Abstract
Microplastics contribute to various environmental issues and serve as carriers for a wide range of toxic compounds such as pesticides, pharmaceuticals, and metal ions. Consequently, there is a gradual shift towards replacing them with biodegradable plastics (bioplastics). However, biodegradable plastics require specific conditions for complete biodegradation, and their biodeterioration often leads to the rapid production of smaller fragments, known as microbioplastics. In this review, we summarize selected issues related to the impact of plastic particles on soil properties and the soil microbiome. Findings from numerous studies indicate that both microplastics and microbioplastics induce adverse changes in soil microbiology, potentially increasing the abundance of soil-borne pathogens. Based on these observations, we argue that plastic particles could serve as carriers for colonies of soil-borne pathogens. Furthermore, the use of bioplastics may exacerbate this issue due to their easier and faster formation, increased support for biofilms, and more pronounced adverse effects on soil biota. However, further research is necessary to either substantiate or refute this perspective.
1. Introduction
Microplastics (MPs) are particulate plastic debris, varying in shape, with dimensions ranging from 0.1 to 5000 µm, according to general agreement []. These particles significantly contaminate environmental compartments such as the atmosphere, pedosphere, hydrosphere, and biosphere, leading to numerous interactions within and across them.
The chemical compositions and shapes of these particles are crucial for their transport and sedimentation processes, as well as their interactions with living and non-living systems. Various types of adsorbed contaminants can be transported over relatively large distances along with MPs, especially substances of organic nature, including persistent organic pollutants, pesticides, and pharmaceuticals such as antibiotics and endocrine disruptors. Some of these substances that are bound to MPs tend to accumulate and manifest their toxicity in living systems at different trophic levels of the food chain. It is currently not possible to precisely estimate the overall environmental impact of these small particles in specific environments and at the global scale [].
Biodegradable bio-based plastics (referred to as bioplastics in this text) are presented as greener alternatives to oil-derived plastics to address environmental concerns associated with conventional plastics, which are typically non-degradable and persist in the environment. Biodegradable plastics are a class of materials designed to decompose naturally in the environment through the action of living organisms, typically microbes []. These plastics are made from renewable raw materials and sometimes include petrochemicals with additives that improve their properties and modify biodegradability []. The chemistry behind biodegradable plastics is focused on ensuring that these materials break down under natural conditions, such as in soil or water, without leaving residues. The biodegradation of these plastics generally involves formation of biofilms on the surface, enzymatic scission of polymer chains, and assimilation of produced fragments. The products typically involve water, carbon dioxide (CO2), under aerobic conditions, and CO2 and methane (CH4) under anaerobic conditions, and microbial biomass []. As bioplastics (also plastics, to a lesser extent) degrade, they release various low molecular weight organic compounds, which some microorganisms can utilize as a source of energy and carbon [].
Biodegradable plastics currently include a specific group of polymeric substances such as polybutylene adipate terephthalate (PBAT); polybutylene succinate (PBS); polylactic acid (PLA); polyhydroxyalkanoates (PHA), mostly polyhydroxybutyrates (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV); and many similar substances.
Bioplastics are mainly promoted in biomedical applications [,], packaging [,,], and cosmetics [], but they are also gradually coming to be used in agricultural applications, such as coatings of fertilisers [], carriers of biologically active compounds and biodegradable plastic mulches (BDMs) []. It is assumed that they will cause lower plastic infestation and will have lower ecological impacts on soil health []. However, in some cases, BDMs have been shown to affect soil organisms and plant growth more than synthetic plastics []. As demonstrated under laboratory and greenhouse conditions, the presence of PHB BDMs increased the activity of soil microorganisms, which had a negative impact on plant growth [] and induced the priming effect []. Nevertheless, the overall environmental impacts of these materials on soil health are still not well understood [].
Depending on the actual conditions (mainly temperature, moisture content, pH, type and abundance of degrading microorganisms), bioplastics may degrade imperfectly, and the biodegradation itself may be slower than anticipated and/or incomplete under actual environmental conditions. As a result, the degradation of bioplastics can lead to the formation of fragments of microscopic size [,,,], and environmental compartments could be exposed to a new type or subgroup of MPs that can be called microbioplastics (MBPs) (see Figure 1). So far, the scientific community has not paid enough attention to the impact of those particles on the environment.
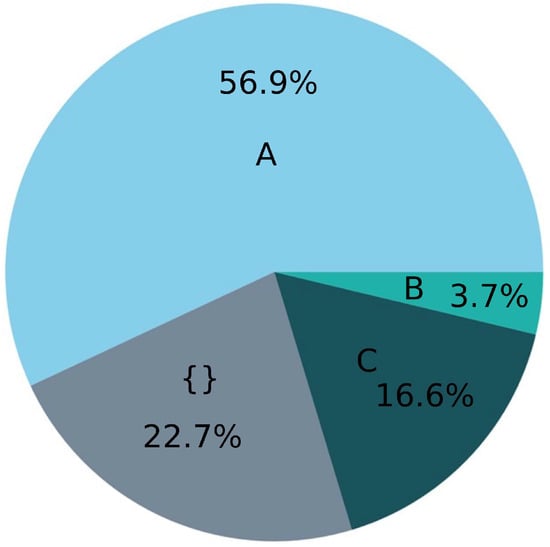
Figure 1.
Summary of 295 studies of microplastic types based on their origin in found literature. A: conventional. B: biodebradable. C: conventional + biodegradable. {}: origin not mentioned. The values are rounded.
In this review, we aim to answer the following questions:
- (i)
- Do conventional microplastics and biodegradable microplastics have adverse effects on soil properties?
- (ii)
- Could conventional and biodegradable microplastics pose a threat to agricultural crops by promoting the proliferation of soil pathogens?
- (iii)
- Could conventional and biodegradable microplastics serve as potential carriers of pathogens? If so, which types of microplastics are more concerning in this regard?
To answer those questions, we summarise the current understanding of the impact of microplastics and microbioplastics on soil properties, sorption and transport of various chemicals, as well as their effects on the soil microbiome and soil metabolism.
As previously noted, the awareness regarding MBPs is still limited; therefore, some of our arguments for BDMs are extrapolated from the behaviour of non-biodegradable MPs. In the review, we also draw attention to the missing information that could potentially explain these unknowns. For research purposes, the findings and references to them that are covered in this review have also been summarised into six tables, which are, due to their extension, attached as supporting material.
2. Methods
This review was designed in several steps to ensure that the search is comprehensive and unbiased and that the hypotheses are justified and clarified. The steps included the following tasks []: (1) Definition of objectives and research questions. We specified the objectives of the review, formulated specific research questions that the review aims to answer, such as ‘How do microplastics and microbioplastics influence soil properties and the activity and spread of soil pathogens?’ (2) Definition of eligibility criteria and inclusion and exclusion criteria for studies to be considered. Criteria included study type (research papers and reviews), relevance to microplastics and soil pathogens, language (we only included studies published in English), and availability of full text. Date range was not a main factor, although the newest studies on the same subject were selected preferentially. (3) Selection of information sources that included identification of databases and search engines. We identified key databases and search engines; primarily, PubMed and Web of Science were used: PubMed, as it is a free database primarily focusing on medicine and healthcare and includes over 30 million citations for biomedical literature from MEDLINE, life science journals, and online books; and Web of Science, as it is a multidisciplinary database covering thousands of scholarly journals across numerous disciplines. Gray literature (like conference papers, thesis, and reports) was excluded. (4) Search strategy definition using relevant keywords and Boolean operators. In particular, for individual chapters and respective tables we used following combination of keywords: Table S1: microplast* AND soil* AND (struct* OR aggreg*); Table S2: microplast* AND soil* AND pH*; Table S3: microplast* AND soil* AND (heavy metal* OR metal*); Table S4: microplast* AND soil* AND (pesticid* OR antibioti* OR pharmac* OR (organic* AND pollutant*)); Table S5: microplast* AND soil* AND (microb* OR pathog*); Table S6: microplast* AND soil* AND transport*. Also, the abbreviations and complete names of the polymers were searched together with the terms ‘microplast*. Search strings were adapted to each database’s syntax and capabilities. (5) Definition of study selection and data extraction. Initial screening was based on titles and abstracts to exclude irrelevant studies. Based on the selection, the full text of selected studies was checked against inclusion criteria. For example, after searching for microplast* AND soil* AND (struct* OR aggreg*) PubMed provided approximately 500 studies, but from these studies, based on the indicators in the table (the column titles in Table S1), we selected only those studies that were most relevant to the specific subtopic. This process significantly narrowed down the large number of studies. Then, based on the selection from the table and the information from the selected studies in the table, the chapter was written. The same procedure was used for each subsection and for each Table. Data were synthesized by qualitative synthesis; i.e., they were presented thematically.
3. Occurrence of Microplastics in Soils
Microplastics are increasingly being detected in terrestrial environments, including soils. The sources, prevalence, and implications of microplastics in soils are areas of active research. The typical abundance of microplastics in soil varies widely depending on factors such as land use, the source of microplastics, and regional practices. Studies have reported a range of concentrations from a few hundred to thousands of microplastic particles per kilogram of soil [].
The use of plastic mulch films in agriculture is a significant source of microplastics. Over time, these films degrade into smaller particles that become incorporated into the soil. The application of biosolids (treated sewage sludge) and composts as fertilizers can also introduce microplastics into agricultural soils. These amendments often contain microplastic particles derived from wastewater and organic waste processing. Also, irrigation with water from sources contaminated with microplastics, such as rivers and lakes, can introduce these particles into agricultural soils []. Therefore, agricultural soils often contain higher concentrations compared to natural soils. However, the data are still sparse and not uniformly collected, making it challenging to provide a precise global average. For instance, data range from hundreds [] to tens of thousands of particles per kilogram []. The most frequent shapes are fibres (from synthetic textiles), followed by fragments and then films (mulching) []. It is estimated that their concentrations could be 4–23 times higher in terrestrial compared to aquatic ecosystems []. Among the most prevalent polymers detected in agricultural soils are polypropylene (PP) and polyethylene (PE), while in industrial soils, polyvinyl chloride (PVC) is more common. These polymers are common in plastic films used in agricultural practices, such as mulching, which contribute significantly to microplastic pollution when they degrade or are mechanically broken down.
The typical abundance of microplastics from biodegradable plastics in soil is not well documented, partly due to the complexity and variability of soil environments, the relatively recent focus on these materials [], and the lack of analytical techniques for their detection []. However, biodegradable plastics, such as polyhydroxyalkanoates (PHAs), polylactic acid (PLA), polybutylene succinate (PBS), and polybutylene adipate terephthalate (PBAT) starches and their blends are increasingly used in applications such as mulch films in agriculture, which will lead to their presence as microplastics in soils []. The fate and biodegradation of most typical bioplastics in soil is extensively reviewed in work of Qin et al. [].
4. Impact of Plastic Particles on Soil Properties
Plastic particles, including microplastics and nanoplastics, have become pervasive in terrestrial environments, leading to significant concerns regarding their impact on soil properties. Recent reviews have highlighted the impact of microplastics (MPs) on soil properties, showing that plastic particles affect a wide range of soil physical, chemical, and microbiological properties, as well as microbial communities and ecological functions []. Therefore, our study will primarily focus on selected parameters such as soil aggregation, pH, and microbiology, which are crucial essential for substantiating the conclusions of this review.
Soil aggregate formation, which is directly related to the rate of surface water infiltration and the availability of organic material in the soil, serves as a key indicator of soil quality and fertility []. Generally, the presence of MPs disrupts soil aggregates, potentially affecting the soil water regime and soil aeration [,]. Additionally, the content of soil organic matter may be influenced, or disturbances may occur in the soil microbiome structure, subsequently impacting soil metabolism []. Consequently, these structural changes may lead to gradual erosion of the soil matrix and induce permanent shifts in soil microbiology [,].
Currently, there is no consistent evaluation on whether and how MPs affect soil aggregate sizes and formation (see Figure 2). Numerous studies (as shown in Table S1, SI) indicate that the presence of MPs tends to increase formation of soil micro-aggregates (250–263 µm) at the expense of macro-aggregates (>2000 µm) [,,,,]. This phenomenon could be due to MPs bound to micro-aggregates interfering with the formation of soil macro-aggregates, likely because of the MPs’ hydrophobic nature and their interaction with soil organic matter via van der Waals forces []. Conversely, plastic microfibres have been reported to promote the formation of soil macro-aggregates by acting as mechanical crosslinkers, causing smaller aggregates to clump together [,,,]. However, according to the literature (as shown in Table S1, SI), the effects of MPs on soil aggregate sizes and stability do not consistently correlate with changes in soil bulk density. In other words, this indicates that alterations in soil compaction or porosity may not always align with the impact of MPs on aggregate sizes [], implicating MPs as potential carriers within the soil [,,].
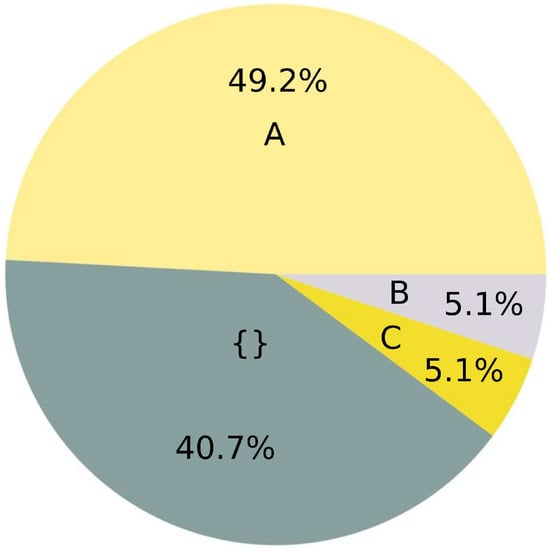
Figure 2.
Summary of 59 studies from Table S1 (SI) related to changes of soil structure by microplastics. A: decrease in soil consistency. B: increase in soil consistency. C: inconclusive. {}: not mentioned. The values were rounded.
The influence of MPs extends beyond soil structure, affecting aspects such as pH, soil metabolism, nutrient cycling, and the distribution and bioavailability of chemical contaminants. Overall, the studies presented in Table S2 (SI) do not reveal a consistent trend regarding the impact of MPs on soil pH (see Figure 3), which varies depending on various factors. These include differences in the chemical composition of the MPs [,], the concentration, shapes and sizes of the MP particles [], their aging and weathering, and the functional groups on their surfaces [], among other variable soil parameters [,]. The majority of the studies in Table S2 (SI) reported a decrease in soil pH due to the presence of various MPs [,,,,,,,,,,], while a few reported an increase [,]. However, some studies showed biased results concerning MPs’ effects on soil pH based on their chemical composition or concentrations [,,,,,]. The scientific literature suggests that the decrease in soil pH due to the presence of MPs is associated with the release of acidic substances during the degradation process in the soil [,], the increase in the soil aggregate size, soil porosity and aeration [] and the increase in soil cation exchange []. Conversely, an increase in soil pH might be associated with the adsorption of metal cations by MPs, potentially creating a more alkaline environment, and to the increase in soil aeration and the changes in soil biota that promote NH4+ formation. The change in soil biota may also result from components leached from the MPs [,].
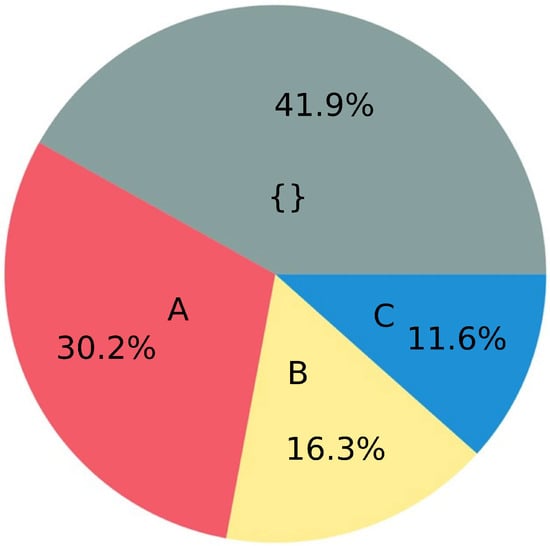
Figure 3.
Summary of 43 studies from Table S2 (SI), related to changes of soil pH by microplastics. A: pH has decreased. B: pH change is inconclusive. C: pH has increased. {}: not mentioned.
Overall, the change in soil pH in response to MPs is reported to be associated with the interaction and alteration of soil microbial activity and soil biota [,,,] as well as the interactions with soil components such as organic matter and microbial exudates []. Additionally, this variable is dependent on the chemical origins of the MPs and overall soil properties [,,]. Changes in soil pH can influence the surface charge of both MPs and soil particles, thereby affecting their interaction and adsorption behaviour [,]. This, in turn, could impact the mobility of the MPs and the soil sorption mechanisms of metal ions and organic pollutants of the MPs, thus influencing the distribution of various toxic pollutants and metal ions by implicating MPs particles as their potential carriers in the soil [,,].
4.1. Interaction of Plastic Particles with Soil Contaminants
Plastic particles can significantly interact with various soil contaminants, influencing their behaviour, mobility, and bioavailability. Understanding these interactions is crucial for assessing the environmental risks associated with plastic pollution in soils. Plastics often contain additives like phthalates, bisphenols, and flame retardants, which can leach into the soil and interact with other contaminants, potentially exacerbating their effects. However, these effects are not discussed in this review. The influence of MPs on the adsorption process and the potential distribution of metal ions has been widely discussed in scientific studies, as outlined in Table S3 (SI). However, only a few studies [,] have considered pH as a factor influencing metal absorption by MP particles. The adsorption processes of metal ions on MPs are positively correlated with the degradation state and morphology of their surfaces, surface areas, and surface chemical functional groups, primarily represented by charged, polar, or oxygen-containing functional groups [,,,,,,]. Li et al. [] observed that bioplastics generally exhibit a higher adsorption capacity for metal ions compared to conventional plastics, likely due to changes in surface properties during degradation.
Organic substances such as pesticides, pharmaceuticals, or flame retardants, which are ubiquitous environmental contaminants, can potentially be adsorbed by MP particles. Thus, MPs can act as vectors, contributing to the environmental distribution and potential bioavailability of these organic contaminants to various organisms, including humans []. The sorption of organic molecules to MPs is predominantly influenced by hydrophobic partitioning and hydrophobic interactions, although this does not uniformly apply to all interactions of organic contaminants with MPs and depends on various factors (as listed in Table S4, SI). Potential binding mechanisms of organic contaminants on MP surfaces include interactions such as π–π conjugation [,], polar interactions such as hydrogen bonding [,], and various electrostatic interactions [] via polar functional groups such as COOH, NH2 and -SO3H []. Tourinho et al. [] suggest that the sorption of organic pollutants to MPs is more influenced by electrostatic interactions at a pH below 6, while at pH 6 and above, hydrophobic forces may be more dominant [,,,]. Other factors that may contribute to the adsorption of organic molecules on MPs are surface morphological features, such as cracks and surface roughness, polymer crystallinity, and particle density [].
The aging process clearly contributes to the sorption of organic substances on MPs, as indicated by several studies [,,]. This suggests that hydrophobic particle formation and hydrophobic interactions might be more significant for the sorption of organic molecules on MP particles, while particle roughness seems to contribute more to metal ion adsorption. However, further investigation is needed to validate these hypotheses.
In terms of the aging process of MP particles, both conventional MPs and MBPs undergo aging processes []. The adsorption capacity of aged MPs is generally higher compared to pristine MPs, and the presence of oxygen-containing functional groups on aged MPs can increase the adsorption affinity for hydrophilic organic pollutants, including some antibiotics []. MBPs may exhibit a higher affinity for hydrophilic compounds and show different sorption capacities compared to conventional MPs. Additionally, MBPs may undergo more significant changes in the aging process due to their faster degradation rates []. These suggestions are based on the differences in crystallinity, specific surface area, and surface structure between MPs and MBPs, although more research is needed to support this finding [].
Chemical contaminants and pathogens, when adhered to MPs, can be transported along with them, potentially leading to contamination of new environments. Only a limited number of studies have investigated the transport of contaminants associated with microplastics directly in soil; some authors involve simulations conducted under conditions that mimic porous media []. Experimentally, MPs of LDPE have been shown to increase the mobility of glyphosate in soils containing microplastics (MP) [], MPs of PE to enhance the mobility of atrazine and 4-(2,4-dichlorophenoxy) butyric acid [], and MPs of PE, PET, PP, PS, PU, PES, and biodegradable PLA to improve the bioavailability of Cu, Pb, Cd, Fe, and Mn []. However, this effect appears to be compound-specific, as in the latter case, the PA MPs had no impact on the bioavailability of metal ions.
However, Gateuille and Naffrechoux noted that due to the limited amount of adsorbed contaminants, MPs as a source of exogenous molecules are negligible compared to endogenous chemicals that are part of plastic composition (i.e., additives such as phthalates, biocides, etc.). Additionally, Castan et al. [] evaluated the diffusion and partitioning coefficients of agrochemicals on tire and PE MPs and found that the contaminants’ desorption was too rapid, implying that MPs are unlikely to facilitate the transport of contaminants in soil unless under conditions with log Kow > 5 and preferential flow.
The transport properties of biodegradable MPs have been less frequently studied, as this is a relatively new area of research. MBPs generally contain more polar binding groups; hence, in these materials, polar interactions such as hydrogen bonding, charge–charge, and electron donor–acceptor (EDA) interactions play a more dominant role []. Although the overall interaction of polar microplastics with environmental constituents has not been extensively studied, existing research indicates that polar microplastics exhibit a higher sorption rate of metals and some organic pollutants compared to their non-polar counterparts. Additionally, the transport processes will be significantly affected by MP aging, which increases the surface hydrophilicity [], thereby affecting combined MP/contaminant transport dynamics within soil profile []. In conclusion, the interaction of plastic particles with soil contaminants is complex and multifaceted, involving adsorption, transport, and potential changes in contaminant bioavailability and toxicity.
4.2. Impact of Plastic Particles on the Soil Microbiome and Implication for Soil Metabolism
When MPs enter the soil ecosystem, they can negatively impact the soil’s microbiome. This disruption can lead to detrimental changes in soil metabolism and an increase in soil pathogens [], significantly affecting agricultural crop production. Therefore, the impact of MPs on soil should not be underestimated. Table S5 (SI) offers a comprehensive summary of the current knowledge on this topic. The interaction between MPs and soil microbiota is complex, influenced by numerous factors. First, factors such as soil structure, pH, and the presence of metal ions and organic chemicals significantly affect the development of microbial biofilms on MP surfaces []. Second, the type, shape, and size of plastic particles play crucial roles in biofilm formation []. Research shows [,,,,] that MPs of different chemical composition origins create habitats for various microorganisms. The properties of these polymers, including surface roughness, free energy, specific surface area, hydrophobicity, and bioavailability influence the differentiation among microbial communities associated with different polymer types [,]. This effect is a form of chemotaxis [].
Studies summarized in Table S5 (SI) indicate that the morphology of microorganisms and their microcolonies on MP surfaces differ from those in bulk soil. The difference arises from the unique properties of MPs, particularly their hydrophobicity [,], which creates distinct habitats for specific bacterial and fungal species []. Additionally, MPs can act as a physical barrier, limiting the movement and dispersal of soil microorganisms. They can also change the availability and distribution of nutrients in the soil [,,]. Consequently, MPs in soil can lead to alterations in the soil microbiome composition by modifying the soil microclimate and creating new ecological niches [,,]. While current data do not precisely describe how MPs impact the soil microbiome and subsequently affect broader properties like soil fertility (Table S5, SI), three related factors must be considered in understanding soil microbiome changes due to MP contamination (see Figure 4).
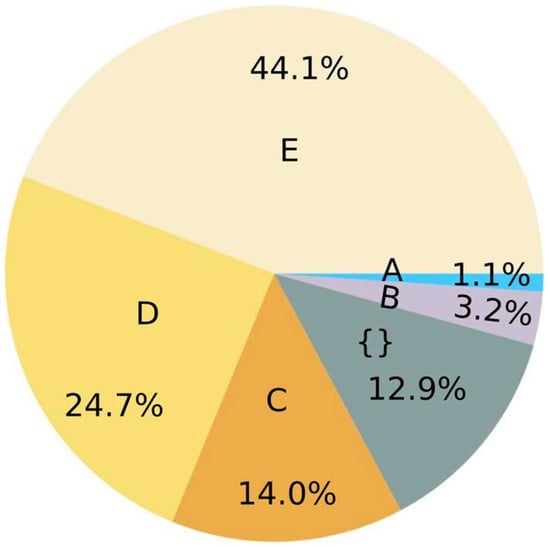
Figure 4.
Summary of 93 studies from Table S5 (SI) related to impacts of microplastics in soil according to three related factors (Microbial resistance—Mr, Microbial diversity—Md, Pathogenic proliferation—P): A: Mr. B: Md + Mr. {}: not mentioned. C: Md + Mr + P. D: Md + P. E: Md.
The first factor concerns the destabilization of the soil microbiome structure, leading to imbalances in soil metabolic pathways. Studies suggest that MPs’ presence tends to reduce the alpha diversity of bacteria and fungi in specific areas of soil while increasing the beta diversity [,,,]. This indicates that while MPs reduce the diversity of bacterial and fungal genera in their immediate vicinity, they do not significantly affect the microbiome in areas without MPs. This supports the idea that MPs create distinct ecological niches with different microbiome structures compared to the surrounding soil, possibly enabling new species to colonize the soil matrix.
The concept of the ‘plastisphere’, microbial communities that form biofilms on MPs’ surface, is a prominent topic in environmental research. It is believed that the plastisphere alters soil metabolism by changing the microbiome structure and composition [,,], leading to significant metabolic differences compared to unaffected soil, impacting nutrient availability, cycling, and microbial activity []. Hu et al. [] reported that the plastisphere can influence soil metabolism by changing the microbiome structure and composition, resulting in significant differences in metabolic pathways and functions compared to the surrounding soil that can lead to changes in nutrient availability, nutrient cycling and microbial activity in the soil. Furthermore, plastisphere bacteria may more significantly influence nutrient cycling processes, while plastisphere fungi may play a greater role in organic matter decomposition and nutrient release []. This suggests a reason why the degradation of biodegradable polyesters like PHB and PBAT is primarily fungal-driven, whereas conventional MPs like polyethylene (PE) more significantly impact soil bacterial biomass []. Therefore, it can be hypothesised that the impact of MPs on fungal communities, especially with bioplastic contamination, could be more pronounced than on bacterial communities.
While there is no conclusive evidence that micro-bioplastics (MBPs) disrupt the soil microbiome structure more than conventional MPs, some studies suggest that MBPs could have a stronger impact [,]. For instance, Hu et al. [] observed that MBPs (PBAT/PLA) might promote beneficial microbial taxa, as opposed to conventional MPs like PE, which could foster more harmful taxa. The question of whether MPs or MBPs have a more severe effect on the soil microbiome remains open to further research. Similarly, some studies [,,] point out that MPs impact soil enzyme activity related to nitrogen fixation, potentially decreasing soil nitrogen bioavailability and causing nutrient imbalances. The effect of MBPs on soil metabolism, particularly in relation to nitrogen fixation, appears to be more significant than that of conventional MPs, but more research is needed to draw definite conclusions [,,].
The second factor is the impact of MPs on soil mycobiome and microbiome relates to the increase in antibiotic resistance genes (ARGs), metal resistance genes (MRGs), and virulence factors (VFs) in soil. ARGs contribute to the emergence of antibiotic-resistant bacteria, MRGs facilitate the spread of metal-resistant bacteria, and VFs are molecules produced by bacteria, enabling them to cause disease []. Previous research indicates [,,] that MPs can act as reservoirs for ARGs and MRGs in the soil microbiome, significantly elevating the abundance and co-selection of these genes. MPs combined with organic and metal pollutants, such as tetracycline or copper, can intensify this effect. Song et al. [] found that MBPs composed of PBS notably increased ARGs and VFs, whereas conventional MPs had minimal impact. These findings imply that MPs could heighten the risk of spreading multi-resistant bacterial species, including pathogenic variants harmful to soil health. Notably, MBPs might pose a greater risk in this context than conventional MPs.
The third factor to consider is the potential of MPs to create natural habitats that foster the proliferation of pathogenic bacteria and fungi in the soil matrix. Various studies [,,,,,,] have linked the presence or increase of soil pathogens to MP contamination. For example, Qi et al. [] observed that plastispheres of LDPE and biodegradable mulch films (starch-based) tend to support higher abundances of certain bacterial phyla (Actinobacteria, Bacteroidetes, and Proteobacteria) and fungal genera (Rhizoctonia and Arthrobotrys), which include facultative plant pathogens. Notable pathogenic species associated with MPs in soil are fungi like Fusarium oxysporum, Corynespora cossicola and Myrothecium roridum [,], and the plant pathogenic bacteria are listed as Acinetobacter johnsonii and Escherichia coli []. Moreover, studies have mentioned viruses adhering to various MP types []. One study recovered infectious virus particles from biofilms on MPs, noting their enhanced survival compared to those in water []. Additionally, irradiation-induced aging of MPs was found to increase the adsorption capacity of viruses on their surfaces []. Given current global health threats, the potential role of MPs in transmitting diseases like SARS-CoV-2 through the ecosystem warrants significant research attention.
Xiang et al. [] highlighted differences between conventional and biodegradable MPs in their interactions with pathogenic microorganisms. Conventional MPs can persist in the environment for extended periods, providing a continual substrate for pathogen growth. In contrast, biodegradable MPs, due to their faster degradation rate, may limit the long-term availability of such substrates. However, as indicated above, the degradation of bioplastics in soil can take several years [,], potentially supporting pathogen proliferation. Karamanlioglu and Robson [] found that PLA materials incubated at 25 °C and 37 °C in compost, soil and sterile water did not significantly degrade over a year. Only at higher temperatures, like 45 °C, did PLA completely degraded in soil within 63 days. In conclusion, the potential impact of bioplastics on the soil microbiome might be more significant than conventional MPs. Their propensity to host more fungal structures could pose a risk for the proliferation and spread of pathogenic fungi. In conclusion, plastic particles in soil significantly impact the soil microbiome and soil metabolism, leading to changes in microbial diversity, function, and overall soil health.
4.3. Microplastic Tranport Pathways in Soil System
Microplastics can move through soil systems via various transport pathways; their transport within the soil matrix is influenced by a variety of factors, including soil aggregation, the shape and size of the MPs, interactions with soil organisms, and environmental conditions [,,], as detailed in studies listed in Table S6 (SI).
Bioturbation plays a pivotal role in both initiating and facilitating the vertical and horizontal movement of MPs within soil systems [,,,]. Soil organisms, notably earthworms, are instrumental in this process. By creating burrows and tunnels, earthworms provide channels that enable MPs to migrate vertically and horizontally, potentially reaching deeper soil layers [,]. Other organisms, such as collembolans, mites, insects, plants, and small animals, also contribute to the transport of MPs. Their ability to ingest and excrete MPs facilitates their movement from shallower to deeper soil layers [], especially in the case of low-density MPs. These particles can penetrate soil pores, and their movement is further amplified by bioturbation activities like plant root growth and earthworm burrowing [].
Abiotic factors play a significant role in the vertical transport of MPs in soil, primarily through soil water infiltration and the formation of soil cracks []. The movement of soil water can cause MPs to migrate vertically into deeper layers, following paths like macropores and cracks, alongside plant roots and through earthworm burrows [].
In contrast, the predominant factor in the horizontal transport of MPs, particularly in soil or sand, is wind erosion []. Wind-driven processes, such as saltation and suspension, are crucial for the horizontal dispersal of MPs in the atmosphere []. However, it is worth noting that soil organisms like collembolans, mites, and other insects also contribute to the horizontal movement of MPs within the soil matrix [,,].
The transportation mechanisms of conventional and biodegradable MPs might differ. Conventional MPs, known for their longevity in the environment, can be transported through various means. Their movement is influenced by factors such as shape and size, soil porosity and permeability, chemical and physical interactions with soil, biofilm formation, environmental conditions including physico-chemical properties of the pore water such as ionic strength, pH, types of dissolved cations, and flow rate []. Biodegradable MPs may exhibit distinct behaviours due to their faster degradation (chemical aging and UV irradiation []) and stronger interactions with soil particles or microorganisms []. In particular, surface hydrophobicity of MP particles is a critical factor. The faster aging of MBPs leads to an abundance of oxygen-containing groups, resulting in the reduction of the zeta potential and increase in the hydrophilicity. This reduces their interaction with soil particles, thereby enhancing their mobility in soil profile [], potentially leading to contamination of ground or surface waters []. Conversely, lower wettability makes MPs more susceptible to interactions with soil particles, reducing their mobility and enhancing their retention in the soil. This MP-induced hetero-aggregation largely depends on the characteristics of the MPs, the soil organic matter content, and biofilm formation []. As a result, hydrophobic (or less aged) particles are less prone to vertical movement by water or bioturbation [] and are more likely to be transported horizontally by wind erosion [].
Experimental studies comparing transport of biodegradable and nondegradable plastics are still rare. Recently, Fei et al. reported a comparative study in which demonstrated that biodegradable PLA was easier to transport in soil than PVC []. The UV-aging of the microplastics increased their mobility. The authors also concluded that increasing pH and flow rate facilitated the transport of MPs, whereas increasing ionic strength and cation valence decreased their mobility.
5. Discussion: Can Microplastics and Microbioplastics Act as Vectors of Pathogens?
After reviewing current literature, the main findings relevant to our topic can be summarized as follows:
- Microplastics (MPs) in soil undergo complex transformation processes that enhance their interaction with soil constituents. Microbial bioplastics (MBPs) are particularly prone to these processes.
- MPs can adsorb contaminants such as metal ions and organic pollutants from the soil, acting as vectors for these substances. MBPs are more effective at adsorbing polar compounds/particles, and aging increases this capability for both MPs and MBPs.
- The mobility of MBPs within the soil matrix is higher than that of MPs, likely because MBPs are more polar and less likely to be trapped within the soil structure.
- MPs create environments favorable for the growth of pathogenic bacteria and fungi. MBPs are especially susceptible to microbial biofilm formation over a shorter timeframe. Additionally, MPs negatively impact the soil microbiome by increasing the prevalence of antibiotic-resistant genes and virulence factors, with more pronounced effects observed in MBPs.
- While not conclusively proven, current theories suggest that microplastic particles may serve as carriers of pathogenic microorganisms, with bioplastics potentially being more effective carriers when not inhibited by interactions with bulk soil.
The introduction of biodegradable plastics as an alternative to conventional plastics aims to balance the benefits of plastic use, such as durability, flexibility, and cost-effectiveness, with the need to protect the environment and reduce the ecological footprint of human activities. However, this effort requires robust data support to prevent future problems. Currently, biodegradable plastics represent one potential solution to mitigate plastic pollution and the persistence of resistant plastics in environmental compartments. Yet, as this work shows, an increasing number of studies on the interactions between soil and other environmental compartments with bioplastics indicate emerging issues, such as nutrient imbalances [], shifts in microbial communities [], and a priming effect [], which appear to intensify with the rate of bioplastics’ biodegradation. This work aimed to draw public attention to other potential threats, such as the support and spread of pathogenic organisms within and beyond the soil matrix by MPs and MBPs.
The increasing frequency and severity of pathogen-induced plant disease outbreaks pose substantial threats to primary productivity, global food security, and biodiversity. These outbreaks lead to significant crop yield losses, estimated to cost around 220 billion USD annually, impacting food security, regional economic stability, and related socio-economic factors [].
In untouched soils, the spread of pathogenic microorganisms is facilitated by several factors and vectors, among the most important belong water, rainfall, and runoff that can mobilize pathogens, leaching them deeper into the soil and affecting groundwater quality or spreading them across landscapes, into surface water bodies []. The second factor is wind [], which can carry soil particles along with attached microorganisms over various distances, depending on the wind speed and soil particle size. This is particularly significant in arid and semi-arid regions where wind erosion is prevalent. The third factor is animals, which can spread soil pathogens through their movement and excreta and during soil disturbance []. Earthworms, insects, birds, and mammals can transport pathogens on their bodies or internally and deposit them in new locations.
Anthropogenic activities have increased the natural spread of pathogens. Agricultural practices, like irrigation, digging, the use of contaminated machinery, tools, and footwear, can mechanically spread pathogens from one field to another. Furthermore, the transportation and application of contaminated manure, compost, or soil amendments can introduce pathogens into the soil. Additionally, the use of infected plant materials, such as seeds, transplants, or debris left in the field after harvest can also contribute to the spread of pathogens. These organisms can survive on plant residues and infect subsequent crops.
Microplastic particles, whether they are made of biodegradable or non-biodegradable polymers, are becoming more prevalent in soil. As a result, these particles can have a significant impact on soil physical and chemical properties, as well as soil ecology. Changes can occur in soil grain size, soil aggregate formation capacity, soil water levels, and soil biodiversity [,,,]. These changes can ultimately lead to issues such as soil erosion, eutrophication and disruption in soil metabolism and nutrient cycling []. It is becoming increasingly evident that the presence of MPs in soil, known as the ‘plastisphere’ or ‘microplastisphere’, can alter the microbial and fungal communities present in the soil, potentially introducing new species and impacting the spread of pathogenic microorganisms in both aquatic and terrestrial ecosystems [,].
The inappropriate soil management exacerbates the problem of pathogen spread. In undisturbed soils, soil-borne pathogens are generally controlled by natural predator–prey relationships and do not pose a significant risk to the environment []. However, soil disturbance through contamination by MPs and MBPs, combined with soil erosion, can disturb this balance, potentially allowing pathogens to proliferate and spread to other ecosystems or infect humans, either directly or indirectly [].
As suggested by the literature reviewed in this work, MBPs are particularly susceptible to microbial biofilm formation, which, together with faster erosion and rapid colonization, could lead to higher risks of disease spread, impacting both plant and animal health. In addition, the biofilm formed on MBPs will have different properties compared to MPs [] and therefore attract a more diverse array of organisms []. As a result, MBP biofilms contain a larger biomass, respond differently to environmental stress, and have a higher potential pathogenic bacteria index compared to MPs (based on the comparison of PLA MBPs with PP and PE MPs []). In marine ecosystems, biofilms on some MPs release noxious signals, known as infochemicals, into the environment, which attract the organisms for ingestion []. It remains unclear whether this attraction also occurs in soil ecosystems, because this would potentially increase the transport of MBPs by soil biota.
Recent reviews have also summarized the uptake of MPs and nanoplastics by plants and their accumulation in plant tissues []. The transport mechanisms include endocytosis, apoplastic transport, crack entry, and stomatal entry. However, current studies do not address the potential uptake of pathogenic microorganisms and viruses to plants, either by MPs or MBPs.
Another factor that must be considered is climate change, which increases outbreak risks by altering pathogen evolution and host–pathogen interactions, facilitating the emergence of new pathogenic strains []. However, the response of pathogenic fungi and microorganisms to altered environmental conditions and elevated atmospheric CO2 concentration could be very diverse [,] As a result, the inclusion of soil-borne pathogens with MPs/MBPs adds to the intricacy of crop–climate–environment relationships, making it challenging to accurately predict the overall impact of climate change on agriculture.
Therefore, the effect of MPs on soil properties may be a critical question with regard to the increasing utilization of biodegradable plastics in agriculture and other fields. Their use in agriculture introduces a large amount of microbiologically labile substrate, which is nutrient-poor and leads to the proliferation of a microbiota that can better lead to stress conditions. Therefore, their possible adverse effect on soil ecosystem services should not be overlooked []. Thus, we hypothesize that these conditions could foster the formation of particles that serve as vectors for pathogenic organisms as depicted in Figure 5. Although the current state of the issue still requires long-term research, it is already possible to promote the idea of a potential solution to this problem, which may be the application of amendments such as biochar or other MP-competing sorbents (e.g., zeolites) [,].
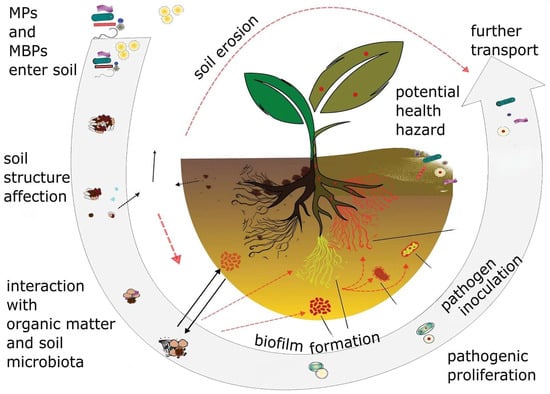
Figure 5.
Illustration of the view described in the review.
In other fields like medicine, bioplastics are preferred for their biodegradability and biocompatibility, often used in lower concentrations and under sterile conditions. However, other applications, such as packaging, may present problems. Bioplastics can support biofilm formation and microbial proliferation under certain conditions, counteracting their purpose of protecting products from microbial contamination. A shift in storage conditions, e.g., water activity, can support this growth. As a result, regulatory frameworks might also need to be adjusted to better manage the lifecycle and environmental footprint of biodegradable plastics.
To summarize the main research gaps following from this review, the future research should focus on:
- Investigating the possible spread of soil-borne pathogens from soil and water contaminated by MPs and MBPs and strategies to prevent their possible transport.
- Developing bioplastics or their blends that minimize ecological impacts while enhancing the beneficial properties of these materials, with careful consideration in their use and disposal.
- Deepening the knowledge on the shift of conditions in plastisphere of MBPs for various bioplastics and their blends in terms of nutrient depletion and potential shift in redox conditions due to oxygen depletion influencing the bacterial and fungal strains.
- Investigating the potential uptake of pathogenic microorganisms and viruses to plants by micro/nano MPs and MBPs.
- Investigating the impact of climatic changes and related changes in ecosystems on proliferation and spread of soil-borne pathogens adhered to MPs and MBPs
- Deepening the knowledge on the impact of MBPs of different types on the microbiome of different soil types, under various crops, and under different soil management practices.
6. Conclusions
This review explores the impact of microplastics (MPs) on soil, highlighting key factors that affect MP transport, as well as soil structure, pH, and microbiome. The studies examined indicate that MP pollution detrimentally affects soil health, leading to alterations in soil structure, pH, and fertility. Additionally, MPs can serve as carriers for contaminants and contribute to an increased presence of antibiotic-resistant genes and virulence factors in the soil microbiome. This study points to a potential emerging threat: MPs acting as vectors for bacterial and fungal soil-borne pathogens. Consequently, this issue should be regarded as a high-priority area for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14114643/s1, Tables S1–S6: Summary of literature used for individual chapters.
Author Contributions
Conceptualization, M.T. and J.K.; methodology, M.T., J.K. and M.B.; software, M.T.; validation, J.H., G.K., J.F., P.P., M.B., H.Z.G. and M.K.; formal analysis, J.H. and M.B.; investigation, M.T., M.B. and J.K.; resources, M.T.; writing—original draft preparation, M.T., M.K., J.H., M.B., G.K. and J.K.; writing—review and editing, M.T., J.K., G.K., M.K. and J.H.; visualization, M.T.; supervision, J.K.; project administration, J.K. and G.K.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Education, Youth and Sports of the Czech Republic [project FCH-S-24-8591].
Acknowledgments
This article is based upon work from COST Action CA20101 Plastics monitoRIng detectiOn RemedIaTion recoverY–PRIORITY, supported by COST (European Cooperation in Science and Technology, www.cost.eu, accessed on 22 May 2024).
Conflicts of Interest
The authors declare no conflict of interest.
References
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Presence of Microplastics and Nanoplastics in Food, with Particular Focus on Seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Fojt, J.; David, J.; Přikryl, R.; Řezáčová, V.; Kučerík, J. A Critical Review of the Overlooked Challenge of Determining Micro-Bioplastics in Soil. Sci. Total Environ. 2020, 745, 140975. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The Microplastisphere: Biodegradable Microplastics Addition Alters Soil Microbial Community Structure and Function. Soil. Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Energy Use Efficiency of Soil Microorganisms: Driven by Carbon Recycling and Reduction. Glob. Chang. Biol. 2023, 29, 6170–6187. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, N.M.; Arteaga-Cardona, F.; de Anda Reyes, M.E.; Gervacio-Arciniega, J.J.; Salazar-Kuri, U. Magnetic Bioplastics Based on Isolated Cellulose from Cotton and Sugarcane Bagasse. Mater. Chem. Phys. 2019, 238, 121921. [Google Scholar] [CrossRef]
- Colnik, M.; Knez-Hrncic, M.; Skerget, M.; Knez, Z. Biodegradable Polymers, Current Trends of Research and Their Applications, a Review. Chem. Ind. Chem. Eng. Q. 2020, 26, 401–418. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in Research and Development of Bioplastic for Food Packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Mlalila, N.; Hilonga, A.; Swai, H.; Devlieghere, F.; Ragaert, P. Antimicrobial Packaging Based on Starch, Poly(3-Hydroxybutyrate) and Poly(Lactic-Co-Glycolide) Materials and Application Challenges. Trends Food Sci. Technol. 2018, 74, 1–11. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Zhila, N.O.; Kiselev, E.G.; Volova, T.G. Constructing Slow-Release Formulations of Metribuzin Based on Degradable Poly(3-Hydroxybutyrate). J. Agric. Food Chem. 2016, 64, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Serrano-Ruiz, H.; Martin-Closas, L.; Pelacho, A.M. Biodegradable Plastic Mulches: Impact on the Agricultural Biotic Environment. Sci. Total Environ. 2021, 750, 141228. [Google Scholar] [CrossRef] [PubMed]
- Brtnicky, M.; Pecina, V.; Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kintl, A.; Fojt, J.; Baltazar, T.; Kucerik, J. Effect of Biodegradable Poly-3-Hydroxybutyrate Amendment on the Soil Biochemical Properties and Fertility under Varying Sand Loads. Chem. Biol. Technol. Agric. 2022, 9, 75. [Google Scholar] [CrossRef]
- Palucha, N.; Fojt, J.; Holátko, J.; Hammerschmiedt, T.; Kintl, A.; Brtnický, M.; Řezáčová, V.; De Winterb, K.; Uitterhaegen, E.; Kučerík, J. Does Poly-3-Hydroxybutyrate Biodegradation Affect the Quality of Soil Organic Matter? Chemosphere 2024, 352, 141300. [Google Scholar] [CrossRef] [PubMed]
- Degli Innocenti, F.; Breton, T. Intrinsic Biodegradability of Plastics and Ecological Risk in the Case of Leakage. ACS Sustain. Chem. Eng. 2020, 8, 9239–9249. [Google Scholar] [CrossRef]
- Šerá, J.; Serbruyns, L.; De Wilde, B.; Koutný, M. Accelerated Biodegradation Testing of Slowly Degradable Polyesters in Soil. Polym. Degrad. Stab. 2020, 171, 109031. [Google Scholar] [CrossRef]
- Polman, E.M.N.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the Aerobic Biodegradation of Biopolymers and the Corresponding Bioplastics: A Review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Templier, M.; Paré, G. A Framework for Guiding and Evaluating Literature Reviews. Commun. Assoc. Inf. Syst. 2015, 37, 6. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Global Concentrations of Microplastics in Soils—A Review. Soil 2020, 6, 649–662. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in Agricultural Soils on the Coastal Plain of Hangzhou Bay, East China: Multiple Sources Other than Plastic Mulching Film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef] [PubMed]
- Rios Mendoza, L.M.; Leon Vargas, D.; Balcer, M. Microplastics Occurrence and Fate in the Environment. Curr. Opin. Green. Sustain. Chem. 2021, 32, 100523. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.; Teng, J.; Wang, Q. Microplastics in Soils: A Review of Possible Sources, Analytical Methods and Ecological Impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Qin, M.; Chen, C.; Song, B.; Shen, M.; Cao, W.; Yang, H.; Zeng, G.; Gong, J. A Review of Biodegradable Plastics to Biodegradable Microplastics: Another Ecological Threat to Soil Environments? J. Clean. Prod. 2021, 312, 127816. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of Microplastics on Soil Properties: Current Knowledge and Future Perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Long, B.; Huang, Q.; Li, J.; Zhou, W.; Yang, C. Integrated Effects of Residual Plastic Films on Soil-Rhizosphere Microbe-Plant Ecosystem. J. Hazard. Mater. 2023, 445, 130420. [Google Scholar] [CrossRef] [PubMed]
- Fojt, J.; Románeková, I.; Procházková, P.; David, J.; Brtnický, M.; Kučerík, J. A Simple Method for Quantification of Polyhydroxybutyrate and Polylactic Acid Micro-Bioplastics in Soils by Evolved Gas Analysis. Molecules 2022, 27, 1898. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of Microplastics on the Terrestrial Environment: A Critical Review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Chah, C.N.; Banerjee, A.; Gadi, V.K.; Sekharan, S.; Katiyar, V. A Systematic Review on Bioplastic-Soil Interaction: Exploring the Effects of Residual Bioplastics on the Soil Geoenvironment. Sci. Total Environ. 2022, 851, 158311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled Effects of Microplastics and Heavy Metals on Plants: Uptake, Bioaccumulation, and Environmental Health Perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Liu, S.; Niu, S.-H.; Xiang, L.; Liao, X.-D.; Xing, S.-C. Effects of the Oversized Microplastic Pollution Layer on Soil Aggregates and Organic Carbon at Different Soil Depths. J. Hazard. Mater. 2023, 450, 131014. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Zhang, F.X.; Li, X.T. Effects of Polyester Microfibers on Soil Physical Properties: Perception from a Field and a Pot Experiment. Sci. Total Environ. 2019, 670, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Battu, A.K.; Varga, T.; Denny, A.C.; Zahid, T.M.; Chowdhury, I.; Flury, M. Minimal Impacts of Microplastics on Soil Physical Properties under Environmentally Relevant Concentrations. Environ. Sci. Technol. 2023, 57, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wang, T.; Cao, F.; Yu, C.; Chu, Q.; Wang, F. A Comparative Study on the Adsorption Behavior of Pesticides by Pristine and Aged Microplastics from Agricultural Polyethylene Soil Films. Ecotoxicol. Environ. Saf. 2021, 209, 111781. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Li, Y.; Jiang, L.; Chen, X.; Wang, L.; An, S.; Zhang, F. Influence of Microplastics Occurrence on the Adsorption of 17β-Estradiol in Soil. J. Hazard. Mater. 2020, 400, 123325. [Google Scholar] [CrossRef] [PubMed]
- Syranidou, E.; Kalogerakis, N. Interactions of Microplastics, Antibiotics and Antibiotic Resistant Genes within WWTPs. Sci. Total Environ. 2022, 804, 150141. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene Microplastics Influence the Transport of Organic Contaminants in Soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, Q.; Wei, Z.; Jiang, J.; Deng, J. Effects of Microplastic Type on Growth and Physiology of Soil Crops: Implications for Farmland Yield and Food Quality. Environ. Pollut. 2023, 326, 121512. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, X.; Zhang, S.; Zhang, S.; Adams, C.A.; Sun, Y. Effects of Co-Contamination of Microplastics and Cd on Plant Growth and Cd Accumulation. Toxics 2020, 8, 36. [Google Scholar] [CrossRef]
- Gharahi, N.; Zamani-Ahmadmahmoodi, R. Effect of Plastic Pollution in Soil Properties and Growth of Grass Species in Semi-Arid Regions: A Laboratory Experiment. Environ. Sci. Pollut. Res. 2022, 29, 59118–59126. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Hou, Y.; Chen, Z.; Bu, Y.; Zhang, X.; Shen, Z.; Chen, Y. Impact of Polyethylene on Soil Physicochemical Properties and Characteristics of Sweet Potato Growth and Polyethylene Absorption. Chemosphere 2022, 302, 134734. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, Y.; Liu, S.; Junaid, M.; Wang, J. Effects of Micro(Nano)Plastics on Higher Plants and the Rhizosphere Environment. Sci. Total Environ. 2022, 807, 150841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Z.; Zhu, D.; Lindhardt, J.H.; Lin, S.-M.; Ke, X.; Cui, L. Long-Term Fertilization History Alters Effects of Microplastics on Soil Properties, Microbial Communities, and Functions in Diverse Farmland Ecosystem. Environ. Sci. Technol. 2021, 55, 4658–4668. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Decrease in Bioavailability of Soil Heavy Metals Caused by the Presence of Microplastics Varies across Aggregate Levels. J. Hazard. Mater. 2020, 395, 122690. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Polyethylene Microplastics Increase Cadmium Uptake in Lettuce (Lactuca sativa L.) by Altering the Soil Microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, Z.; Zhang, Y.; Fan, P.; Xi, B.; Tan, W. Metal Type and Aggregate Microenvironment Govern the Response Sequence of Speciation Transformation of Different Heavy Metals to Microplastics in Soil. Sci. Total Environ. 2021, 752, 141956. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, P.; Han, S.; Jin, Y.; Nan, Y.; Deng, J.; He, J.; Wu, Y.; Chen, S. Distribution Characteristics of Microplastics in the Soil of Mangrove Restoration Wetland and the Effects of Microplastics on Soil Characteristics. Ecotoxicology 2022, 31, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L. Short-Term Effects of Polyethene and Polypropylene Microplastics on Soil Phosphorus and Nitrogen Availability. Chemosphere 2022, 291, 132984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, R.; Wang, X.; Zhang, J.; Wang, J.; Cao, B.; Zhao, Y.; Xu, L.; Chen, Y.; Zou, G. How Do Controlled-Release Fertilizer Coated Microplastics Dynamically Affect Cd Availability by Regulating Fe Species and DOC Content in Soil? Sci. Total Environ. 2022, 850, 157886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pei, L.; Zhao, Y.; Shan, J.; Zheng, X.; Xu, G.; Sun, Y.; Wang, F. Effects of Microplastics and Nitrogen Deposition on Soil Multifunctionality, Particularly C and N Cycling. J. Hazard. Mater. 2023, 451, 131152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, L.; Wang, X.; Li, C.; Zhao, Y.; Cao, B.; Zhang, C.; Zhang, J.; Wang, J.; Chen, Y.; et al. Microplastics Promoted Cadmium Accumulation in Maize Plants by Improving Active Cadmium and Amino Acid Synthesis. J. Hazard. Mater. 2023, 447, 130788. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Jadhav, B. Influence of Different Microplastic Forms on PH and Mobility of Cu2+ and Pb2+ in Soil. Molecules 2022, 27, 1744. [Google Scholar] [CrossRef]
- Chen, S.; Feng, T.; Lin, X.; Hou, Z.; Chao, L.; Zhang, X.; Liu, Y. Effects of Microplastics and Cadmium on the Soil-Wheat System as Single and Combined Contaminants. Plant Physiol. Biochem. 2023, 196, 291–301. [Google Scholar] [CrossRef]
- Liu, X.; Lin, H.; Xu, S.; Yan, Y.; Yu, R.; Hu, G. Occurrence, Distribution, and Characteristics of Microplastics in Agricultural Soil around a Solid Waste Treatment Center in Southeast China. J. Soils Sediments 2023, 23, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Cao, F.; Cao, L.; Wang, T.; Yu, C.; Wang, F. A Comparative Study on the Adsorption Behavior and Mechanism of Pesticides on Agricultural Film Microplastics and Straw Degradation Products. Chemosphere 2022, 303, 135058. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Gowda, N.K.; Mahesh, S. Identification of Microplastics from Urban Informal Solid Waste Landfill Soil; MP Associations with COD and Chloride. Water Sci. Technol. 2023, 87, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, X.; Liu, Y.; Cui, W.; Sun, Y.; Zhang, S.; Wang, F. Effects of Microplastics and Carbon Nanotubes on Soil Geochemical Properties and Bacterial Communities. J. Hazard. Mater. 2022, 433, 128826. [Google Scholar] [CrossRef] [PubMed]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of Chemical Contaminants to Microplastics: Sorption Mechanisms, Environmental Distribution and Effects on Toxicity and Bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. Adsorption Mechanism of As(III) on Polytetrafluoroethylene Particles of Different Size. Environ. Pollut. 2019, 254, 112950. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) Adsorption onto Different-Sized Polystyrene Microplastic Particles and Its Mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Tan, X.; Ye, S.; Ma, L.; Gu, Y.; Zhang, P.; Chen, Q.; Yang, Y.; Tang, Y. Mechanism Analysis of Heavy Metal Lead Captured by Natural-Aged Microplastics. Chemosphere 2021, 270, 128624. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Li, X.; Liu, H. Source, Occurrence, Migration and Potential Environmental Risk of Microplastics in Sewage Sludge and during Sludge Amendment to Soil. Sci. Total Environ. 2020, 742, 140355. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Li, F.; Xiao, H.-C.; Liu, B.-L.; Feng, L.-N.; Yu, P.-F.; Meng, C.; Zhao, H.-M.; Feng, N.-X.; Li, Y.-W.; et al. Polyethylene and Polypropylene Microplastics Reduce Chemisorption of Cadmium in Paddy Soil and Increase Its Bioaccessibility and Bioavailability. J. Hazard. Mater. 2023, 449, 130994. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A.; Fatima Rizvi, Z. Impact of Plastic Mulching as a Major Source of Microplastics in Agroecosystems. J. Hazard. Mater. 2023, 445, 130455. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, D.; Wu, D.; Guo, H.; Han, S. Influence of Polyethylene-Microplastic on Environmental Behaviors of Metals in Soil. Environ. Sci. Pollut. Res. 2021, 28, 28329–28336. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Zhang, J.; Liu, H. Response of Occurrence in Microplastics and Its Adsorped Cadmium Capacity to Simulated Agricultural Environmental Scenarios in Sludge-Amended Soil. Environ. Res. 2023, 222, 115346. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, Y.; Sheng, Y.; Xiang, Q.; Zhou, Y.; Cizdziel, J.V. Effect of Prothioconazole on the Degradation of Microplastics Derived from Mulching Plastic Film: Apparent Change and Interaction with Heavy Metals in Soil. Environ. Pollut. 2020, 260, 113988. [Google Scholar] [CrossRef]
- Šunta, U.; Prosenc, F.; Trebše, P.; Bulc, T.G.; Kralj, M.B. Adsorption of Acetamiprid, Chlorantraniliprole and Flubendiamide on Different Type of Microplastics Present in Alluvial Soil. Chemosphere 2020, 261, 127762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Huang, P.; Sun, H.; Ma, J.; Li, B. The Structure of Agricultural Microplastics (PT, PU and UF) and Their Sorption Capacities for PAHs and PHE Derivates under Various Salinity and Oxidation Treatments. Environ. Pollut. 2020, 257, 113525. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qian, X.; Wang, C.; Zhang, C.; Tang, T.; Zhao, X.; Li, L. Environmentally Relevant Concentrations of Microplastic Exhibits Negligible Impacts on Thiacloprid Dissipation and Enzyme Activity in Soil. Environ. Res. 2020, 189, 109892. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, B.; Li, B.; Zhao, T.; Cai, Y.; Luo, Y.; Zhang, H. A Contrasting Alteration of Sulfamethoxazole Bioaccessibility in Two Different Soils Amended with Polyethylene Microplastic: In-Situ Measurement Using Diffusive Gradients in Thin Films. Sci. Total Environ. 2022, 808, 152187. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics Play a Minor Role in Tetracycline Sorption in the Presence of Dissolved Organic Matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zeng, F.; Ma, H.; Xing, B. Identification of the Aged Microplastics Film and Its Sorption of Antibiotics and Bactericides in Aqueous and Soil Compartments. Mar. Pollut. Bull. 2022, 185, 114312. [Google Scholar] [CrossRef]
- Černá, T.; Pražanová, K.; Beneš, H.; Titov, I.; Klubalová, K.; Filipová, A.; Klusoň, P.; Cajthaml, T. Polycyclic Aromatic Hydrocarbon Accumulation in Aged and Unaged Polyurethane Microplastics in Contaminated Soil. Sci. Total Environ. 2021, 770, 145254. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, H.; Sun, X.; Wang, Y.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Wang, G. Distinct Influence of Conventional and Biodegradable Microplastics on Microbe-Driving Nitrogen Cycling Processes in Soils and Plastispheres as Evaluated by Metagenomic Analysis. J. Hazard. Mater. 2023, 451, 131097. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, X.; Xu, L.; Zhang, J.; Wang, Y.; Zhou, Z. Atrazine Sorption on Biodegradable Microplastics: Significance of Microbial Aging. Sci. Total Environ. 2023, 862, 160904. [Google Scholar] [CrossRef]
- Castan, S.; Henkel, C.; Hüffer, T.; Hofmann, T. Microplastics and Nanoplastics Barely Enhance Contaminant Mobility in Agricultural Soils. Commun. Earth Environ. 2021, 2, 193. [Google Scholar] [CrossRef]
- Zhao, L.; Rong, L.; Xu, J.; Lian, J.; Wang, L.; Sun, H. Sorption of Five Organic Compounds by Polar and Nonpolar Microplastics. Chemosphere 2020, 257, 127206. [Google Scholar] [CrossRef] [PubMed]
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic Aging Processes: Environmental Relevance and Analytical Implications. TrAC Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Beriot, N.; Corradini, F.; Silva, V.; Yang, X.; Baartman, J.; Rezaei, M.; van Schaik, L.; Riksen, M.; Geissen, V. Review of Microplastic Sources, Transport Pathways and Correlations with Other Soil Stressors: A Journey from Agricultural Sites into the Environment. Chem. Biol. Technol. Agric. 2022, 9, 20. [Google Scholar] [CrossRef]
- Qin, P.; Li, T.; Cui, Z.; Zhang, H.; Hu, X.; Wei, G.; Chen, C. Responses of Bacterial Communities to Microplastics: More Sensitive in Less Fertile Soils. Sci. Total Environ. 2023, 857, 159440. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ou, Q.; He, Q.; Wu, Z.; Ma, J.; Huangfu, X. Influence of Dissolved Black Carbon on the Aggregation and Deposition of Polystyrene Nanoplastics: Comparison with Dissolved Humic Acid. Water Res. 2021, 196, 117054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Gao, N.; Li, Y.; Dou, S.; Liu, Z.; Chen, Y.; Ma, C.; Zhang, H. Responses of Maize (Zea Mays L.) Seedlings Growth and Physiological Traits Triggered by Polyvinyl Chloride Microplastics Is Dominated by Soil Available Nitrogen. Ecotoxicol. Environ. Saf. 2023, 252, 114618. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Hou, D. Soil Plastisphere: Exploration Methods, Influencing Factors, and Ecological Insights. J. Hazard. Mater. 2022, 430, 128503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; O’Connor, P.; Zhu, Y.-G. Microbial Communities on Biodegradable Plastics under Different Fertilization Practices in Farmland Soil Microcosms. Sci. Total Environ. 2022, 809, 152184. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, J.; Wang, X.; Ding, C.; Wang, J. Deciphering the Mechanisms Shaping the Plastisphere Microbiota in Soil. mSystems 2022, 7, e0035222. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Sun, Y.; Li, X.; Ding, C.; Huang, Y.; Du, X.; Wang, J. Biodegradable Microplastics Induced the Dissemination of Antibiotic Resistance Genes and Virulence Factors in Soil: A Metagenomic Perspective. Sci. Total Environ. 2022, 828, 154596. [Google Scholar] [CrossRef]
- Šerá, J.; Huynh, F.; Ly, F.; Vinter, Š.; Kadlečková, M.; Krátká, V.; Máčalová, D.; Koutný, M.; Wallis, C. Biodegradable Polyesters and Low Molecular Weight Polyethylene in Soil: Interrelations of Material Properties, Soil Organic Matter Substances, and Microbial Community. Int. J. Mol. Sci. 2022, 23, 15976. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Dai, Y.; Ren, J.; Li, Y.; Wang, X.; Zhang, P.; Peng, C. Effects of Co-Loading of Polyethylene Microplastics and Ciprofloxacin on the Antibiotic Degradation Efficiency and Microbial Community Structure in Soil. Sci. Total Environ. 2020, 741, 140463. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Lv, J.; Wang, Z.; Peng, Y.; Wang, X. Microplastic Presence Significantly Alters Soil Nitrogen Transformation and Decreases Nitrogen Bioavailability under Contrasting Temperatures. J. Environ. Manag. 2022, 317, 115473. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhu, D.; Wang, Y.; Wang, H.; Liang, A.; Sun, H.; Chen, Q.; Lassen, S.B.; Lv, M.; Chen, L. Exposure to Heavy Metal and Antibiotic Enriches Antibiotic Resistant Genes on the Tire Particles in Soil. Sci. Total Environ. 2021, 792, 148417. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, Y.; Wang, X.; Wang, J. Microplastics Reduce Soil Microbial Network Complexity and Ecological Deterministic Selection. Environ. Microbiol. 2022, 24, 2157–2169. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Li, X.; Liu, H.; Lu, G.; Dang, Z.; Yin, H. Bacterial Communities on Soil Microplastic at Guiyu, an E-Waste Dismantling Zone of China. Ecotoxicol. Environ. Saf. 2020, 195, 110521. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, X.; Cao, N.; Duan, C.; Ding, C.; Huang, Y.; Wang, J. Biodegradable Microplastics Enhance Soil Microbial Network Complexity and Ecological Stochasticity. J. Hazard. Mater. 2022, 439, 129610. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yue, L.; Zhao, Y.; Li, J.; Fu, Y.; Deng, H.; Feng, D.; Li, Q.; Yu, H.; Zhang, Y.; et al. Changes in Bacterial Community Structures in Soil Caused by Migration and Aging of Microplastics. Sci. Total Environ. 2022, 848, 157790. [Google Scholar] [CrossRef]
- Yin, W.; Zhang, B.; Zhang, H.; Zhang, D.; Leiviskä, T. Vertically Co-Distributed Vanadium and Microplastics Drive Distinct Microbial Community Composition and Assembly in Soil. J. Hazard. Mater. 2022, 440, 129700. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qu, J.; Yang, J. Microplastics Distribution and Microbial Community Characteristics of Farmland Soil under Different Mulch Methods. J. Hazard. Mater. 2023, 445, 130408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liu, S.; Wang, H.; Wang, D.; Zhu, Y.; Wang, J.; He, Y.; Zheng, Q.; Zhan, X. Microplastic Particles Alter Wheat Rhizosphere Soil Microbial Community Composition and Function. J. Hazard. Mater. 2022, 436, 129176. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gu, H.; Wang, Y.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Dai, Q.; Wang, G. Succession of Soil Bacterial Communities and Network Patterns in Response to Conventional and Biodegradable Microplastics: A Microcosmic Study in Mollisol. J. Hazard. Mater. 2022, 436, 129218. [Google Scholar] [CrossRef] [PubMed]
- Gkoutselis, G.; Rohrbach, S.; Harjes, J.; Obst, M.; Brachmann, A.; Horn, M.A.; Rambold, G. Microplastics Accumulate Fungal Pathogens in Terrestrial Ecosystems. Sci. Rep. 2021, 11, 13214. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Z.; Peng, Y.; Zhang, Z.; Fan, Z.; Wang, J.; Wang, X. Microbes Drive Metabolism, Community Diversity, and Interactions in Response to Microplastic-Induced Nutrient Imbalance. Sci. Total Environ. 2023, 877, 162885. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jia, W.; Xu, L.; Zhang, M.; Huang, Y. The Plastisphere of Biodegradable and Conventional Microplastics from Residues Exhibit Distinct Microbial Structure, Network and Function in Plastic-Mulching Farmland. J. Hazard. Mater. 2023, 442, 130011. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, W.; Ai, F.; Xu, F.; Zhu, J.; Yin, Y.; Ji, R.; Guo, H. Polystyrene Microplastics Alleviate the Effects of Sulfamethazine on Soil Microbial Communities at Different CO2 Concentrations. J. Hazard. Mater. 2021, 413, 125286. [Google Scholar] [CrossRef]
- Rohrbach, S.; Gkoutselis, G.; Hink, L.; Weig, A.R.; Obst, M.; Diekmann, A.; Ho, A.; Rambold, G.; Horn, M.A. Microplastic Polymer Properties as Deterministic Factors Driving Terrestrial Plastisphere Microbiome Assembly and Succession in the Field. Environ. Microbiol. 2022, 25, 2681–2697. [Google Scholar] [CrossRef]
- Qi, Y.; Ossowicki, A.; Yergeau, É.; Vigani, G.; Geissen, V.; Garbeva, P. Plastic Mulch Film Residues in Agriculture: Impact on Soil Suppressiveness, Plant Growth, and Microbial Communities. FEMS Microbiol Ecol 2022, 98, fiac017. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cao, N.; Duan, C.; Wang, Q.; Ding, C.; Wang, J. Selection of Antibiotic Resistance Genes on Biodegradable and Non-Biodegradable Microplastics. J. Hazard. Mater. 2021, 409, 124979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Liu, P.; Li, H.; Hu, F. The Formation of Specific Bacterial Communities Contributes to the Enrichment of Antibiotic Resistance Genes in the Soil Plastisphere. J. Hazard. Mater. 2022, 436, 129247. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, W.; Wang, C.; Gao, M. Distinct Distribution Patterns and Functional Potentials of Rare and Abundant Microorganisms between Plastisphere and Soils. Sci. Total Environ. 2023, 873, 162413. [Google Scholar] [CrossRef] [PubMed]
- Moresco, V.; Oliver, D.M.; Weidmann, M.; Matallana-Surget, S.; Quilliam, R.S. Survival of Human Enteric and Respiratory Viruses on Plastics in Soil, Freshwater, and Marine Environments. Environ. Res. 2021, 199, 111367. [Google Scholar] [CrossRef] [PubMed]
- Moresco, V.; Charatzidou, A.; Oliver, D.M.; Weidmann, M.; Matallana-Surget, S.; Quilliam, R.S. Binding, Recovery, and Infectiousness of Enveloped and Non-Enveloped Viruses Associated with Plastic Pollution in Surface Water. Environ. Pollut. 2022, 308, 119594. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yu, Z.; Ngiam, L.; Guo, J. Microplastics as Potential Carriers of Viruses Could Prolong Virus Survival and Infectivity. Water Res. 2022, 225, 119115. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Chen, Q.-L.; Yang, X.-R.; Li, G.; Zhu, D. Soil Mesofauna Alter the Balance between Stochastic and Deterministic Processes in the Plastisphere during Microbial Succession. Sci. Total Environ. 2022, 849, 157820. [Google Scholar] [CrossRef]
- Tanunchai, B.; Ji, L.; Schröder, O.; Gawol, S.J.; Geissler, A.; Wahdan, S.F.M.; Buscot, F.; Kalkhof, S.; Schulze, E.-D.; Noll, M.; et al. Fate of a Biodegradable Plastic in Forest Soil: Dominant Tree Species and Forest Types Drive Changes in Microbial Community Assembly, Influence the Composition of Plastisphere, and Affect Poly(Butylene Succinate-Co-Adipate) Degradation. Sci. Total Environ. 2023, 873, 162230. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Robson, G.D. The Influence of Biotic and Abiotic Factors on the Rate of Degradation of Poly(Lactic) Acid (PLA) Coupons Buried in Compost and Soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Abbasi, S.; Rezaei, M.; Mina, M.; Sameni, A.; Oleszczuk, P.; Turner, A.; Ritsema, C. Entrainment and Horizontal Atmospheric Transport of Microplastics from Soil. Chemosphere 2023, 322, 138150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Xu, X.; Shao, L.; Che, Z.; Lu, B.; Ye, J.; He, P. A Neglected Transport of Plastic Debris to Cities from Farmland in Remote Arid Regions. Sci. Total Environ. 2022, 807, 150982. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, W.; Hu, A.; Wang, J.; Huang, Y.; Xu, J.; He, Y.; Lu, Z. Co-Occurrence of Light Microplastics and Phthalate Esters in Soils of China. Sci. Total Environ. 2022, 852, 158384. [Google Scholar] [CrossRef]
- Jin, T.; Tang, J.; Lyu, H.; Wang, L.; Gillmore, A.B.; Schaeffer, S.M. Activities of Microplastics (MPs) in Agricultural Soil: A Review of MPs Pollution from the Perspective of Agricultural Ecosystems. J. Agric. Food Chem. 2022, 70, 4182–4201. [Google Scholar] [CrossRef] [PubMed]
- Rolf, M.; Laermanns, H.; Kienzler, L.; Pohl, C.; Möller, J.N.; Laforsch, C.; Löder, M.G.J.; Bogner, C. Flooding Frequency and Floodplain Topography Determine Abundance of Microplastics in an Alluvial Rhine Soil. Sci. Total Environ. 2022, 836, 155141. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants—A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Nghiem, L.D.; Gao, L.; Zamyadi, A.; Zhang, Z.; Sun, J.; Wang, Q. Solid-Embedded Microplastics from Sewage Sludge to Agricultural Soils: Detection, Occurrence, and Impacts. ACS ES&T Water 2021, 1, 1322–1333. [Google Scholar] [CrossRef]
- Büks, F.; Loes Van Schaik, N.; Kaupenjohann, M. What Do We Know about How the Terrestrial Multicellular Soil Fauna Reacts to Microplastic? SOIL 2020, 6, 245–267. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Xie, H.; Zhao, Y.; Zhou, X.; Sun, H.; Wang, N.; Wang, J.; Yin, X. Transport of Degradable/Nondegradable and Aged Microplastics in Porous Media: Effects of Physicochemical Factors. Sci. Total Environ. 2022, 851, 158099. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of Microplastics and Interaction with Other Coexisting Constituents in Terrestrial and Aquatic Environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Song, J.; Shentu, J.; Chen, Q.; Lin, K. Attachment and Detachment of Large Microplastics in Saturated Porous Media and Its Influencing Factors. Chemosphere 2022, 305, 135322. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Pan, S.; Li, P.; Wang, L.; Hou, R.; Wu, W.-M.; Luo, J.; Hou, D. Vertical Migration of Microplastics in Porous Media: Multiple Controlling Factors under Wet-Dry Cycling. J. Hazard. Mater. 2021, 419, 126413. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate Change Impacts on Plant Pathogens, Food Security and Paths Forward. Nat. Rev. Microbiol. 2023, 21, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Derby, J.A.; Brevik, E.C. Soil Pathogens That May Potentially Cause Pandemics, Including Severe Acute Respiratory Syndrome (SARS) Coronaviruses. Curr. Opin. Environ. Sci. Health 2020, 17, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, Y.; Zhu, Y.-G. Human Pathogens in the Soil Ecosystem: Occurrence, Dispersal, and Study Method. Curr. Opin. Environ. Sci. Health 2023, 33, 100471. [Google Scholar] [CrossRef]
- Xu, J.; Zuo, R.; Shang, J.; Wu, G.; Dong, Y.; Zheng, S.; Xu, Z.; Liu, J.; Xu, Y.; Wu, Z.; et al. Nano- and Micro-Plastic Transport in Soil and Groundwater Environments: Sources, Behaviors, Theories, and Models. Sci. Total Environ. 2023, 904, 166641. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, X.; Zhang, X.; Liu, C.; Chen, Z.; Sun, H.; Wang, L. Bacterial Community under the Influence of Microplastics in Indoor Environment and the Health Hazards Associated with Antibiotic Resistance Genes. Environ. Sci. Technol. 2022, 56, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wang, J.; Liu, X.; Wang, L. Differences in the Plastispheres of Biodegradable and Non-Biodegradable Plastics: A Mini Review. Front. Microbiol. 2022, 13, 849147. [Google Scholar] [CrossRef] [PubMed]
- Botterell, Z.L.R.; Beaumont, N.; Cole, M.; Hopkins, F.E.; Steinke, M.; Thompson, R.C.; Lindeque, P.K. Bioavailability of Microplastics to Marine Zooplankton: Effect of Shape and Infochemicals. Environ. Sci. Technol. 2020, 54, 12024–12033. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and Transport of Micro/Nanoplastics in Terrestrial Plants: Detection, Mechanisms, and Influencing Factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, A.; Garrido-Chamorro, S.; Barreiro, C. Microorganisms and Climate Change: A Not so Invisible Effect. Microbiol. Res. 2023, 14, 918–947. [Google Scholar] [CrossRef]
- Seidel, D.; Wurster, S.; Jenks, J.D.; Sati, H.; Gangneux, J.-P.; Egger, M.; Alastruey-Izquierdo, A.; Ford, N.P.; Chowdhary, A.; Sprute, R.; et al. Impact of Climate Change and Natural Disasters on Fungal Infections. Lancet Microbe 2024. [Google Scholar] [CrossRef] [PubMed]
- Fojt, J.; Denková, P.; Brtnický, M.; Holátko, J.; Řezáčová, V.; Pecina, V.; Kučerík, J. Influence of Poly-3-Hydroxybutyrate Micro-Bioplastics and Polyethylene Terephthalate Microplastics on the Soil Organic Matter Structure and Soil Water Properties. Environ. Sci. Technol. 2022, 56, 10732–10742. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, H.; Li, D.; Tan, Z.; Hou, J. Impact of Coexistence of Microplastics and Biochar on the Abundance and Structure of Soil Fungal Communities. J. Biobased Mater. Bioenergy 2023, 17, 404–412. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Sang, M.K.; Igalavithana, A.D.; Zhang, M.; Hou, D.; Oleszczuk, P.; Sung, J.; Ok, Y.S. Biochar Alters Chemical and Microbial Properties of Microplastic-Contaminated Soil. Environ. Res. 2022, 209, 112807. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).