The Influence of Cooking Methods and Muscle on Beef Aroma Profile and Consumer Satisfaction: Insights from Volatile Compound Analysis
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Meat Sample Preparation
2.2. Consumer Panels
2.3. Basic Composition Analysis
2.4. Volatile Compound Analysis
3. Results and Discussion
3.1. Composition of Meat Samples
3.2. Consumers’ Palatability Scores
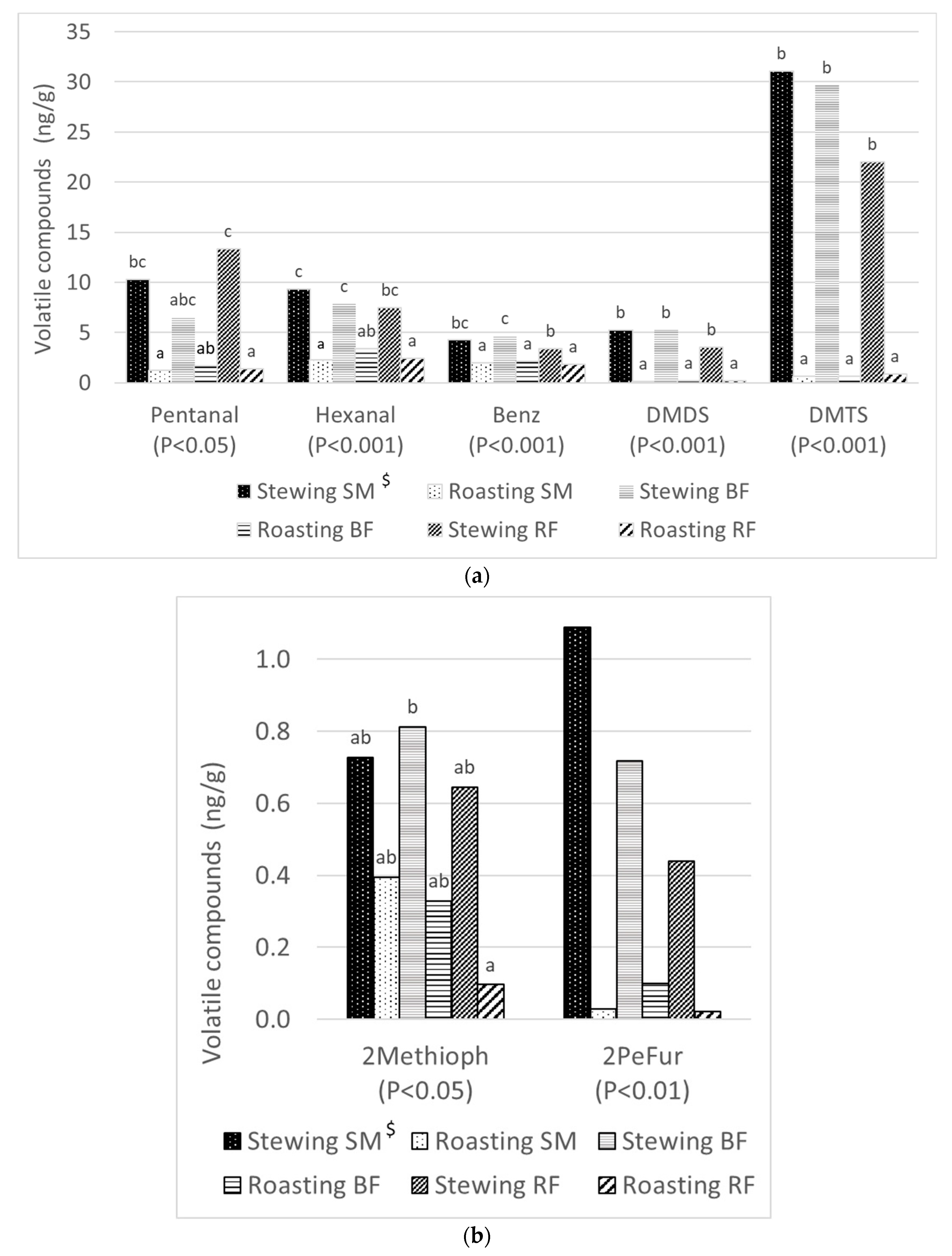
3.3. Volatile Compounds
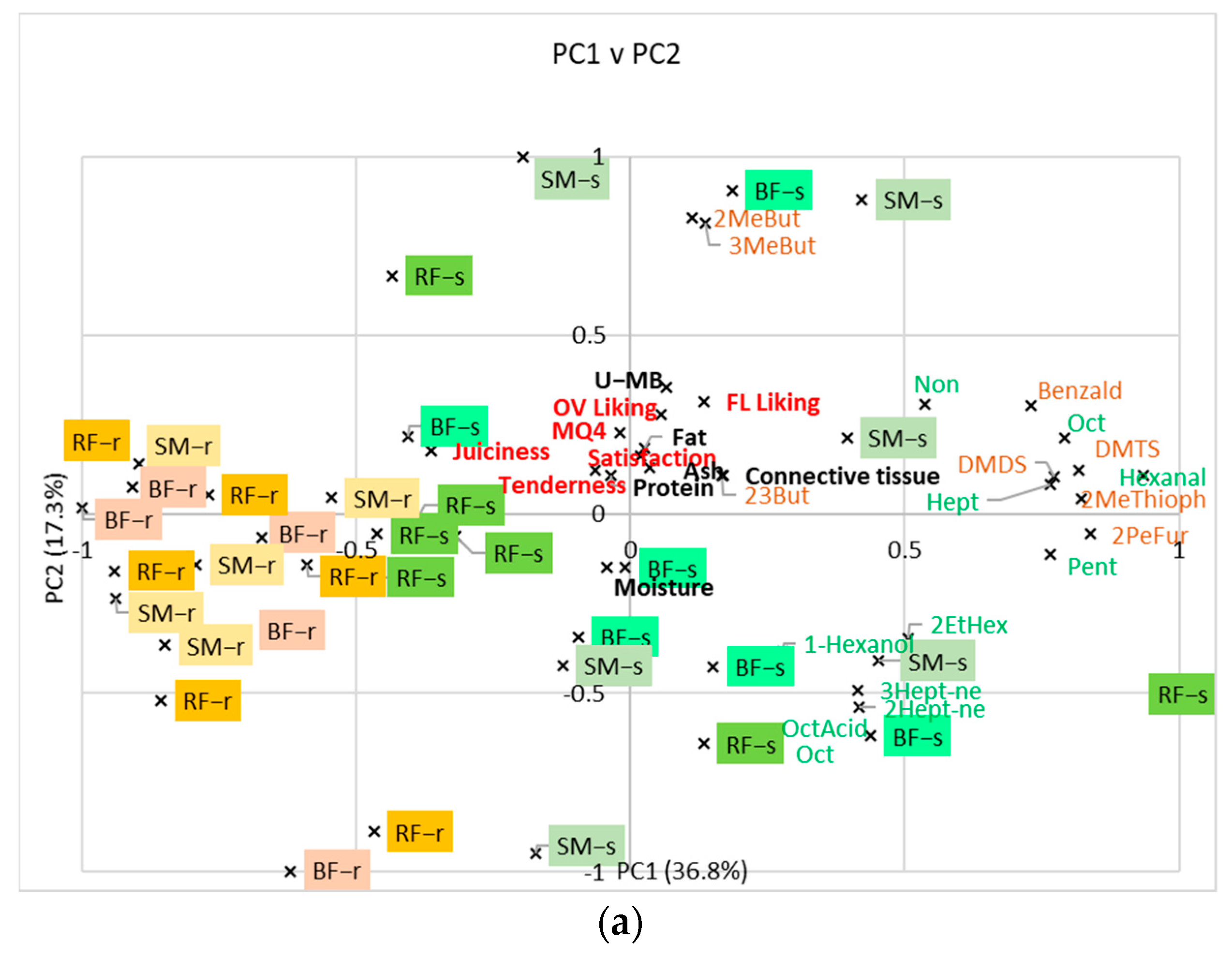
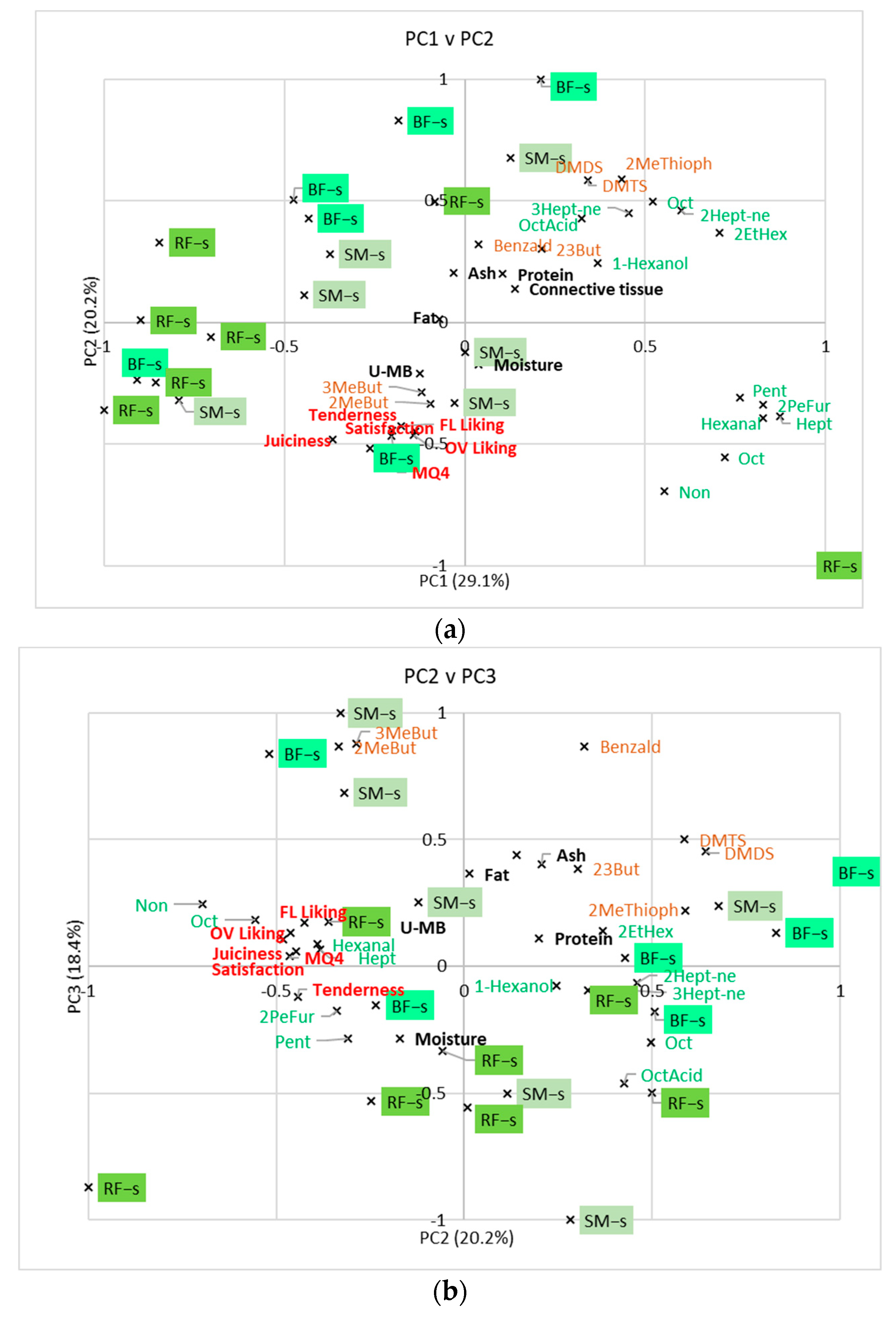
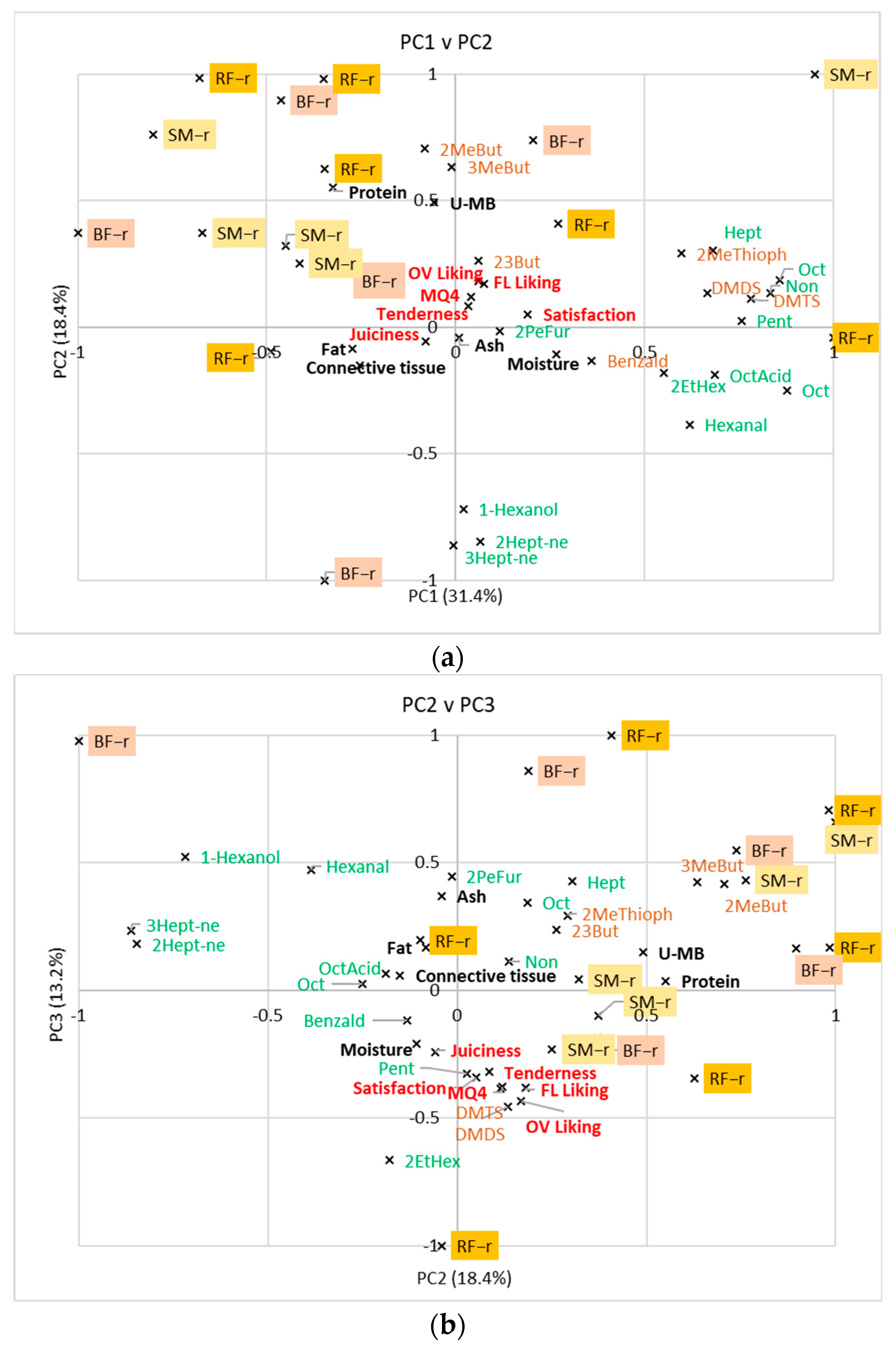
3.4. Relationship between Volatile Compounds, Consumer Scores, and Compositional Analyses
- n-aldehydes
- Strecker aldehydes
- Ketones
- Sulfur compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbin, D.F.; Elmasry, G.; Sun, D.; Allen, P. Non-Destructive Determination of Chemical Composition in Intact and Minced Pork Using near-Infrared Hyperspectral Imaging. Food Chem. 2013, 138, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Polkinghorne, R.A.; Thompson, J.M.B.; Watson, R.C.; Gee, A.D.; Porter, M.E. Evolution of the Meat Standards Australia (MSA) Beef Grading System. Aust. J. Exp. Agric. 2008, 48, 1351–1359. [Google Scholar] [CrossRef]
- Huffman, K.L.; Miller, M.F.; Hoover, L.C.; Wu, C.K.; Brittin, H.C.; Ramsey, C.B. Effect of Beef Tenderness on Consumer Satisfaction with Steaks Consumed in the Home and Restaurant. J. Anim. Sci. 1996, 74, 8778116. [Google Scholar] [CrossRef]
- Miller, M.F.; Carr, M.A.; Ramsey, C.B.; Crockett, K.L.; Hoover, L.C. Consumer Thresholds for Establishing the Value of Beef Tenderness. J. Anim. Sci. 2001, 79, 3062–3068. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelski, G.; Woźniak, K.; Polkinghorne, R.; Półtorak, A.; Wierzbicka, A. Polish Consumer Categorisation of Grilled Beef at 6 Mm and 25 mm Thickness into Quality Grades, Based on Meat Standards Australia Methodology. Meat Sci. 2020, 161, 107953. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of Thermal Treatment on Formation of Volatile Compounds, Cooking Loss and Lipid Oxidation in Foal Meat. LWT—Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Chiavaro, E.; Rinaldi, M.; Vittadini, E.; Barbanti, D. Cooking of Pork Longissimus Dorsi at Different Temperature and Relative Humidity Values: Effects on Selected Physico-Chemical Properties. J. Food Eng. 2009, 93, 158–165. [Google Scholar] [CrossRef]
- Meinert, L.; Andersen, L.T.; Bredie, W.L.P. Chemical and Sensory Characterisation of Pan-Fried Pork Flavour: Interactions between Raw Meat Quality, Ageing and Frying Temperature. Meat Sci. 2007, 75, 229–242. [Google Scholar] [CrossRef]
- Meinert, L.; Schäfer, A.; Bjergegaard, C.; Aaslyng, M.D.; Bredie, W.L.P. Comparison of Glucose, Glucose 6-Phosphate, Ribose, and Mannose as Flavour Precursors in Pork; the Effect of Monosaccharide Addition on Flavour Generation. Meat Sci. 2009, 81, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Van Ba, H.; Amna, T.; Hwang, I. Significant in Fl Uence of Particular Unsaturated Fatty Acids and PH on the Volatile Compounds in Meat-like Model Systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Majcher, M.A.; Jeleń, H.H. Effect of Cysteine and Cystine Addition on Sensory Profile and Potent Odorants of Extruded Potato Snacks. J. Agric. Food Chem. 2007, 55, 5754–5760. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C.; Davidek, T. Formation of Aroma Compounds from Ribose and Cysteine during the Maillard Reaction. J. Agric. Food Chem. 2003, 51, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Claeys, E.; De Smet, S.; Balcaen, A.; Raes, K.; Demeyer, D. Quantification of Fresh Meat Peptides by SDS—PAGE in Relation to Ageing Time and Taste Intensity. Meat Sci. 2004, 67, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Katsumata, T.; Egi, M. Taste Properties of Maillard-Reaction Products Prepared from 1000 to 5000 Da Peptide. Food Chem. 2006, 99, 600–604. [Google Scholar] [CrossRef]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The Influence of Cooking and Fat Trimming on the Actual Nutrient Intake from Meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mottram, D.S. Flavour Formation in Meat and Meat Products. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Soncin, S.; Chiesa, L.M.; Cantoni, C.; Biondi, P.A. Preliminary Study of the Volatile Fraction in the Raw Meat of Pork, Duck and Goose. J. Food Compos. Anal. 2007, 20, 436–439. [Google Scholar] [CrossRef]
- Sitz, B.M.; Calkins, C.R.; Feuz, D.M.; Umberger, W.J.; Eskridge, K.M. Consumer Sensory Acceptance and Value of Domestic, Canadian, and Australian Grass-Fed Beef Steaks 1. J. Anim. Sci. 2005, 83, 2863–2868. [Google Scholar] [CrossRef]
- Chevance, F.F.; Farmer, L.J. Release of Volatile Odor Compounds from Full-Fat and Reduced-Fat. J. Agric. Food Chem. 1999, 47, 5161–5168. [Google Scholar] [CrossRef]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Effect of Different Cooking Methods on Lipid Oxidation and Formation of Volatile Compounds in Foal Meat. Meat Sci. 2014, 97, 223–230. [Google Scholar] [CrossRef]
- Dubost, A.; Micol, D.; Picard, B.; Lethias, C.; Andueza, D.; Bauchart, D.; Listrat, A. Structural and Biochemical Characteristics of Bovine Intramuscular Connective Tissue and Beef Quality. Meat Sci. 2013, 95, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Zhou, G.H.; Xu, X.L. Dynamical Changes of Beef Intramuscular Connective Tissue and Muscle Fiber during Heating and Their Effects on Beef Shear Force. Food Bioprocess Technol. 2010, 3, 521–527. [Google Scholar] [CrossRef]
- Rasinska, E.; Rutkowska, J.; Czarniecka-Skubina, E.; Tambor, K. Effects of Cooking Methods on Changes in Fatty Acids Contents, Lipid Oxidation and Volatile Compounds of Rabbit Meat. LWT—Food Sci. Technol. 2019, 110, 64–70. [Google Scholar] [CrossRef]
- Farmer, L.; Hagan, T.D.J.; Devlin, Y.; Gordon, A.; Oltra, O. Relationships between Beef Aroma Compounds and Other Quality Attributes. In Proceedings of the International Congress on Meat Science and Technology, Montreal, QC, Canada, 12–17 August 2012; p. 2012. [Google Scholar]
- Watson, R. Consumer Assessment of Eating Quality—Development of Protocols for Meat Consumer Assessment of Eating Quality—Development of Protocols for Meat Standards Australia (MSA) Testing. Aust. J. Exp. Agric. 2008, 48, 1360–1367. [Google Scholar] [CrossRef]
- USDA United States Standards for Grades of Carcass Beef; Agricultural Marketing Service, Ed.; U.S. Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Wyrwisz, J.; Moczkowska; Kurek, M.; Stelmasiak, A.; Półtorak, A.; Wierzbicka, A. Influence of 21 Days of Vacuum-Aging on Color, Bloom Development, and WBSF of Beef Semimembranosus. Meat Sci. 2016, 122, 48–54. [Google Scholar] [CrossRef]
- Legako, J.F.; Brooks, J.C.; Quinn, T.G.O.; Hagan, T.D.J.; Polkinghorne, R.; Farmer, L.J.; Miller, M.F. Consumer Palatability Scores and Volatile Beef Flavor Compounds of Five USDA Quality Grades and Four Muscles. Meat Sci. 2015, 100, 291–300. [Google Scholar] [CrossRef]
- Modzelewska-Kapituła, M.; Dąbrowska, E.; Jankowska, B.; Kwiatkowska, A.; Cierach, M. The Effect of Muscle, Cooking Method and Final Internal Temperature on Quality Parameters of Beef Roast. Meat Sci. 2012, 91, 195–202. [Google Scholar] [CrossRef]
- Jeremiah, L.E.; Dugan, M.E.R.; Aalhus, J.L.; Gibson, L.L. Assessment of the Chemical and Cooking Properties of the Major Beef Muscles and Muscle Groups. Meat Sci. 2003, 65, 985–992. [Google Scholar] [CrossRef]
- Offer, G.; Knight, P. The Structural Basis of Water-Holding in Meat. Part 2: Drip Loss; Lawrie, R., Ed.; Elsevier Applied Science: London, UK, 1988; Volume 4. [Google Scholar]
- Huff-Lonergan Elisabeth, L.S.M. Mechanisms of Water-Holding Capacity of Meat: The Role of Postmortem Biochemical and Structural Changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Chen, J.; Stokes, J.R. Rheology and Tribology: Two Distinctive Regimes of Food Texture Sensation. Trends Food Sci. Technol. 2012, 25, 4–12. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Song, Y.; Qiang, Y.; Han, D.; Zhang, C. Changes Provoked by Altitudes and Cooking Methods in Physicochemical Properties, Volatile Profile, and Sensory Characteristics of Yak Meat. Food Chem. X 2023, 20, 101019. [Google Scholar] [CrossRef] [PubMed]
- Stahnke, L.H. Aroma Components from Dried Sausages Fermented with Staphylococcus Xylosus. Meat Sci. 1994, 38, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Granvogl, M.; Beksan, E.; Schieberle, P. New Insights into the Formation of Aroma-Active Strecker Aldehydes from 3-Oxazolines as Transient Intermediates. J. Agric. Food Chem. 2012, 60, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.T.; Baek, K.H.; Jeong, H.S.; Yoon, S.K.; Joo, S.; Lee, S.K. Effects of Cooking Method and Final Core-Temperature on Cooking Loss, Lipid Oxidation, Nucleotide-Related Compounds and Aroma Volatiles of Hanwoo Brisket. Asian-Australas. J. Anim. Sci. 2018, 31, 293. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Parkanyi, C. Mechanisms of Formation of Heterocyclic Compounds in Maillard and Pyrolysis Reactions. In Chemistry of Heterocyclic Compounds in Flavors and Aromas; Vernin, G., Ed.; Ellis Horwood: Chichester, UK, 1982; pp. 151–207. [Google Scholar]
- Mottram, D.S. Meat In Volatile Compounds in Foods and Beverages; Dekker, M., Ed.; Imprint Routledge: New York, NY, USA, 1991. [Google Scholar]
- Zhang, Z.; Wang, B.; Cao, Y. Effect of Maillard Reaction Products Derived From Cysteine On the Formation of Dimethyl Disulfide and Dimethyl Trisulfide During Storage. J. Agric. Food Chem. 2023, 71, 13043–13053. [Google Scholar] [CrossRef] [PubMed]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper (II) Mediated Hydrogen Sulfide and Thiol Oxidation to Disulfides and Organic Polysulfanes, and Their Reductive Cleavage in Wine: Mechanistic Elucidation and Potential Applications Copper (II) Mediated Hydrogen Sulfide and Thiol Oxidation to D. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Hartman, G.J.; Scheide, J.D.; Ho, C.-T. Effect of Water Activity on the Major Volatiles Produced in a Model System Approximating Cooked Meat. J. Food Sci. 1984, 49, 607–613. [Google Scholar] [CrossRef]
- Grosch, W. Lipid Degradation Products and Flavour in Food Flavours; Morton, I.D., Mac-Lend, A.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Hernández, M.L.; Sicardo, M.D.; Belaj, A. The Oleic/Linoleic Acid Ratio in Olive (Olea europaea L.) Fruit Mesocarp Is Mainly Controlled by OeFAD2-2 and OeFAD2-5 Genes Together With the Different Specificity of Extraplastidial Acyltransferase Enzymes. Front. Plant Sci. 2021, 12, 653997. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ho, C.T. The Flavour of Poultry Meat. In Flavor of Meat and Meat ProductsMeat Products; Shahidi, F., Ed.; Blackie Academic and Professional: Glasgow, UK, 1994; pp. 52–69. [Google Scholar]
- Górska, E.; Nowicka, K.; Jaworska, D.; Przybylski, W.; Tambor, K. Relationship between Sensory Attributes and Volatile Compounds of Polish Dry-Cured Loin. Asian-Australas. J. Anim. Sci. 2017, 30, 720–727. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, D.; Wang, X.; Zhu, Y.; Chen, G.; Liu, Y. Analysis of Aroma-Active Compounds in Four Chinese Dry-Cured Hams Based on GC-O Combined with AEDA and Frequency Detection Methods. LWT—Food Sci. Technol. 2022, 153, 112497. [Google Scholar] [CrossRef]
- Farmer, L.; Hagan, T.D.J.; Oltra, O.R.; Devlin, Y.; Gordon, A. Relating Beef Aroma Compounds to Flavour Precursors and Other Measures of Quality. In Current Topics in Flavor Chemistry & Biology, Proceedings of the Wartburg Flavour Symposium Wartburg Symposium on Flavor Chemistry and Biology, Eisenach, Germany, 16–19 April 2013; Hofmann, T., Krautwurst, D., Schieberle, P., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2013. [Google Scholar]
- Manley, C.H. Progress in the Science of Thermal Generation of Aromas: A Review. In Thermal Generation of Aromas; Parliment, T.H., McGorrin, R.J., Ho, C.T., Eds.; American Chemical Society: Washington, DC, USA, 1989; Volume 409, pp. 12–22. ISBN 9780841216822. [Google Scholar]
- Insausti, K.; Gon, V.; Petri, E.; Gorraiz, C.; Beriain, M.J. Effect of Weight at Slaughter on the Volatile Compounds of Cooked Beef from Spanish Cattle Breeds. Meat Sci. 2005, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]



| Cooking Method: | Stewing | Roasting |
|---|---|---|
| Total number of consumers | 180 | 180 |
| Gender [%] | ||
| Women | 57 | 54 |
| Men | 43 | 46 |
| Age [%] | ||
| <20 | 10 | 6 |
| 20–25 | 50 | 45 |
| 26–30 | 13 | 12 |
| 31–39 | 11 | 15 |
| 40–50 | 10 | 9 |
| >50 | 6 | 13 |
| Professions [%] | ||
| Students | 19 | 19 |
| Office worker | 23 | 31 |
| Laboratory technicians | 6 | 7 |
| Sales/Services | 12 | 7 |
| Worker | 9 | 6 |
| Teacher | 10 | 5 |
| Trader | 3 | 4 |
| Housewives | 1 | 3 |
| Other occupational | 16 | 16 |
| Unemployed | 1 | 2 |
| Eating beef frequency [%] | ||
| Daily | 1 | 0 |
| 4–5 per week | 0 | 2 |
| 2–3 per week | 13 | 12 |
| Once a week | 33 | 24 |
| Once two weeks | 19 | 24 |
| Once per month | 32 | 35 |
| Never | 2 | 3 |
| Preferred degree of doneness [%] | ||
| Blue | 3 | 3 |
| Rare | 2 | 1 |
| Medium rare | 2 | 4 |
| Medium | 32 | 23 |
| Medium well | 47 | 51 |
| Well done | 14 | 18 |
| Muscle | SM $ | BF | RF | SEM | p-Value |
|---|---|---|---|---|---|
| Fat | 2.3 b | 2.8 b | 1.1 a | 0.16 | <0.001 |
| Moisture | 73.3 a | 73.2 a | 75.4 b | 0.22 | <0.001 |
| Protein | 23.8 b | 23.2 ab | 22.9 a | 0.12 | <0.05 |
| Ash | 1.4 b | 1.5 b | 1.0 a | 0.05 | <0.001 |
| Connective tissue | 1.3 a | 1.6 b | 1.1 a | 0.05 | <0.001 |
| Factors | Cooking Method (C) | Muscle (M) | C×M | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stewing | Roasting | SEM | p-v | SM $ | BF | RF | SEM | p-v | p-v | |
| Flavor liking | 53.1 | 47.6 | 1.77 | 0.120 | 47.8 a | 43.8 a | 59.2 b | 1.81 | <0.001 | <0.01 |
| Tenderness | 45.6 | 43.6 | 2.87 | 0.729 | 35.4 a | 33.3 a | 64.3 b | 2.89 | <0.001 | <0.001 |
| Juiciness | 45.6 | 53.4 | 2.41 | 0.103 | 42.8 a | 43.3 a | 61.8 b | 2.46 | <0.001 | <0.01 |
| Overall liking | 52.0 | 47.9 | 1.93 | 0.291 | 46.6 a | 41.6 a | 61.5 b | 1.97 | <0.001 | <0.001 |
| MQ4 | 49.8 | 47.1 | 2.07 | 0.519 | 43.2 a | 40.0 a | 61.7 b | 2.10 | <0.001 | <0.001 |
| Satisfaction | 3.0 | 2.9 | 0.08 | 0.576 | 2.8 a | 2.6 a | 3.5 b | 2.10 | <0.001 | <0.001 |
| Factors | Cooking Method (C) | Muscle (M) | C.M | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stewing | Roasting | SEM | p-v | SM $ | BF | RF | SEM | p-v | p-v | |
| n-Aldehydes | ||||||||||
| Pentanal | 10.2 b | 1.5 a | 1.37 | <0.001 | 5.8 | 3.9 | 7.8 | 1.37 | 0.505 | <0.05 |
| Hexanal | 8.2 b | 2.7 a | 0.60 | <0.001 | 5.8 | 5.5 | 5.2 | 0.60 | 0.909 | <0.001 |
| Heptanal | 0.2 | 0.5 | 0.10 | 0.230 | 0.4 | 0.5 | 0.2 | 0.10 | 0.496 | NS |
| Octanal | 0.2 | 0.2 | 0.00 | 0.378 | 0.2 | 0.2 | 0.2 | 0.03 | 0.857 | NS |
| Nonanal | 4.6 | 3.8 | 0.02 | 0.389 | 4.0 | 4.2 | 4.4 | 0.41 | 0.941 | NS |
| Strecker aldehydes | ||||||||||
| 2-Methyl butanal | 15.9 | 15.9 | 1.42 | 0.988 | 16.3 | 18.2 | 13.2 | 1.42 | 0.355 | NS |
| 3-Methyl butanal | 28.1 | 27.3 | 2.23 | 0.857 | 28.9 | 32.2 | 22.2 | 2.23 | 0.167 | NS |
| Benzaldehyde | 4.1 b | 2.0 a | 0.20 | <0.001 | 3.1 | 3.3 | 2.7 | 0.20 | 0.431 | <0.001 |
| Thiophenes | ||||||||||
| 2-Methyl thiophene | 0.7 b | 0.3 a | 0.07 | <0.001 | 0.6 | 0.6 | 0.4 | 0.07 | 0.572 | <0.01 |
| Ketones | ||||||||||
| 2.3-Butanedione | 35.6 | 35.5 | 4.42 | 0.991 | 36.8 | 40.8 | 29.3 | 4.42 | 0.563 | NS |
| 2-Heptanone | 3.9 | 3.2 | 0.20 | 0.086 | 3.1 | 3.9 | 3.5 | 0.20 | 0.366 | NS |
| 3-Heptanone | 1.4 | 1.6 | 0.12 | 0.623 | 1.6 | 1.6 | 1.3 | 0.12 | 0.569 | NS |
| Sulfides | ||||||||||
| Dimethyl disulfide | 4.6 b | 0.2 a | 0.41 | <0.001 | 2.7 | 2.5 | 2.0 | 0.41 | 0.786 | <0.001 |
| Dimethyl trisulfide | 27.3 b | 0.7 a | 2.42 | <0.001 | 15.9 | 14.0 | 12.2 | 2.42 | 0.839 | <0.001 |
| Furans | ||||||||||
| 2-Pentyl furan | 0.9 b | 0.1 a | 0.12 | <0.001 | 0.6 | 0.4 | 0.5 | 0.12 | 0.837 | <0.01 |
| Alcohols | ||||||||||
| 1-Hexanol | 0.3 | 0.3 | 0.04 | 0.625 | 0.3 | 0.4 | 0.2 | 0.05 | 0.203 | NS |
| 2-Ethyl hexanol | 0.0 | 0.4 | 0.15 | 0.176 | 0.3 | 0.4 | 0.0 | 0.15 | 0.615 | NS |
| Acids | ||||||||||
| Octanoic acid | 0.2 | 0.2 | 0.02 | 0.876 | 0.2 | 0.2 | 0.1 | 0.02 | 0.798 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtasik-Kalinowska, I.; Farmer, L.J.; Hagan, T.D.J.; Gordon, A.W.; Polkinghorne, R.; Pogorzelski, G.; Wierzbicka, A.; Poltorak, A. The Influence of Cooking Methods and Muscle on Beef Aroma Profile and Consumer Satisfaction: Insights from Volatile Compound Analysis. Appl. Sci. 2024, 14, 4477. https://doi.org/10.3390/app14114477
Wojtasik-Kalinowska I, Farmer LJ, Hagan TDJ, Gordon AW, Polkinghorne R, Pogorzelski G, Wierzbicka A, Poltorak A. The Influence of Cooking Methods and Muscle on Beef Aroma Profile and Consumer Satisfaction: Insights from Volatile Compound Analysis. Applied Sciences. 2024; 14(11):4477. https://doi.org/10.3390/app14114477
Chicago/Turabian StyleWojtasik-Kalinowska, Iwona, Linda J. Farmer, Terence D. J. Hagan, Alan W. Gordon, Rod Polkinghorne, Grzegorz Pogorzelski, Agnieszka Wierzbicka, and Andrzej Poltorak. 2024. "The Influence of Cooking Methods and Muscle on Beef Aroma Profile and Consumer Satisfaction: Insights from Volatile Compound Analysis" Applied Sciences 14, no. 11: 4477. https://doi.org/10.3390/app14114477
APA StyleWojtasik-Kalinowska, I., Farmer, L. J., Hagan, T. D. J., Gordon, A. W., Polkinghorne, R., Pogorzelski, G., Wierzbicka, A., & Poltorak, A. (2024). The Influence of Cooking Methods and Muscle on Beef Aroma Profile and Consumer Satisfaction: Insights from Volatile Compound Analysis. Applied Sciences, 14(11), 4477. https://doi.org/10.3390/app14114477








