically slowed down the degradation rate of polybutylene adipate terephthalate (PBAT)/PHA mulching films when subjected to accelerated aging test (AAT). On the other hand, the addition of a hydrolysis resistant additive was found to be ineffective in protecting the mulching film from degradation [110]. Othman et al. [111] tested different combinations of PHA/polycaprolactone (PCL) films for active mulching purposes and found them to be a good and biodegradable alternative to traditional ones, as they improved rice seed germination.
2.7. Polylactic Acid (PLA): A Non-Toxic Alternative for Mulching Films and Food Packaging
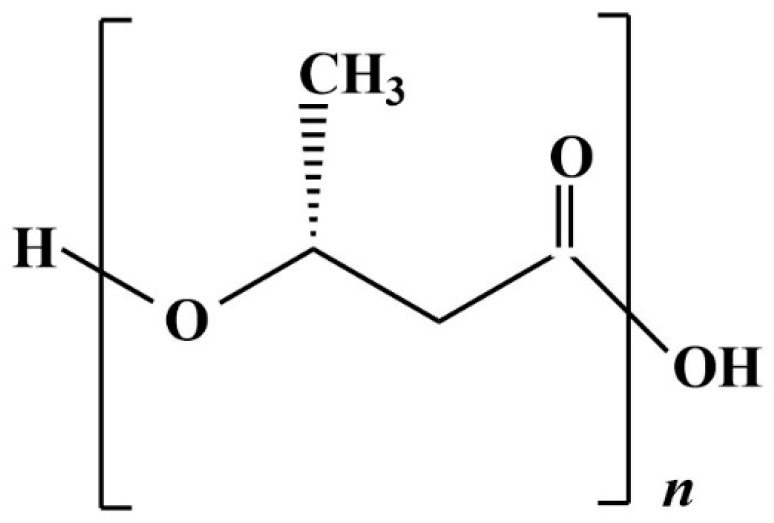
PLA is a biopolymer with properties like those of conventional plastics deriving from fossil fuels; the PLA chemical structure can be seen in Figure 7. Since the properties of PLA are better when the polymer consists exclusively of the stereoisomer L- (L-LA) [112], the cheaper way is trough fermentation. Lactic acid (LA) can derive from the fermentation catalysed by a wide range of microorganisms that can, consequently, grow on and exploit a wide range of substrates; for this reason, a broad spectrum of AFW finds its usage in this stream of production. The optimization of the use of AFW as fermentation substrates to produce LA is crucial for both a waste valorization approach and for the cost reduction of the process [113]. Before the fermentation step, the biomass typically undergoes a pre-treatment, which may be physical, biological, chemical, or a combination of these methods: this is because agricultural by-products, which are usually lignocellulosic, need to be delignified and saccharified for the microorganism to use them as substrates [114]. Alternatively, the process needs to employ a microorganism with both the capacity to degrade complex lignocellulosic biomasses and to produce LA; this approach is called consolidated bioprocessing (CBP) [115].
Figure 7.
Polylactic acid chemical structure.
In Table 7, some of the agricultural waste biomasses that have been investigated as substrates to produce LA are reported. In this case, different microorganisms can afford different yields of LA.

Table 7.
Biotransformation of biomass in LA.
An example of this was the study elaborated by D’Ambrosio et al. [116] (Table 7, Entry 1) where a maximum theoretical yield of LA from glucose was obtained; however, the LA yield calculated on the starting biomass was affected by a lower yield in the previous step (38.1%, enzymatic saccharification). Entries 2 and 3 [117] report a non-sterile B. coagulans DSM2314 fermentation that led to satisfying yields when fermenting xylose-rich hydrolysates; in fact, they were very similar to the one obtained when fermenting pure xylose (0.77 g LA/g Xyl); moreover, the lack of sterility is an advantage as it greatly reduces operation costs.
Liu et al. [119] (Table 7, Entry 5) followed a different path when treating corncob residue, as they applied an alkali delignification followed by a whole-cell enzymatic saccharification with C. thermocellum ΔpyrF: p2638-BGL, an engineered strain with enhanced β-glucosidase activity [120]. The saccharified fraction was then fermented by G. stearothermophilus 2H-3, a contaminant strain found in corncob residue that can produce high quantities of LA with very little substrate competition by other fermentation products. Another advantage of this fermentation method is that saccharification and fermentation can be carried out in a one-pot successive fermentation.
Despite the advantage of not requiring additional pre-treatments, CBP with R. oryzae [118] (Table 7, Entry 4) afforded a much lower LA final titer and yield on biomass, but it is worthy to note that this bioprocess mainly aimed to produce bioethanol, which was obtained with a final titer of 18.83 g/L.
Overall, to enhance the competitiveness of PLA compared to fossil-derived plastics, a thorough optimization process is required, encompassing biomass pre-treatment, fermentation parameters, and, ultimately, the selection of bacterial and fungal strains [121].
PLA, being both biodegradable and non-toxic, is a particularly useful biopolymer in agricultural applications. To be specific, PLA has been widely investigated in its role in the formation of mulch films [122] and food packaging [123] and has been proposed for the construction of shade nets and greenhouse covers [124].
The use of lignocellulosic waste such as wood flour [125,126], cork [125], rubber wood sawdust [127], bark [128], spinach stems, tomato pomace, cocoa shells [122], and orange peels [129] as fillers in PLA films successfully improved its mechanical characteristics.
Poly (lactic acid) (PLA) is a biodegradable, bio-based polymer that has attracted a lot of attention as an alternative to conventional petroleum-based plastics [112]. However, it is not the only sustainable alternative. Others include polybutylene succinate (PBS) and polybutylene adipate terephthalate (PBAT), which have several advantages and limitations compared to PLA [130,131].
PBS is a biodegradable polyester that has properties similar to polypropylene. It is derived from succinic acid and 1,4-butanediol, which can be produced from renewable resources. PBS offers good thermal and mechanical properties, making it suitable for various applications, such as packaging films, bags, and disposable items. One advantage of PBS over PLA is its greater flexibility and strength, which can be an advantage in certain applications. However, PBS biodegrades at a slower rate than PLA and can be more expensive to produce [130].
PBAT, on the other hand, is another biodegradable polyester that is often used in blends with PLA to improve its flexibility and strength [132]. PBAT is synthesized from adipic acid, 1,4-butanediol, and terephthalic acid, all of which can be obtained from renewable resources. PBAT offers good flexibility and elongation properties [132], making it suitable for applications that require elasticity [131], such as compostable bags and films [130]. Compared to PLA, PBAT generally has better elongation at break and toughness. However, depending on the specific formulation, it may have lower stiffness and heat resistance than PLA [130].
Although both PBS and PBAT offer advantages over PLA in terms of certain mechanical properties and flexibility, they can also present challenges, such as slower biodegradation rates, higher production costs, and potential compatibility issues in blends [130]. In addition, the choice between these polymers often depends on application-specific requirements, including mechanical performance, biodegradability, and cost considerations. However, PLA is currently most used in agricultural applications due to its high biodegradability and biocompatibility, making it more suitable for sustainable agricultural applications [130].
3. Sustainable Strategies and Future Challenges for AFW Disposal
The sustainability of agricultural supply chains has become a priority concern in recent years, driven by growing awareness of environmental issues, resource scarcity, and the need to feed a growing world population [133].
The challenge at hand involves managing waste generated throughout the supply chain, from farm to fork. Innovative waste management models are emerging as important tools to address this issue and improve the sustainability of food supply chains [31]. AFW recovery holds great potential for economic, social, and environmental benefits. Many countries around the world have implemented food and waste recovery strategies. The reuse of AFW encompasses the conversion into useful products, such as biofuels and chemicals [134]. To achieve this goal, various methods have been exploited to add value to AFW, including pyrolysis, anaerobic digestion, decomposition, chemical and thermal treatment, solid catalysis, extraction, and fermentation [31,135].
Biorefineries are an innovative approach to waste management in the agricultural food supply chain, aiming for the complete utilization of all the components of AFW. This goal represents an environmentally friendly solution, as it encompasses advanced biochemical and biotechnological processes to convert organic waste streams into high-value products such as biofuels, biochemicals, and bioplastics. By utilizing by-products that would otherwise be discarded, biorefineries not only reduce waste, but also contribute to the development of a more sustainable and resource-efficient supply chain. Governments play a critical role in facilitating the transition to innovative waste management models in agri-food supply chains. By implementing supportive policies, regulations, and financial incentives, policy makers can encourage investment in sustainable waste management infrastructure, drive research and development of waste reduction technologies, and create a favourable environment for innovation and collaboration [136].
The development toward the sustainability of food supply chains depends on the adoption of innovative waste management models that prioritize resource efficiency, minimize waste generation, and create value from waste streams. Converting agricultural waste into potential valuable products and closing the loop aligns with the principles of the circular economy in agriculture and requires collaboration among all actors in the food supply chain.
Adopting a circular economy approach, harnessing technological advances, promoting biorefineries and by-product recovery, fostering collaborative partnerships, engaging consumers, and providing policy support and incentives is crucial to build resilient, sustainable, and environmentally friendly agri-food supply chains.
4. Conclusions
Fossil-based plastics represent one of the greatest problems of this last century; being fabricated from non-renewable resources and being (mostly) non-biodegradable, they contribute to GHG emissions and pollution both at the beginning and at the end of their life [137].
This review explores the feasibility of converting AFW into biopolymers and the possible employment of this in agriculture as mulch films, hydrogels, biocides, biostimulants, soil stabilizers, and for seed, fruit, and vegetable coatings [14,15]. Biopolymers can represent a good alternative to fossil-derived plastic, as they are renewable and often biodegradable. Replacing conventional plastics with biopolymers is desirable, as it represents a significant step towards sustainability, given the ubiquitous use of polymers across various industries.
The agricultural sector, and, more broadly, the food industry, would be one of the first fields to benefit from the use of biopolymers, as they can help mitigate the environmental footprint of an industry already heavily impacting the environment. Moreover, the employment of biopolymers would improve the safety of the food produced, as microplastics are a potential health hazard that is becoming more and more alarming [138]. Furthermore, the capacity of biopolymers to biodegrade helps to simplify the agricultural process (e.g., mulching films composed by biopolymers do not have to be removed anymore since they naturally biodegrade). However, some gaps still need to be filled for biopolymers to be considered a valid commercial alternative to fossil-based plastic; further research should be done on the improvement of mechanical properties of biopolymers. Indeed, since they are often too fragile for their intended use, mixtures of biopolymers pose as a feasible alternative to modulate the polymer features, making them suitable for the intended application.
According to an economic point of view, the employment of AFW helps with cutting the cost of operations, but not enough to compete with traditional plastic biopolymers. An overall process optimization is necessary to improve employment and popularity of biopolymers in the agricultural field. The employment of a cascade process where more than one compound is produced from AFW can help to cut the cost of production, by producing value-added products. In processes where microbial production is concerned, optimization of the fermentation parameters and genetic strain improvement are crucial for the economic feasibility of the process.
Future actions should focus on researching ways to optimize processes, in order to make them more economically viable and more environmentally friendly. Each region should focus on local AFW production to reduce costs and greenhouse gas emissions caused by transportation. Furthermore, investing in renewable energy sources, such as solar or wind energy to enhance AFW, can further strengthen sustainability efforts, ensuring that our actions can pave the way for a greener and more sustainable future.
When the process of converting AFW into biopolymers is optimized and scaled at the industrial level, the costs of the final product will be reduced, and biopolymers can become more competitive than traditional plastic, thus making the agro-industrial sector more sustainable.
Author Contributions
Writing—original draft preparation, C.V. and M.V.; writing—review and editing, S.T., E.C.G. and G.C.; supervision, C.F. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Turin (Ricerca Locale 2023), and the Agritech National Research Center and received funding from the European Union Next-GenerationEU (Piano Nazionale di Ripresa e Resilienza (PNRR)—missione 4 componente 2, investimento 1.4—D.D. 1032 17/06/2022, CN00000022).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AFW | Agri-food waste |
| NaDES | Natural deep eutectic solvents |
| UA | Ultrasound assisted |
| US | Ultrasounds |
| ChLA | Choline chloride-lactic acid |
| ChLevA | Choline chloride-levulinic acid |
| ChLAGly | Choline chloride-lactic acid-glycine |
| PLA | Polylactic acid |
| SWE | Subcritical water extraction |
| MAE | Microwave assisted extraction |
| PUAE | Pulsed ultrasound assisted extraction |
| HAE | Hot acid extraction |
| BC | Bacterial cellulose |
| CMC | Carboxymethyl cellulose |
| PVA | Polyvinyl alcohol |
| HDPE | High density polyethylene |
| GlcNAC | N-Acetylglucosamine |
| GlcN | Glucosamine |
| FF | Feast and famine |
| SBR | Sequencing batch reactor |
| ORL | Organic loading rate |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| SFP | Soluble fermentation products |
| PBAT | Polybutylene adipate terephthalate |
| AAT | Accelerated aging test |
| PCV | Polycaprolactone |
| LA | Lactic acid |
| GHG | Greenhouse gases |
References
- Bioplastics Market Development Update 2023. Available online: https://www.european-bioplastics.org/market/ (accessed on 19 February 2024).
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.R.; Barlow, C.Y. Life Cycle Assessments of Biodegradable, Commercial Biopolymers—A Critical Review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Jorge, A.M.S.; Gaspar, M.C.; Henriques, M.H.F.; Braga, M.E.M. Edible Films Produced from Agrifood By-Products and Wastes. Innov. Food Sci. Emerg. Technol. 2023, 88, 103442. [Google Scholar] [CrossRef]
- Ensure Sustainable Consumption and Production Patterns. Available online: https://sdgs.un.org/goals/goal12 (accessed on 20 February 2024).
- Voss, M.; Gaudino, E.C.; Tabasso, S.; Forte, C.; Cravotto, G. Current Emerging Green Technologies for the Valorization of Grape and Cherry Wastes. Curr. Food Sci. Technol. Rep. 2023, 1, 47–61. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Use of Herbs, Spices and Their Bioactive Compounds in Active Food Packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef]
- Bolaji, I.; Nejad, B.; Billham, M.; Mehta, N.; Smyth, B.; Cunningham, E. Multi-Criteria Decision Analysis of Agri-Food Waste as a Feedstock for Biopolymer Production. Resour. Conserv. Recycl. 2021, 172, 105671. [Google Scholar] [CrossRef]
- Nabuurs, G.-J.; Aoki, L.; Humpenöder, F.; Boone Kauffman, J.; Ayala-Niño, F.; Emmet-Booth, J.P.; Mrabet, R.; Abu Hatab, A.; Bustamante, M.; Clark, H.; et al. 7—Agriculture, Forestry and Other Land Uses (AFOLU). In Climate Change 2022—Mitigation of Climate Change: Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023. [Google Scholar] [CrossRef]
- Fahim, I.S.; Chbib, H.; Mahmoud, H.M. The Synthesis, Production & Economic Feasibility of Manufacturing PLA from Agricultural Waste. Sustain. Chem. Pharm. 2019, 12, 100142. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Pu, W.; Shen, C.; Wei, B.; Yang, Y.; Li, Y. A Comprehensive Review of Polysaccharide Biopolymers for Enhanced Oil Recovery (EOR) from Flask to Field. J. Ind. Eng. Chem. 2018, 61, 1–11. [Google Scholar] [CrossRef]
- Maraveas, C. Environmental Sustainability of Greenhouse Covering Materials. Sustainability 2019, 11, 6129. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L. Biopolymers: Biomedical and Environmental Applications; Scrivener Publishing: Austin, TX, USA, 2011; ISBN 978-0-470-63923-8. [Google Scholar]
- Klein, M.; Poverenov, E. Natural Biopolymer-Based Hydrogels for Use in Food and Agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Brunow, G. Lignin Chemistry and Its Role in Biomass Conversion. In Biorefineries-Industrial Processes and Products: Status Quo and Future Directions; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; Volume 2, pp. 151–163. ISBN 3527310274. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Systematic Review on Lignin Valorization in the Agro-Food System: From Sources to Applications. J. Environ. Manag. 2022, 317, 115258. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A Review on Lignin Pyrolysis: Pyrolytic Behavior, Mechanism, and Relevant Upgrading for Improving Process Efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Peng, X.; Xu, Y.; Tang, T.; Wei, S.; Liu, B.; Wang, D.; Wang, H.M.; Jiang, J. Macromolecular Structural Characteristics and Functional Potential of Tobacco Stalk Lignin from the Phosphotungstic Acid-Assisted Delignification Process. Biomass Bioenergy 2023, 170, 106706. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Tabasso, S.; Calcio Gaudino, E.; Moreno, A.; Mariatti, F.; Cravotto, G. An Innovative, Green Cascade Protocol for Grape Stalk Valorization with Process Intensification Technologies. Appl. Sci. 2022, 12, 7417. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhu, L.; Liang, J.; Zhu, J. Production of Hemicellulose Sugars Combined with the Alkaline Extraction Lignin Increased the Hydro-Depolymerization of Cellulose from Corn Cob. Sustainability 2023, 15, 9041. [Google Scholar] [CrossRef]
- Fialho, J.; Moniz, P.; Duarte, L.C.; Carvalheiro, F. Green Fractionation Approaches for the Integrated Upgrade of Corn Cobs. ChemEngineering 2023, 7, 35. [Google Scholar] [CrossRef]
- Banerjee, R.; Chintagunta, A.D.; Ray, S. Laccase Mediated Delignification of Pineapple Leaf Waste: An Ecofriendly Sustainable Attempt towards Valorization. BMC Chem. 2019, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Hernes, P.J.; Kaiser, K.; Dyda, R.Y.; Cerli, C. Molecular Trickery in Soil Organic Matter: Hidden Lignin. Environ. Sci. Technol. 2013, 47, 9077–9085. [Google Scholar] [CrossRef] [PubMed]
- Yiamsawas, D.; Kangwansupamonkon, W.; Kiatkamjornwong, S. Lignin-Based Nanogels for the Release of Payloads in Alkaline Conditions. Eur. Polym. J. 2021, 145, 110241. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Tian, D.; Shen, F.; Wan, X.; Xu, L.; Chen, Y.; Zhang, H.; Hu, J.; Shen, F. Fabrication and Characterization of Lignin–Xylan Hybrid Nanospheres as Pesticide Carriers with Enzyme-Mediated Release Property. Soft Matter 2020, 16, 9083–9093. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Beckers, S.J.; Yiamsawas, D.; Thines, E.; Landfester, K.; Wurm, F.R. Targeted Drug Delivery in Plants: Enzyme-Responsive Lignin Nanocarriers for the Curative Treatment of the Worldwide Grapevine Trunk Disease Esca. Adv. Sci. 2019, 6, 1802315. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.; Valle, C.; Gaudino, E.C.; Tabasso, S.; Forte, C.; Cravotto, G. Unlocking the Potential of Agrifood Waste for Sustainable Innovation in Agriculture. Recycling 2024, 9, 25. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, X.; Tong, Z.; Liu, G.; Meng, S.; Yang, Y.; Helal, M.I.D.; Li, Y.C. Lignin-Clay Nanohybrid Biocomposite-Based Double-Layer Coating Materials for Controllable-Release Fertilizer. ACS Sustain. Chem. Eng. 2020, 8, 18957–18965. [Google Scholar] [CrossRef]
- Muhammed, A.P.; Thangarasu, S.; Oh, T.H. Green Interconnected Network Structure of Chitosan-Microcrystalline Cellulose-Lignin Biopolymer Film for Active Packaging Applications. Int. J. Biol. Macromol. 2023, 253, 127471. [Google Scholar] [CrossRef]
- Rodrigues, J.S.; de Freitas, A.D.S.; Lopes, H.S.M.; Pires, A.A.F.; Lemes, A.P.; Ferreira, M.; Botaro, V.R. Improvement of UV Stability of Thermoplastic Starch Matrix by Addition of Selected Lignin Fraction—Photooxidative Degradation. Int. J. Biol. Macromol. 2023, 230, 123142. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, M.; Huo, W.; Cai, D.; Qin, P.; Cao, H.; Tan, T. Fractionation and Oxypropylation of Corn-Stover Lignin for the Production of Biobased Rigid Polyurethane Foam. Ind. Crops Prod. 2020, 143, 111887. [Google Scholar] [CrossRef]
- Xie, J.; Hse, C.Y.; Shupe, T.F.; Hu, T. Physicochemical Characterization of Lignin Recovered from Microwave-Assisted Delignified Lignocellulosic Biomass for Use in Biobased Materials. J. Appl. Polym. Sci. 2015, 132, 42635. [Google Scholar] [CrossRef]
- Morandim-Giannetti, A.A.; Agnelli, J.A.M.; Lanças, B.Z.; Magnabosco, R.; Casarin, S.A.; Bettini, S.H.P. Lignin as Additive in Polypropylene/Coir Composites: Thermal, Mechanical and Morphological Properties. Carbohydr. Polym. 2012, 87, 2563–2568. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, W.; Liu, Y.; Hu, H.; Cochran, E.; Bai, X. Agricultural Residue-Derived Lignin as the Filler of Polylactic Acid Composites and the Effect of Lignin Purity on the Composite Performance. J. Appl. Polym. Sci. 2019, 136, 47915. [Google Scholar] [CrossRef]
- Rizal, S.; Alfatah, T.; Abdul Khalil, H.P.S.; Yahya, E.B.; Abdullah, C.K.; Mistar, E.M.; Ikramullah, I.; Kurniawan, R.; Bairwan, R.D. Enhanced Functional Properties of Bioplastic Films Using Lignin Nanoparticles from Oil Palm-Processing Residue. Polymers 2022, 14, 5126. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and Antibacterial Lignin Nanoparticles in Polyvinyl Alcohol/Chitosan Films for Active Packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Rai, S.; Dutta, P.K.; Mehrotra, G.K. Natural Antioxidant and Antimicrobial Agents from Agrowastes: An Emergent Need to Food Packaging. Waste Biomass Valorization 2020, 11, 1905–1916. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent Progress in Pectin Extraction and Their Applications in Developing Films and Coatings for Sustainable Food Packaging: A Review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lü, X. Pectin Extracted from Apple Pomace and Citrus Peel by Subcritical Water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Podetti, C.; Riveros-Gomez, M.; Román, M.C.; Zalazar-García, D.; Fabani, M.P.; Mazza, G.; Rodríguez, R. Polyphenol-Enriched Pectin from Pomegranate Peel: Multi-Objective Optimization of the Eco-Friendly Extraction Process. Molecules 2023, 28, 7656. [Google Scholar] [CrossRef] [PubMed]
- Vakilian, K.; Nateghi, L.; Javadi, A.; Anarjan, N. Optimization of Conventional and Ultrasound-Assisted Extraction of Pectin from Unripe Grape Pomace: Extraction Yield, Degree of Esterification, and Galacturonic Acid Content. J. Food Meas. Charact. 2023, 17, 5777–5793. [Google Scholar] [CrossRef]
- Arora, S.; Kataria, P.; Ahmad, W.; Mishra, R.; Upadhyay, S.; Dobhal, A.; Bisht, B.; Hussain, A.; Kumar, V.; Kumar, S. Microwave Assisted Green Extraction of Pectin from Citrus Maxima Albedo and Flavedo, Process Optimization, Characterisation and Comparison with Commercial Pectin. Food Anal. Methods 2023, 17, 105–118. [Google Scholar] [CrossRef]
- Zakaria, N.A.; Abd Rahman, N.H.; Rahman, R.A.; Zaidel, D.N.A.; Hasham, R.; Illias, R.M.; Mohamed, R.; Ahmad, R.A. Extraction Optimization and Physicochemical Properties of High Methoxyl Pectin from Ananas Comosus Peel Using Microwave-Assisted Approach. J. Food Meas. Charact. 2023, 17, 3354–3367. [Google Scholar] [CrossRef]
- Figueira, O.; Pereira, V.; Castilho, P.C. A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods 2023, 12, 3834. [Google Scholar] [CrossRef]
- Şen, E.; Göktürk, E.; Hajiyev, V.; Uğuzdoğan, E. Comparisons of Pulsed Ultrasound-Assisted and Hot-Acid Extraction Methods for Pectin Extraction under Dual Acid Mixtures from Onion (Allium Cepa L.) Waste. Food Sci. Nutr. 2023, 11, 7320–7329. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Cervone, F.; Bellincampi, D. Methyl Esterification of Pectin Plays a Role during Plant–Pathogen Interactions and Affects Plant Resistance to Diseases. J. Plant Physiol. 2012, 169, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, N.E.; Naguib, D.M. Agricultural Wastes Polysaccharides Promising Soil Fertilizer Improves Plant Growth and Resistance against Soil-Borne Pathogens. Plant Soil. 2024, 495, 675–697. [Google Scholar] [CrossRef]
- Kraut-Cohen, J.; Shapiro, O.H.; Dror, B.; Cytryn, E. Pectin Induced Colony Expansion of Soil-Derived Flavobacterium Strains. Front. Microbiol. 2021, 12, 651891. [Google Scholar] [CrossRef]
- Sayed, A.; Mohamed, M.M.; Abdel-raouf, M.E.S.; Mahmoud, G.A. Radiation Synthesis of Green Nanoarchitectonics of Guar Gum-Pectin/Polyacrylamide/Zinc Oxide Superabsorbent Hydrogel for Sustainable Agriculture. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4589–4600. [Google Scholar] [CrossRef]
- Sharma, R.; Bajpai, J.; Bajpai, A.K.; Acharya, S.; Kumar, B.; Singh, R.K. Assessment of Water Retention Performance of Pectin-Based Nanocarriers for Controlled Irrigation in Agriculture. Agric. Res. 2017, 6, 139–149. [Google Scholar] [CrossRef]
- Pholsin, R.; Shiekh, K.A.; Jafari, S.; Kijpatanasilp, I.; Na Nan, T.; Suppavorasatit, I.; Assatarakul, K. Impact of Pectin Edible Coating Extracted from Cacao Shell Powder on Postharvest Quality Attributes of Tomato (Lycopersicon esculentum Mill.) Fruit during Storage. Food Control 2024, 155, 110023. [Google Scholar] [CrossRef]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent Advances in Understanding the Roles of Pectin as an Active Participant in Plant Signaling Networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef]
- Wu, M.C.; Li, H.C.; Wu, P.H.; Huang, P.H.; Wang, Y.T. Assessment of Oligogalacturonide from Citrus Pectin as a Potential Antibacterial Agent against Foodborne Pathogens. J. Food Sci. 2014, 79, M1541–M1544. [Google Scholar] [CrossRef]
- Sun, X.; Wall, M.; Follett, P.; Liang, P.; Xu, S.; Zhong, T. Effect of Pectin Coatings Containing Trans-Cinnamaldehyde on the Postharvest Quality of Rambutan. HortScience 2023, 58, 11–15. [Google Scholar] [CrossRef]
- Gamage, A.; Liyanapathiranage, A.; Manamperi, A.; Gunathilake, C.; Mani, S.; Merah, O.; Madhujith, T. Applications of Starch Biopolymers for a Sustainable Modern Agriculture. Sustainability 2022, 14, 6085. [Google Scholar] [CrossRef]
- Hernández-Carmona, F.; Morales-Matos, Y.; Lambis-Miranda, H.; Pasqualino, J. Starch Extraction Potential from Plantain Peel Wastes. J. Environ. Chem. Eng. 2017, 5, 4980–4985. [Google Scholar] [CrossRef]
- Kaur, J.; Borah, A.; Chutia, H.; Gupta, P. Extraction, Modification, and Characterization of Native Litchi Seed (Litchi chinesis Sonn.) Starch. J. Sci. Food Agric. 2024, 104, 215–224. [Google Scholar] [CrossRef]
- Weligama Thuppahige, V.T.; Moghaddam, L.; Welsh, Z.G.; Wang, T.; Xiao, H.W.; Karim, A. Extraction and Characterisation of Starch from Cassava (Manihot esculenta) Agro-Industrial Wastes. LWT 2023, 182, 114787. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and Potential Utilizations of Starch from Pineapple Stem Waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Mieles-Gómez, L.; Quintana, S.E.; García-Zapateiro, L.A. Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties. Gels 2023, 9, 136. [Google Scholar] [CrossRef]
- Rosseto, M.; Krein, D.D.C.; Balbé, N.P.; Dettmer, A. Starch–Gelatin Film as an Alternative to the Use of Plastics in Agriculture: A Review. J. Sci. Food Agric. 2019, 99, 6671–6679. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Hassanisaadi, M.; Vatankhah, M.; Kennedy, J.F. Encapsulating Biocontrol Bacteria with Starch as a Safe and Edible Biopolymer to Alleviate Plant Diseases: A Review. Carbohydr. Polym. 2023, 302, 120384. [Google Scholar] [CrossRef]
- Supare, K.; Mahanwar, P.A. Starch-Derived Superabsorbent Polymers in Agriculture Applications: An Overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem.—Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- El-Bestawy, E.; Eltaweil, A.S.; Khallaf, N.S. Effective Production of Bacterial Cellulose Using Acidic Dairy Industry By-Products and Agro Wastes. Sustain. Chem. Pharm. 2023, 33, 101064. [Google Scholar] [CrossRef]
- Sagdic-Oztan, C.; Koschella, A.; Heinze, T.; Karaguler, N.G.; Tuter, M. Preparation of Bacterial Cellulose Using Enzymatic Hydrolysate of Olive Pomace as Carbon Source. Bioresources 2023, 18, 4168–4181. [Google Scholar] [CrossRef]
- Yekta, R.; Abedi-Firoozjah, R.; Azimi Salim, S.; Khezerlou, A.; Abdolmaleki, K. Application of Cellulose and Cellulose Derivatives in Smart/Intelligent Bio-Based Food Packaging. Cellulose 2023, 30, 9925–9953. [Google Scholar] [CrossRef]
- Chen, R.; Hou, Y.; Zhang, J.; Cui, J.; Li, G. Feasibility for the Preparation of Aerogels with Celluloses Extracted Mildly from Waste Palm Leaves. Nord. Pulp Pap. Res. J. 2023, 38, 197–207. [Google Scholar] [CrossRef]
- Klunklin, W.; Hinmo, S.; Thipchai, P.; Rachtanapun, P. Effect of Bleaching Processes on Physicochemical and Functional Properties of Cellulose and Carboxymethyl Cellulose from Young and Mature Coconut Coir. Polymers 2023, 15, 3376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, R.; Guo, Q.; Xu, G.; Zhang, L.; Sun, P.; Cao, Y.; Hu, S. The Use of Newly Isolated Fungal Cultures for the Selective Delignification of Bamboo Culms. Front. Bioeng. Biotechnol. 2023, 11, 1265420. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, I.K.; Anbu, P.V.; Parthiban, K.T.; Uthandi, S. A New Enzolv Process for Simultaneous Delignification and Lignin-Derived Chemical Production from the Woody Biomass of Melia Dubia. Biomass Convers. Biorefin. 2023, 13, 14557–14571. [Google Scholar] [CrossRef]
- Karanicola, P.; Patsalou, M.; Stergiou, P.Y.; Kavallieratou, A.; Evripidou, N.; Christou, P.; Panagiotou, G.; Damianou, C.; Papamichael, E.M.; Koutinas, M. Ultrasound-Assisted Dilute Acid Hydrolysis for Production of Essential Oils, Pectin and Bacterial Cellulose via a Citrus Processing Waste Biorefinery. Bioresour. Technol. 2021, 342, 126010. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef]
- Ahmad, D.F.B.A.; Wasli, M.E.; Tan, C.S.Y.; Musa, Z.; Chin, S.F. Eco-Friendly Cellulose-Based Hydrogels Derived from Wastepapers as a Controlled-Release Fertilizer. Chem. Biol. Technol. Agric. 2023, 10, 36. [Google Scholar] [CrossRef]
- Callaghan, C.; Califano, D.; Feresin Gomes, M.H.; Pereira de Carvalho, H.W.; Edler, K.J.; Mattia, D. Cellulose Acetate Microbeads for Controlled Delivery of Essential Micronutrients. ACS Sustain. Chem. Eng. 2023, 11, 4749–4758. [Google Scholar] [CrossRef]
- Ali, S.; Akbar Anjum, M.; Sattar Khan, A.; Nawaz, A.; Ejaz, S.; Khaliq, G.; Iqbal, S.; Ullah, S.; Naveed Ur Rehman, R.; Moaaz Ali, M.; et al. Carboxymethyl Cellulose Coating Delays Ripening of Harvested Mango Fruits by Regulating Softening Enzymes Activities. Food Chem. 2022, 380, 131804. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bhattacharjee, S.; Bhaladhare, S. Preparation of Cellulose Hydrogels and Hydrogel Nanocomposites Reinforced by Crystalline Cellulose Nanofibers (CNFs) as a Water Reservoir for Agriculture Use. ACS Appl. Polym. Mater. 2023, 5, 2895–2904. [Google Scholar] [CrossRef]
- Das, D.; Prakash, P.; Rout, P.K.; Bhaladhare, S. Synthesis and Characterization of Superabsorbent Cellulose-Based Hydrogel for Agriculture Application. Starch/Staerke 2021, 73, 1900284. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Man, L. Bamboo-Derived Carboxymethyl Cellulose for Liquid Film as Renewable and Biodegradable Agriculture Mulching. Int. J. Biol. Macromol. 2021, 192, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Duan, Y.; Huang, Y.; Teng, Y.; Li, C.; Tao, Y.; Lu, J.; Du, J.; Wang, H. Multifunctional Soybean Protein Isolate-Graft-Carboxymethyl Cellulose Composite as All-Biodegradable and Mechanically Robust Mulch Film for “Green” Agriculture. Carbohydr. Polym. 2024, 323, 121410. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.; Qiu, Z.; Li, Y.; Qiu, F.; Zhang, T. Fabrication of Flexible AgNW/Cellulose Hybrid Film with Heat Preservation and Antibacterial Properties for Agriculture Application. Cellulose 2021, 28, 8693–8704. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Peniche Covas, C.A.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Fook, B.R.P.L.; Lima, V.A.D.O.; Rached, R.Í.D.F.; Lima, E.P.N.; Lima, R.J.D.S.; Covas, C.A.P.; Fook, M.V.L. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Moussa, S.H.; El-Tras, W.F.; Elguindy, N.M.; Opwis, K. Antimicrobial Textile Treated with Chitosan from Aspergillus Niger Mycelial Waste. Int. J. Biol. Macromol. 2011, 49, 241–245. [Google Scholar] [CrossRef]
- Zininga, J.T.; Puri, A.K.; Dlangamandla, N.; Wang, Z.; Singh, S.; Permaul, K. Integrated Biorefinery of Mucor Circinelloides Biomass and Sugarcane Bagasse for Application of High-Value Biopolymers. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Habibi, A.; Karami, S.; Varmira, K.; Hadadi, M. Key Parameters Optimization of Chitosan Production from Aspergillus Terreus Using Apple Waste Extract as Sole Carbon Source. Bioprocess. Biosyst. Eng. 2021, 44, 283–295. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.F.; Galindo, H.M.; de Lima, M.A.B.; Ribeaux, D.R.; Rodríguez, D.M.; da Silva Andrade, R.F.; Gusmão, N.B.; de Campos-Takaki, G.M. Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates. Int. J. Mol. Sci. 2020, 21, 4286. [Google Scholar] [CrossRef]
- Romanazzi, G.; Moumni, M. Chitosan and Other Edible Coatings to Extend Shelf Life, Manage Postharvest Decay, and Reduce Loss and Waste of Fresh Fruits and Vegetables. Curr. Opin. Biotechnol. 2022, 78, 102834. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, R.; Jha, R.; Singh, P. Heritable Priming by Trichoderma: A Sustainable Approach for Wheat Protection against Bipolaris Sorokiniana. Front. Plant Sci. 2022, 13, 1050765. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.D.; Chandrika, K.S.V.P.; Godbole, V. A Novel Chitosan Biopolymer Based Trichoderma Delivery System: Storage Stability, Persistence and Bio Efficacy against Seed and Soil Borne Diseases of Oilseed Crops. Microbiol. Res. 2020, 237, 126487. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-José, N.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Nanoparticles Based on Chitosan as Carriers for the Combined Herbicides Imazapic and Imazapyr. Sci. Rep. 2016, 6, 19768. [Google Scholar] [CrossRef] [PubMed]
- Możejko-Ciesielska, J.; Kiewisz, R. Bacterial Polyhydroxyalkanoates: Still Fabulous? Microbiol. Res. 2016, 192, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Campanari, S.; E Silva, F.A.; Bertin, L.; Villano, M.; Majone, M. Effect of the Organic Loading Rate on the Production of Polyhydroxyalkanoates in a Multi-Stage Process Aimed at the Valorization of Olive Oil Mill Wastewater. Int. J. Biol. Macromol. 2014, 71, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Verdini, F.; Tabasso, S.; Mariatti, F.; Bosco, F.; Mollea, C.; Calcio Gaudino, E.; Cirio, A.; Cravotto, G. From Agri-Food Wastes to Polyhydroxyalkanoates through a Sustainable Process. Fermentation 2022, 8, 556. [Google Scholar] [CrossRef]
- Razzaq, S.; Shahid, S.; Farooq, R.; Noreen, S.; Perveen, S.; Bilal, M. Sustainable Bioconversion of Agricultural Waste Substrates into Poly (3-Hydroxyhexanoate) (Mcl-PHA) by Cupriavidus Necator DSM 428. Biomass Convers. Biorefin. 2022, 14, 9429–9439. [Google Scholar] [CrossRef]
- Unrean, P.; Napathorn, S.C.; Tee, K.L.; Wong, T.S.; Champreda, V. Lignin to Polyhydroxyalkanoate Bioprocessing by Novel Strain of Pseudomonas Monteilii. Biomass Convers. Biorefin. 2023, 12, 4651–4657. [Google Scholar] [CrossRef]
- Matos, M.; Cruz, R.A.P.; Cardoso, P.; Silva, F.; Freitas, E.B.; Carvalho, G.; Reis, M.A.M. Combined Strategies to Boost Polyhydroxyalkanoate Production from Fruit Waste in a Three-Stage Pilot Plant. ACS Sustain. Chem. Eng. 2021, 9, 8270–8279. [Google Scholar] [CrossRef]
- Kag, S.; Kumar, P.; Kataria, R. Potato Peel Waste as an Economic Feedstock for PHA Production by Bacillus Circulans. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Cascone, G.; D’Emilio, A.; Buccellato, E.; Mazzarella, R. New Biodegradable Materials for Greenhouse Soil Mulching. Acta Hortic. 2008, 801 Pt 1, 283–290. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, J.H. Polyhydroxyalkanoates for Biodegradable Mulch Films Applications. ACS Symp. Ser. 2020, 1373, 145–160. [Google Scholar] [CrossRef]
- Botta, L.; Mistretta, M.C.; Palermo, S.; Fragalà, M.; Pappalardo, F. Characterization and Processability of Blends of Polylactide Acid with a New Biodegradable Medium-Chain-Length Polyhydroxyalkanoate. J. Polym. Environ. 2015, 23, 478–486. [Google Scholar] [CrossRef]
- Wang, J.H.; Tian, Y.; Zhou, B. Degradation and Stabilization of Poly(Butylene Adipate-Co-Terephthalate)/Polyhydroxyalkanoate Biodegradable Mulch Films Under Different Aging Tests. J. Polym. Environ. 2022, 30, 1366–1379. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Seko, N. Biodegradable Dual-Layer Polyhydroxyalkanoate (Pha)/Polycaprolactone (Pcl) Mulch Film for Agriculture: Preparation and Characterization. Energy Nexus 2022, 8, 100137. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of Poly(Lactic Acid): A Review. J. Macromol. Sci. Part C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A Review on the Lactic Acid Fermentation from Low-Cost Renewable Materials: Recent Developments and Challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Liu, S. Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste. Fermentation 2023, 9, 765. [Google Scholar] [CrossRef]
- Mazzoli, R. Current Progress in Production of Building-Block Organic Acids by Consolidated Bioprocessing of Lignocellulose. Fermentation 2021, 7, 248. [Google Scholar] [CrossRef]
- D’ambrosio, S.; Zaccariello, L.; Sadiq, S.; D’Albore, M.; Battipaglia, G.; D’Agostino, M.; Battaglia, D.; Schiraldi, C.; Cimini, D. Grape Stalk Valorization: An Efficient Re-Use of Lignocellulosic Biomass through Hydrolysis and Fermentation to Produce Lactic Acid from Lactobacillus Rhamnosus IMC501. Fermentation 2023, 9, 616. [Google Scholar] [CrossRef]
- Cox, R.; Narisetty, V.; Castro, E.; Agrawal, D.; Jacob, S.; Kumar, G.; Kumar, D.; Kumar, V. Fermentative Valorisation of Xylose-Rich Hemicellulosic Hydrolysates from Agricultural Waste Residues for Lactic Acid Production under Non-Sterile Conditions. Waste Manag. 2023, 166, 336–345. [Google Scholar] [CrossRef]
- Ozer Uyar, G.E.; Uyar, B. Potato Peel Waste Fermentation by Rhizopus Oryzae to Produce Lactic Acid and Ethanol. Food Sci. Nutr. 2023, 11, 5908–5917. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Y.; Chi, F.; Chen, C.; Wan, W.; Feng, Y.; Song, X.; Cui, Q. Integrated Lactic Acid Production from Lignocellulosic Agricultural Wastes under Thermal Conditions. J. Environ. Manag. 2023, 342, 118281. [Google Scholar] [CrossRef]
- Qi, K.; Chen, C.; Yan, F.; Feng, Y.; Bayer, E.A.; Kosugi, A.; Cui, Q.; Liu, Y.J. Coordinated β-Glucosidase Activity with the Cellulosome Is Effective for Enhanced Lignocellulose Saccharification. Bioresour. Technol. 2021, 337, 125441. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mothar, W.A.Q.I.; Khalid, N.I.; Rahim, M.H.A.; Luthfi, A.A.I.; Zaini, N.S.M.; Din, N.A.S.; Zaini, N.A.M. Underutilized Malaysian Agro-Industrial Wastes as Sustainable Carbon Sources for Lactic Acid Production. Fermentation 2023, 9, 905. [Google Scholar] [CrossRef]
- Merino, D.; Zych, A.; Athanassiou, A. Biodegradable and Biobased Mulch Films: Highly Stretchable PLA Composites with Different Industrial Vegetable Waste. ACS Appl. Mater. Interfaces 2022, 14, 46920–46931. [Google Scholar] [CrossRef] [PubMed]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. 4D Printing: Perspectives for the Production of Sustainable Plastics for Agriculture. Biotechnol. Adv. 2022, 54, 107785. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, J.; Szostak, M.; Barczewski, M.; Łuczak, P. Cork-Wood Hybrid Filler System for Polypropylene and Poly(Lactic Acid) Based Injection Molded Composites. Structure Evaluation and Mechanical Performance. Compos. B Eng. 2019, 163, 655–668. [Google Scholar] [CrossRef]
- Dalu, M.; Temiz, A.; Altuntaş, E.; Demirel, G.K.; Aslan, M. Characterization of Tanalith E Treated Wood Flour Filled Polylactic Acid Composites. Polym. Test. 2019, 76, 376–384. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S. Mechanical and Morphological Properties of Wood Plastic Biocomposites Prepared from Toughened Poly(Lactic Acid) and Rubber Wood Sawdust (Hevea brasiliensis). J. Bionic Eng. 2014, 11, 630–637. [Google Scholar] [CrossRef]
- Borysiuk, P.; Boruszewski, P.; Auriga, R.; Danecki, L.; Auriga, A.; Rybak, K.; Nowacka, M. Influence of a Bark-Filler on the Properties of PLA Biocomposites. J. Mater. Sci. 2021, 56, 9196–9208. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Lin, W.Y.; Harun, N.Y.; Mutiari, D. Modification of Poly(Lactic Acid) with Orange Peel Powder as Biodegradable Composite. Polymers 2022, 14, 4126. [Google Scholar] [CrossRef] [PubMed]
- Coiai, S.; Di Lorenzo, M.L.; Cinelli, P.; Righetti, M.C.; Passaglia, E. Binary Green Blends of Poly(Lactic Acid) with Poly(Butylene Adipate-Co-Butylene Terephthalate) and Poly(Butylene Succinate-Co-Butylene Adipate) and Their Nanocomposites. Polymers 2021, 13, 2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hirschberg, V.; Rodrigue, D. Mechanical Fatigue of Biodegradable Polymers: A Study on Polylactic Acid (PLA), Polybutylene Succinate (PBS) and Polybutylene Adipate Terephthalate (PBAT). Int. J. Fatigue 2022, 159, 106798. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural Waste: Review of the Evolution, Approaches and Perspectives on Alternative Uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A Review on the Valorisation of Food Waste as a Nutrient Source and Soil Amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef]
- Ong, K.L.; Kaur, G.; Pensupa, N.; Uisan, K.; Lin, C.S.K. Trends in Food Waste Valorization for the Production of Chemicals, Materials and Fuels: Case Study South and Southeast Asia. Bioresour. Technol. 2018, 248, 100–112. [Google Scholar] [CrossRef]
- Biobased, Biodegradable and Compostable Plastics. Available online: https://environment.ec.europa.eu/topics/plastics/biobased-biodegradable-and-compostable-plastics_en#:~:text=Biobased%20plastics%20are%20fully%20or,not%20necessarily%20biodegradable%20or%20compostable (accessed on 9 February 2024).
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The Potential Toxicity of Microplastics on Human Health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).