Evaluation of the Suitability of Selecting a Faecal Microbiota Transplant: Bacterial Composition and Subsequent Long-Term Monitoring of the Viability of Its Frozen and Lyophilised Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Faecal Microbiota Transplant Donor
2.2. Processing of Faecal Microbiota Transplant
2.3. Analysis of FMT Bacterial Composition Using Next-Generation Sequencing (NGS)
2.3.1. Sequencing Data Processing
2.3.2. Taxonomic Annotation
2.4. Determination of Microbial Viability in the Faecal Microbiota Transplant
2.5. Statistical Analysis
3. Results
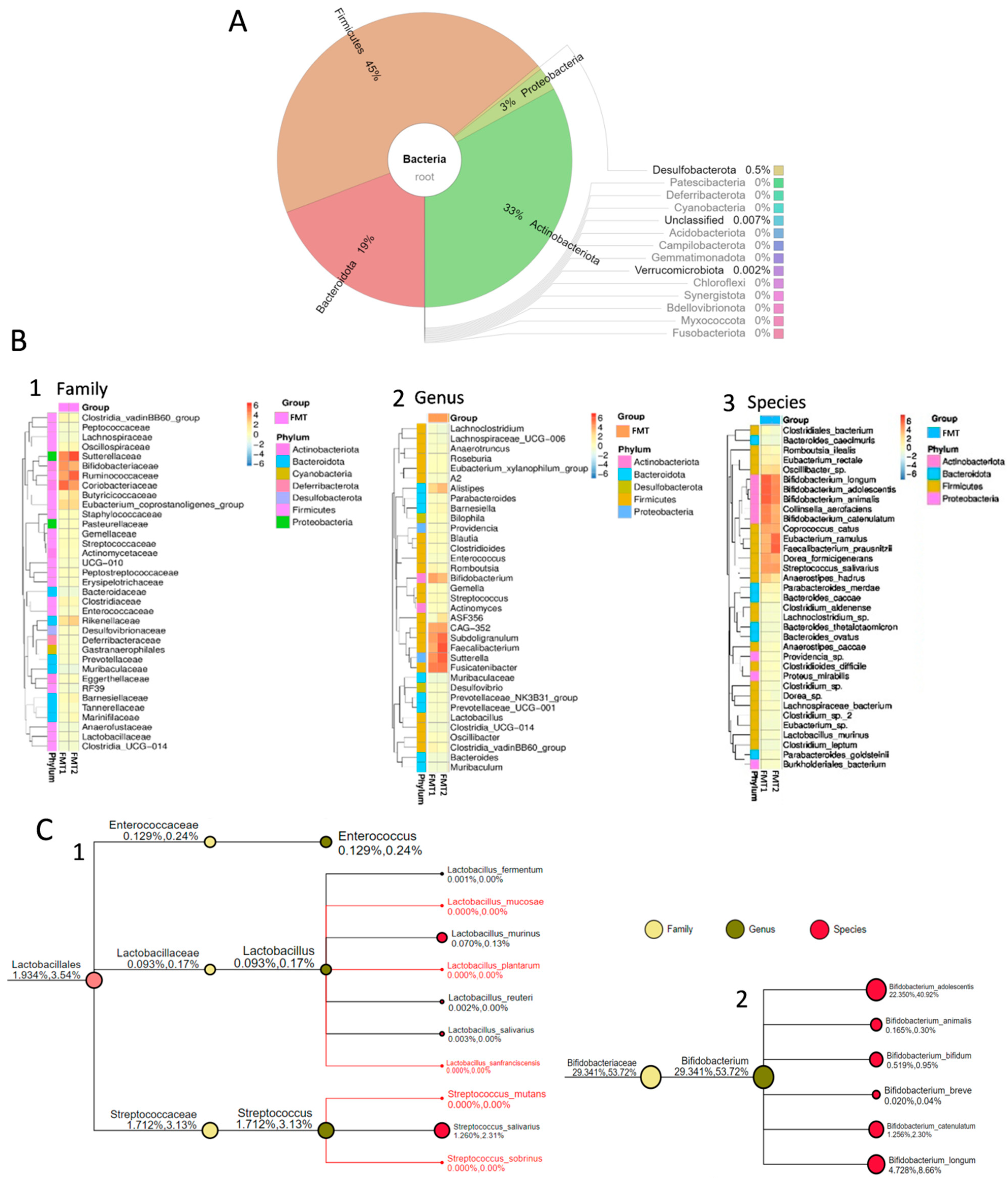
3.1. Bacterial Composition of Faecal Microbiota Transplant from the Healthy Donor
3.2. Monitoring the Metabolic Activity (Viability) Dynamics of Frozen and Lyophilised Forms of FMT across Prolonged Storage Periods
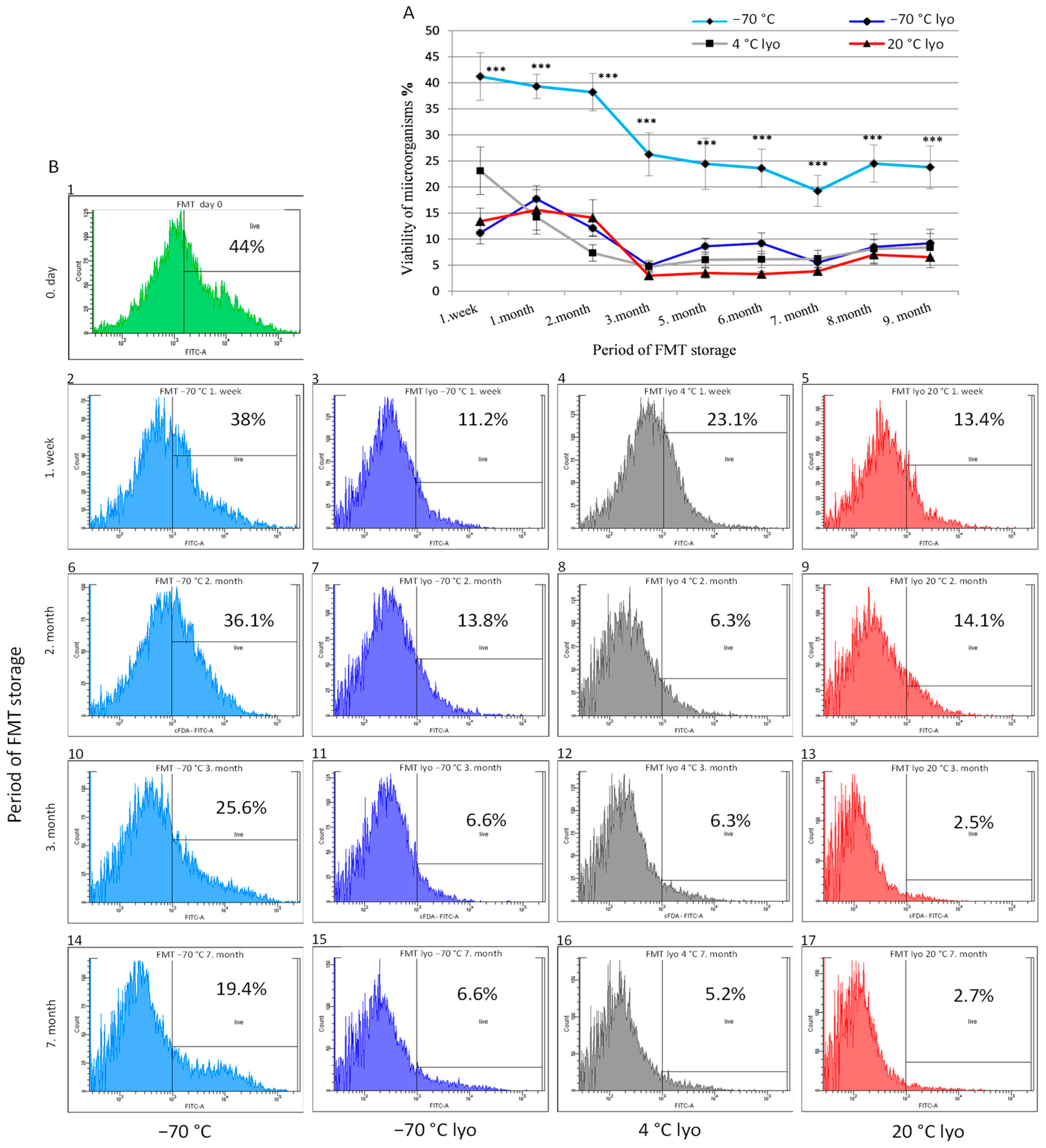
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allegretti, J.R.; Mullish, B.H.; Kelly, C.; Fischer, M. The evolution of the use of faecal microbiota transplantation and emerging therapeutic indications. Lancet 2019, 394, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Saha, S.; Khanna, S. Therapies to modulate gut microbiota: Past, present and future. World J. Gastroenterol. 2020, 26, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Blanchaert, C.; Strubbe, B.; Peeters, H. Fecal microbiota transplantation in ulcerative colitis. Acta Gastro-Enterol. Belg. 2019, 82, 519–528. [Google Scholar]
- Rossen, N.; Fuentes, S.; Van Der Spek, M.J.; Tijssen, J.G.P.; Hartman, J.; Duflou, A.; Löwenberg, M.; Van Den Brink, G.R.; Mathus-Vliegen, E.M.H.; De Vos, W.M.; et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D.; Feng, B.; Chen, D.; Ren, J.; Deng, M.; et al. Microbiota transplantation: Concept, methodology and strategy for its modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ding, C.; Gong, J.; Ge, X.; McFarland, L.V.; Gu, L.; Yao, W.; Chen, Q.; Zhu, W.; Li, J.; et al. Treatment of slow transit constipation with fecal microbiota transplantation. J. Clin. Gastroenterol. 2016, 50, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.M.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft- versus -host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, X.; Luo, X.; Shi, Y.; Peng, L.; Wang, C.; Zou, L.; Yang, Y. Mo1667 Fecal microbiota transplantation for children with autism spectrum disorder. Gastrointest. Endosc. 2019, 89, AB512–AB513. [Google Scholar] [CrossRef]
- Lü, C.; Rong, J.; Fu, C.; Wang, W.; Xu, J.; Ju, X. Overall rebalancing of gut microbiota is key to autism intervention. Front. Psychol. 2022, 13, 862719. [Google Scholar] [CrossRef]
- He, Z.; Cui, B.; Zhang, T.; Li, P.; Long, C.; Ji, G.; Zhang, F. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s disease: The first report. World J. Gastroenterol. 2017, 23, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.I.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.; Sérino, M.; Planas-Fèlix, M.; et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Borody, T.J.; Campbell, J. Fecal microbiota transplantation: Current status and future directions. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 653–655. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Coy, K.D.M.; Frisoni, G.B.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Lauko, S.; Gancarčíková, S.; Hrčková, G.; Hajdučková, V.; Andrejčáková, Z.; Fecskeová, L.K.; Bertková, I.; Hijová, E.; Kamlárová, A.; Janíčko, M.; et al. Beneficial effect of faecal microbiota transplantation on mild, moderate and severe dextran sodium sulphate-induced ulcerative colitis in a pseudo germ-free animal model. Biomedicines 2023, 12, 43. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, O.I.; Sunbare-Funto, O.J.; Mbah, C.E.; Ajibade, O.A.; Oyawoye, O.M.; Aborode, A.T.; Ogunleye, S.C.; Jamiu, A.T.; Bolarinwa, B.; Abanikannda, M.F.; et al. Faecal microbial transplant. Adv. Biomark. Sci. Technol. 2024, 6, 20–34. [Google Scholar]
- Rosen, C.E.; Palm, N.W. Navigating the microbiota seas: Triangulation finds a way forward. Cell Host Microbe 2018, 23, 1–3. [Google Scholar] [CrossRef]
- Nishida, A.; Inoüe, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, M.; Guo, J.; Wang, J. Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin. Med. J. 2019, 132, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii produces butyrate to maintain th17/treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; De Sousa Faria, A.V.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Ng, S.C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.Y.; Inohara, N.; Núñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Leong, R.W.; Paramsothy, S. The role of faecal microbiota transplantation in the treatment of inflammatory bowel disease. Curr. Opin. Pharmacol. 2020, 55, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Jie, Z.; Cui, B.; Wang, H.; Feng, Q.; Zou, Y.; Zhang, X.; Yang, H.; Wang, J.; Zhang, F.; et al. Fecal microbiota transplantation results in bacterial strain displacement in patients with inflammatory bowel diseases. FEBS Open Bio 2019, 10, 41–55. [Google Scholar] [CrossRef]
- Kump, P.; Wurm, P.; Gröchenig, H.; Wenzl, H.; Petritsch, W.; Halwachs, B.; Wagner, M.A.; Stadlbauer, V.; Eherer, A.; Hoffmann, K.M.; et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 47, 67–77. [Google Scholar] [CrossRef]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal microbiota transplantation: Screening and selection to choose the optimal donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.; Van Den Bogaerde, J.; Samuel, D.B.; et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, S.; Qin, H.; Li, N.; Chen, Q. Long-term safety of faecal microbiota transplantation for gastrointestinal diseases in China. Lancet Gastroenterol. Hepatol. 2022, 7, 702–703. [Google Scholar] [CrossRef] [PubMed]
- Haindl, R.; Totzauer, L.; Kulozik, U. Preservation by lyophilization of a human intestinal microbiota: Influence of the cultivation pH on the drying outcome and re-establishment ability. Microb. Biotechnol. 2022, 15, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, X.; Fang, Z.; Li, L.; Wu, C.; Bi, D.; Li, N.; Chen, Q.; Qin, H. Fecal microbiota transplantation in clinical practice: Present controversies and future prospects. hLife 2024, in press. [Google Scholar] [CrossRef]
- Fouhy, F.; Deane, J.; Rea, M.C.; O’Sullivan, Ó.; Ross, R.P.; O’Callaghan, G.; Plant, B.J.; Stanton, C. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS ONE 2015, 10, e0119355. [Google Scholar] [CrossRef] [PubMed]
- Burz, S.D.; Abraham, A.L.; Fonseca, F.; David, O.; Chapron, A.; Béguet-Crespel, F.; Cénard, S.; Le Roux, K.; Patrascu, O.; Levenez, F.; et al. A guide for ex vivo handling and storage of stool samples intended for fecal microbiota transplantation. Sci. Rep. 2019, 9, 8897. [Google Scholar] [CrossRef]
- Reygner, J.; Charrueau, C.; Delannoy, J.; Mayeur, C.; Robert, V.; Cuinat, C.; Meylheuc, T.; Mauras, A.; Augustin, J.; Nicolis, I.; et al. Freeze-dried fecal samples are biologically active after long-lasting storage and suited to fecal microbiota transplantation in a preclinical murine model of Clostridioides difficile infection. Gut Microbes 2020, 11, 1405–1422. [Google Scholar] [CrossRef] [PubMed]
- Papanicolas, L.E.; Wang, Y.; Choo, J.M.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Optimisation of a propidium monoazide based method to determine the viability of microbes in faecal slurries for transplantation. J. Microbiol. Methods 2019, 156, 40–45. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.X.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial viability in faecal transplants: Which bacteria survive. eBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Alexander, A.; Ke, S.; Valilis, E.M.; Hu, S.; Li, B.; DuPont, H.L. Stability and efficacy of frozen and lyophilized fecal microbiota transplant (FMT) product in a mouse model of Clostridium difficile infection (CDI). Anaerobe 2017, 48, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.D.; Smith, M.B.; Perrotta, A.R.; Kassam, Z.; Alm, E.J. Profiling living bacteria informs preparation of fecal microbiota transplantations. PLoS ONE 2017, 12, e0170922. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: A randomized clinical trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.J.; Weingarden, A.R.; Sadowsky, M.J.; Khoruts, A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 2012, 107, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Frozen faecal matter works as well as fresh for transplantation in C. difficile patients. BMJ 2016, 352, i138. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yin, W.; Liu, W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory Clostridium difficile infection: A meta-analysis? Diagn. Microbiol. Infect. Dis. 2017, 88, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fu, L.; Wang, J. Protocol for fecal microbiota transplantation in inflammatory bowel disease: A systematic review and meta-analysis. Biomed. Res. Int. 2018, 2018, 8941340. [Google Scholar] [CrossRef] [PubMed]
- Hirotaka, S.; Katsuhiro, A.; Ichiro, T.; Takuya, T.; Takashi, A.; Hirokazu, T.; Satoshi, M.; Yuichiro, Y. Anaerobic stool preparation method for fecal microbiota transplantation is not superior to conventional aerobic method in preserving anaerobic bacteria: P-125. Off. J. Am. College Gastroenterol. 2018, 113, 29. [Google Scholar] [CrossRef]
- Mendolia, G.; Kassam, Z.; McClure, E.L.; Bi, H.S. Mo1954 Anaerobic fecal microbiota transplantation preparations are not necessary for treatment successful engraftment microbial in recurrent C. difficile infection. Gastroenterology 2020, 158, 991–992. [Google Scholar] [CrossRef]
- Brunse, A.; Deng, L.; Pan, X.; Hui, Y.; Castro-Mejía, J.L.; Kot, W.; Nguyen, D.N.; Secher, J.B.; Nielsen, D.S.; Thymann, T. Fecal filtrate transplantation protects against necrotizing enterocolitis. ISME J. 2021, 16, 686–694. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.L.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of sterile fecal filtrate transfer for treating patients with Clostridium difficile infection. Gastroenterology 2017, 152, 799–811.e797. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, T.; Desmons, A.; Krasniqi, P.; Lacorte, J.-M.; Kapel, N.; Lamazière, A.; Fourati, S.; Eguether, T. Effect of stool sampling on a routine clinical method for the quantification of six short chain fatty acids in stool using gas chromatography–mass spectrometry. Microorganisms 2024, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, J.; Oda, M.; Hirayama, M.; Sugitate, K.; Sakui, N.; Hamada, R.; Ito, M.; Saito, I.; Ohno, K. Freeze-drying enables homogeneous and stable sample preparation for determination of fecal short-chain fatty acids. Anal. Biochem. 2020, 589, 113508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Qiu, Y.; Zhong, W.; Baxter, S.; Su, M.; Li, Q.; Xie, G.; Ore, B.M.; Qiao, S.; Spencer, M.D.; et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 2013, 9, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Ajami, N.J.; Petrosino, J.F.; Jun, G.; Hanis, C.L.; Shah, M.; Hochman, L.; Ankoma-Sey, V.; DuPont, A.W.; Wong, M.C.; et al. Randomised clinical trial: Faecal microbiota transplantation for recurrent clostridum difficile infection—Fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment. Pharmacol. Ther. 2017, 45, 899–908. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Jenq, R.R.; Ajami, N.J.; Petrosino, J.F.; Alexander, A.A.; Ke, S.; Iqbal, T.; DuPont, A.W.; Muldrew, K.; Shi, Y.; et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: A randomized clinical trial. PLoS ONE 2018, 13, e0205064. [Google Scholar] [CrossRef]
| Anamnesis |
|
| Complex laboratory examination | |
| Bacterial examination |
|
| Virological examination |
|
| Serological examination |
|
| Parasitic examination |
|
| Immunological examination |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacuta, I.; Gancarcikova, S.; Lauko, S.; Hajduckova, V.; Janicko, M.; Demeckova, V.; Rynikova, M.; Adamkova, P.; Mudronova, D.; Ambro, L.; et al. Evaluation of the Suitability of Selecting a Faecal Microbiota Transplant: Bacterial Composition and Subsequent Long-Term Monitoring of the Viability of Its Frozen and Lyophilised Forms. Appl. Sci. 2024, 14, 4856. https://doi.org/10.3390/app14114856
Pacuta I, Gancarcikova S, Lauko S, Hajduckova V, Janicko M, Demeckova V, Rynikova M, Adamkova P, Mudronova D, Ambro L, et al. Evaluation of the Suitability of Selecting a Faecal Microbiota Transplant: Bacterial Composition and Subsequent Long-Term Monitoring of the Viability of Its Frozen and Lyophilised Forms. Applied Sciences. 2024; 14(11):4856. https://doi.org/10.3390/app14114856
Chicago/Turabian StylePacuta, Ivan, Sona Gancarcikova, Stanislav Lauko, Vanda Hajduckova, Martin Janicko, Vlasta Demeckova, Maria Rynikova, Petra Adamkova, Dagmar Mudronova, Lubos Ambro, and et al. 2024. "Evaluation of the Suitability of Selecting a Faecal Microbiota Transplant: Bacterial Composition and Subsequent Long-Term Monitoring of the Viability of Its Frozen and Lyophilised Forms" Applied Sciences 14, no. 11: 4856. https://doi.org/10.3390/app14114856
APA StylePacuta, I., Gancarcikova, S., Lauko, S., Hajduckova, V., Janicko, M., Demeckova, V., Rynikova, M., Adamkova, P., Mudronova, D., Ambro, L., Fialkovicova, M., Nemetova, D., & Bertkova, I. (2024). Evaluation of the Suitability of Selecting a Faecal Microbiota Transplant: Bacterial Composition and Subsequent Long-Term Monitoring of the Viability of Its Frozen and Lyophilised Forms. Applied Sciences, 14(11), 4856. https://doi.org/10.3390/app14114856








