Microplastics’ Detection in Honey: Development of Protocols in a Simulation
Abstract
:1. Introduction
2. Materials and Methods
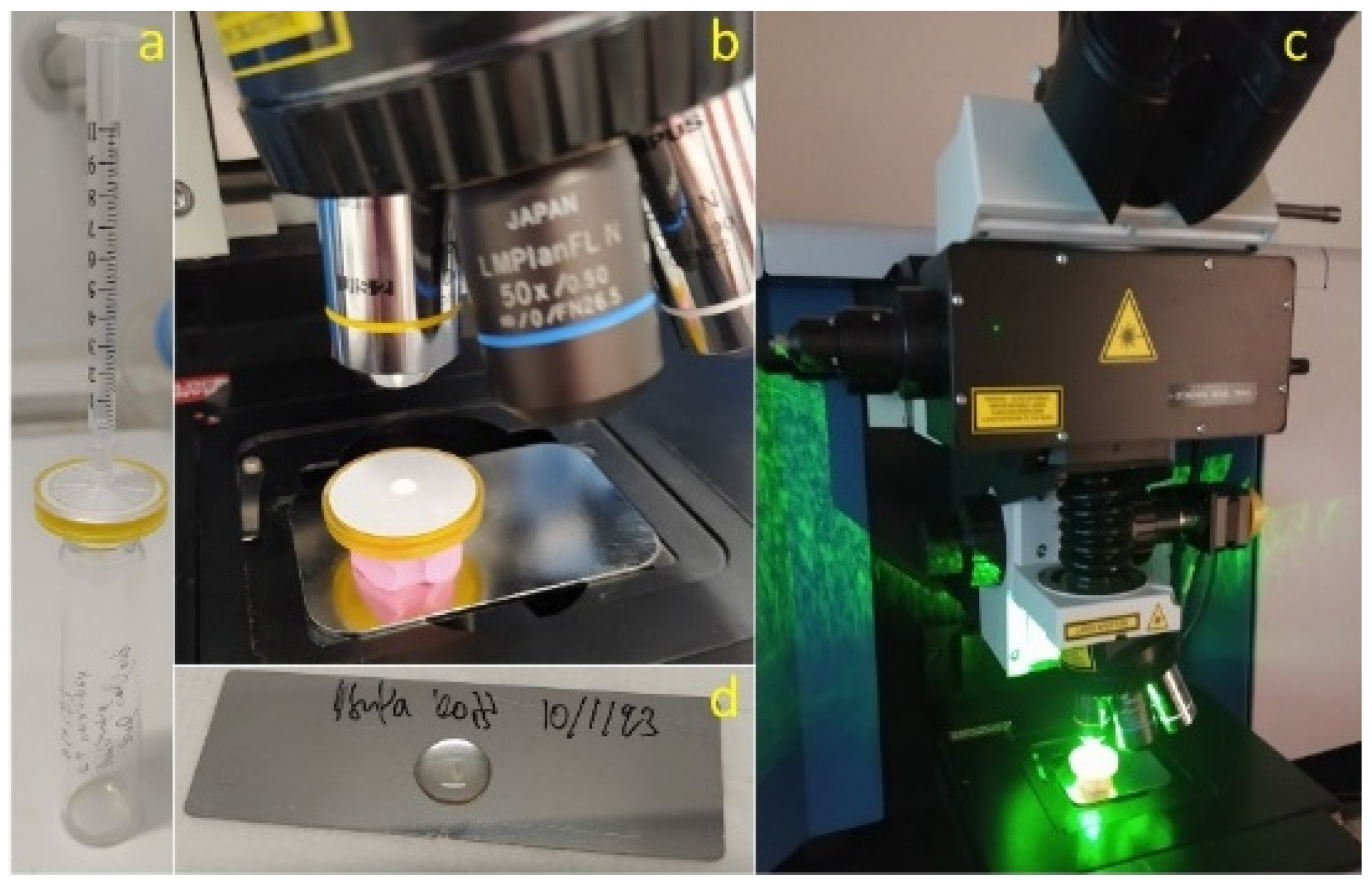
2.1. Plastic Packages of Honey
2.2. MP Pulverization Protocol
2.3. Protocol 1: PET MPs Detection in Deionized Water through Organic Solvents
2.4. Protocol 2: Methodological Experiment: Distributions of PET MPs into Different Organic Phases according to Their Dimensions
2.5. Protocol 3: Honey Samples Dyed with DANS Dye
2.5.1. Pre-DANS Test Protocol
2.5.2. First Experiment with Dyed MPs in Honey Protocol
2.5.3. Second Experiment with Dyed MPs in Honey Protocol
2.6. Material Characterization
2.6.1. Raman Spectroscopy and Mapping
2.6.2. Optical and Fluorescence Microscopy
2.6.3. Sample Preparation and Mass Spectrometry Analysis
- Ionization: Positive [MH]+
- ESI source Parameters:
- ○
- Spray Voltage: 3.6 kV
- ○
- Capilary Voltage: 37 V
- ○
- Capilary Temp: 275 °C
- ○
- Tube Lens: 130 V
- AGC Settings:
- ○
- Full MS 5 × 105
- ○
- Flow rate: 3 μL/min
- ○
- Resolution: 80,000
3. Results and Discussion
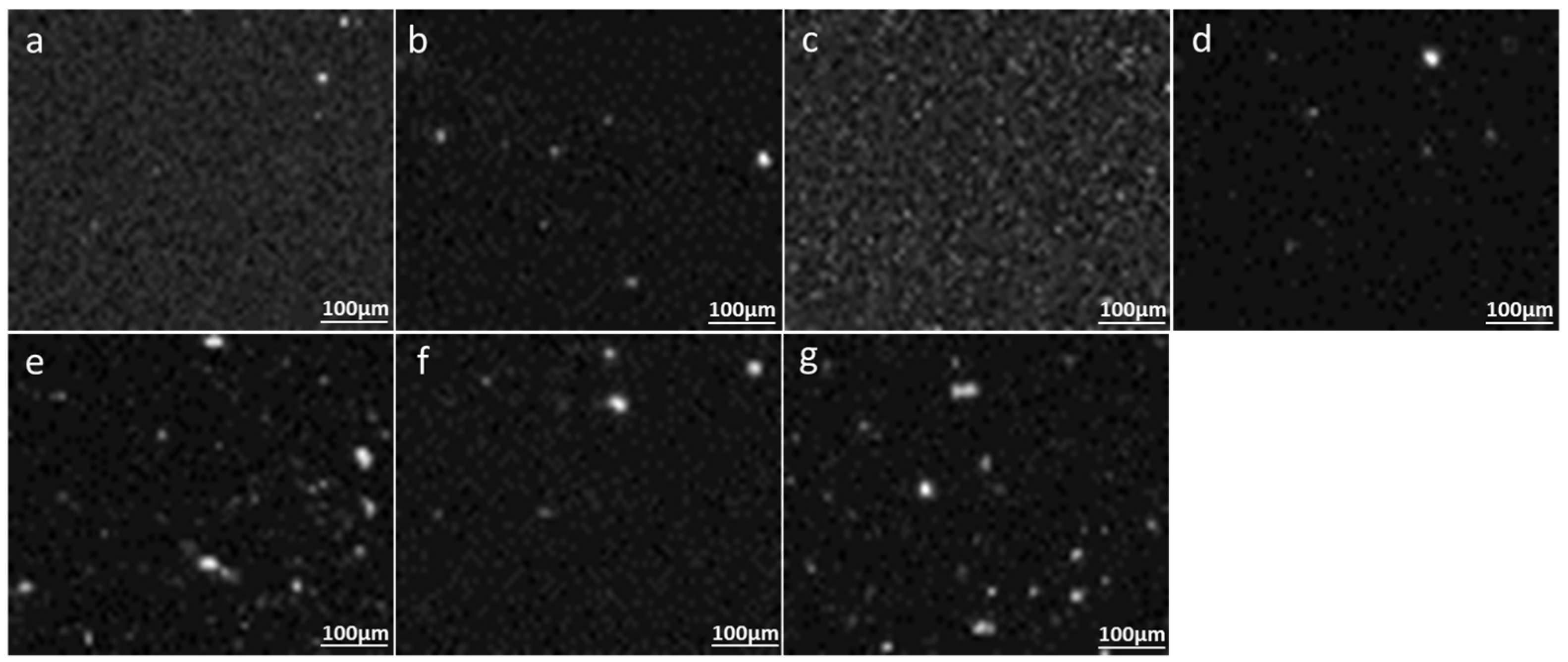
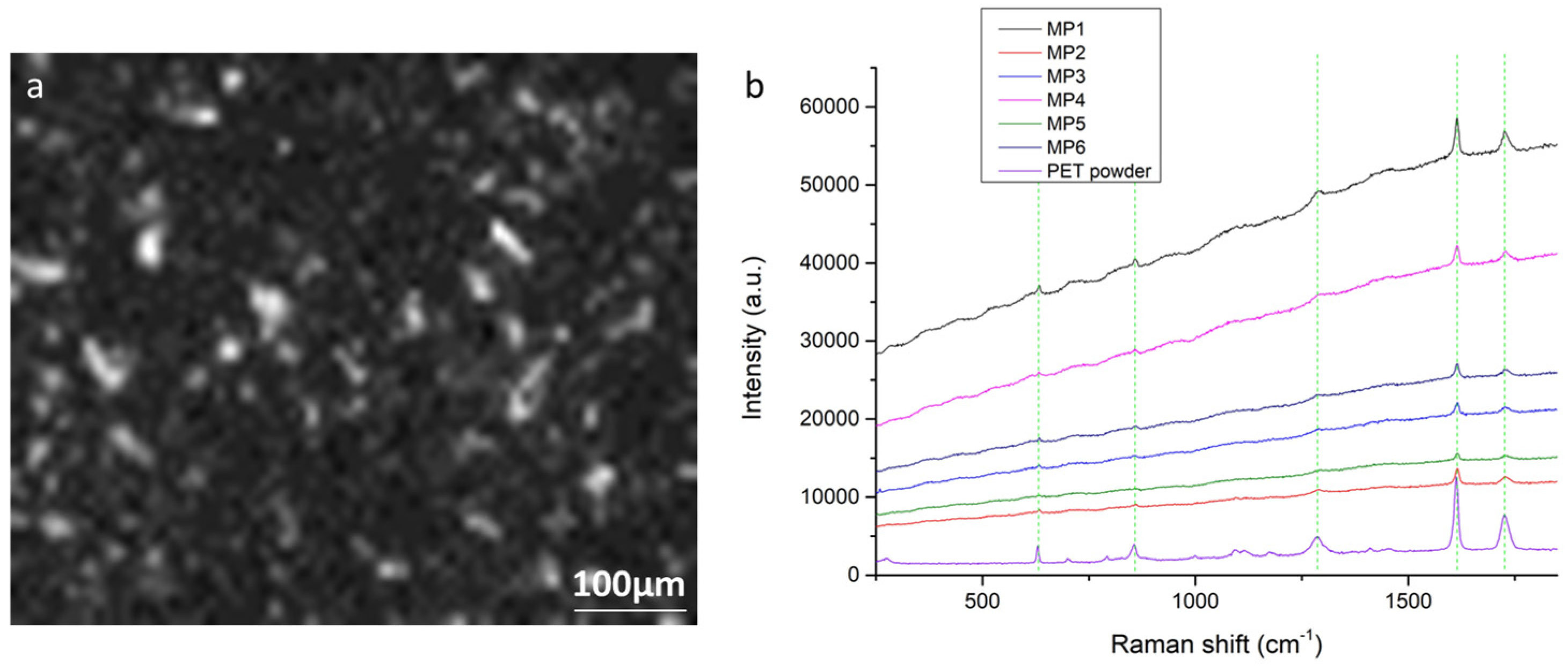
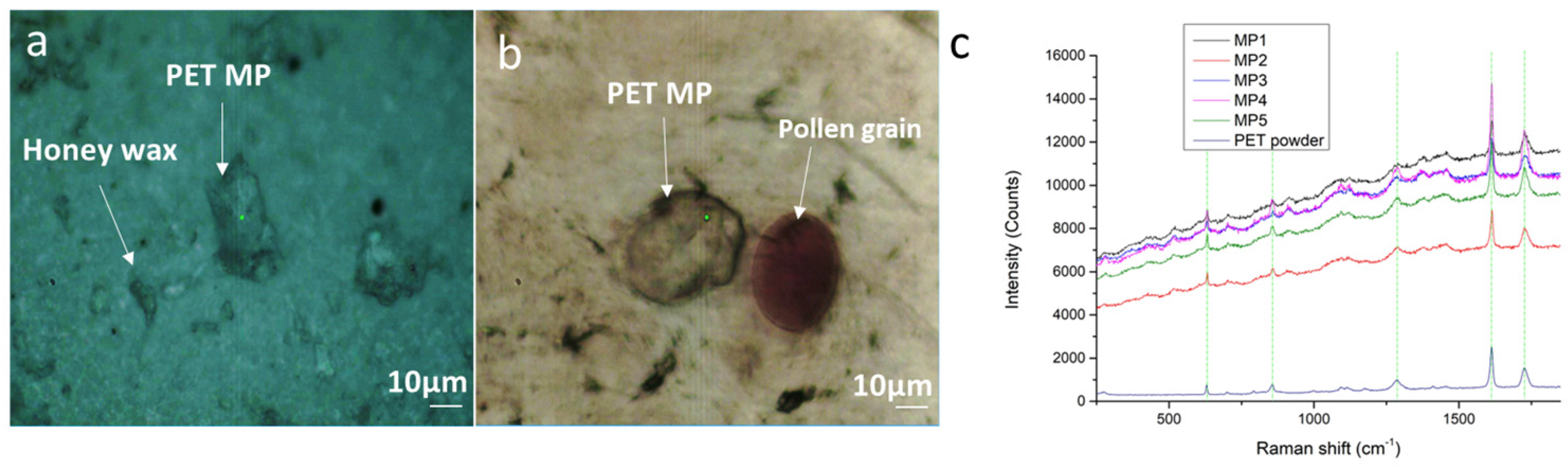
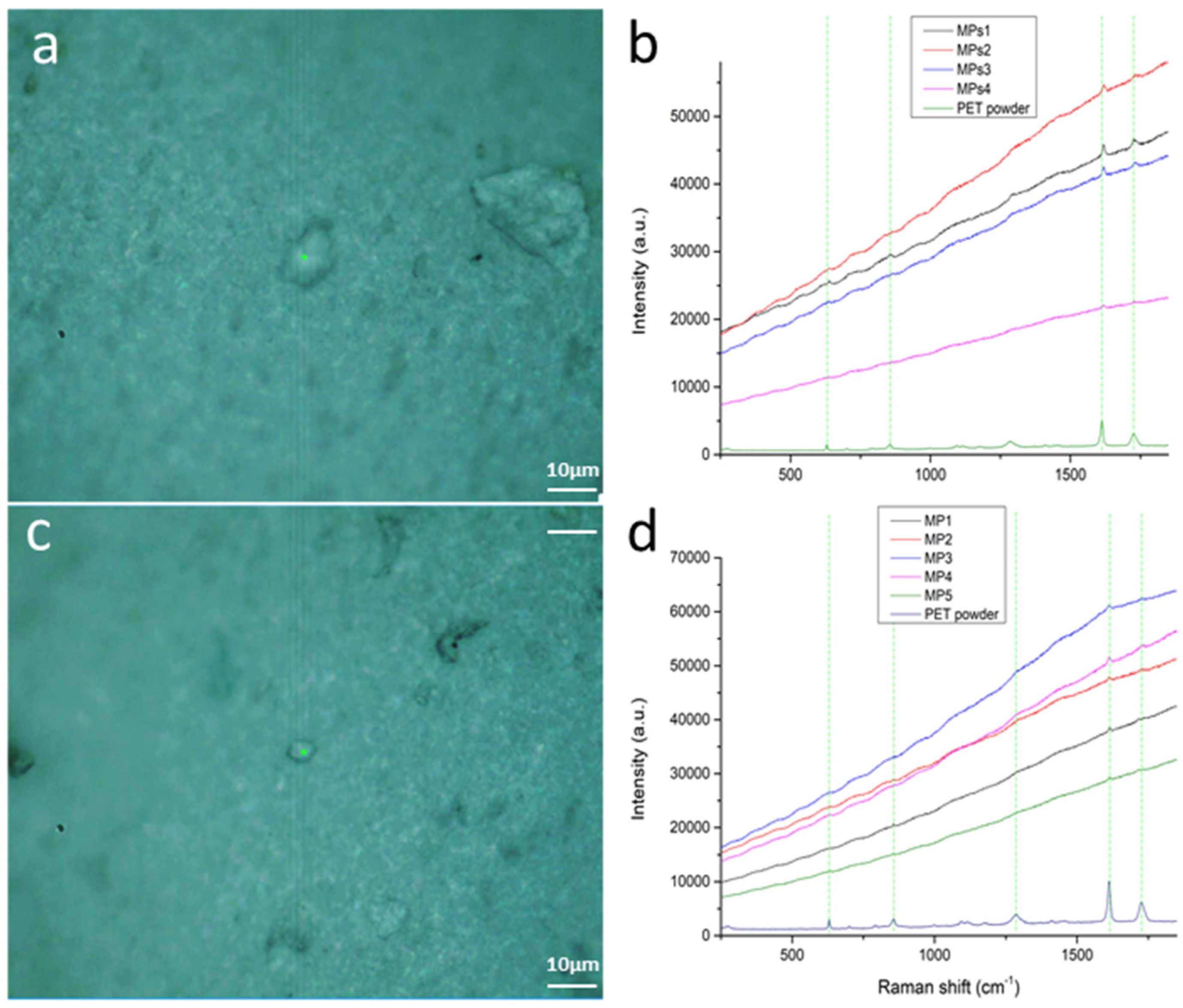
3.1. Method 1: PET MPs Detection in Honey Solution through Raman Mapping
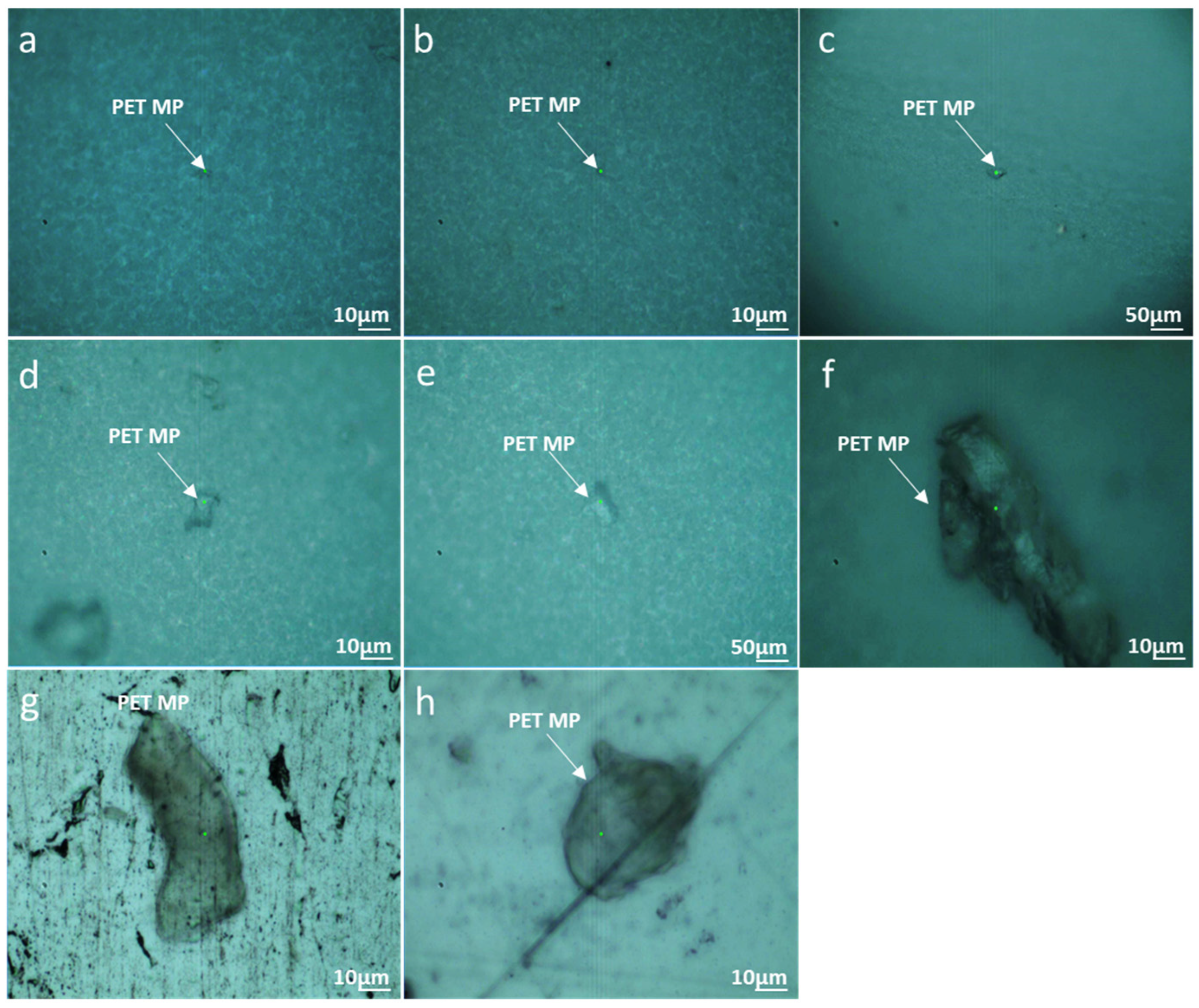
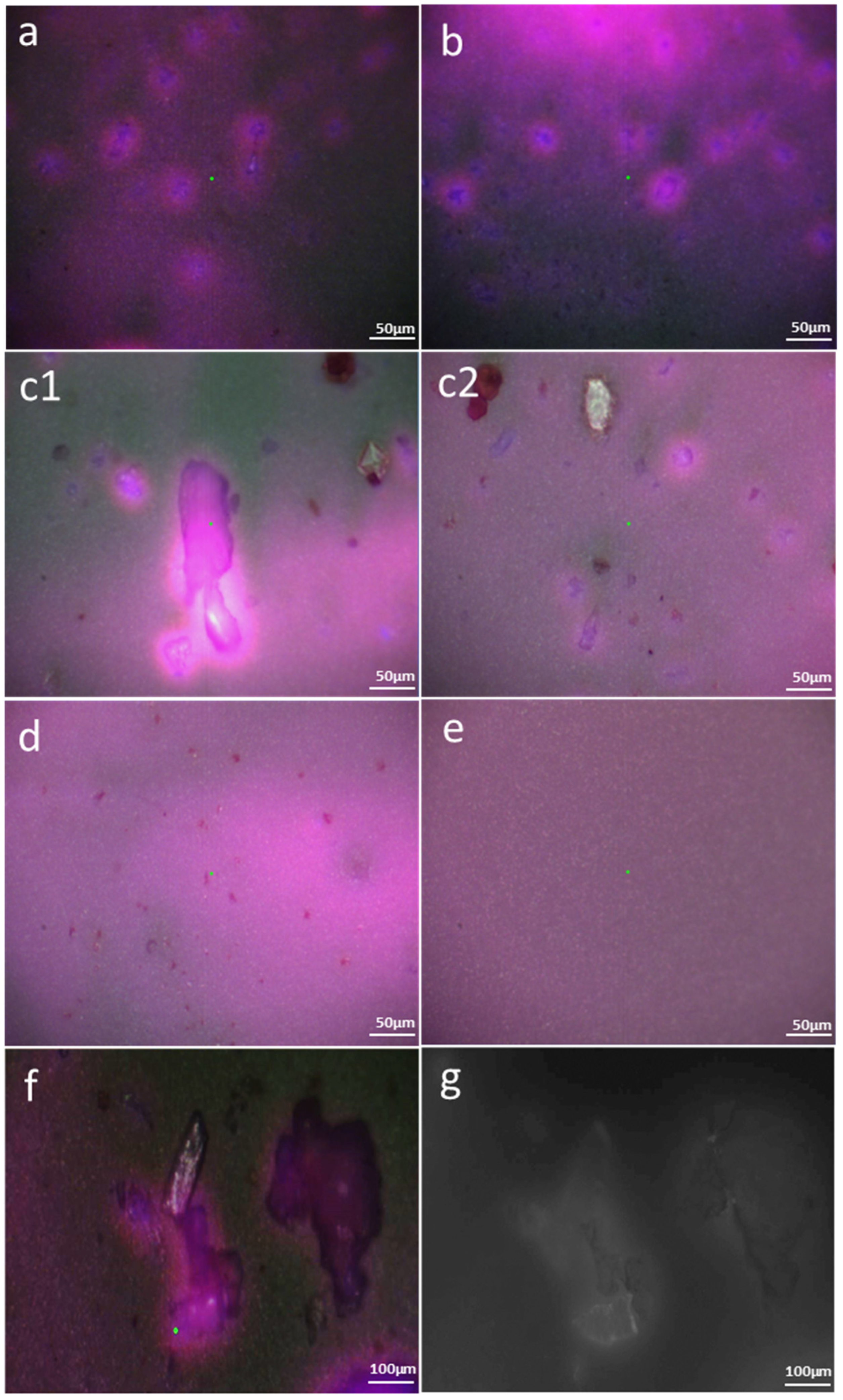
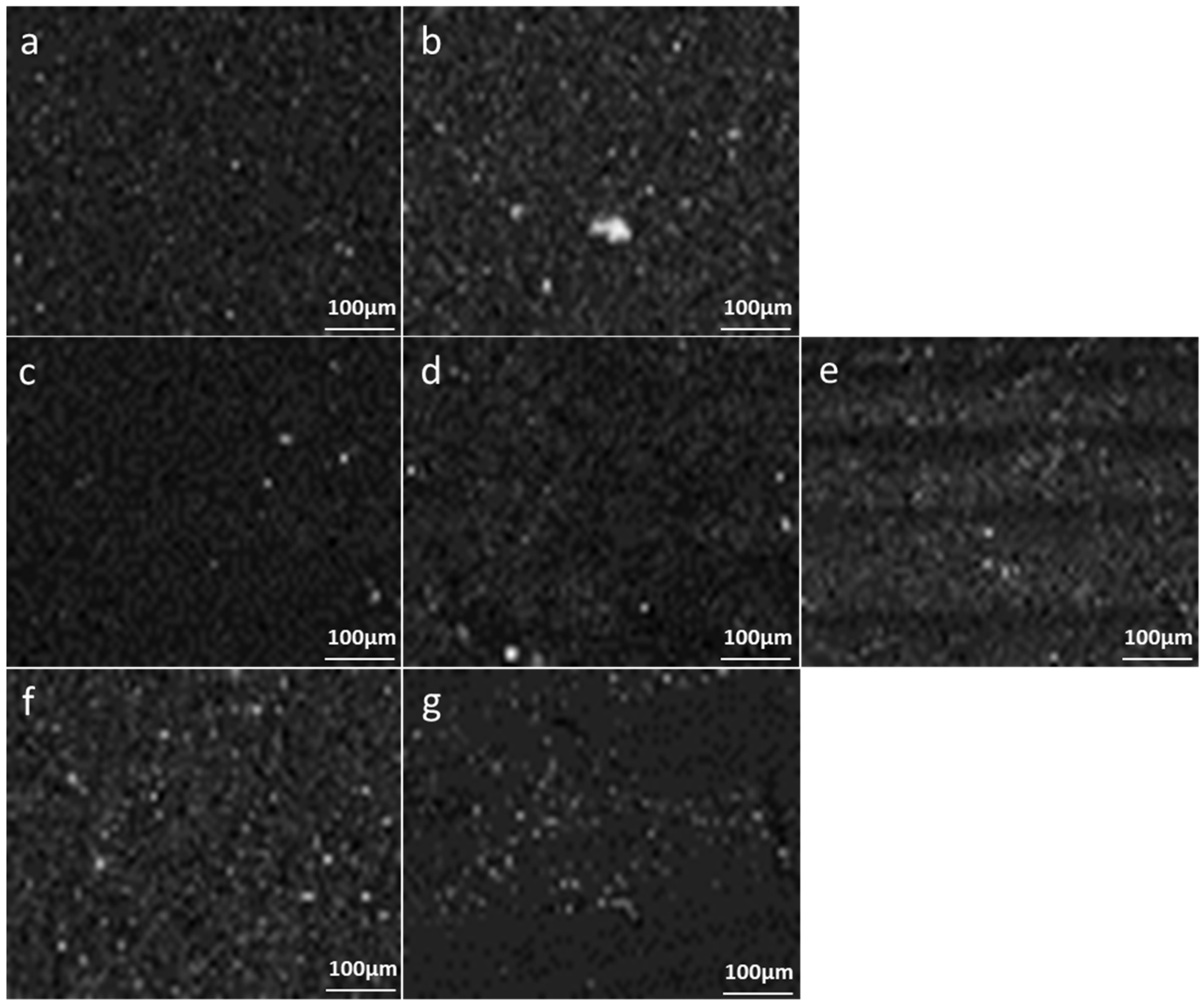
3.2. Method 2: PET MPs Detection in Honey through Optical Observation of Fluorescence
3.2.1. Optical Microscopy
3.2.2. Enhanced Optical and Fluorescence Microscopy with DANS Dye under UV Radiation and Multispectral Imaging
3.3. PET MP Detection in Deionized Water through Organic Solvents
3.4. Contamination Check
3.4.1. Reference Spectra and Assignments
3.4.2. Contamination Due to Materials Used
3.5. Methodological Experiment: Distributions of PET MPs into Different Organic Phases according to Their Dimensions
3.6. Analysis of Mass Spectrometry and Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Islam, M.K.; Lawag, I.L.; Green, K.J.; Sostaric, T.; Hammer, K.A.; Lim, L.Y.; Locher, C. An investigation of the suitability of melissopalynology to authenticate Jarrah honey. Curr. Res. Food Sci. 2022, 5, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Gkoutzouvelidou, M.; Panos, G.; Xanthou, M.N.; Papachristoforou, A.; Giaouris, E. Comparing the Antimicrobial Actions of Greek Honeys from the Island of Lemnos and Manuka Honey from New Zealand against Clinically Important Bacteria. Foods 2021, 10, 1402. [Google Scholar] [CrossRef] [PubMed]
- Balzani, P.; Galeotti, G.; Scheggi, S.; Masoni, A.; Santini, G.; Baracchi, D. Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honey bee health and cognition. Environ. Pollut. 2022, 305, 119318. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef]
- Edo, C.; Fernández-Alba, A.R.; Vejsnæs, F.; van der Steen, J.J.M.; Fernández-Piñas, F.; Rosal, R. Honeybees as active samplers for microplastics. Sci. Total Environ. 2021, 767, 144481. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.; La Spina, R.; Mehn, D.; Fumagalli, F.; Ceccone, G.; Valsesia, A.; Gilliland, D. Detecting Micro- and Nanoplastics Released from Food Packaging: Challenges and Analytical Strategies. Polymers 2022, 14, 1238. [Google Scholar] [CrossRef]
- Penalver, R.; Arroyo-Manzanares, N.; Campillo, N.; Vinas, P. Targeted and untargeted gas chromatography-mass spectrometry analysis of honey samples for determination of migrants from plastic packages. Food Chem. 2021, 334, 127547. [Google Scholar] [CrossRef]
- Diaz-Basantes, M.F.; Conesa, J.A.; Fullana, A. Microplastics in Honey, Beer, Milk and Refreshments in Ecuador as Emerging Contaminants. Sustainability 2020, 12, 5514. [Google Scholar] [CrossRef]
- Muhlschlegel, P.; Hauk, A.; Walter, U.; Sieber, R. Lack of evidence for microplastic contamination in honey. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Katsara, K.; Kenanakis, G.; Alissandrakis, E.; Papadakis, V.M. Honey Quality and Microplastic Migration from Food Packaging: A Potential Threat for Consumer Health? Microplastics 2022, 1, 406–427. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Ren, J. Analysis of Microplastics in Takeaway Food Containers in China Using FPA-FTIR Whole Filter Analysis. Molecules 2022, 27, 2646. [Google Scholar] [CrossRef] [PubMed]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Katsara, K.; Psatha, K.; Kenanakis, G.; Aivaliotis, M.; Papadakis, V.M. Subtyping on Live Lymphoma Cell Lines by Raman Spectroscopy. Materials 2022, 15, 546. [Google Scholar] [CrossRef]
- Roeffaers, M.B.; Zhang, X.; Freudiger, C.W.; Saar, B.G.; van Ruijven, M.; van Dalen, G.; Xiao, C.; Xie, X.S. Label-free imaging of biomolecules in food products using stimulated Raman microscopy. J. Biomed. Opt. 2011, 16, 021118. [Google Scholar] [CrossRef]
- Lotfi Choobbari, M.; Ferguson, J.; Van den Brande, N.; Smith, T.; Chalyan, T.; Meulebroeck, W.; Ottevaere, H. Studying the concentration of polymers in blended microplastics using 2D and 3D Raman mapping. Sci. Rep. 2023, 13, 7771. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta 2019, 1077, 191–199. [Google Scholar] [CrossRef]
- Leung, M.M.; Ho, Y.W.; Maboloc, E.A.; Lee, C.H.; Wang, Y.; Hu, M.; Cheung, S.G.; Fang, J.K. Determination of microplastics in the edible green-lipped mussel Perna viridis using an automated mapping technique of Raman microspectroscopy. J. Hazard. Mater. 2021, 420, 126541. [Google Scholar] [CrossRef]
- Park, H.M.; Park, S.; de Guzman, M.K.; Baek, J.Y.; Cirkovic Velickovic, T.; Van Messem, A.; De Neve, W. MP-Net: Deep learning-based segmentation for fluorescence microscopy images of microplastics isolated from clams. PLoS ONE 2022, 17, e0269449. [Google Scholar] [CrossRef]
- Zada, L.; Leslie, H.A.; Vethaak, A.D.; Tinnevelt, G.H.; Jansen, J.J.; de Boer, J.F.; Ariese, F. Fast microplastics identification with stimulated Raman scattering microscopy. J. Raman Spectrosc. 2018, 49, 1136–1144. [Google Scholar] [CrossRef]
- Sancataldo, G.; Ferrara, V.; Bonomo, F.P.; Chillura Martino, D.F.; Licciardi, M.; Pignataro, B.G.; Vetri, V. Identification of microplastics using 4-dimethylamino-4′-nitrostilbene solvatochromic fluorescence. Microsc. Res. Tech. 2021, 84, 2820–2831. [Google Scholar] [CrossRef]
- Peng, C.; Tang, X.; Gong, X.; Dai, Y.; Sun, H.; Wang, L. Development and Application of a Mass Spectrometry Method for Quantifying Nylon Microplastics in Environment. Anal. Chem. 2020, 92, 13930–13935. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Kelkar, V.; Kumar, R.; Halden, R.U. Methods and challenges in the detection of microplastics and nanoplastics: A mini-review. Polym. Int. 2021, 71, 543–551. [Google Scholar] [CrossRef]
- Sunta, U.; Trebse, P.; Kralj, M.B. Simply Applicable Method for Microplastics Determination in Environmental Samples. Molecules 2021, 26, 1840. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Llorca, M.; Seró, R.; Moyano, E.; Barceló, D.; Abad, E.; Farré, M. Trace analysis of polystyrene microplastics in natural waters. Chemosphere 2019, 236, 124321. [Google Scholar] [CrossRef]
- Tian, L.; Skoczynska, E.; van Putten, R.J.; Leslie, H.A.; Gruter, G.M. Quantification of polyethylene terephthalate micro- and nanoplastics in domestic wastewater using a simple three-step method. Sci. Total Environ. 2023, 857, 159209. [Google Scholar] [CrossRef]
- Prosenc, F.; Leban, P.; Šunta, U.; Bavcon Kralj, M. Extraction and Identification of a Wide Range of Microplastic Polymers in Soil and Compost. Polymers 2021, 13, 4069. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-J.; Lee, K.-T. Determination of monomers and oligomers in polyethylene terephthalate trays and bottles for food use by using high performance liquid chromatography-electrospray ionization-mass spectrometry. Polym. Test. 2012, 31, 490–499. [Google Scholar] [CrossRef]
- Wilm, M. Principles of electrospray ionization. Mol. Cell. Proteom. 2011, 10, M111.009407. [Google Scholar] [CrossRef]
- von Eyken, A.; Ramachandran, S.; Bayen, S. Suspected-target screening for the assessment of plastic-related chemicals in honey. Food Control 2020, 109, 106941. [Google Scholar] [CrossRef]
- Yan, X.T.; Zhang, Y.; Zhou, Y.; Li, G.H.; Feng, X.S. Technical Overview of Orbitrap High Resolution Mass Spectrometry and Its Application to the Detection of Small Molecules in Food (Update Since 2012). Crit. Rev. Anal. Chem. 2022, 52, 593–626. [Google Scholar] [CrossRef]
- Li, F.; Song, N.; Li, X.; Jirigalantu; Mi, X.; Sun, C.; Sun, Y.; Feng, S.; Wang, G.; Qiu, J.; et al. Detection of microplastics via a confocal-microscope spatial-heterodyne Raman spectrometer with echelle gratings. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 313, 124099. [Google Scholar] [CrossRef]
- Schymanski, D.; Ossmann, B.E.; Benismail, N.; Boukerma, K.; Dallmann, G.; von der Esch, E.; Fischer, D.; Fischer, F.; Gilliland, D.; Glas, K.; et al. Analysis of microplastics in drinking water and other clean water samples with micro-Raman and micro-infrared spectroscopy: Minimum requirements and best practice guidelines. Anal. Bioanal. Chem. 2021, 413, 5969–5994. [Google Scholar] [CrossRef]
- Ossmann, B.E.; Sarau, G.; Schmitt, S.W.; Holtmannspotter, H.; Christiansen, S.H.; Dicke, W. Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4099–4109. [Google Scholar] [CrossRef]
- Murray, A.; Örmeci, B. Removal Effectiveness of Nanoplastics (<400 nm) with Separation Processes Used for Water and Wastewater Treatment. Water 2020, 12, 635. [Google Scholar] [CrossRef]
- Kniggendorf, A.-K.; Wetzel, C.; Roth, B. Microplastics Detection in Streaming Tap Water with Raman Spectroscopy. Sensors 2019, 19, 1839. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Alam, I.; Chowdhury, I. Aggregation and stability of nanoscale plastics in aquatic environment. Water Res. 2020, 171, 115401. [Google Scholar] [CrossRef] [PubMed]
- Bistričić, L.; Borjanović, V.; Leskovac, M.; Mikac, L.; McGuire, G.E.; Shenderova, O.; Nunn, N. Raman spectra, thermal and mechanical properties of poly(ethylene terephthalate) carbon-based nanocomposite films. J. Polym. Res. 2015, 22, 39. [Google Scholar] [CrossRef]
- Huzortey, A.A.; Arefi, A.; Anderson, B.; Khadem, H.; Sackey, S.S.; Mahmoodi-Khaledi, E.; Tavassoli, S.H. 532-nm Laser-Excited Raman Spectroscopic Evaluation of Iranian Honey. Food Anal. Methods 2021, 15, 772–782. [Google Scholar] [CrossRef]
- Perret, E.; Braun, O.; Sharma, K.; Tritsch, S.; Muff, R.; Hufenus, R. High-resolution 2D Raman mapping of mono- and bicomponent filament cross-sections. Polymer 2021, 229, 124011. [Google Scholar] [CrossRef]
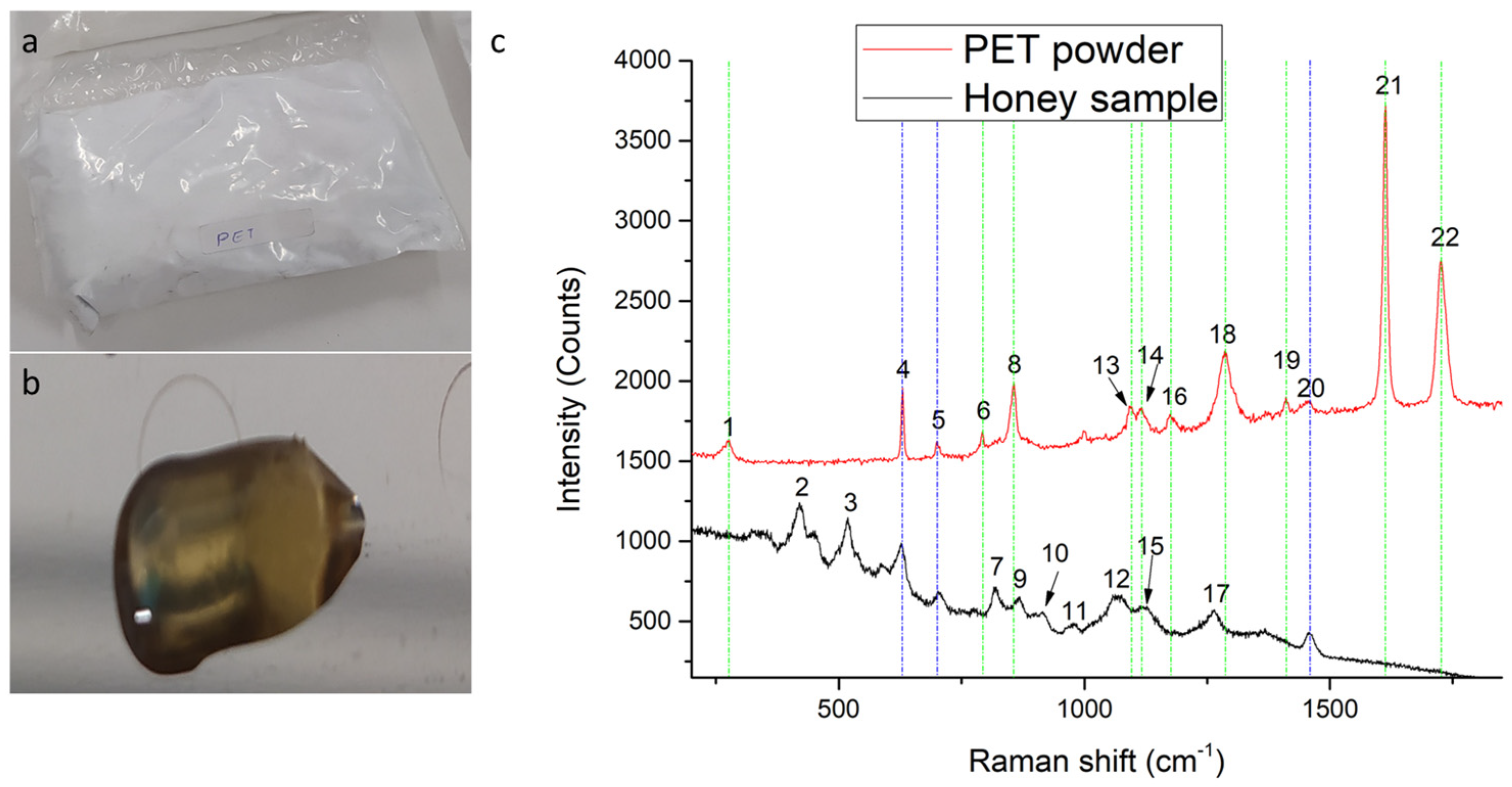
| Peak Number | Raman Shift (cm−1) | Assignments | Peak Strength | ||
|---|---|---|---|---|---|
| Honey | PET | Honey | PET | ||
| 1 | - | 277 | - | C-C stretching (ring), CCC bending (ring) [41] | Weak |
| 2 | 420 | - | C–C-O and C–C-C bending of major Fructose or minor Glucose [42] | - | Strong |
| 3 | 518 | - | C–C-O and C–C-C deformation of minor Fructose or major Glucose [42] | - | Strong |
| 4 | 628 | 630 | Ring deformation of major Fructose or minor Sucrose [42] | CCC in-plane bending (ring) [41] | Common (strong) |
| 5 | 706 | 700 | C-O and C–C-O stretching, O-C-O bending of Fructose [42] | - | Common (weak) |
| 6 | - | 792 | - | CH out of plane bending (ring) [41] | Weak |
| 7 | 820 | - | C–OH bending of Fructose [42] | - | Medium |
| 8 | - | 857 | - | C–C stretching (ring breathing), C–O stretching [41] | Strong |
| 9 | 869 | - | C–O–C Cyclic alkyl ethers of major Fructose or minor Glucose [42] | - | Medium weak |
| 10 | 916 | - | CH, COH bending of major Glucose or minor Maltose [42] | - | Weak |
| 11 | 980 | - | Ring breathing of Fructose [42] | - | Weak |
| 12 | 1075 | - | C–O–C stretching, C-N vibration of proteins in major Fructose or minor Glucose [42] | - | Medium |
| 13 | - | 1095 | - | Ring C–C, ester C(O)–O, ethylene glycol C–C (trans) [43] | Weak |
| 14 | - | 1115 | - | CH in-plane bending (ring), C–O stretching [41] | Weak |
| 15 | 1127 | - | C–OH deformation of major Glucose or minor Maltose [42] | Medium | |
| 16 | - | 1174 | - | CH in-plane bending (ring) [41] | Weak |
| 17 | 1264 | - | C–O–C Cyclic alkyl ethers of Fructose [42] | Medium | |
| 18 | - | 1285 | - | C–C stretching (ring), C–O stretching [41] | Strong |
| 19 | - | 1411 | - | C–C stretching (ring) [41] | Weak |
| 20 | 1457 | 1455 | CH2 bending of Fructose or minor Glucose [42] | CH deformation [41] | Common (weak) |
| 21 | - | 1613 | - | C=C stretching (ring) [41] | Very Strong |
| 22 | - | 1726 | - | C=O stretching [41] | Strong |
| First Extraction Phase | Second Extraction Phase | ||||||
|---|---|---|---|---|---|---|---|
| Optical Microscopy/Raman Mapping | ImageJ | Optical Microscopy/Raman Mapping | ImageJ | MP Dimensions and Quantity Notes | |||
| Phase | MP Dimension | MP Count | Average Dimension (μm2) | MP Dimension | MP Count | Average Dimension (μm2) | |
| D_Y (1,2) | 1–20 μm | 54 | 12 | 1–20 μm | 41 | 19 | Many small and medium MPs |
| D_YC (1,2) | - | - | - | 18–50 μm A few 2–3 μm | 27 | 28 | Medium MPs |
| D_C (1,2) | 1–30 μm | 111 | 30 | Many 18–25 μm 100 μm clusters Some 10 μm | 74 | 25 | First extraction → Many small and medium MPs Second extraction → Many medium and large MPs |
| Sediment of colloidal phase | No MPs < 8–74 μm | No red map | No red map | 75 μm and >100 μm clusters Some 5 μm | No red map | No red map | First extraction → Large MPs Second extraction → Huge clusters > CA filter pore size |
| D_Final | 3, 5, 10, 20 μm 55–75 μm | 121 | 29 | The most MPs found. MPs < 1–2 μm not found | |||
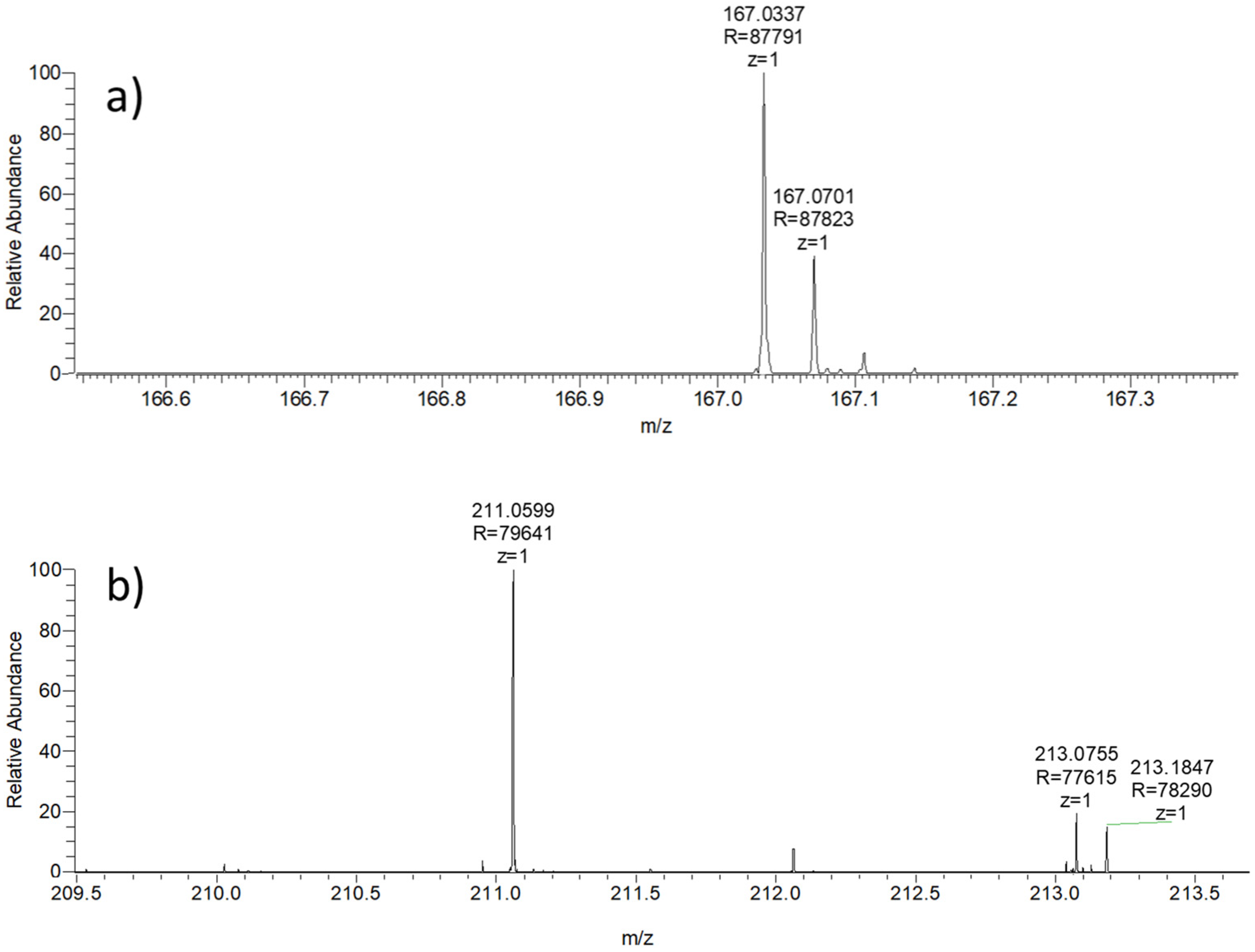
| PET Fragments in MPs_H2O | ||
|---|---|---|
| 167.0337 MH+ (±10 ppm) | 211.0599 MH+ (±10 ppm) | |
| Samples | PET Fragments in Samples | |
| Deionized water (H2O) | 167.0338, z = 1 | - |
| (ACN)-0.5% formic acid | 167.0337, z = 1 | - |
| (ACN)-0.5% formic acid–pentane | 167.0339, z = 1 | - |
| (ACN)-0.5% formic acid–(H2O) | 167.0337, z = 1 | - |
| C_C1 | - | - |
| C_C2 | - | - |
| C_Final | - | - |
| D_C1 | 167.0341, z = 1 | 211.0605, z = 1 |
| D_C2 | 167.0342, z = 1 | 211.0605, z = 1 |
| D_Final | 167.0339, z = 1 | 211.0607, z = 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsara, K.; Viskadourakis, Z.; Alissandrakis, E.; Kountourakis, N.; Kenanakis, G.; Papadakis, V.M. Microplastics’ Detection in Honey: Development of Protocols in a Simulation. Appl. Sci. 2024, 14, 4720. https://doi.org/10.3390/app14114720
Katsara K, Viskadourakis Z, Alissandrakis E, Kountourakis N, Kenanakis G, Papadakis VM. Microplastics’ Detection in Honey: Development of Protocols in a Simulation. Applied Sciences. 2024; 14(11):4720. https://doi.org/10.3390/app14114720
Chicago/Turabian StyleKatsara, Klytaimnistra, Zacharias Viskadourakis, Eleftherios Alissandrakis, Nikos Kountourakis, George Kenanakis, and Vassilis M. Papadakis. 2024. "Microplastics’ Detection in Honey: Development of Protocols in a Simulation" Applied Sciences 14, no. 11: 4720. https://doi.org/10.3390/app14114720






