Production of Transportation Fuels from Fischer–Tropsch Waxes: Distillation, Blending, and Hydrocracking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstocks and Catalyst
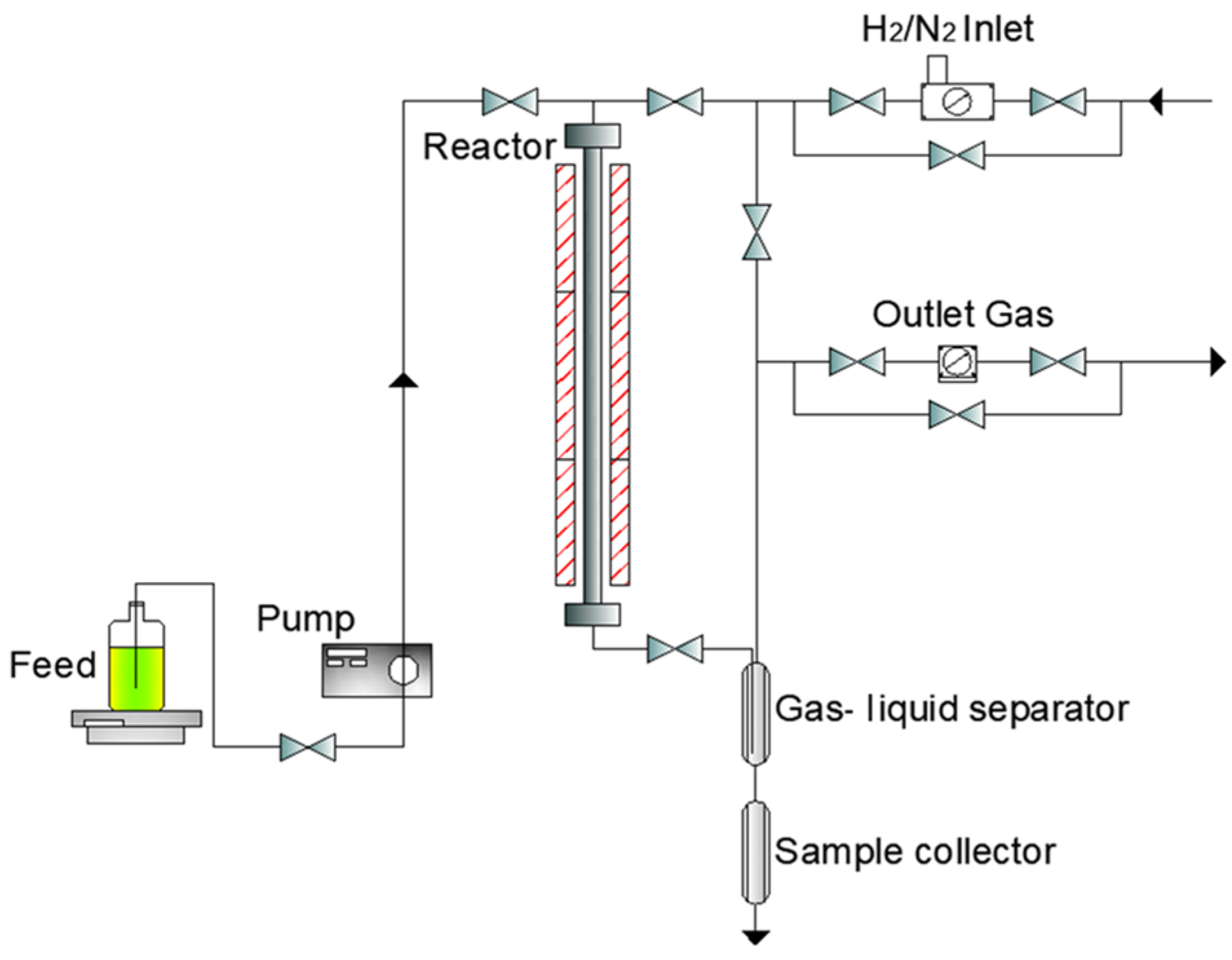
2.2. Experimental Setup and Experiments
2.3. Blending and Hydrocracking Products Analyses
- (i)
- HayeSep Q column with a thermal conductivity detector (TCD) to measure H2, with N2 used as the carrier gas.
- (ii)
- HayeSep Q column with TCD to measure O2, N2, CO, CO2, H2S, and C1–C2 hydrocarbons, with He used as the carrier gas.
- (iii)
- 5 A molecular sieve column with a flame ionisation detector to measure C1–C7 hydrocarbons, with He used as the carrier gas.
3. Results and Discussion
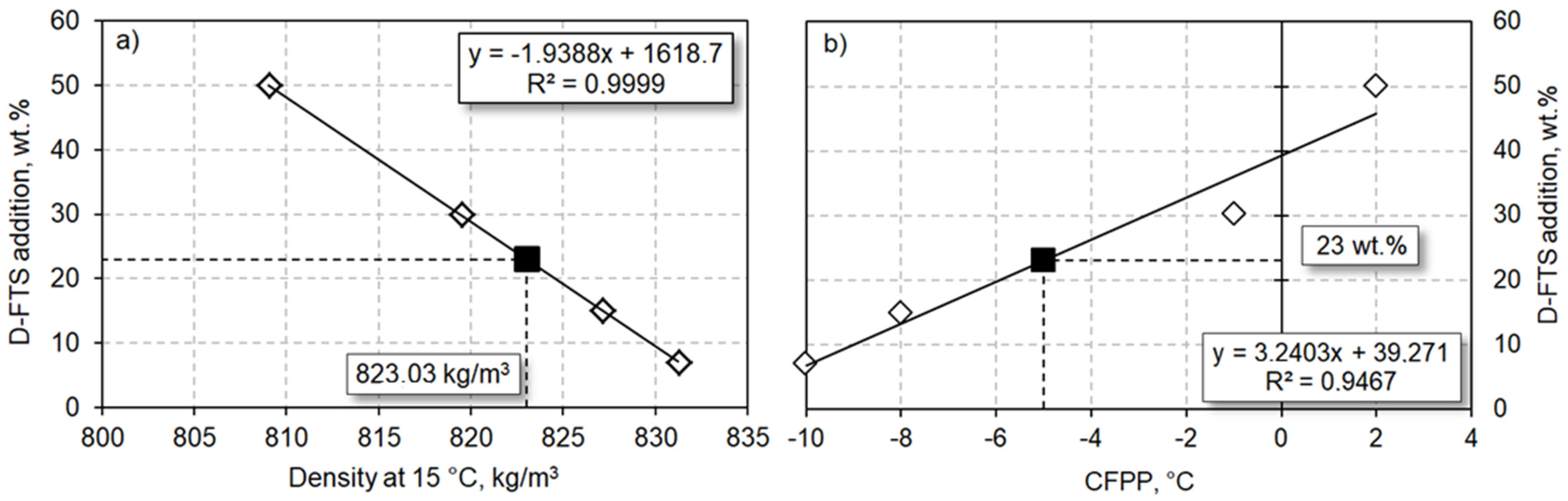
3.1. Blending Experiments
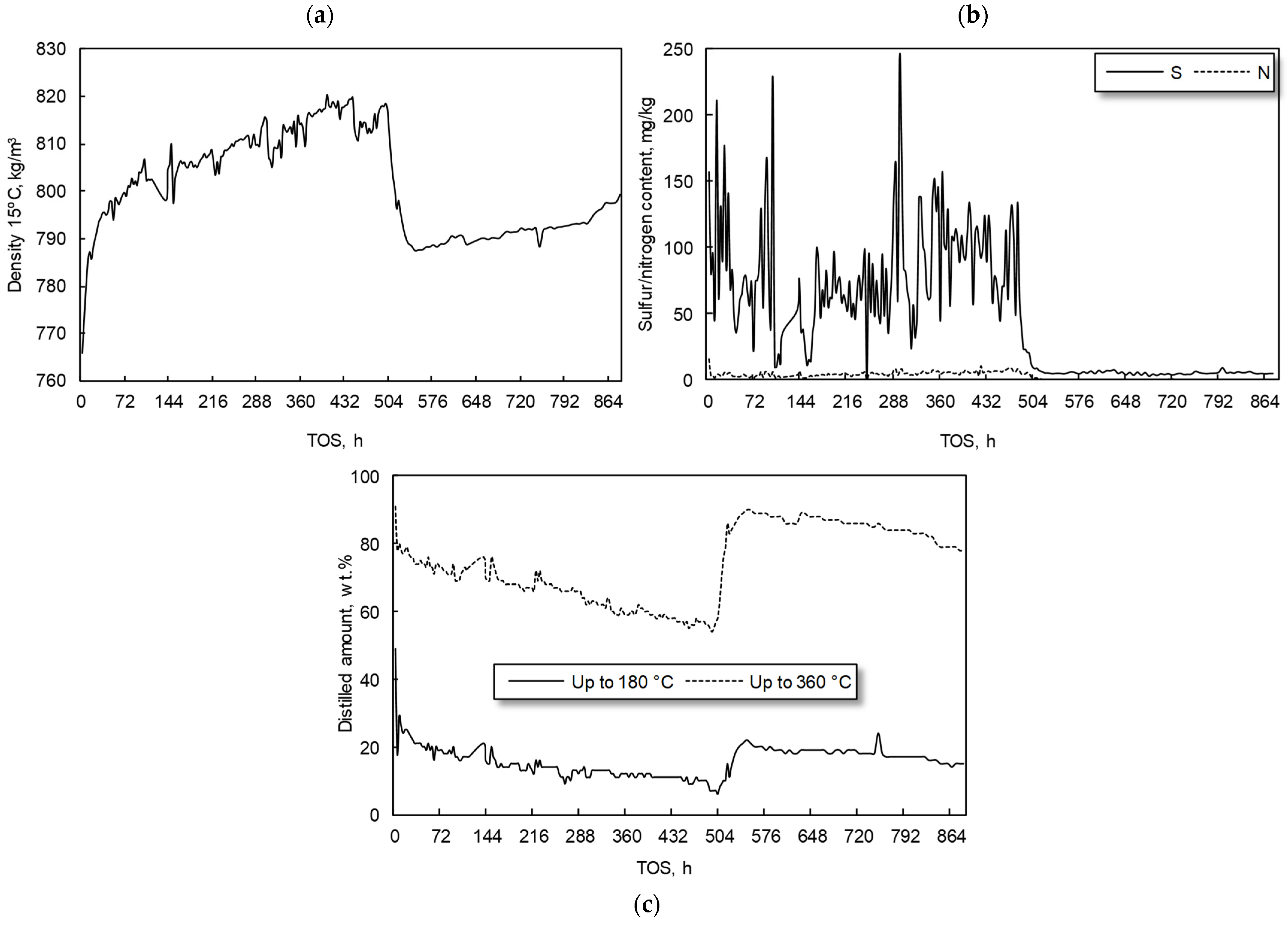
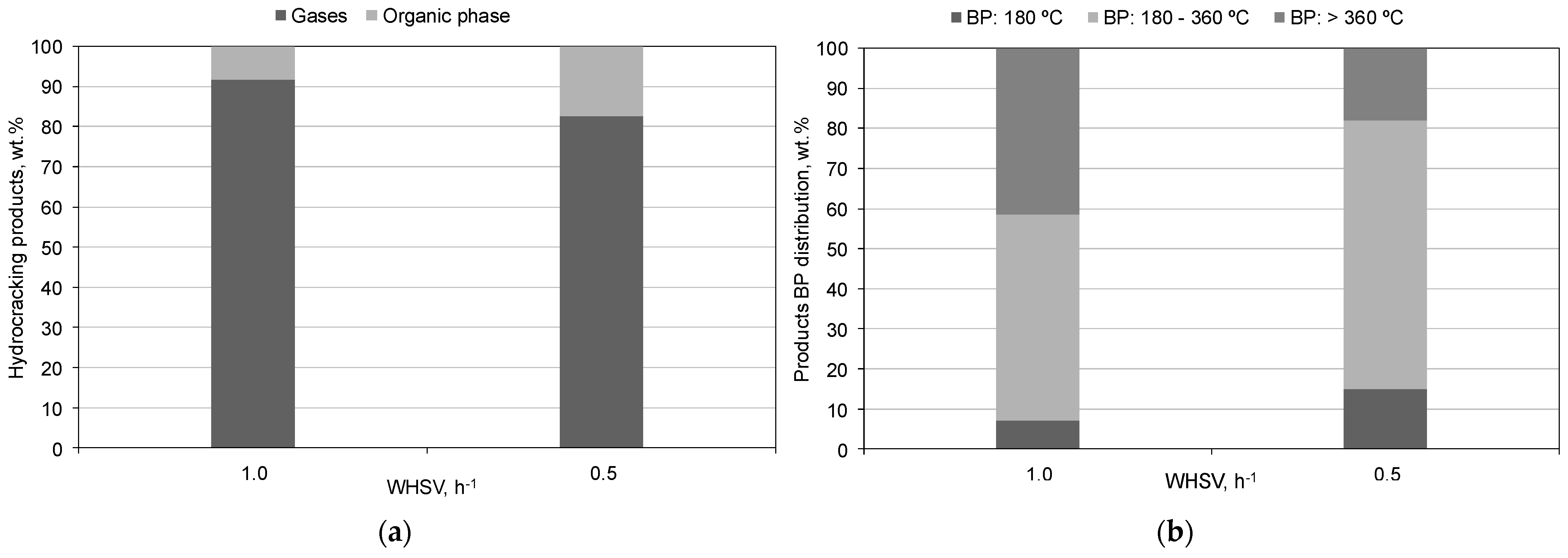
3.2. Hydrocracking Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Rosa, M.; Gainsford, K.; Pallonetto, F.; Finn, D.P. Diversification, concentration and renewability of the energy supply in the European Union. Energy 2022, 253, 124097. [Google Scholar] [CrossRef]
- Solarin, S.A.; Sahu, P.K. Sectoral foreign direct investment and environmental degradation: New insights from diversification of energy mix containing fossil fuels and renewable energy. Environ. Sci. Pollut. Res. Int. 2023, 30, 91853–91873. [Google Scholar] [CrossRef] [PubMed]
- Aitken, C.; Ersoy, E. War in Ukraine: The options for Europe’s energy supply. World Econ. 2023, 46, 887–896. [Google Scholar] [CrossRef]
- Sotiriou, C.; Zachariadis, T. Optimal Timing of Greenhouse Gas Emissions Abatement in Europe. Energies 2019, 12, 1872. [Google Scholar] [CrossRef]
- Kazancoglu, Y.; Ozbiltekin-Pala, M.; Ozkan-Ozen, Y.D. Prediction and evaluation of greenhouse gas emissions for sustainable road transport within Europe. Sustain. Cities Soc. 2021, 70, 102924. [Google Scholar] [CrossRef]
- Jankuj, V.; Spitzer, S.H.; Krietsch, A.; Štroch, P.; Bernatík, A. Safety of alternative energy sources: A review. Chem. Eng. Trans. 2022, 90, 115–120. [Google Scholar] [CrossRef]
- Hagem, C.; Kallbekken, S.; Mæstad, O.; Westskog, H. Enforcing the Kyoto Protocol: Sanctions and strategic behavior. Energy Policy 2005, 33, 2112–2122. [Google Scholar] [CrossRef]
- Wysokińska, Z. A Review of Transnational Regulations in Environmental Protection and the Circular Economy. Comp. Econ. Res. Cent. East. Eur. 2020, 23, 149–168. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Z.; Cao, J.; Yang, L.; Guan, D. Environmental Regulation and Enterprise Innovation: A Review. Bus. Strateg. Environ. 2020, 29, 1465–1478. [Google Scholar] [CrossRef]
- European Commission. Commission Communication: Stepping up Europe’s 2030 Climate Ambition Investing in a Climate-Neutral Future for the Benefit of Our People. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:749e04bb-f8c5-11ea-991b-01aa75ed71a1.0001.02/DOC_2&format=PDF (accessed on 9 May 2024).
- European Environment Agency. Greenhouse Gas Emissions from Transport in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/greenhouse-gas-emissions-from-transport (accessed on 13 July 2021).
- Martinelli, M.; Gnanamani, M.K.; LeViness, S.; Jacobs, G.; Shafer, W.D. An overview of Fischer-Tropsch Synthesis: XtL processes, catalysts and reactors. Appl. Catal. A Gen. 2020, 608, 117740. [Google Scholar] [CrossRef]
- Van Vliet, O.P.R.; Faaij, A.P.C.; Turkenburg, W.C. Fischer-Tropsch diesel production in a well-to-wheel perspective: A carbon, energy flow and cost analysis. Energy Convers. Manag. 2009, 50, 855–876. [Google Scholar] [CrossRef]
- Vosoughi, V.; Badoga, S.; Dalai, A.K.; Abatzoglou, N. Modification of mesoporous alumina as a support for cobalt-based catalyst in Fischer-Tropsch synthesis. Fuel Process. Technol. 2017, 162, 55–65. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A. Comparison between different types of renewable diesel. Renew. Sustain. Energy Rev. 2013, 21, 110–116. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.; Zhu, G.; Xie, C.; Zhang, X.; Zhang, X. Catalytic Cracking of Fischer-Tropsch Wax on Different Zeolite Catalysts. Catalysts 2023, 13, 1223. [Google Scholar] [CrossRef]
- Karaba, A.; Rozhon, J.; Patera, J.; Hájek, J.; Zámostný, P. Fischer-Tropsch Wax from Renewable Resources as An Excellent Feedstock For The Steam-Cracking Process. Chem. Eng. Technol. 2021, 44, 329–338. [Google Scholar] [CrossRef]
- Murat, M.; Gholami, Z.; Šimek, J.; Rodríguez-Padrón, D.; Hidalgo-Herrador, J.M. Rendering Fat and Heavy Fischer-Tropsch Waxes Mixtures (0–100%) Fast Pyrolysis Tests for the Production of Ethylene and Propylene. Processes 2021, 9, 367. [Google Scholar] [CrossRef]
- Kubička, D.; Černý, R. Upgrading of Fischer–Tropsch Waxes by Fluid Catalytic Cracking. Ind. Eng. Chem. Res. 2012, 51, 8849–8857. [Google Scholar] [CrossRef]
- Leckel, D. Noble Metal Wax Hydrocracking Catalysts Supported on High-Siliceous Alumina. Ind. Eng. Chem. Res. 2007, 46, 3505–3512. [Google Scholar] [CrossRef]
- Peng, C.; Du, Y.; Feng, X.; Hu, Y.; Fang, X. Research and development of hydrocracking catalysts and technologies in China. Front. Chem. Sci. Eng. 2018, 12, 867–877. [Google Scholar] [CrossRef]
- Frątczak, J.; de Paz Carmona, H.; Tišler, Z.; Hidalgo Herrador, J.M.; Gholami, Z. Hydrocracking of Heavy Fischer–Tropsch Wax Distillation Residues and Its Blends with Vacuum Gas Oil Using Phonolite-Based Catalysts. Molecules 2021, 26, 7172. [Google Scholar] [CrossRef]
- Pleyer, O.; Vrtiška, D.; Straka, P.; Šimáček, P. Co-processing of BTL Fischer-Tropsch wax and heavy vacuum gas oil. Renew. Energy 2024, 225, 120276. [Google Scholar] [CrossRef]
- Šimáček, P.; Kubička, D.; Pospíšil, M.; Rubáš, V.; Hora, L.; Šebor, G. Fischer–Tropsch Product as A Co-Feed for Refinery Hydrocracking Unit. Fuel 2013, 105, 432–439. [Google Scholar] [CrossRef]
- Halmenschlager, C.M.; Brar, M.; Apan, I.T.; de Klerk, A. Hydrocracking vacuum gas oil with wax. Catal. Today 2020, 353, 187–196. [Google Scholar] [CrossRef]
- Hodala, J.L.; Jung, J.-S.; Yang, E.-H.; Hong, G.H.; Noh, Y.S.; Moon, D.J. Hydrocracking of FT-wax to fuels over non-noble metal catalysts. Fuel 2016, 185, 339–347. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Georgiev, B.; Argirov, G.; Atanassova, V.; Vassilev, P.; Atanassov, K.; Yordanov, D.; et al. Catalytic Cracking of Diverse Vacuum Residue Hydrocracking Gas Oils. Chem. Eng. Technol. 2021, 44, 997–1008. [Google Scholar] [CrossRef]
- Xing, T.; De Crisci, A.G.; Chen, J. Hydrocracking of Fischer-Tropsch wax and its mixtures with heavy vacuum gas oil. Can. J. CHem. Eng. 2019, 97, 1515–1524. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Wang, G.; Chen, Z.; Han, J.; Gao, C.; Gao, J. Fischer-Tropsch wax catalytic cracking for the production of low olefin and high octane number gasoline: Experiment and molecular level kinetic modeling study. Fuel 2021, 303, 121226. [Google Scholar] [CrossRef]
- ČSN EN 590; Motor fuels - Diesel fuel - Technical requirements and test methods ČSN EN 590 CORRECTION 1 65 6506. Original in Czech language: ČSN EN 590, Motorová paliva – Motorové nafty – Technické požadavky a metody zkoušení ČSN EN 590 OPRAVA 1 65 6506; Czech Standardization Agency: Prague, Czech Republic.
- ASTM D4052; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D5291; Standard test methods for instrumental determination of carbon, hydrogen, and nitrogen in petroleum products and lubricants. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D5453; Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel, and Engine Oil by Ultraviolet Fluorescence. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D4629; Standard Test Method for Trace Nitrogen in Liquid Petroleum Hydrocarbons by Syringe/Inlet Oxidative Combustion and Chemiluminescence Detection. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D2887; Standard Test Method for Boiling Range Distribution of Petroleum Fractions by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- Hidalgo, J.M.; Horaček, J.; Matoušek, L.; Vráblík, A.; Tišler, Z.; Černý, R. Catalytic Hydrocracking of Vacuum Residue and Waste Cooking Oil Mixtures. Monatsh. Chem. 2018, 149, 1167–1177. [Google Scholar] [CrossRef]
- Hidalgo Herrador, J.M.; Psenička, M.; Horaček, J.; Tišler, Z.; Vráblík, A.; Černý, R.; Murat, M. Co-Processing Of Waste Cooking Oil And Light Cycle Oil With Niw/(Pseudoboehmite + Sba-15). Catalyst. Chem. Eng. Technol. 2019, 42, 512–517. [Google Scholar] [CrossRef]
- ASTM D3238; Standard Test Method for Calculation of Carbon Distribution and Structural Group Analysis of Petroleum Oils bu the n-d-M Method. ASTM International: West Conshohocken, PA, USA, 2017.
- IP 391; Petroleum Products—Determination of Aromatic Hydrocarbon Types in Middle Distillates—High Performance Liquid Chromatography Method with Refractive Index Detection. Energy Institute: London, UK, 2019.
- de Paz Carmona, H.; Vráblík, A.; Hidalgo Herrador, J.M.; Velvarská, R.; Cerný, R. Animal fats as a suitable feedstock for co-processing with atmospheric gas oil. Sustain. Energy 2021, 5, 4955–4964. [Google Scholar] [CrossRef]
- Bezergianni, S.; Dimitriadis, A.; Kikhtyanin, O.; Kubička, D. Refinery co-processing of renewable feeds. Prog. Energ. Combust. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Kubička, D.; Horáček, J. Deactivation of HDS catalysts in deoxygenation of vegetable oils. Appl. Catal. A Gen. 2011, 394, 9–17. [Google Scholar] [CrossRef]
- Horáček, J.; Kubička, D. Bio-oil Hydrotreating over conventional CoMo & NiMo catalysts: The role of reaction conditions and additives. Fuel 2017, 198, 49–57. [Google Scholar] [CrossRef]
- Hosseinifar, P.; Shahverdi, H. Development of A Generalized Model for Predicting the Composition of Homologous Groups Derived from Molecular Type Analyses to Characterize Petroleum Fractions. J. Petrol. Sci. Eng. 2021, 204, 108774. [Google Scholar] [CrossRef]
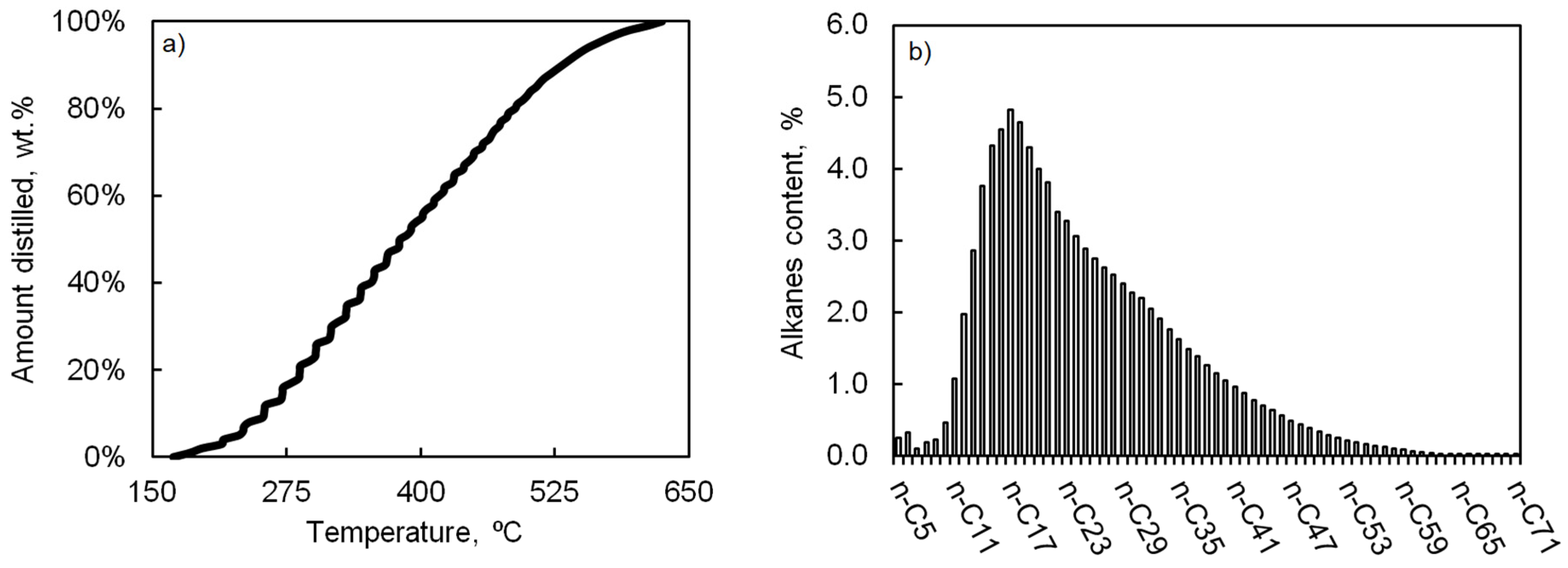
| Properties | FTS Wax Raw Material * | FTS Wax without Light Compounds and Water * | FTS Wax Distillation Product ** |
|---|---|---|---|
| S content, mg/kg | 2.2 | N/A | N/A |
| N content, mg/kg | 83.7 | N/A | N/A |
| Elemental analysis | |||
| C content, wt.% | 80.8 | N/A | N/A |
| H content, wt.% | 14.1 | N/A | N/A |
| Boiling range fraction | |||
| Gasoline (<180 °C) | 1.0 | 0.0 | 0.0 |
| Diesel (180–360 °C) | 42.0 | 39.0 | 38.0 |
| Heavy/residue (>360 °C) | 57.0 | 61.0 | 58.0 |
| Losses (wt.%) | 0.0 | 0.0 | 4.0 |
| Analysis | Fossil Diesel | Mix 1 D-FTS:FD 7:93 | Mix 2 D-FTS:FD 15:85 | Mix 3 D-FTS:FD 30:70 | Mix 4 D-FTS:FD 50:50 | Diesel Fuel Standards EN590 | ||
|---|---|---|---|---|---|---|---|---|
| Min. | Max. | |||||||
| Density at 15 °C, kg/m3 | 834.9 | 831.2 | 827.2 | 819.5 | 809.1 | 820 | 845 | |
| Kinematic viscosity at 40 °C, mm2/s | 2.6 | 2.6 | 2.7 | 2.7 | 2.9 | 2.0 | 4.5 | |
| Sulphur content, mg/kg | 8.1 | 6.3 | 5.3 | 5.1 | 4.4 | N/A | 10.0 | |
| Nitrogen content, mg/kg | 20.9 | 23.8 | 25.7 | 30.8 | 38.0 | N/A | N/A | |
| Poliaromatics, wt.% | 8.0 | 7.3 | 7.0 | 6.7 | 5.3 | N/A | 8.0 | |
| Elemental analysis (wt.%) | ||||||||
| Carbon content | 86.7 | 86.4 | 86.6 | 86.4 | 85.9 | N/A | N/A | |
| Hydrogen content | 13.2 | 13.6 | 13.3 | 13.5 | 14.2 | N/A | N/A | |
| Distillation | ||||||||
| Evaporates at 250 °C: | 40 | 40 | 40 | 30 | 30 | N/A | 65 | |
| Evaporates at 350 °C: | 100 | 100 | 100 | 97 | 100 | 85 | N/A | |
| 95% evaporated at: | 345.8 | 345.5 | 342.5 | 342.3 | 339.6 | N/A | 360 | |
| Cetane index | 50 | 53 | 55 | 60 | 67 | 46 | N/A | |
| Cetane number | 53 | 54 | 57 | 64 | 73 | 51 | N/A | |
| Flash point, °C | 68 | 68 | 71 | 73 | 77 | 55 | N/A | |
| Refractive index | 1.4624 | 1.4611 | 1.4595 | 1.4537 | 1.4494 | N/A | N/A | |
| Water content, wt.% | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | N/A | 0.02 | |
| Impurities, mg/kg | 0.81 | 1.19 | 0.62 | 0.83 | 0.84 | N/A | 24 | |
| Oxidity stabilisation, h | 28.6 | 29.3 | 23.3 | 23.7 | 29.4 | 20 | N/A | |
| Colour ASTM D 1500 | 0.9 | 0.9 | 0.9 | 0.8 | 0.7 | N/A | 2.0 | |
| CPFF | −12 | −10 | −8 | −1 | 2 | N/A | * | |
| Analysis | VGO | VGO:H-FTS (10:90) | VGO:H-FTS (20:80) | VGO:H-FTS (30:70) | VGO:H-FTS (40:60) | VGO:H-FTS (50:50) |
|---|---|---|---|---|---|---|
| Density at 15 °C, kg/m3 | 905.0 | 908.6 | 908.2 | 907.8 | 907.4 | 907.0 |
| Kinematic viscosity at 100 °C, mm2/s | N/A | 10.30 | 8.87 | 7.73 | 6.67 | 5.51 |
| Sulphur content, mg/kg | 1.92 | <0.05 | <0.05 | 0.18 | 0.26 | 0.63 |
| Nitrogen content, mg/kg | 847 | N/A | N/A | N/A | N/A | N/A |
| Elemental analysis (wt.%) | ||||||
| Carbon content | 84.9 | 86.0 | 85.8 | 86.3 | 86.1 | 86.2 |
| Hydrogen content | 12.1 | 14.0 | 14.0 | 13.9 | 14.0 | 13.7 |
| H/C ratio | 1.4 | 1.94 | 1.95 | 1.92 | 1.94 | 1.89 |
| Distillation | ||||||
| IBP, °C | 284.0 | 315.1 | 303.7 | 298.2 | 294.2 | 293.8 |
| FBP, °C | 510.3 | 719.3 | 719.7 | 708.4 | 689.0 | 643.7 |
| BMCI/CI * | N/A | 39.2 | 40.0 | 40.8 | 42.4 | 42.7 |
| Analysis | Diesel Fraction HC Product WHSV 0.5 h−1 | Diesel Fraction HC Product WHSV 1.0 h−1 | Diesel Fuel Standards EN590 | |
|---|---|---|---|---|
| Min. | Max. | |||
| Density at 15 °C, kg/m3 | 799.3 | 811.5 | 820 | 820 |
| Kinematic viscosity at 40 °C, mm2/s | 2.77 | 3.38 | 2.0 | 2.0 |
| Sulphur content, mg/kg | 2.5 | 38.1 | N/A | N/A |
| Nitrogen content, mg/kg | 1.02 | 2.69 | N/A | N/A |
| Elemental analysis, wt.% | ||||
| Carbon content | 85.6 | 86.5 | N/A | N/A |
| Hydrogen content | 14.5 | 13.4 | N/A | N/A |
| Distillation | ||||
| -Evaporates at 250 °C: | 30 | 20 | N/A | 65 |
| Evaporates at 350 °C: | 100 | 90 | 85 | N/A |
| 95% evaporated at: | 337.3 | 356.2 | N/A | 360 |
| Cetane index | 70 | 68 | 46 | N/A |
| Cetane number | 68 | 67 | 51 | N/A |
| Flash point, °C | 88 | 93 | 55 | N/A |
| Refractive index | 1.4431 | 1.4498 | N/A | N/A |
| Water content, wt.% | <0.01 | <0.01 | N/A | 0.02 |
| Impurities, mg/kg | 2.04 | 4.18 | N/A | 24 |
| Oxidity stabilisation, h | >48 | >48 | 20 | N/A |
| Colour ASTM D 1500 | 0.3 | 0.5 | N/A | 2.0 |
| CPFF | −20 | −1 | N/A | * |
| n-d m method, wt.% | ||||
| Aromatic Carbon | 4.6 | 6.6 | N/A | N/A |
| Naphthenic Carbon | 25.5 | 22.4 | N/A | N/A |
| Paraffinic Carbon | 55.5 | 57.5 | N/A | N/A |
| Mono-aromatics | 7.7 | 12.3 | N/A | N/A |
| Di-aromatics | 1.0 | 1.7 | N/A | N/A |
| Poly-aromatics | 0.2 | 0.4 | N/A | 8.0 |
| Total aromatics | 8.9 | 14.4 | N/A | N/A |
| GCxGC-MS analysis, % | ||||
| n-alkanes | 13.29 | 17.71 | N/A | N/A |
| i-alkanes | 66.17 | 63.98 | N/A | N/A |
| Alkenes + cycloalkanes | 16.03 | 12.22 | N/A | N/A |
| Aromatics | 3.28 | 4.86 | N/A | N/A |
| Undefined compounds | 1.24 | 1.24 | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frątczak, J.; Górska, J.; Babor, M.; Gholami, Z.; Hidalgo Herrador, J.M.; de Paz Carmona, H. Production of Transportation Fuels from Fischer–Tropsch Waxes: Distillation, Blending, and Hydrocracking. Appl. Sci. 2024, 14, 4656. https://doi.org/10.3390/app14114656
Frątczak J, Górska J, Babor M, Gholami Z, Hidalgo Herrador JM, de Paz Carmona H. Production of Transportation Fuels from Fischer–Tropsch Waxes: Distillation, Blending, and Hydrocracking. Applied Sciences. 2024; 14(11):4656. https://doi.org/10.3390/app14114656
Chicago/Turabian StyleFrątczak, Jakub, Joanna Górska, Martin Babor, Zahra Gholami, José Miguel Hidalgo Herrador, and Héctor de Paz Carmona. 2024. "Production of Transportation Fuels from Fischer–Tropsch Waxes: Distillation, Blending, and Hydrocracking" Applied Sciences 14, no. 11: 4656. https://doi.org/10.3390/app14114656








