Novel Yellow Aromatic Imine Derivative Incorporating Oxazolone Moiety for Color Resist Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
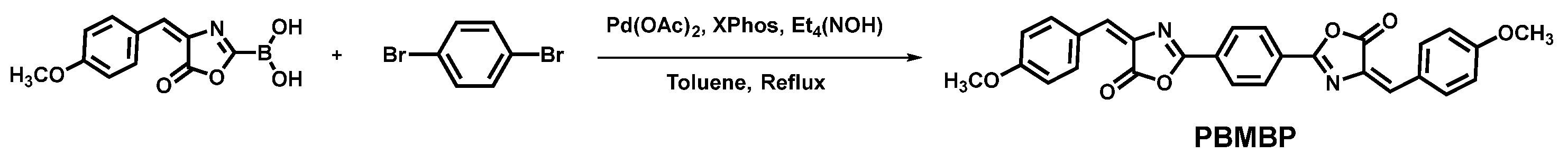
2.2. Synthesis of 2,2′-(1,4-Phenylene)bis [4-[(4-Methoxyphenyl)methylene]-5(4H)-oxazolone] (PBMBO)
2.3. Fabrication of Film and Color Filters
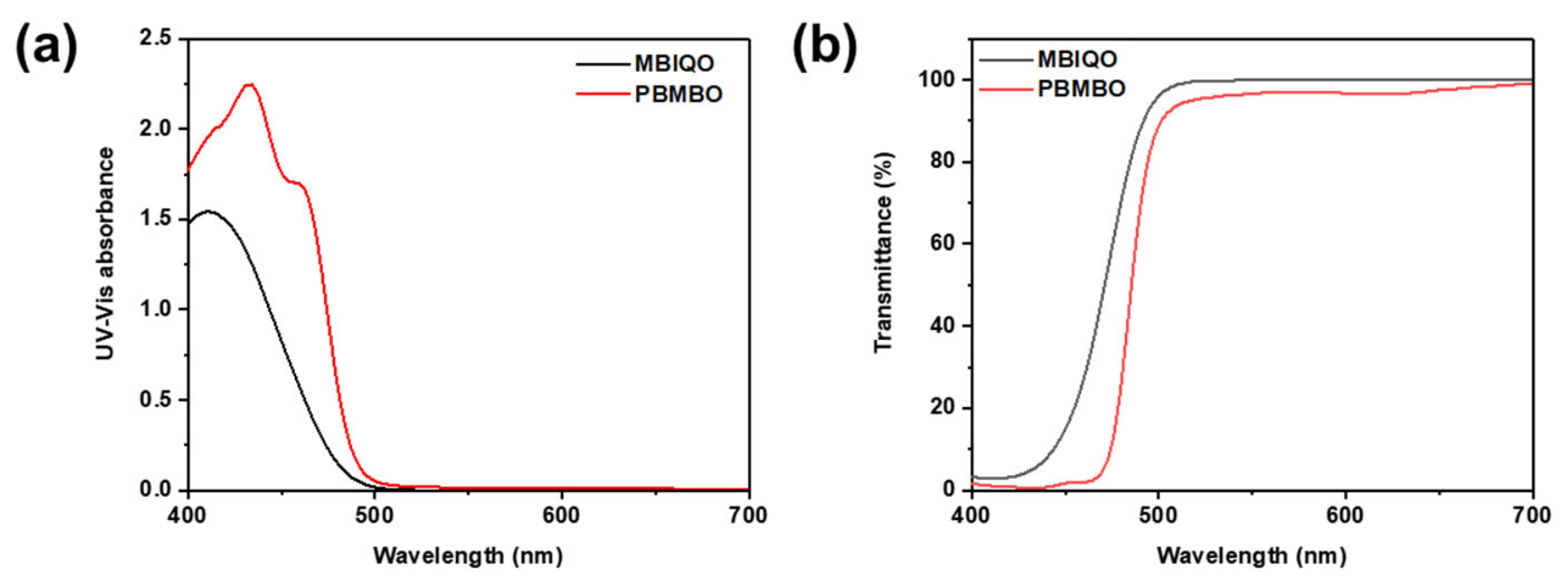
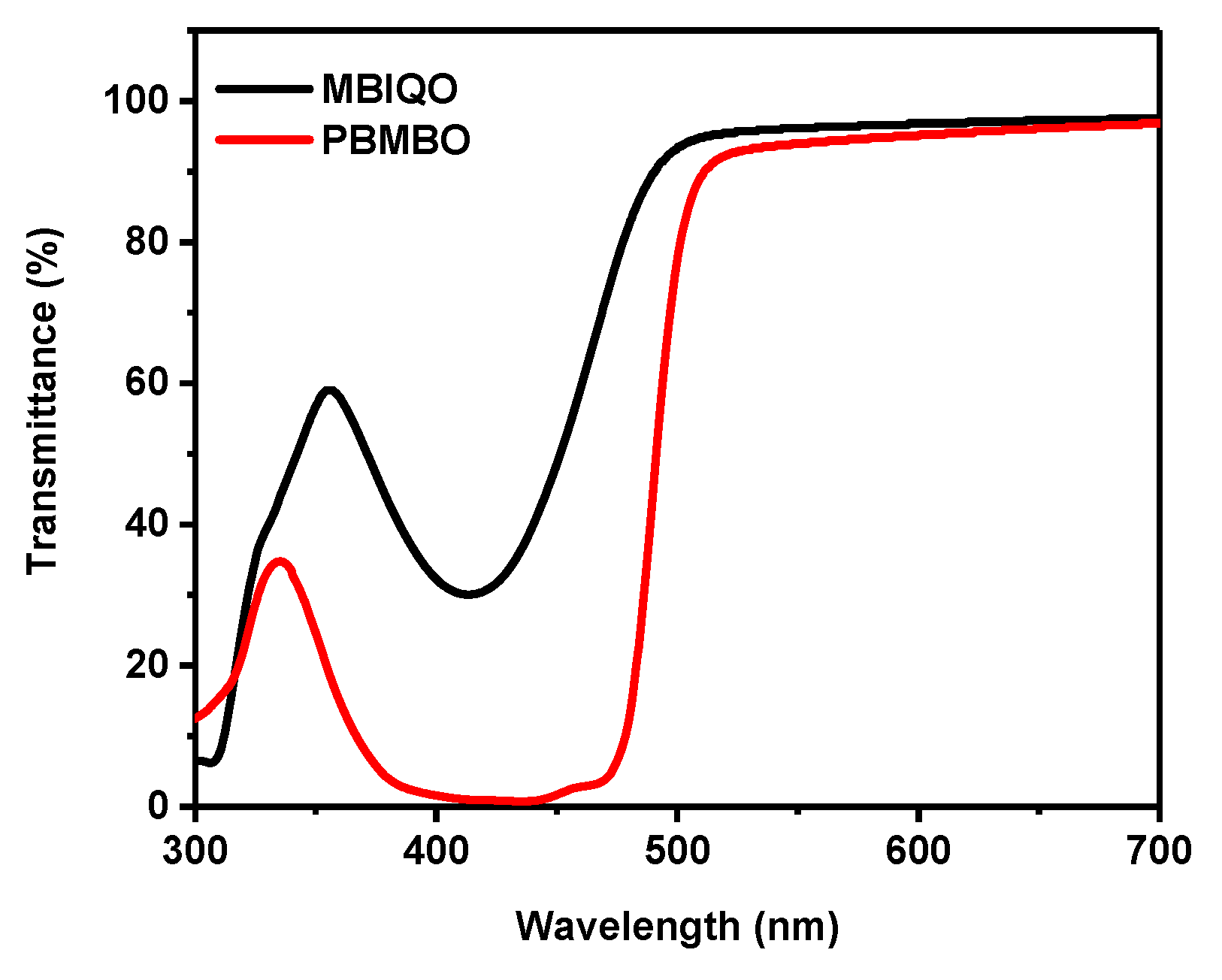
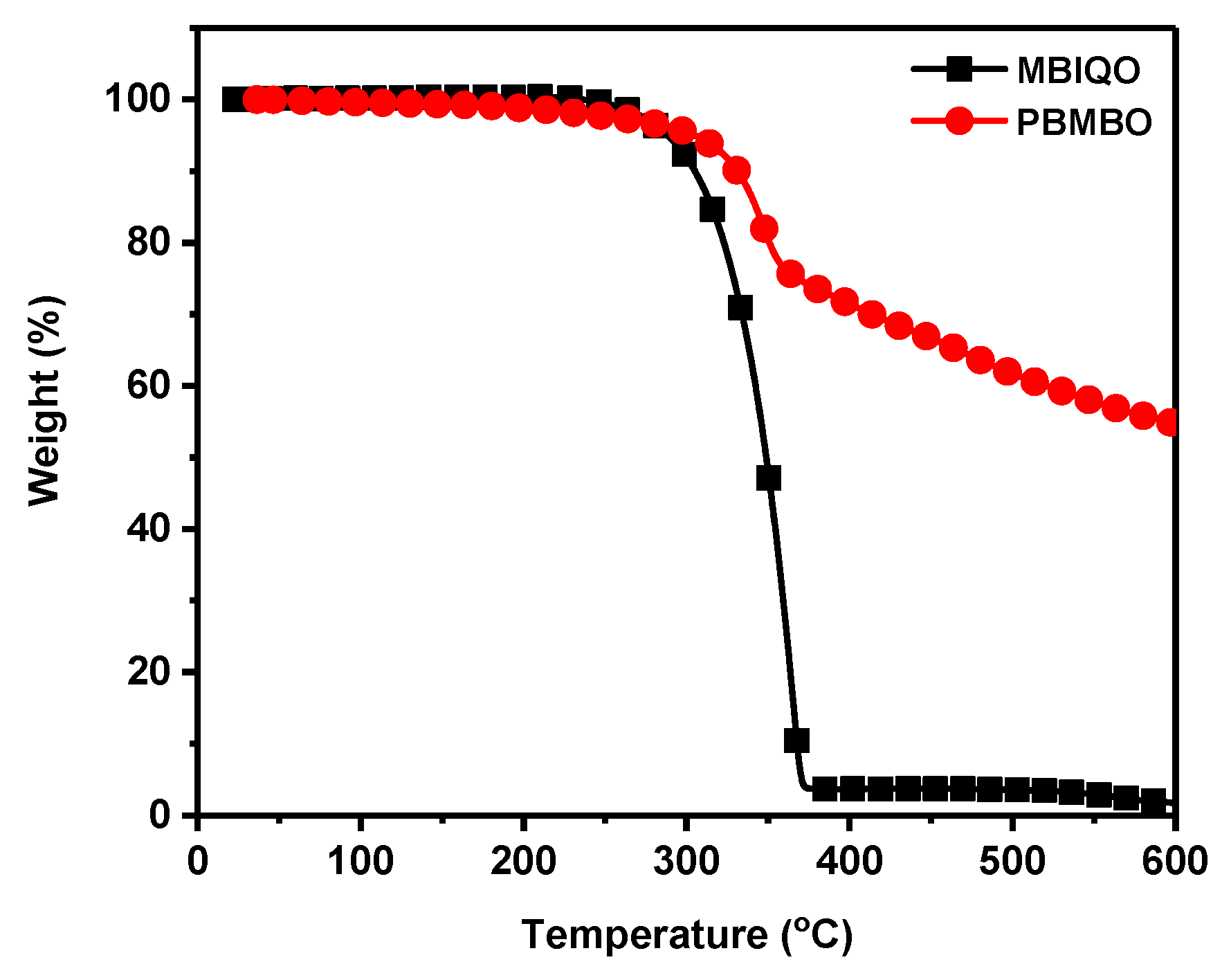
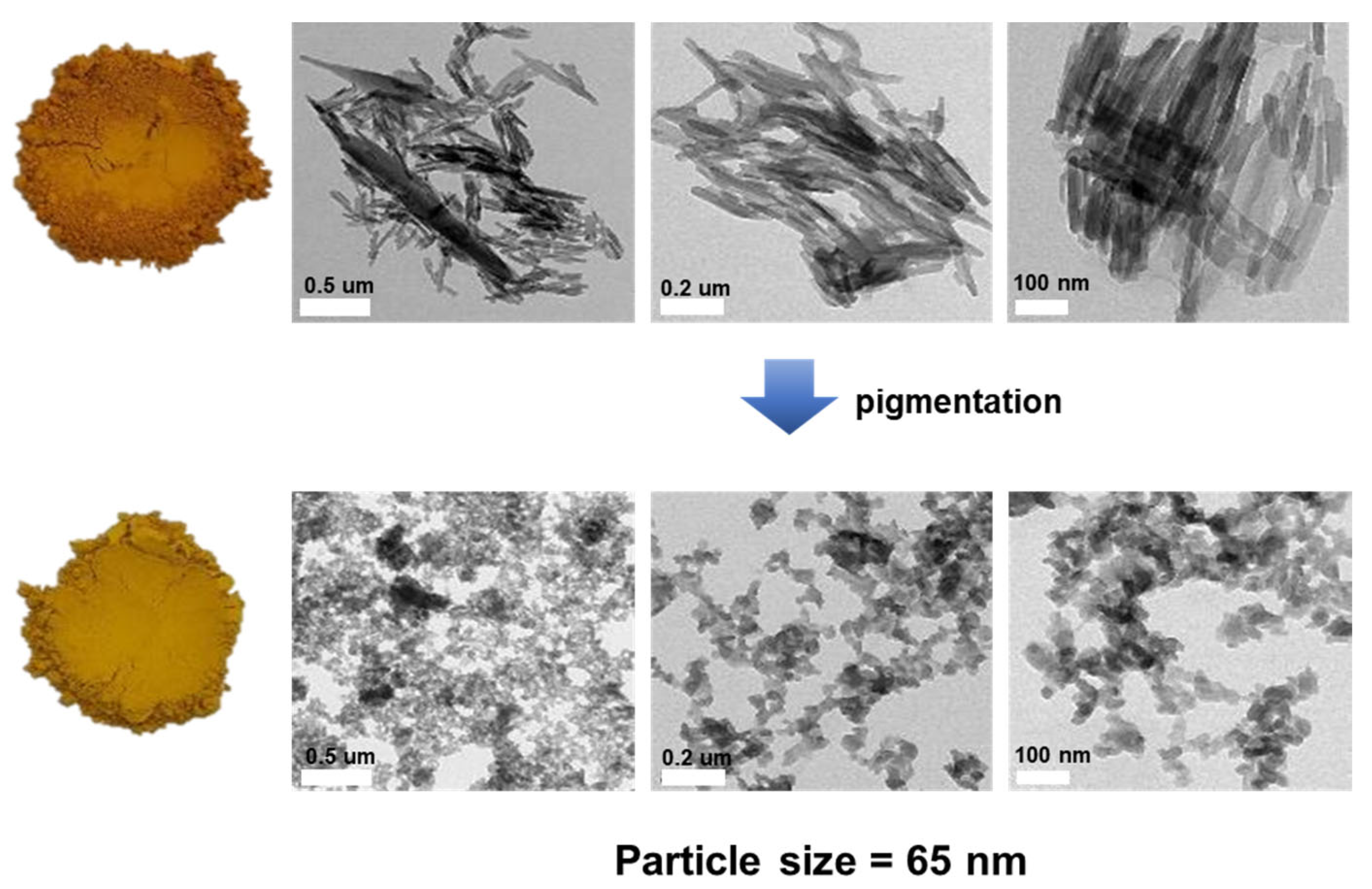
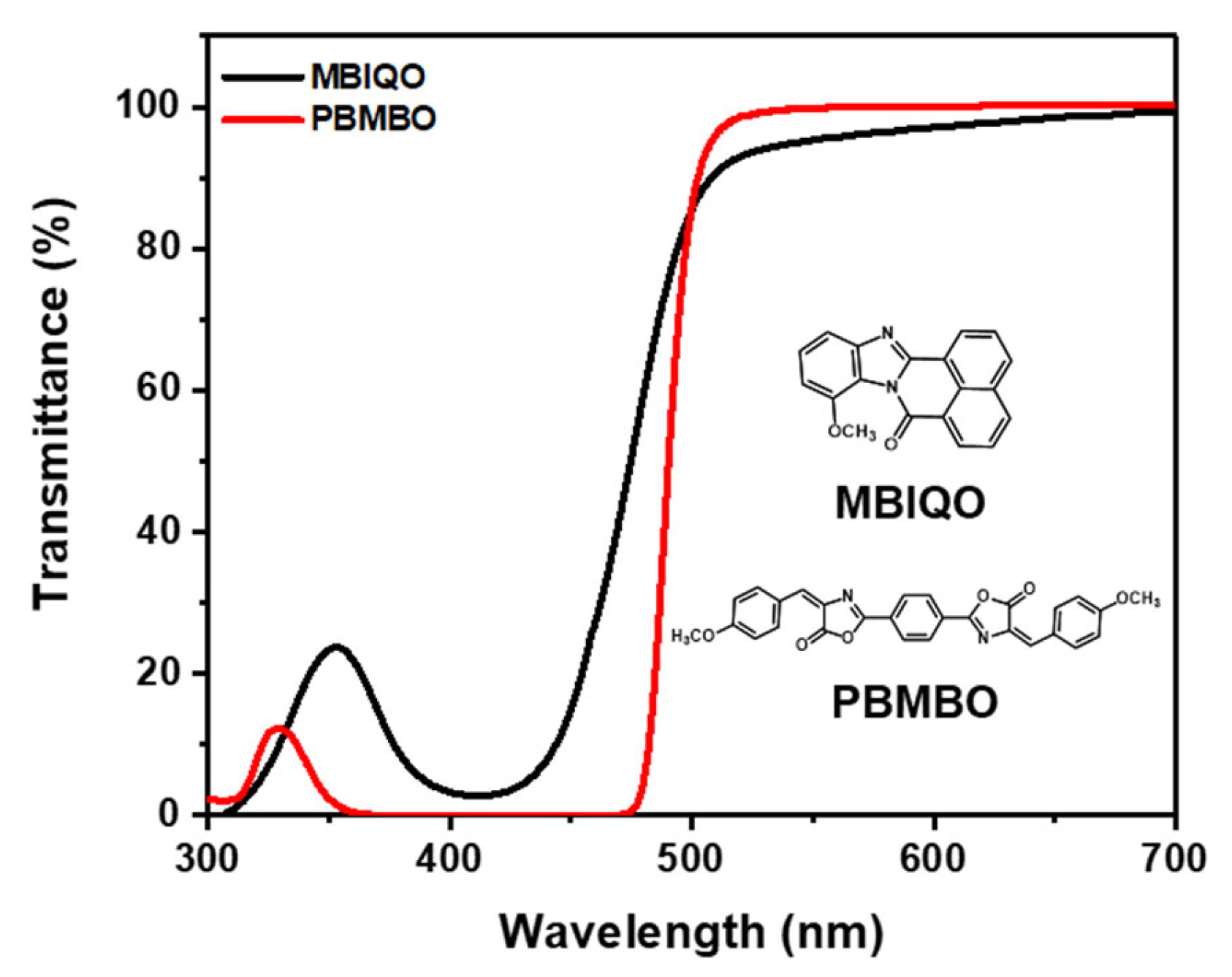
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yokogawa, S.; Burgos, S.P.; Atwater, H.A. Plasmonic color filters for CMOS image sensor applications. Nano Lett. 2012, 12, 4349–4354. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.-S.; Chen, M.; Pan, P.-C. LCD-based color filter films fabricated by a pigment-based colorant photo resist inks and printing technology. Thin Solid Film. 2006, 515, 896–901. [Google Scholar] [CrossRef]
- Meng, F.; Chang, Y.; Pan, C.; Li, C. Nanostructured Color Filters for CMOS Image Sensors. IEEE Photonics Technol. Lett. 2023, 35, 641–644. [Google Scholar] [CrossRef]
- Zollinger, H. Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ashida, T. Development of color resists containing novel dyes for liquid crystal displays. Sumitomo Kagaku 2013, 1, 521–523. [Google Scholar]
- Miyamichi, A.; Ono, A.; Kagawa, K.; Yasutomi, K.; Kawahito, S. Plasmonic Color Filter Array with High Color Purity for CMOS Image Sensors. Sensors 2019, 19, 1750. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, J.; Wu, S.T. Recent Advances on Quantum-Dot-Enhanced Liquid-Crystal Displays. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1900611. [Google Scholar] [CrossRef]
- Chen, H.W.; Zhu, R.D.; He, J.; Duan, W.; Hu, W.; Lu, Y.Q.; Li, M.C.; Lee, S.L.; Dong, Y.J.; Wu, S.T. Going beyond the limit of an LCD’s color gamut. Light Sci. Appl. 2017, 6, e17043. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Hallas, G. Dyeing and fastness properties of benzodifuranones, naphthodifuranones and naphthofuranonepyrrolidones. Color. Technol. 2006, 118, 125–130. [Google Scholar] [CrossRef]
- Hallas, G.; Yoon, C. The synthesis and properties of naphthodifuranones and naphthofuranonepyrrolidones. Dye. Pigment. 2012, 48, 121–132. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, B.-J.; An, S.-O.; Lee, J.-H.; Choi, J.-H. The synthesis of red dyes based on diketo-pyrrolo-pyrrole chromophore to improve heat stability and solubility for colour filter fabrication. Dye. Pigment. 2020, 174, 108053. [Google Scholar] [CrossRef]
- Namgoong, J.W.; Kim, H.M.; Kim, S.H.; Yuk, S.B.; Choi, J.; Kim, J.P. Synthesis and characterization of metal phthalocyanine bearing carboxylic acid anchoring groups for nanoparticle dispersion and their application to color filters. Dye. Pigment. 2021, 184, 108737. [Google Scholar] [CrossRef]
- Kong, N.S.; Jung, H.; Kim, B.; Lee, C.K.; Kong, H.; Jun, K.; Kim, J.C.; Noh, S.M.; Cheong, I.W.; Park, J.; et al. Development of dimeric triarylmethine derivatives with improved thermal and photo stability for color filters. Dye. Pigment. 2017, 144, 242–248. [Google Scholar] [CrossRef]
- Do Kim, Y.; Kim, J.P.; Kwon, O.S.; Cho, I.H. The synthesis and application of thermally stable dyes for ink-jet printed LCD color filters. Dye. Pigment. 2009, 81, 45–52. [Google Scholar] [CrossRef]
- Choi, J.; Lee, W.; Sakong, C.; Yuk, S.B.; Park, J.S.; Kim, J.P. Facile synthesis and characterization of novel coronene chromophores and their application to LCD color filters. Dye. Pigment. 2012, 94, 34–39. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.H.; Lee, W.; Yoon, C.; Kim, J.P. Synthesis and characterization of thermally stable dyes with improved optical properties for dye-based LCD color filters. New J. Chem. 2012, 36, 812–818. [Google Scholar] [CrossRef]
- Park, S.; Kang, Y.; Kwon, H.; Kim, H.; Kang, S.; Lee, H.; Yoon, C.; Park, J. Novel Yellow Azo Pyridone Derivatives with Different Halide Atoms for Image-Sensor Color Filters. Molecules 2022, 27, 6601. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-Q.; Feng, Y.-Q.; Li, X.-G.; Bai, Y.; Li, X.-X.; An, J.; Lu, M. Surface modification of organic pigment particles for microencapsulated electrophoretic displays. Dye. Pigment. 2012, 92, 554–562. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Chiang, T.-Y.; Huang, Y.-C.; Chang, C.-Y.; Kuo, M.-T. Preparation, Particle Characterizations and Application of Nano-Pigment Suspension. Polym. Plast. Technol. Eng. 2010, 49, 1552–1562. [Google Scholar] [CrossRef]
- Rizk, H.F.; Ibrahim, S.A.; El-Borai, M.A. Synthesis, fastness properties, color assessment and antimicrobial activity of some azo reactive dyes having pyrazole moiety. Dye. Pigment. 2015, 112, 86–92. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Rahimi, S.; Pasandideh Nadamani, M. Microwave-assisted synthesis and photochromic properties of new azo-imidazoles. Dye. Pigment. 2017, 143, 387–392. [Google Scholar] [CrossRef]
- Aysha, T.S.; Mohamed, M.B.I.; El-Sedik, M.S.; Youssef, Y.A. Multi-functional colorimetric chemosensor for naked eye recognition of Cu2+, Zn2+ and Co2+ using new hybrid azo-pyrazole/pyrrolinone ester hydrazone dye. Dye. Pigment. 2021, 196, 109795. [Google Scholar] [CrossRef]
- Bagheri, M.; Mirzaee, M.; Hosseini, S.; Gholamzadeh, P. The photochromic switchable imidazoles: Their genesis, development, synthesis, and characterization. Dye. Pigment. 2022, 203, 110322. [Google Scholar] [CrossRef]
- Gonciarz, A.; Pich, R.; Bogdanowicz, K.A.; Jewloszewicz, B.; Przybył, W.; Dysz, K.; Dylong, A.; Kwak, A.; Kaim, A.; Iwan, A.; et al. UV–Vis Absorption Properties of New Aromatic Imines and Their Compositions with Poly({4,8-bis[(2-Ethylhexyl)oxy]Benzo[1,2-b:4,5-b′]Dithiophene-2,6-diyl}{3-Fluoro-2-[(2-Ethylhexyl)Carbonyl]Thieno[3,4-b]Thiophenediyl}). Materials 2019, 12, 4191. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.; Vieira, B.; Alves, C.; Silva, B.; Pinto, E.; Cerqueira, F.; Silva, R.; Remião, F.; Shvalya, V.; Cvelbar, U.; et al. Halochromic Silk Fabric as a Reversible pH-Sensor Based on a Novel 2-Aminoimidazole Azo Dye. Polymers 2023, 15, 1730. [Google Scholar] [CrossRef]
- Dias, G.G.; ORodrigues, M.; Paz, E.R.; PNunes, M.; Araujo, M.H.; Rodembusch, F.S.; da Silva Júnior, E.N. Aryl-Phenanthro[9,10-d]imidazole: A Versatile Scaffold for the Design of Optical-Based Sensors. ACS Sens. 2022, 7, 2865–2919. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Cui, Y.-P. The synthesis, structure and spectroscopic properties of novel oxazolone-, pyrazolone- and pyrazoline-containing heterocycle chromophores. Dye. Pigment. 2009, 81, 27–34. [Google Scholar] [CrossRef]
- Jędrzejewska, B.; Gordel, M.; Szeremeta, J.; Krawczyk, P.; Samoć, M. Synthesis and Linear and Nonlinear Optical Properties of Three Push–Pull Oxazol-5(4H)-one Compounds. J. Org. Chem. 2015, 80, 9641–9651. [Google Scholar] [CrossRef] [PubMed]
- Ozturk Urut, G.; Alp, S.; Topkaya, D. Synthesis and characterization of new green and orange region emitting anthracene based oxazol-5-one dyes. Dye. Pigment. 2017, 145, 103–109. [Google Scholar] [CrossRef]
- Gordel-Wójcik, M.; Nyk, M.; Śmiałek, P.; Petrusevich, E.F.; Bartkowiak, W.; Samoć, M.; Jędrzejewska, B. Linear and nonlinear absorption properties of hemicyanine dyes containing 5-(4H)-oxazolone as heterocyclic ring. Dye. Pigment. 2023, 208, 110772. [Google Scholar] [CrossRef]
- Park, S.; Godi, M.; Khairnar, N.; Dae, S.; Kwon, H.; Park, S.; Lee, H.; Lee, K.; Park, J. Enhanced Optical and Thermal Properties of a Novel Yellow Quinophthalone Derivative for Color Filter Colorants in Image Sensors. Phys. Status Solidi A-Appl. Mat. 2024, 2300888. [Google Scholar] [CrossRef]
- Park, S.; Jillella, R.; Kwon, H.; Park, S.; Lee, H.; Lee, K.; Park, J. Enhancement of Optical and Chemical Resistance Properties with a Novel Yellow Quinophthalone Derivative for Image Sensor Colorants. Molecules 2024, 29, 1100. [Google Scholar] [CrossRef] [PubMed]

| MBIQO | PBMBO | ||
|---|---|---|---|
| Transmittance [%] | 435 nm | 4.30 | 0.58 |
| 530 nm | 99.80 | 95.90 | |
| Molar extinction coefficient a [L/mol·cm] | 1.23 × 104 | 2.24 × 104 | |
| MBIQO | PBMBO | |
|---|---|---|
| 435 nm | 36.6% | 0.82% |
| 530 nm | 95.7% | 93.1% |
| MBIQO | PBMBO | |
|---|---|---|
| Solubility | 0.15 | 0.32 |
| MBIQO | PBMBO | |
|---|---|---|
| 435 nm | 3.63% | 0.03% |
| 530 nm | 94.1% | 99.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Park, S.; Oh, S.; Heo, Y.; Lee, H.; Park, J. Novel Yellow Aromatic Imine Derivative Incorporating Oxazolone Moiety for Color Resist Applications. Appl. Sci. 2024, 14, 4362. https://doi.org/10.3390/app14114362
Park S, Park S, Oh S, Heo Y, Lee H, Park J. Novel Yellow Aromatic Imine Derivative Incorporating Oxazolone Moiety for Color Resist Applications. Applied Sciences. 2024; 14(11):4362. https://doi.org/10.3390/app14114362
Chicago/Turabian StylePark, Sunwoo, Sangwook Park, Seyoung Oh, Yeongjae Heo, Hayoon Lee, and Jongwook Park. 2024. "Novel Yellow Aromatic Imine Derivative Incorporating Oxazolone Moiety for Color Resist Applications" Applied Sciences 14, no. 11: 4362. https://doi.org/10.3390/app14114362
APA StylePark, S., Park, S., Oh, S., Heo, Y., Lee, H., & Park, J. (2024). Novel Yellow Aromatic Imine Derivative Incorporating Oxazolone Moiety for Color Resist Applications. Applied Sciences, 14(11), 4362. https://doi.org/10.3390/app14114362







