Effect of EAF Slag on the Performance of Wollastonite Mixes Inspired by CO2 Curing Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Test Methods
2.3. Characterization Studies
3. Results and Discussion
3.1. Sample Properties
3.2. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashraf, W.; Olek, J.; Tian, N. Multiscale characterization of carbonated wollastonite paste and application of homogenization schemes to predict its effective elastic modulus. Cem. Concr. Compos. 2016, 72, 284–298. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, J.; Xu, H.; Cheng, X.; Guo, X. Mechanical properties and mechanism of wollastonite fibers reinforced oil well cement. Constr. Build. Mater. 2020, 260, 120461. [Google Scholar] [CrossRef]
- Khan, R.I.; Ashraf, W. Effects of ground wollastonite on cement hydration kinetics and strength development. Constr. Build. Mater. 2019, 218, 150–161. [Google Scholar] [CrossRef]
- Nair, N.A.; Sairam, V. Research initiatives on the influence of wollastonite in cement-based construction material—A review. J. Clean. Prod. 2021, 283, 124665. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Mathematical modelling and experimental study of carbonation of wollastonite in the aqueous media. J. CO2 Util. 2019, 31, 181–191. [Google Scholar]
- Abbass, A.M.; Elrahman, M.A.; Sikora, P.; Strzałkowski, J.; Stephan, D.; Abdel-Gawwad, H.A. From dolomite waste to katoite-based binder: Synthesis, performance and characterization. J. Build. Eng. 2023, 75, 106971. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Xuan, D.; Shen, P.; Zhu, J.; Guan, X.; Shi, C. Enhanced carbonation reactivity of wollastonite by rapid cooling process: Towards an ultra-low calcium CO2 sequestration binder. Constr. Build. Mater. 2021, 299, 124336. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar]
- Zhu, D.; Wen, A.; Tang, A. Mechanical properties, durability and environmental assessment of low-carbon cementitious composite with natural fibrous wollastonite. Environ. Res. 2023, 234, 116552. [Google Scholar] [CrossRef]
- Öz, H.Ö.; Güneş, M. The effects of synthetic wollastonite developed with calcite and quartz on high performance mortars. Struct. Concr. 2021, 22, E257–E272. [Google Scholar] [CrossRef]
- Saxena, S.K.; Kumar, M.; Chundawat, D.S.; Singh, N.B. Utilization of wollastonite in cement manufacturing. Mater. Today Proc. 2020, 29, 733–737. [Google Scholar] [CrossRef]
- Mun, S.-J.; Han, S.-J.; Wee, J.-H. CO2 mineralization of double decomposition suspension of Ca-leached wollastonite solutions. Miner. Eng. 2022, 176, 107315. [Google Scholar]
- Lackner, K.S. Carbonate chemistry for sequestering fossil carbon. Annu. Rev. Energy Environ. 2002, 27, 193–232. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.-J.; Comans, R.N.J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- Zulumyan, N.; Isahakyan, A.; Beglaryan, H.; Melikyan, S. The influence of NaOH on the synthesis of calcium silicates. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1323–1332. [Google Scholar] [CrossRef]
- EPA Report. Sustainable Materials Management. Available online: https://www.epa.gov/smm (accessed on 21 May 2024).
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Hajiaghamemar, M.; Mostofinejad, D.; Bahmani, H. A high-strength concrete resistant to elevated temperatures using steel slag aggregates. Struct. Concr. 2023, 24, 3162–3177. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, H.-G.; Kim, M.-J.; Kim, D.-H.; Kim, D.-S.; Kim, K.-H. Bond performance of reinforced concrete beams with electric arc furnace slag aggregates. Constr. Build. Mater. 2020, 244, 118366. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Mei, H.; Rao, L.; Wang, H.; Lv, N. Generation, utilization, and environmental impact of ladle furnace slag: A minor review. Sci. Total Environ. 2023, 895, 165070. [Google Scholar]
- Kim, J.; Azimi, G. The CO2 sequestration by supercritical carbonation of electric arc furnace slag. J. CO2 Util. 2021, 52, 101667. [Google Scholar] [CrossRef]
- González-Ortega, M.A.; Cavalaro, S.H.P.; Rodríguez de Sensale, G.; Aguado, A. Durability of concrete with electric arc furnace slag aggregate. Constr. Build. Mater. 2019, 217, 543–556. [Google Scholar] [CrossRef]
- Amani, A.; Ramezanianpour, A.M.; Palassi, M. Investigation on the sustainable use of electric arc furnace slag aggregates in eco-friendly alkali-activated low fineness slag concrete as a green construction composite. J. Clean. Prod. 2021, 307, 127257. [Google Scholar] [CrossRef]
- Chang, J.J.; Yeih, W.; Chung, T.J.; Huang, R. Properties of pervious concrete made with electric arc furnace slag and alkali-activated slag cement. Constr. Build. Mater. 2016, 109, 34–40. [Google Scholar] [CrossRef]
- Nair, A.T.; Mathew, A.; Archana, A.R.; Akbar, M.A. Use of hazardous electric arc furnace dust in the construction industry: A cleaner production approach. J. Clean. Prod. 2022, 377, 134282. [Google Scholar] [CrossRef]
- Bheel, N.; Awoyera, P.; Shar, I.A.; Kırgız, M.S. 16—Self-compacting concrete blended with fly ash and ground granulated blast furnace slag. In Advance Upcycling of By-Products in Binder and Binder-Based Materials; Kırgız, M.S., Ed.; Woodhead Publishing: Sawston, UK, 2024; pp. 309–334. [Google Scholar]
- Lee, J.-Y.; Choi, J.-S.; Yuan, T.-F.; Yoon, Y.-S.; Mitchell, D. Comparing properties of concrete containing electric arc furnace slag and granulated blast furnace slag. Materials 2019, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Hekal, E.E.; Abo-El-Enein, S.A.; El-Korashy, S.A.; Megahed, G.M.; El-Sayed, T.M. Hydration characteristics of Portland cement—Electric arc furnace slag blends. HBRC J. 2013, 9, 118–124. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-S.; Jung, S.S.; Hwang, J.I.; Choi, J.-S.; Sohn, I. Valorization of electric arc furnace primary steelmaking slags for cement applications. Waste Manag. 2015, 41, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.; Sun, P.; Elgowainy, A.; Bobba, P.; McMillan, C.; Ma, O.; Podkaminer, K.; Rustagi, N.; Melaina, M.; Koleva, M. Cost and life cycle analysis for deep CO2 emissions reduction of steelmaking: Blast furnace-basic oxygen furnace and electric arc furnace technologies. Int. J. Greenh. Gas Control 2023, 128, 103958. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve´, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- EN 1097-6; Tests for Mechanical and Physical Properties of Aggregates: Determination of Particle Density and Water Absorption. British Standard Institution: London, UK, 2013.
- ASTM C1437; Standard Test Method for Flow of Hydraulic Cement Mortar. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM: West Conshohocken, PA, USA, 2016.
- ASTM C348; Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. ASTM: West Conshohocken, PA, USA, 2021.
- Dong, Q.; Wang, G.; Chen, X.; Tan, J.; Gu, X. Recycling of steel slag aggregate in portland cement concrete: An overview. J. Clean. Prod. 2021, 282, 124447. [Google Scholar] [CrossRef]
- Plattenberger, D.A.; Ling, F.T.; Tao, Z.; Peters, C.A.; Clarens, A.F. Calcium silicate crystal structure impacts reactivity with CO2 and precipitate chemistry. Environ. Sci. Technol. Lett. 2018, 5, 558–563. [Google Scholar] [CrossRef]
- Goodbrake, C.J.; Young, J.F.; Berger, R.L. Reaction of hydraulic calcium silicates with carbon dioxide and water. J. Am. Ceram. Soc. 1979, 62, 488–491. [Google Scholar] [CrossRef]
- Jagadisha, A.; Rao, K.B.; Nayak, G.; Kamath, M. Influence of nano-silica on the microstructural and mechanical properties of high-performance concrete of containing EAF aggregate and processed quarry dust. Constr. Build. Mater. 2021, 304, 124392. [Google Scholar] [CrossRef]
- Christensen, A.N.; Jensen, T.R.; Hanson, J.C. Formation of ettringite, Ca6Al2(SO4)3(OH)12·26H2O, AFt, and monosulfate, Ca4Al2O6(SO4)·14H2O, AFm-14, in hydrothermal hydration of Portland cement and of calcium aluminum oxide—Calcium sulfate dihydrate mixtures studied by in situ synchrotron X-ray powder diffraction. J. Solid State Chem. 2004, 177, 1944–1951. [Google Scholar]
- Češnovar, M.; Traven, K.; Horvat, B.; Ducman, V. The potential of ladle slag and electric arc furnace slag use in synthesizing alkali activated materials; the influence of curing on mechanical properties. Materials 2019, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.S.; El-Gamal, S.M.A.; Abo-El-Enein, S.A.; El-Hosiny, F.I.; Ramadan, M. Physico-chemical characteristics of blended cement pastes containing electric arc furnace slag with and without silica fume. HBRC J. 2015, 11, 321–327. [Google Scholar] [CrossRef]
- Monkman, S.; Shao, Y. Assessing the carbonation behavior of cementitious materials. J. Mater. Civ. Eng. 2006, 18, 768–776. [Google Scholar] [CrossRef]
- Hara, N.; Inoue, N. Formation of jennite from fumed silica. Cem. Concr. Res. 1980, 10, 677–682. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; Routledge: London, UK, 2005; p. 432. [Google Scholar]
- Murugan, M.; Santhanam, M.; Gupta, S.S.; Pradeep, T.; Shah, S.P. Influence of 2D rGO nanosheets on the properties of OPC paste. Cem. Concr. Compos. 2016, 70, 48–59. [Google Scholar] [CrossRef]
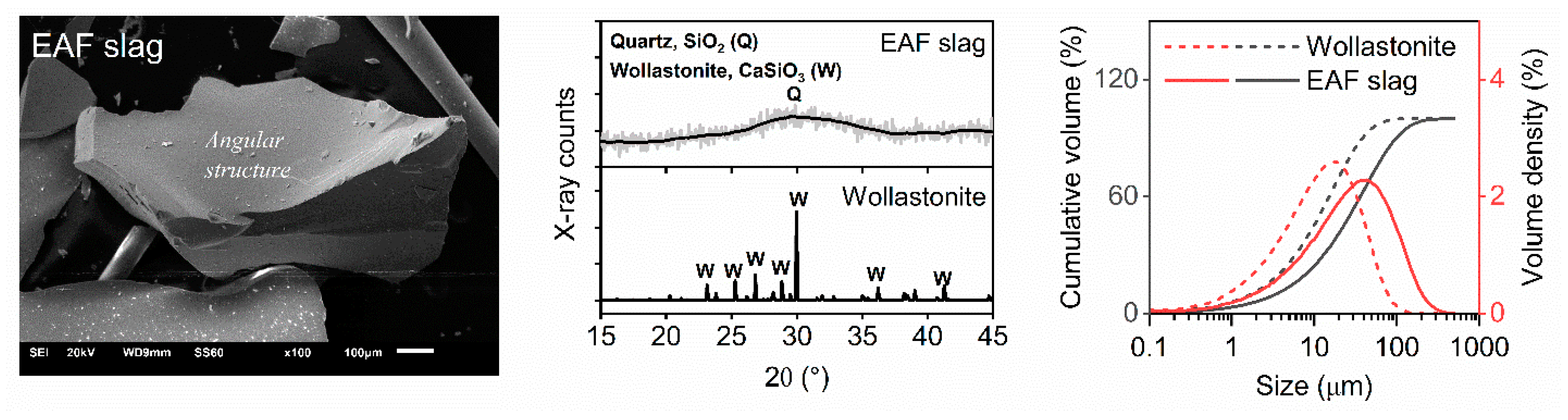
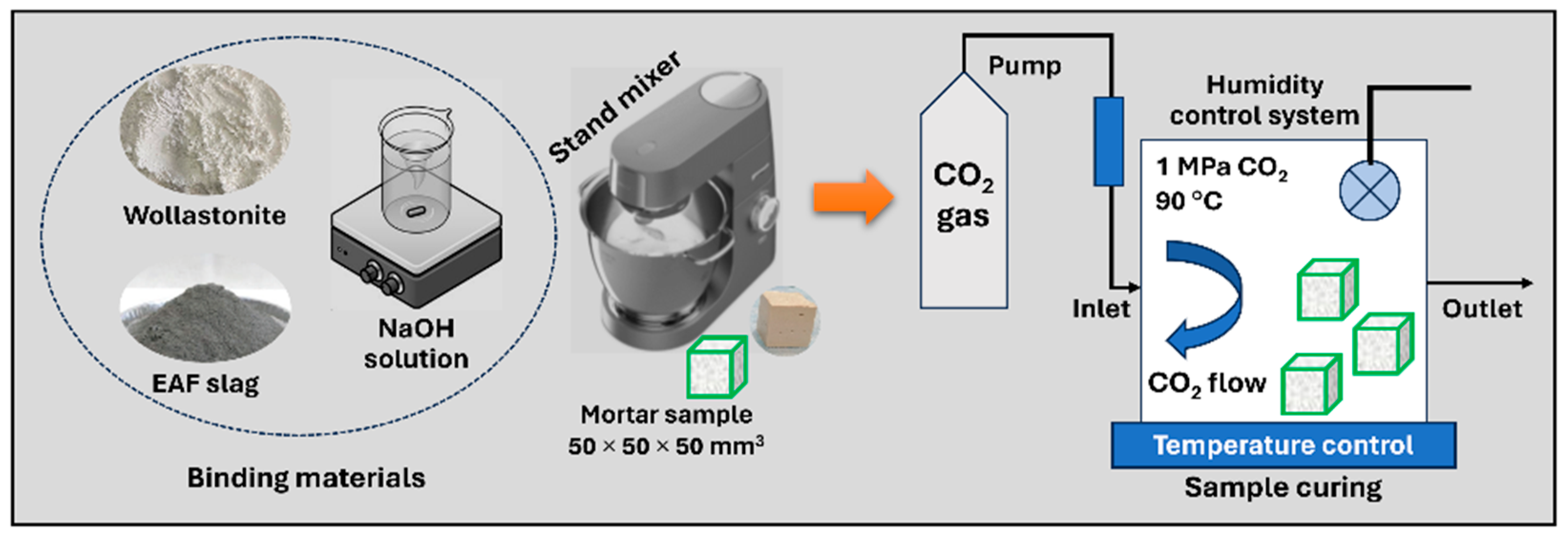
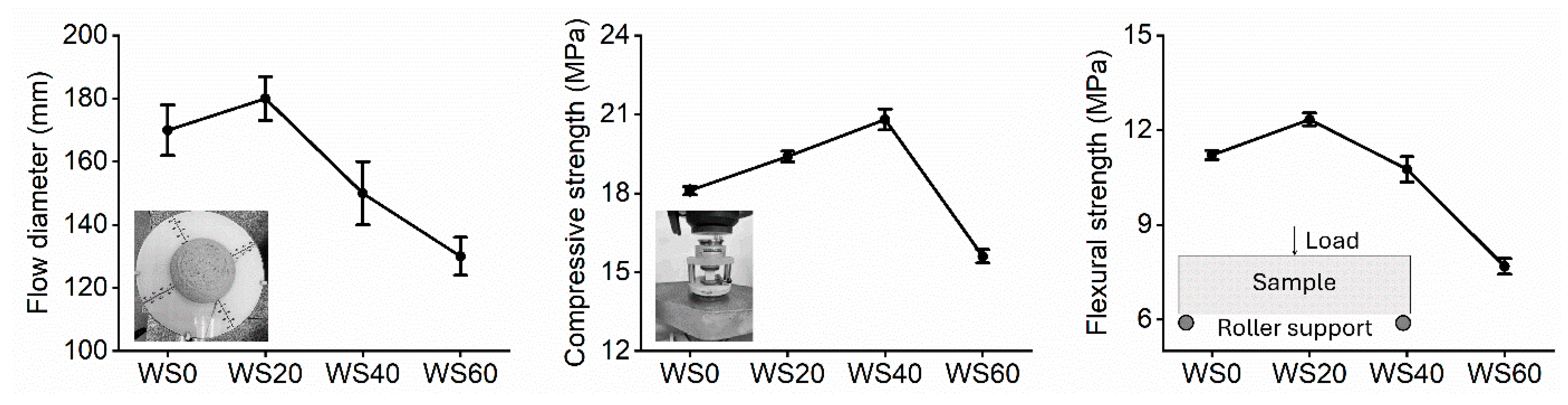

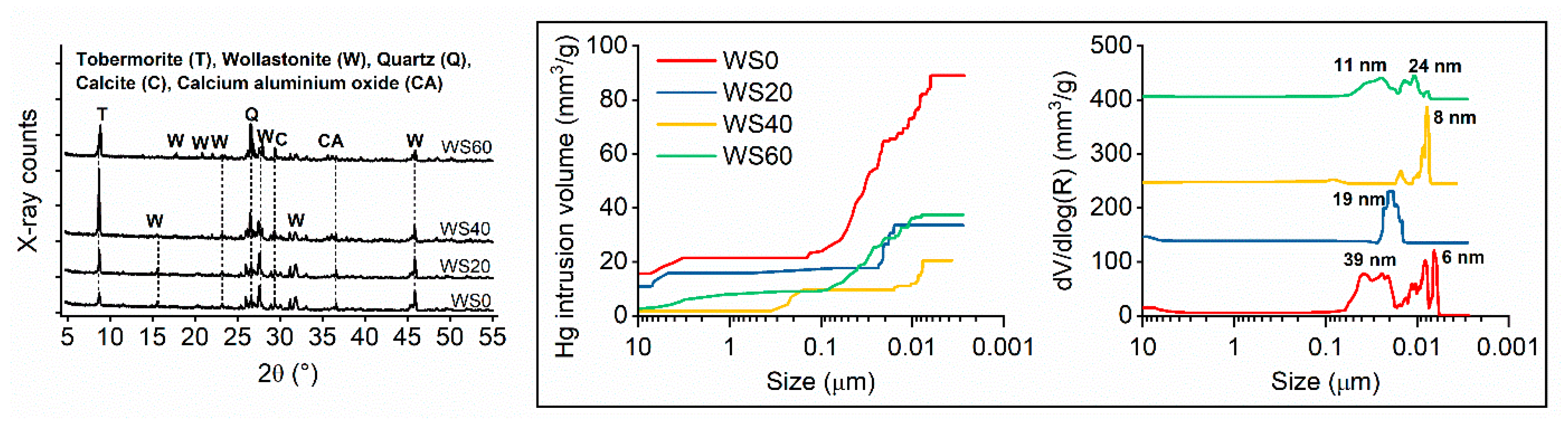
| Sample (%) | CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | MgO | TiO2 | MnO | SO3 | Cr3O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wollastonite | 39.89 | 47.12 | 3.91 | 2.76 | 0.23 | 1.46 | 0.21 | - | - | - | 4.42 |
| EAF slag | 36.02 | 12.86 | 7.52 | 31.07 | 0.13 | 1.73 | 0.63 | 2.68 | 0.32 | 2.46 | 4.58 |
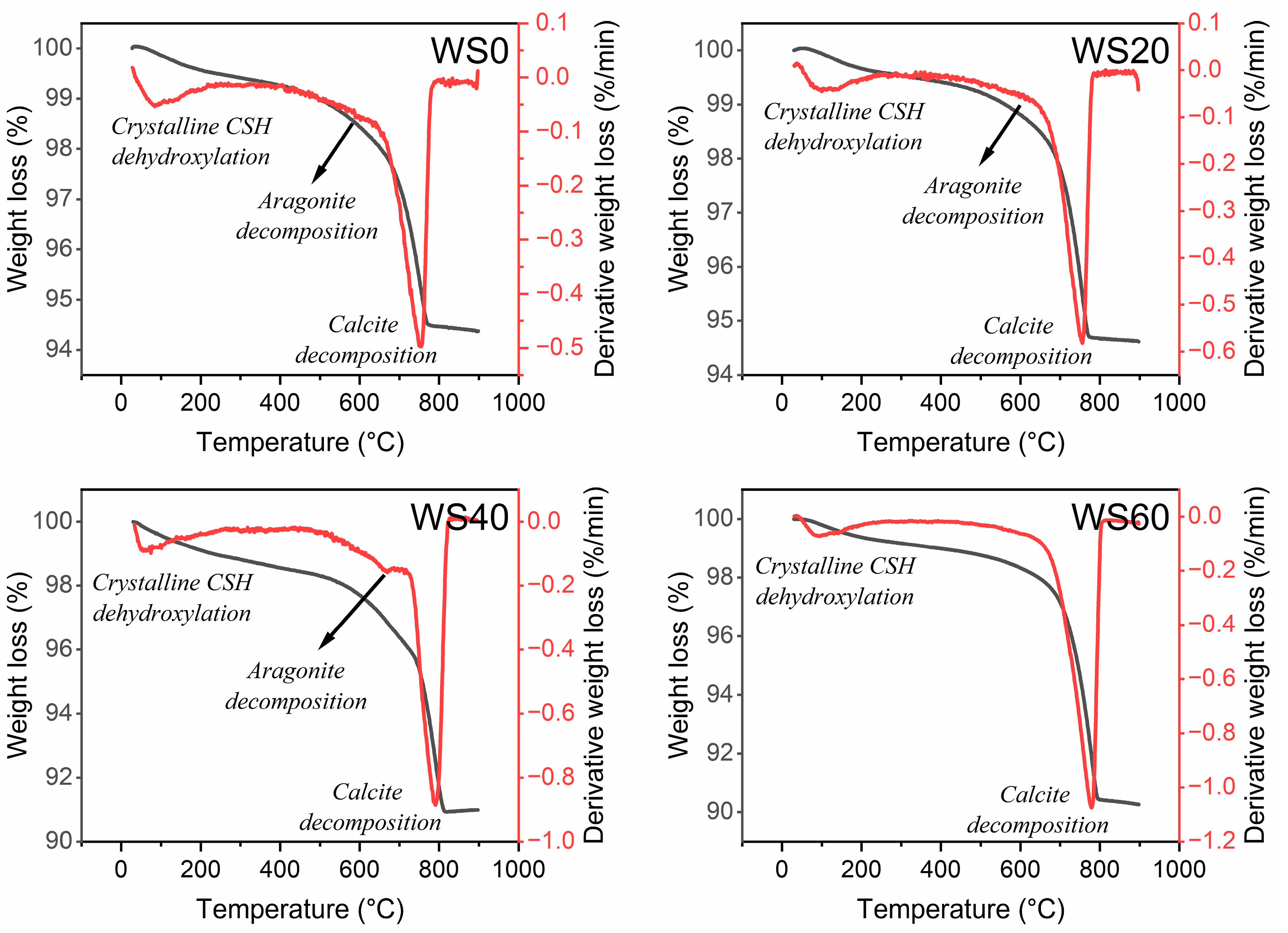
| Sample | WS0 | WS20 | WS40 | WS60 |
|---|---|---|---|---|
| Total weight loss (%) | 5.6 | 5.4 | 9 | 9.7 |
| Weight loss due to CSH dehydroxylation 30–260 °C (%) | 0.5 | 0.4 | 1.1 | 0.8 |
| Weight loss due to aragonite decomposition 650–750 °C (%) | 2.6 | 2.6 | 1.7 | 3.3 |
| Weight loss due to calcite decomposition 750–1000 °C (%) | 1 | 1.2 | 4.3 | 4.3 |
| Gel pore volume ~<10 nm (%) | 18 | 0 | 42 | 4 |
| Capillary pore volume ~10–100 nm (%) | 56 | 48 | 11 | 71 |
| Macropore volume ~>100 nm (%) | 26 | 52 | 47 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthu, M.; Kumar, S.; Chajec, A.; Sadowski, Ł. Effect of EAF Slag on the Performance of Wollastonite Mixes Inspired by CO2 Curing Technology. Appl. Sci. 2024, 14, 4485. https://doi.org/10.3390/app14114485
Muthu M, Kumar S, Chajec A, Sadowski Ł. Effect of EAF Slag on the Performance of Wollastonite Mixes Inspired by CO2 Curing Technology. Applied Sciences. 2024; 14(11):4485. https://doi.org/10.3390/app14114485
Chicago/Turabian StyleMuthu, Murugan, Sanjeev Kumar, Adrian Chajec, and Łukasz Sadowski. 2024. "Effect of EAF Slag on the Performance of Wollastonite Mixes Inspired by CO2 Curing Technology" Applied Sciences 14, no. 11: 4485. https://doi.org/10.3390/app14114485







