Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Treatments and Olive Leaf Sampling
- -
- Control treatment (C): water
- -
- Si1 treatment: water and Silitec (Kimitec Agro®) at a concentration of 4.25 mL per litre of water (0.55 g Si/L)
- -
- Si2 treatment: water and Silitec at a concentration of 8.5 mL per litre of water (1.1 g Si/L)
- -
- Si3 treatment: water and Silitec at a concentration of 17 mL per litre of water (2.2 g Si/L).
2.2. Chemicals
2.3. Identification and Quantification of Phenolic Compounds by High-Performance Liquid Chromatography (HPLC)
2.4. Elemental Analysis
2.5. MTS-Based Cell Proliferation Assay
2.6. Statistical Analysis
3. Results
3.1. Effect of Si Foliar Biostimulant Application on Phenolic Content in Olive Leaf
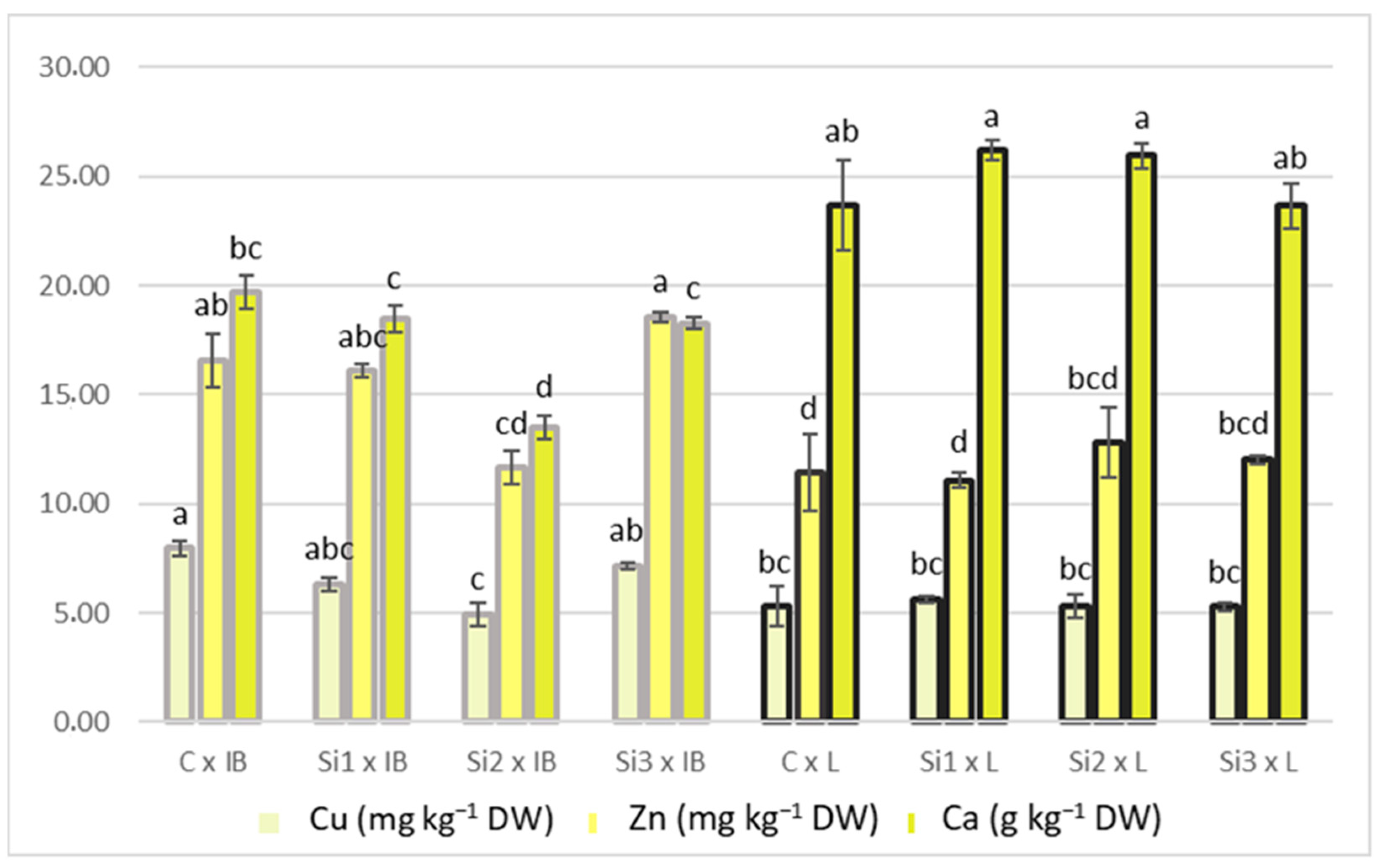
3.2. Effect of Si Foliar Biostimulant Application on Elemental Concentration in Olive Leaf
3.3. Effect of Extracted Polyphenols from Si3 Treatment on Cancer Cell Line Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic Compounds in Olive Leaves: Analytical Determination, Biotic and Abiotic Influence, and Health Benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean Diet and Cancer: Epidemiological Evidence and Mechanism of Selected Aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef]
- Fares, R.; Bazzi, S.; Baydoun, S.E.; Abdel-Massih, R.M. The Antioxidant and Anti-proliferative Activity of the Lebanese Olea europaea Extract. Plant Foods Hum. Nutr. 2011, 66, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a Phenolic Compound of Virgin Olive Oil, Activates AMP-activated Protein kinase to Inhibit Carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Zang, H.; Yin, Z.; Guan, P.; Yu, C.; Shan, A.; Feng, X. Dietary Pterostilbene Exerts Potential Protective Effects by Regulating Lipid Metabolism and Enhancing Antioxidant Capacity on Liver in Broilers. J. Anim. Physiol. Anim. Nutr. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Yu, H.; Wang, S.; Sun, J.; Chai, X.; Sun, X.; Qi, X.; Zhang, R.; Jiao, Y.; Li, Z.; et al. Dietary Rutin Alleviated the Damage by Cold Stress on Inflammation Reaction, Tight Junction Protein and Intestinal Microbial Flora in the Mice Intestine. J. Nutr. Biochem. 2024, 130, 109658. [Google Scholar] [CrossRef]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A.A. New HPLC-MS Method for Measuring Maslinic Acid and Oleanolic Acid in HT29 and HepG2 Human Cancer Cells. Int. J. Mol. Sci. 2015, 16, 21681. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological Activities of the Natural Antioxidant Oleuropein: Exceeding the Expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major Phenolic Compounds in Olive Oil: Metabolism and Health Effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant Polyphenol Content, Soil Fertilization and Agricultural Management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Majetić Germek, V.; Ban, D.; Klanjac, J.; Franić, M.; et al. Boron Foliar Application Enhances Oleuropein Level and Modulates Volatile Compound Composition in Olive Leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of Nitrogen Fertilization on the Phenolic Composition and Antioxidant Properties of Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.S.; Leal-Costa, M.V.; Coutinho, M.A.S.; Moreira, N.S.; Lage, C.L.S.; Barbi, N.S.; Costa, S.S.; Tavares, E.S. Increased Antioxidant Activity and Changes in Phenolic Profile of Kalanchoe pinnata (Lamarck) Persoon (Crassulaceae) Specimens Grown under Supplemental Blue Light. Photochem. Photobiol. 2013, 89, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Tekaya, M.; Mechri, B.; Bchir, A.; Attia, F.; Cheheb, H.; Daassa, M.; Hammami, M. Effect of Nutrient-based Fertilisers of Olive Trees on Olive Oil Quality. J. Sci. Food Agric. 2013, 93, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.Q.; Rodrigues, M.Â.; Moutinho-Pereira, J.M.; Correia, C.M.; Arrobas, M. Olive Tree Response to Applied Phosphorus in Field and Pot Experiments. Sci. Hortic. 2018, 234, 236–244. [Google Scholar] [CrossRef]
- Roussos, P.A.; Gasparatos, D.; Kechrologou, K.; Katsenos, P.; Bouchagier, P. Impact of Organic Fertilization on Soil Properties, Plant Physiology and Yield in Two Newly Planted Olive (Olea europaea L.) Cultivars under Mediterranean Conditions. Sci. Hortic. 2017, 220, 11–19. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon Application Alleviates Drought Stress in Wheat Through Transcriptional Regulation of Multiple Antioxidant Defense Pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Hassan, I.F.; Ajaj, R.; Gaballah, M.S.; Ogbaga, C.C.; Kalaji, H.M.; Hatterman-Valenti, H.M.; Alam-Eldein, S.M. Foliar Application of Nano-Silicon Improves the Physiological and Biochemical Characteristics of ‘Kalamata’ Olive Subjected to Deficit Irrigation in a Semi-Arid Climate. Plants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Nascimento-Silva, K.; Benlloch-González, M.; Fernández-Escobar, R. Silicon Nutrition in Young Olive Plants: Effect of Dose, Application Method, and Cultivar. HortScience 2022, 57, 1534–1539. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic Variation in the Silicon Composition of Plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Martos-García, I.; Fernández-Escobar, R.; Benlloch-González, M. Silicon is a Non-essential Element but Promotes Growth in Olive Plants. Sci. Hortic. 2024, 323, 112541. [Google Scholar] [CrossRef]
- Marcelić, Š.; Vidović, N.; Pasković, I.; Lukić, M.; Jukić Špika, M.; Palčić, I.; LukiĆ, I.; Petek, M.; Pecina, M.; Herak Custić, M.; et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae 2022, 8, 203. [Google Scholar] [CrossRef]
- Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Polić Pasković, M.; Palčić, I.; Major, N.; Goreta Ban, S.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. [Google Scholar] [CrossRef]
- Stateras, D.C.; Moustakas, N.K. Seasonal Changes of Macro- and Micro-nutrients Concentration in Olive Leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Fredotović, Ž.; Puizina, J.; Nazlić, M.; Maravić, A.; Ljubenkov, I.; Soldo, B.; Vuko, E.; Bajić, D. Phytochemical Characterization and Screening of Antioxidant, Antimicrobial and Antiproliferative Properties of Allium × cornutum Clementi and Two Varieties of Allium cepa L. Peel Extracts. Plants 2021, 10, 832. [Google Scholar] [CrossRef]
- Statistica, Version 14.1.0.8.; TIBCO Software Inc.: Palo Alto, CA, USA, 2017.
- Hammer, D.A.T.; Ryan, P.D.; Hammer, Ø.; Harper, D.A.T. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant Activity of Phenolics Extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and Bioactive Properties of Olive (Olea europaea L.) Leaves. Eur. Food Res. Technol. 2016, 243, 89–99. [Google Scholar] [CrossRef]
- González-Báide, A.; Gómez, P. Dysfunctionality of the Xylem in Olea europaea L. Plants Associated with the Infection Process by Verticillium dahliae Kleb. Role of Phenolic Compounds in Plant Defense Mechanism. Artic. J. Agric. Food Chem. 2007, 55, 3373–3377. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Liber, Z.; Montemurro, C.; Miazzi, M.M.; Ljubenkov, I.; Soldo, B.; Žanetić, M.; Vitanović, E.; Politeo, O.; Škevin, D. Quantitatively Unraveling Hierarchy of Factors Impacting Virgin Olive Oil Phenolic Profile and Oxidative Stability. Antioxidants 2022, 11, 594. [Google Scholar] [CrossRef]
- Talhaoui, N. Analytical, Agronomic, and Biological Evaluation of Phenolic Compounds in Olea europaea Products and By-Products. Ph.D. Thesis, University of Granada, Faculty of Sciences, Granada, Spain, 8 January 2016. [Google Scholar]
- Mujić, I.; Živković, J.; Nikolić, G.; Vidović, S.; Trutić, N.; Kosić, U.; Jokić, S.; Ruznić, A. Phenolic Compounds in Olive Leaf Extract as a Source of Useful Antioxidants. Croat. J. Food Technol. Biotehnol. Nutr. 2011, 6, 129–133. [Google Scholar]
- Pasković, I.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Koprivnjak, O.; Major, N.; Grozić, K.; Franić, M.; Ban, D.; et al. Temporal Variation of Phenolic and Mineral Composition in Olive Leaves Is Cultivar Dependent. Plants 2020, 9, 1099. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.; et al. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae 2023, 9, 594. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Majetić, V.; Brkić Bubola, K.; Kosić, U. Variability of Phenolic and Volatile Compounds in Virgin Olive Oil from Leccino and Istarska Bjelica Cultivars in Relation to Their Fruit Mixtures. Food Technol. Biotechnol. 2012, 50, 216–221. [Google Scholar]
- Baiano, A.; Terracone, C.; Viggiani, I.; Del Nobile, M.A. Effects of Cultivars and Location on Quality, Phenolic Content and Antioxidant Activity of Extra-Virgin Olive Oils. J. Am. Oil Chem. Soc. 2013, 90, 103–111. [Google Scholar] [CrossRef]
- Ortega-García, F.; Peragón, J. The Response of Phenylalanine Ammonia-lyase, Polyphenol oxidase and Phenols to Cold Stress in the Olive Tree (Olea europaea L. cv. Picual). J. Sci. Food Agric. 2009, 89, 1565–1573. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; Koundouras, S.; Giannakoula, A. Effect of Water Deficit on Leaf Phenolic Composition, Gas Exchange, Oxidative Damage and Antioxidant Activity of Four Greek Olive (Olea europaea L.) Cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Cui, Y.; Ding, X.; Dai, Q. Stimulation of Phenolic Metabolism by Silicon Contributes to Rice Resistance to Sheath Blight. J. Plant Nutr. Soil Sci. 2013, 176, 118–124. [Google Scholar] [CrossRef]
- Schaller, J.; Brackhage, C.; Dudel, E.G. Silicon Availability Changes Structural Carbon Ratio and Phenol Content of Grasses. Environ. Exp. Bot. 2012, 77, 283–287. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Ashour, H.A.; Mahmoud, A.W.M. Response of Jatropha integerrima Plants Irrigated with Different Levels of Saline Water to Nano Silicon and Gypsum. J. Agric. Stud. 2017, 5, 136–160. [Google Scholar] [CrossRef]
- Therios, I. Mineral nutrition of the olive. In Olives, 1st ed.; Atheron, J., Rees, A., Eds.; Cabi: Wallingford, UK, 2008; pp. 179–209. [Google Scholar]
- Cavalheiro, C.V.; Picoloto, R.S.; Cichoski, A.J.; Wagner, R.; Menezes, C.R.; Zepka, L.Q.; Da Croce, D.M.; Barin, J.S. Olive Leaves Offer More Than Phenolic Compounds—Fatty Acids and Mineral Composition of Varieties from Southern Brazil. Ind. Crops Prod. 2015, 71, 122–127. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Yao, J.; Wu, J.; Yang, X.; Yang, J.; Zhang, Y.; Du, L. Oleuropein Induced Apoptosis in HeLa Cells via a Mitochondrial Apoptotic Cascade Associated with Activation of the c-Jun NH2-terminal kinase. J. Pharmacol. Sci. 2014, 125, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Vizza, D.; Lupinacci, S.; Toteda, G.; Puoci, F.; Ortensia, I.P.; De Bartolo, A.; Lofaro, D.; Scrivano, L.; Bonofiglio, R.; La Russa, A.; et al. An Olive Leaf Extract Rich in Polyphenols Promotes Apoptosis in Cervical Cancer Cells by Upregulating p21Cip/WAF1 Gene Expression. Nutr. Cancer 2019, 71, 320–333. [Google Scholar] [CrossRef]
- Zhang, X.; Reinsmoen, N.L. Impact of Non-human Leukocyte Antigen-specific Antibodies in Kidney and Heart Transplantation. Front. Immunol. 2017, 8, 434. [Google Scholar] [CrossRef][Green Version]
- Panduranga, A.K.; Ganapasam, S.; Kumar, A. Cytotoxic Effect of Luteolin on Human Colorectal Cancer Cell Line (HCT-15): Crucial Involvement of Reactive Oxygen Species. Middle East J. Cancer 2013, 4, 175–180. [Google Scholar]
- Potočnjak, I.; Šimić, L.; Vukelić, I.; Batičić, L.; Domitrović, R. Oleanolic Acid Induces HCT116 Colon Cancer Cell Death Through the p38/FOXO3a/Sirt6 Pathway. Chem. Biol. Interact. 2022, 363, 110010. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Jo, H.J.; Lee, K.J.; Choi, J.W.; An, J.H. Oleanolic acid induces p53-dependent apoptosis via the ERK/JNK/AKT pathway in cancer cell lines in prostatic cancer xenografts in mice. Oncotarget 2018, 9, 26370–26386. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Cecchi, L.; Bellumori, M.; Balli, D.; Giovannelli, L.; Huang, L.; Mulinacci, N. Phenolic Compounds and Triterpenes in Different Olive Tissues and Olive Oil By-Products, and Cytotoxicity on Human Colorectal Cancer Cells: The Case of Frantoio, Moraiolo and Leccino Cultivars (Olea europaea L.). Foods 2021, 10, 2823. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic Acid and its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a Quercetin Glycoside, Restores Chemosensitivity in Human Breast Cancer Cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]




| Treatment | Cultivar | T x Cv. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenolic Compounds (mg 100 g−1 DW) | C | Si1 | Si2 | Si3 | p Value | IB | L | p Value | p Value |
| Rutin | 116.63 ± 13.13 b | 118.51 ± 12.13 b | 131.76 ± 11.53 ab | 151.17 ± 13.82 a | 0.004 | 158.87 ± 5.77 b | 100.16 ± 5.23 a | <0.001 | 0.963 |
| Luteolin-7-O-glucoside | 632.38 ± 23.99 b | 628.74 ± 28.05 b | 716.21 ± 57.45 ab | 758.39 ± 70.88 a | 0.008 | 585.3 ± 14.18 b | 782.55 ± 34.23 a | <0.001 | 0.026 |
| Apigenin-7-O-glucoside | 44.28 ± 2.64 b | 46.09 ± 3.95 b | 54.74 ± 2.01 ab | 65.73 ± 9.11 a | 0.015 | 47.3 ± 2.03 b | 58.13 ± 5.21 a | 0.031 | 0.195 |
| Luteolin-4-O-glucoside | 33.98 ± 1.3 ab | 31.51 ± 0.92 b | 35.81 ± 1.5 ab | 37.25 ± 2.28 a | 0.034 | 32.71 ± 0.79 b | 36.56 ± 1.35 a | 0.009 | 0.114 |
| Luteolin | 9.44 ± 1 | 7.48 ± 1.3 | 7.83 ± 1.05 | 6.89 ± 1.18 | 0.244 | 6.03 ± 0.69 b | 9.79 ± 0.61 a | <0.001 | 0.342 |
| Apigenin | 0.02 ± 0.02 | 0.21 ± 0.1 | 0.1 ± 0.08 | 0.24 ± 0.22 | 0.615 | 0.07 ± 0.04 | 0.22 ± 0.12 | 0.254 | 0.681 |
| Catechin | 21.83 ± 3.35 | 24.49 ± 3.3 | 22.99 ± 3.52 | 31.99 ± 4.34 | 0.157 | 22.86 ± 2.64 | 27.79 ± 2.6 | 0.151 | 0.080 |
| Tyrosol | 18.73 ± 2.09b | 15.38 ± 1.66 ab | 19.04 ± 2.51 ab | 25.2 ± 3.2 a | 0.019 | 16.14 ± 1.43 b | 23.03 ± 1.89 a | 0.002 | 0.243 |
| Verbascoside | 133.57 ± 10.07 | 110.37 ± 6.26 | 137.44 ± 9.65 | 118.3 ± 11.26 | 0.108 | 111.13 ± 4.62 b | 138.71 ± 7.33 a | 0.003 | 0.923 |
| Oleuropein | 2422.23 ± 136.22 b | 2531.43 ± 241.5 b | 3041.13 ± 197.68 ab | 3752.62 ± 304.05 a | 0.001 | 3037.28 ± 178.64 | 2836.42 ± 228.52 | 0.385 | 0.340 |
| Oleacein | 36.24 ± 9.65 | 49.9 ± 10.35 | 56.36 ± 11.64 | 56.86 ± 9.29 | 0.441 | 50.56 ± 6.43 | 49.12 ± 8.14 | 0.886 | 0.152 |
| Oleuropein aglycone | 22.69 ± 3.85 a | 18.45 ± 4.87 ab | 11.06 ± 2.85 c | 12.49 ± 3.58 ab | 0.027 | 9.00 ± 1.32 b | 23.35 ± 2.86 a | <0.001 | 0.516 |
| Oleanolic acid | 559.1 ± 41.05 | 529.03 ± 63.45 | 535.28 ± 51.71 | 571.69 ± 50.7 | 0.892 | 615.93 ± 33.29 a | 481.62 ± 29.78 b | 0.006 | 0.144 |
| Statistic | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 5.88 | 3.70 | 2.33 |
| % variance | 28.02 | 17.63 | 11.11 |
| Cumulative% | 28.02 | 45.65 | 56.76 |
| Factor loadings/eigenvector | |||
| Rutin | −0.47 | 0.74 | −0.20 |
| Luteolin-7-O-glucoside | 0.89 | 0.23 | 0.14 |
| Apigenin-7-O-glucoside | 0.56 | 0.35 | −0.35 |
| Luteolin-4-O-glucoside | 0.69 | 0.46 | −0.13 |
| Luteolin | 0.50 | −0.47 | 0.03 |
| Apigenin | 0.44 | 0.31 | 0.27 |
| Catechin | 0.35 | 0.12 | −0.50 |
| Tyrosol | 0.67 | 0.31 | 0.03 |
| Verbascoside | 0.67 | −0.08 | −0.06 |
| Oleuropein | 0.35 | 0.80 | −0.11 |
| Oleacein | 0.30 | 0.48 | 0.50 |
| Oleuropein aglycone | 0.37 | −0.71 | 0.20 |
| Oleanolic acid | −0.49 | 0.43 | 0.04 |
| Cu | −0.55 | 0.31 | 0.46 |
| Zn | −0.58 | 0.52 | 0.42 |
| Se | −0.17 | 0.33 | 0.30 |
| Si | 0.52 | 0.35 | 0.26 |
| I | 0.64 | 0.03 | 0.33 |
| K | 0.39 | 0.06 | 0.37 |
| Mg | −0.42 | −0.23 | 0.66 |
| Ca | 0.58 | −0.36 | 0.52 |
| Treatment | Cultivar | T x Cv. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Microelements (mg kg−1 DW) | C | Si1 | Si2 | Si3 | p | IB | L | p | p |
| Cu | 6.63 ± 0.55 a | 5.96 ± 0.29 ab | 5.12 ± 0.44 b | 6.21 ± 0.44 ab | 0.026 | 6.59 ± 0.4 a | 5.37 ± 0.1 b | 0.001 | 0.019 |
| Zn | 14.01 ± 1.13 ab | 13.6 ± 1.02 ab | 12.23 ± 0.85 b | 15.3 ± 1.44 a | 0.041 | 15.73 ± 0.9 b | 11.84 ± 0.21 a | <0.001 | 0.004 |
| Se | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.13 ± 0.01 | 0.130 | 0.11 ± 0.01 | 0.1 ± 0.01 | 0.735 | 0.156 |
| Si | 123.26 ± 6.36 b | 259.35 ± 32.15 a | 287.11 ± 39.01 a | 347.68 ± 35.77 a | <0.001 | 206.51 ± 23.79 b | 302.19 ± 30.54 a | <0.001 | 0.040 |
| I | 0.48 ± 0.05 | 0.51 ± 0.06 | 0.6 ± 0.07 | 0.64 ± 0.08 | 0.183 | 0.46 ± 0.04 b | 0.65 ± 0.04 a | 0.003 | 0.790 |
| Macroelements (g kg−1 DW) | |||||||||
| K | 11.8 ± 0.35 | 11.64 ± 0.44 | 25.01 ± 14.96 | 12.16 ± 0.52 | 0.506 | 10.42 ± 0.53 | 19.88 ± 7.31 | 0.210 | 0.350 |
| Mg | 2.43 ± 0.15 | 2.56 ± 0.12 | 2.25 ± 0.22 | 2.1 ± 0.09 | 0.167 | 2.43 ± 0.14 | 2.23 ± 0.06 | 0.192 | 0.359 |
| Ca | 21.67 ± 0.88 | 22.31 ± 1.48 | 19.72 ± 2.55 | 20.95 ± 1.15 | 0.068 | 17.47 ± 0.81 b | 24.85 ± 0.43 a | <0.001 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasković, I.; Franić, M.; Polić Pasković, M.; Talhaoui, N.; Marcelić, Š.; Lukić, I.; Fredotović, Ž.; Žurga, P.; Major, N.; Goreta Ban, S.; et al. Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells. Appl. Sci. 2024, 14, 4669. https://doi.org/10.3390/app14114669
Pasković I, Franić M, Polić Pasković M, Talhaoui N, Marcelić Š, Lukić I, Fredotović Ž, Žurga P, Major N, Goreta Ban S, et al. Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells. Applied Sciences. 2024; 14(11):4669. https://doi.org/10.3390/app14114669
Chicago/Turabian StylePasković, Igor, Mario Franić, Marija Polić Pasković, Nassima Talhaoui, Šime Marcelić, Igor Lukić, Željana Fredotović, Paula Žurga, Nikola Major, Smiljana Goreta Ban, and et al. 2024. "Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells" Applied Sciences 14, no. 11: 4669. https://doi.org/10.3390/app14114669
APA StylePasković, I., Franić, M., Polić Pasković, M., Talhaoui, N., Marcelić, Š., Lukić, I., Fredotović, Ž., Žurga, P., Major, N., Goreta Ban, S., Vidović, N., Rončević, S., Nemet, I., Džafić, N., & Soldo, B. (2024). Silicon Foliar Fertilisation Ameliorates Olive Leaves Polyphenolic Compounds Levels and Elevates Its Potential towards Different Cancer Cells. Applied Sciences, 14(11), 4669. https://doi.org/10.3390/app14114669











