Acute Effects of Caffeine and Taurine on Linear and Nonlinear Measures of the Cardiovascular System in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Denoising
2.4. Data Acquisition
2.5. Nonlinear Analysis
Phase Space Reconstruction
2.6. Linear Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.E. Energy drinks, race, and problem behaviors among college students. J. Adolesc. Health 2008, 43, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Rehman, H.; Babayan, Z.; Stapleton, D.; Joshi, D.D. Energy drinks and their adverse health effects: A systematic review of the current evidence. Postgrad. Med. 2015, 127, 308–322. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 85, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Lindsey, T. Fatal caffeine overdose: Two case reports. Forensic Sci. Int. 2005, 153, 67–69. [Google Scholar] [CrossRef]
- Bichler, A.; Swenson, A.; Harris, M.A. A combination of caffeine and taurine has no effect on short term memory but induces changes in heart rate and mean arterial blood pressure. Amino Acids 2006, 31, 471–476. [Google Scholar] [CrossRef]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Schaffer, S.W.; Shimada, K.; Jong, C.J.; Ito, T.; Azuma, J.; Takahashi, K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids 2014, 46, 1147–1157. [Google Scholar] [CrossRef]
- Ellermann, C.; Hakenes, T.; Wolfes, J.; Wegner, F.K.; Willy, K.; Leitz, P.; Rath, P.; Eckardt, L.; Frommeyer, G. Cardiovascular risk of energy drinks: Caffeine and taurine facilitate ventricular arrhythmias in a sensitive whole-heart model. J. Cardiovasc. Electrophysiol. 2022, 33, 1290–1297. [Google Scholar] [CrossRef]
- Caliskan, S.G.; Kilic, M.A.; Bilgin, M.D. Acute effects of energy drink on hemodynamic and electrophysiologic parameters in habitual and non-habitual caffeine consumers. Clin. Nutr. ESPEN 2021, 42, 333–338. [Google Scholar] [CrossRef]
- Doerner, J.M.; Kuetting, D.L.; Luetkens, J.A.; Naehle, C.P.; Dabir, D.; Homsi, R.; Nadal, J.; Schild, H.H.; Thomas, D.K. Caffeine and taurine containing energy drink increases left ventricular contractility in healthy volunteers. Int. J. Cardiovasc. Imaging 2015, 31, 595–601. [Google Scholar] [CrossRef]
- Jeffries, O.; Hill, J.; Patterson, S.D.; Waldron, M. Energy drink doses of caffeine and taurine have a null or negative effect on sprint performance. J. Strength Cond. Res. 2020, 34, 3475–3481. [Google Scholar] [CrossRef]
- Benjamim, C.J.R.; Kliszczewicz, B.; Garner, D.M.; Cavalcante, T.C.F.; da Silva, A.A.M.; Santana, M.D.R.; Valenti, V.E. Is caffeine recommended before exercise? A systematic review to investigate its impact on cardiac autonomic control via heart rate and its variability. J. Am. Coll. Nutr. 2020, 39, 563–573. [Google Scholar] [CrossRef]
- Porto, A.A.; Benjamim, C.J.R.; Gonzaga, L.A.; Luciano de Almeida, M.; Bueno Júnior, C.R.; Garner, D.M.; Valenti, V.E. Caffeine intake and its influences on heart rate variability recovery in healthy active adults after exercise: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1071–1082. [Google Scholar] [CrossRef]
- Porto, A.A.; Benjamim, C.J.R.; Gonzaga, L.A.; Garner, D.M.; Valenti, V.E. Acute effects of energy drink on heart rate variability recovery after exercise: A systematic review and meta-analysis. Sci. Sports 2022, 8067, 109–216. [Google Scholar] [CrossRef]
- Baum, M.; Weiβ, M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids 2001, 20, 75–82. [Google Scholar] [CrossRef]
- Hasty, F.; García, G.; Dávila, C.H.; Wittels, S.H.; Hendricks, S.; Chong, S. Heart rate variability as a possible predictive marker for acute inflammatory response in COVID-19 patients. Mil. Med. 2020, 186, e34–e38. [Google Scholar] [CrossRef]
- Germán-Salló, Z.; Germán-Salló, M. Non-linear methods in HRV analysis. Proc. Technol. 2016, 22, 645–651. [Google Scholar] [CrossRef]
- Ernst, G.; Watne, L.O.; Rostrup, M.; Neerland, B.E. Delirium in patients with hip fracture is associated with increased heart rate variability. Aging Clin. Exp. Res. 2020, 32, 2311–2318. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Nejadgohli, I.; Moradi, M.H.; Abdolali, F. Using phase space reconstruction for patient independent heartbeat classification in comparison with some benchmark methods. Comput. Biol. Med. 2011, 41, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Das, S.; Cappielo, G.; Mazomenos, E.B.; Maharatna, K.; Puddu, P.E.; Morgan, J.M. Prompt and accurate diagnosis of ventricular arrhythmias with a novel index based on phase space reconstruction of ECG. Int. J. Cardiol. 2015, 182, 38–43. [Google Scholar] [CrossRef]
- Wiklund, U.; Karlsson, M.; Oström, M.; Messner, T. Influence of energy drinks and alcohol on post-exercise heart rate recovery and heart rate variability. Clin. Physiol. Funct. Imaging 2009, 29, 74–80. [Google Scholar] [CrossRef]
- An, S.M.; Park, J.S.; Kim, S.H. Effect of energy drink dose on exercise capacity, heart recovery and heart rate variability after high-intensity exercise. J. Exerc. Nutr. Biochem. 2014, 18, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, S.G.; Bilgin, M.D. Non-linear analysis of heart rate variability for evaluating the acute effects of caffeinated beverages in young adults. Cardiol. Young 2020, 30, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Yaregani, V.K.; Krishnan, S.; Engels, H.J.; Gretebeck, R. Effects of caffeine on linear and nonlinear measures of heart rate variability before and after exercise. Depress. Anxiety 2005, 21, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Vlachopoulos, C.; Ioakeimidis, N.; Alexopoulos, N.; Stefanadis, C. Nonlinear dynamics of blood pressure variability after caffeine consumption. Clin. Med. Res. 2006, 4, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Seljuq, U.; Himayun, F.; Rasheed, H. Selection of an optimal mother wavelet basis function for ECG signal denoising. In Proceedings of the 17th IEEE International Multi Topic Conference, Karachi, Pakistan, 8–10 December 2014; pp. 26–30. [Google Scholar]
- Pan, J.; Thompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Practical method for determining the minimum embedding dimension of a scalar time series. Physics D 1997, 110, 43–50. [Google Scholar] [CrossRef]
- Fraser, A.M.; Swinney, H.L. Independent coordinates for strange attractors from mutual information. Phys. Rev. A 1986, 33, 1134–1140. [Google Scholar] [CrossRef]
- Caliskan, S.G.; Polatli, M.; Bilgin, M.D. Nonlinear analysis of heart rate variability of healthy subjects and patients with chronic obstructive pulmonary disease. J. Med. Eng. Technol. 2018, 42, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Artalejo, F.; López-García, E. coffee consumption and cardiovascular disease: A condensed review of epidemiological evidence and mechanisms. J. Agric. Food Chem. 2018, 66, 5257–5263. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.R.; Sung, B.H.; Pincomb, G.A.; Whitsett, T.L.; Wilson, M.F.; Lovallo, W.R. Hypertension risk status and effect of caffeine on blood pressure. Hypertension 2000, 36, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Chei, C.L.; Loh, J.K.; Soh, A.; Yuan, J.M.; Koh, W.P. Coffee, tea, caffeine, and risk of hypertension: The Singapore Chinese Health Study. Eur. J. Nutr. 2018, 57, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Geethavani, G.; Rameswarudu, M.; Rameshwari, R.R. Effect of caffeine on heart rate and blood pressure. Int. J. Sci. Res. 2014, 4, 1–2. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Scientific opinion on the safety and efficacy of taurine as a feed additive for all animal species. EFSA J. 2012, 10, 2736. [CrossRef]
- Ghandforoush-Sattari, M.; Mashayekhi, S.; Krishna, C.V.; Thompson, J.P.; Routledge, P.A. Pharmacokinetics of oral taurine in healthy volunteers. J. Amino Acids 2010, 2010, 346237. [Google Scholar] [CrossRef]
- Palatini, P.; Ceolotto, G.; Ragazzo, F.; Dorigatti, F.; Saladini, F.; Papparella, I.; Mos, L.; Zanta, G.; Santonastaso, M. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J. Hypertens. 2009, 27, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Hano, T.; Kasano, M.; Tomari, H.; Iwane, N. Taurine suppresses pressor response through the inhibition of sympathetic nerve activity and the improvement in baro-reflex sensitivity of spontaneously hypertensive rats. Adv. Exp. Med. Biol. 2009, 643, 57–63. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Nelson, M.T.; Biltz, G.R.; Dengel, D.R. Cardiovascular and ride time-to-exhaustion effects of an energy drink. J. Int. Soc. Sports Nutr. 2014, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Çalışkan, Ş.G.; Bilgin, M.D. Acute effects of caffeinated beverages on electrocardiographic and hemodynamic parameters in young adults. Cukurova Med. J. 2022, 47, 972–980. [Google Scholar] [CrossRef]
- Flueck, J.L.; Schaufelberger, F.; Lienert, M.; Schäfer Olstad, D.; Wilhelm, M.; Perret, C. Acute effects of caffeine on heart rate variability, blood pressure and tidal volume in paraplegic and tetraplegic compared to able-bodied individuals: A randomized, blinded trial. PLoS ONE 2016, 11, e0165034. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Newell, K.M. Changing complexity in human behavior and physiology through aging and disease. Neurobiol. Aging 2002, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, S.; Akyol, L.; Şahin, Ş.; Ede, H.; Börekçi, E.; Keser Yılmaz, Y.; Bolat, A.; Erbay, A.R. Clinical approach to patients with supraventricular tachycardia. Bozok Tıp Derg. 2013, 3, 51–58. [Google Scholar]
- Fuchs, F.D.; Whelton, P.K. High blood pressure and cardiovascular disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Arnaud, M.J.; Welsch, C. Theophylline and Caffeine Metabolism in Man; Friedr. Vieweg and Sons: Zurich, Switzerland, 1982. [Google Scholar]


| Caffeine | Taurine | Caffeine + Taurine | Control | ||
|---|---|---|---|---|---|
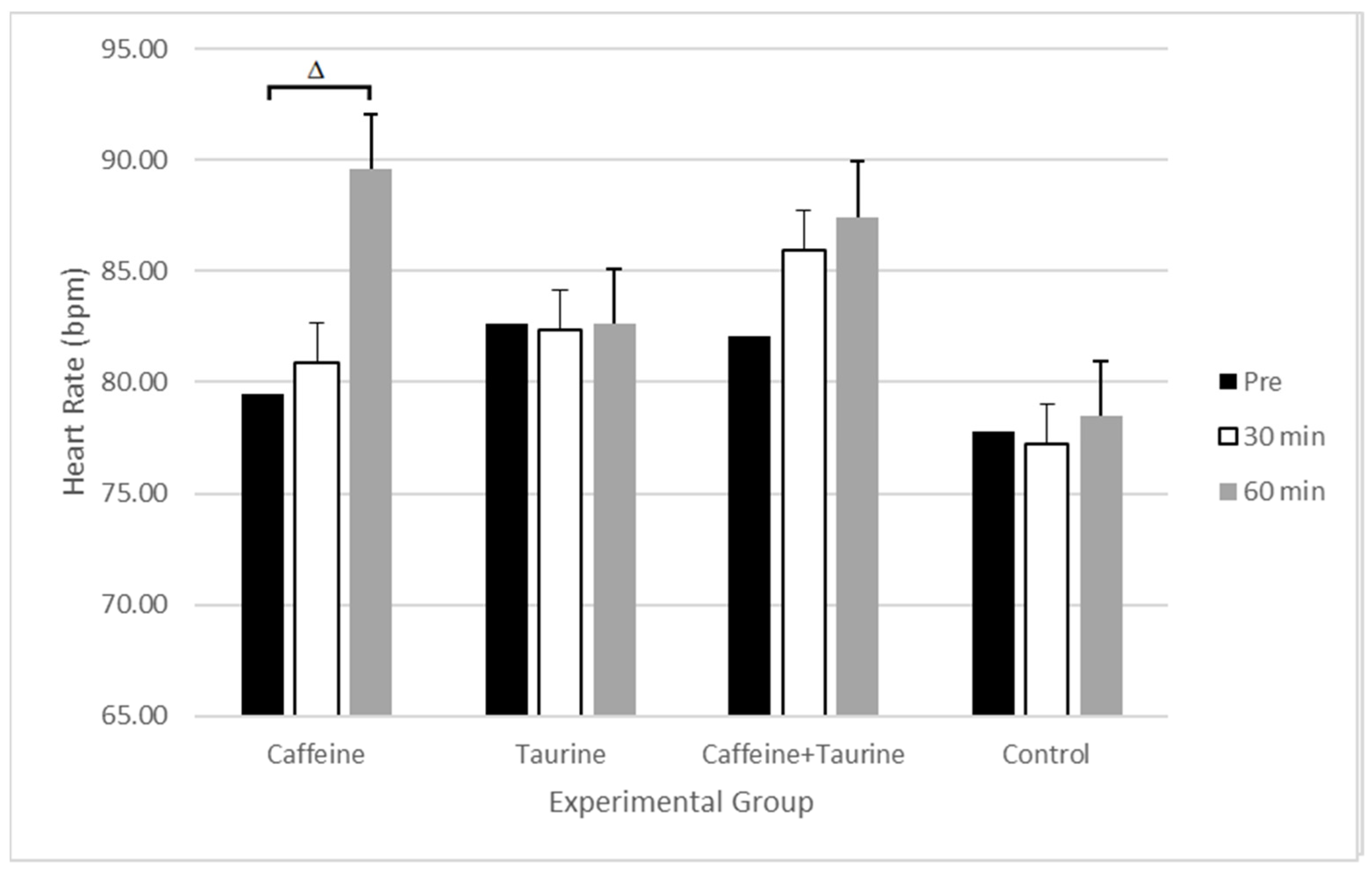
| Heart Rate (bpm) | Pre | 79.50 ± 2.11 | 82.62 ± 3.09 | 82.07 ± 2.81 | 77.75 ± 2.34 |
| 30 min | 80.86 ± 2.20 | 82.36 ± 3.21 | 85.93 ± 2.33 | 77.24 ± 2.51 | |
| 60 min | 89.57 ± 2.06 | 82.64 ± 3.17 | 87.43 ± 2.12 | 78.49 ± 3.01 | |
| p-value | 0.013 | 0.401 | 0.474 | 0.315 | |
| Systolic Blood Pressure (mm Hg) | Pre | 111.43 ± 3.13 | 111.00 ± 3.36 | 111.62 ± 2.70 | 110.92 ± 2.22 |
| 30 min | 112.50 ± 3.42 | 112.21 ± 3.11 | 114.21 ± 3.33 | 111.62 ± 3.64 | |
| 60 min | 119.43 ± 2.97 | 111.08 ± 4.22 | 116.29 ± 3.09 | 110.38 ± 3.99 | |
| p-value | 0.019 | 0.253 | 0.621 | 0.778 | |
| Diastolic Blood Pressure (mm Hg) | Pre | 76.29 ± 2.01 | 75.71 ± 2.50 | 75.93 ± 2.94 | 75.23 ± 2.66 |
| 30 min | 75.29 ± 1.63 | 75.86 ± 2.12 | 76.07 ± 1.77 | 75.15 ± 2.42 | |
| 60 min | 76.20 ± 2.09 | 76.14 ± 1.29 | 76.36 ± 2.31 | 74.36 ± 3.14 | |
| p-value | 0.151 | 0.636 | 0.119 | 0.294 | |
| SDNN (ms) | Pre | 52.323 ± 10.125 | 53.469 ± 9.336 | 53.254 ± 10.512 | 52.585 ± 9.801 |
| 30 min | 48.723 ± 7.197 | 53.979 ± 7.461 | 49.471 ± 5.755 | 51.754 ± 8.266 | |
| 60 min | 42.900 ± 6.874 | 53.571 ± 5.914 | 44.971 ± 5.911 | 52.823 ± 8.348 | |
| p-value | 0.764 | 0.823 | 0.791 | 0.193 | |
| RMSSD (ms) | Pre | 55.438 ± 10.215 | 57.095 ± 12.361 | 56.486 ± 14.399 | 56.592 ± 15.804 |
| 30 min | 67.493 ± 13.966 | 58.940 ± 8.643 | 60.332 ± 15.121 | 56.946 ± 10.988 | |
| 60 min | 73.836 ± 16.821 | 58.377 ± 10.393 | 63.407 ± 11.302 | 56.908 ± 9.622 | |
| p-value | 0.392 | 0.496 | 0.163 | 0.512 | |
| NN50 (beat) | Pre | 8.781 ± 2.326 | 8.819 ± 2.194 | 8.429 ± 2.061 | 8.507 ± 2.094 |
| 30 min | 10.643 ± 2.115 | 9.692 ± 1.116 | 9.643 ± 1.169 | 8.577 ± 1.288 | |
| 60 min | 13.000 ± 2.734 | 9.615 ± 2.730 | 11.923 ± 1.334 | 8.231 ± 1.966 | |
| p-value | 0.460 | 0.911 | 0.137 | 0.714 | |
| LF (Hz) | Pre | 545.071 ± 161.337 | 548.286 ± 87.667 | 541.143 ± 126.995 | 543.308 ± 117.241 |
| 30 min | 496.357 ± 129.724 | 547.357 ± 62.308 | 516.214 ± 114.364 | 541.769 ± 83.774 | |
| 60 min | 482.426 ± 174.611 | 544.429 ± 57.328 | 503.286 ± 101.252 | 548.769 ± 92.601 | |
| p-value | 0.518 | 0.334 | 0.816 | 0.433 | |
| HF (Hz) | Pre | 695.857 ± 162.664 | 704.846 ± 171.911 | 695.750 ± 234.552 | 696.154 ± 183.732 |
| 30 min | 725.000 ± 209.768 | 703.077 ± 224.413 | 716.857 ± 211.783 | 698.846 ± 196.912 | |
| 60 min | 776.923 ± 200.301 | 713.000 ± 200.184 | 749.231 ± 241.219 | 698.538 ± 201.744 | |
| p-value | 0.381 | 0.347 | 0.607 | 0.115 | |
| LF/HF | Pre | 0.783 ± 0.115 | 0.777 ± 0.677 | 0.778 ± 0.971 | 0.780 ± 0.384 |
| 30 min | 0.685 ± 0.264 | 0.779 ± 0.236 | 0.720 ± 0.116 | 0.775 ± 0.994 | |
| 60 min | 0.621 ± 0.820 | 0.764 ± 0.591 | 0.672 ± 0.534 | 0.786 ± 0.163 | |
| p-value | 0.633 | 0.396 | 0.674 | 0.116 | |
| Caffeine | Taurine | Caffeine + Taurine | Control | ||
|---|---|---|---|---|---|
| Embedding Dimension | Pre | 3 ± 0.688 | 3 ± 0.641 | 3 ± 0.194 | 3 ± 0.326 |
| 30 min | 3 ± 0.109 | 3 ± 0.113 | 3 ± 0.677 | 3 ± 0.773 | |
| 60 min | 3 ± 0.341 | 3 ± 0.651 | 3 ± 0.382 | 3 ± 0.161 | |
| p-value | 0.445 | 0.943 | 0.111 | 0.444 | |
| Time Delay | Pre | 2 ± 0.120 | 2 ± 0.663 | 2 ± 0.798 | 4 ± 0.391 |
| 30 min | 3 ± 0.251 | 3 ± 0.214 | 3 ± 0.379 | 3 ± 0.832 | |
| 60 min | 3 ± 0.188 | 3 ± 0.216 | 3 ± 0.256 | 2 ± 0.166 | |
| p-value | 0.337 | 0.910 | 0.963 | 0.558 | |
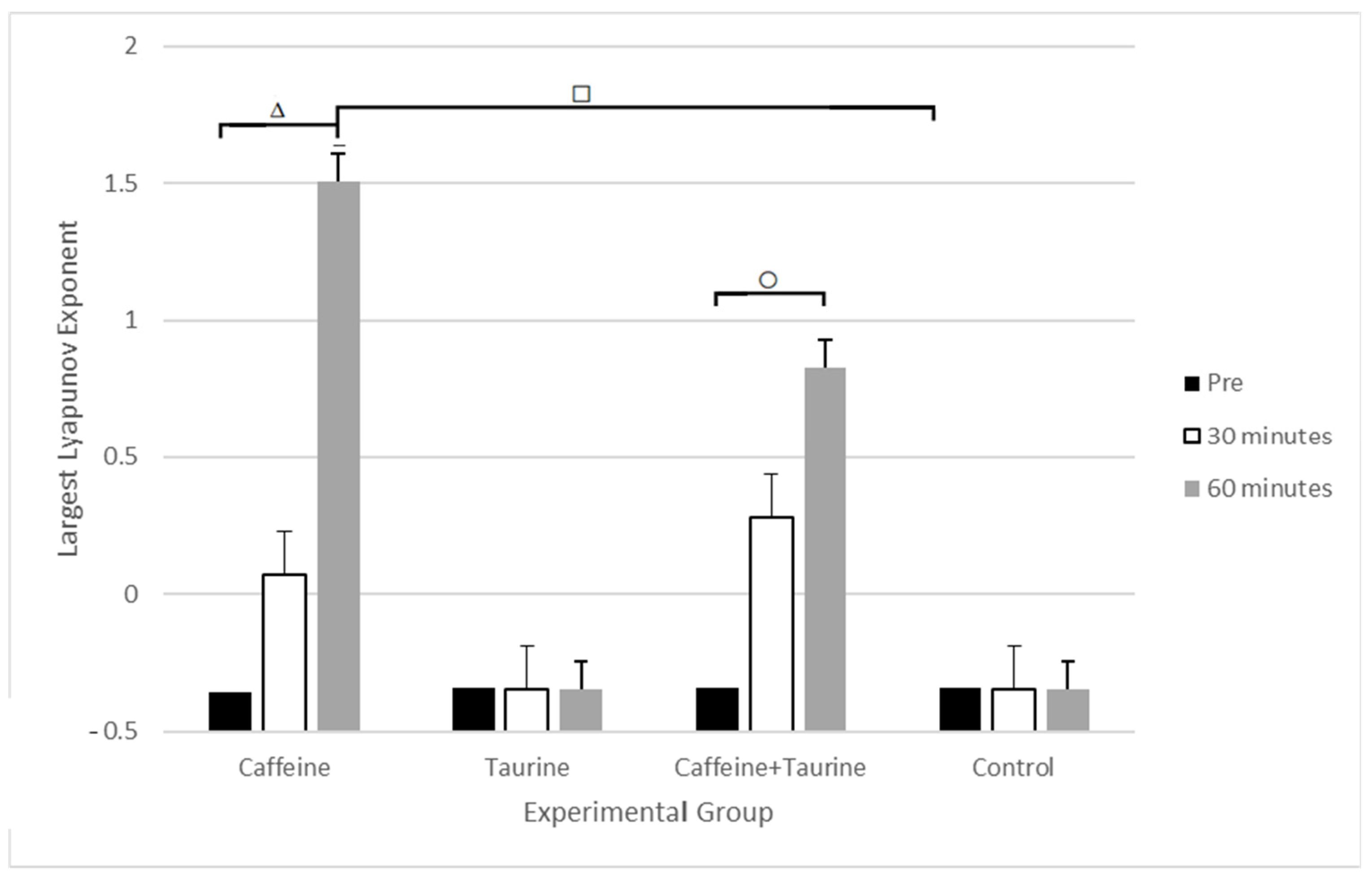
| Largest Lyapunov Exponent | Pre | −0.357 ± 1.193 | −0.341 ± 1.184 | −0.343 ± 1.246 | −0.343 ± 0.167 |
| 30 min | 0.069 ± 1.166 | −0.345 ± 1.108 | 0.280 ± 1.013 | −0.347 ± 0.995 | |
| 60 min | 1.507 ± 1.391 | −0.347 ± 1.825 | 0.829 ± 1.211 | −0.346 ± 0.571 | |
| p-value | 0.009 | 0.101 | 0.021 | 0.609 | |
| Correlation Dimension | Pre | 0.987 ± 0.016 | 1.001 ± 0.017 | 0.995 ± 0.013 | 0.986 ± 0.021 |
| 30 min | 1.029 ± 0.053 | 0.990 ± 0.064 | 1.023 ± 0.091 | 0.984 ± 0.013 | |
| 60 min | 1.140 ± 0.078 | 0.864 ± 0.048 | 1.086 ± 0.087 | 0.984 ± 0.029 | |
| p-value | 0.261 | 0.811 | 0.113 | 0.716 | |
| Approximate Entropy | Pre | 0.089 ± 0.052 | 0.090 ± 0.026 | 0.091 ± 0.067 | 0.090 ± 0.096 |
| 30 min | 0.119 ± 0.043 | 0.093 ± 0.080 | 0.108 ± 0.084 | 0.090 ± 0.023 | |
| 60 min | 0.136 ± 0.086 | 0.093 ± 0.007 | 0.127 ± 0.030 | 0.092 ± 0.089 | |
| p-value | 0.035 | 0.959 | 0.143 | 0.517 | |
| Hurst Exponent | Pre | 0.793 ± 0.012 | 0.787 ± 0.181 | 0.787 ± 0.109 | 0.793 ± 0.145 |
| 30 min | 0.823 ± 0.073 | 0.785 ± 0.129 | 0.825 ± 0.141 | 0.791 ± 0.166 | |
| 60 min | 0.858 ± 0.091 | 0.785 ± 0.117 | 0.849 ± 0.132 | 0.789 ± 0.219 | |
| p-value | 0.079 | 0.704 | 0.228 | 0.900 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çalışkan, Ş.G. Acute Effects of Caffeine and Taurine on Linear and Nonlinear Measures of the Cardiovascular System in Young Adults. Appl. Sci. 2024, 14, 4912. https://doi.org/10.3390/app14114912
Çalışkan ŞG. Acute Effects of Caffeine and Taurine on Linear and Nonlinear Measures of the Cardiovascular System in Young Adults. Applied Sciences. 2024; 14(11):4912. https://doi.org/10.3390/app14114912
Chicago/Turabian StyleÇalışkan, Şerife Gökçe. 2024. "Acute Effects of Caffeine and Taurine on Linear and Nonlinear Measures of the Cardiovascular System in Young Adults" Applied Sciences 14, no. 11: 4912. https://doi.org/10.3390/app14114912
APA StyleÇalışkan, Ş. G. (2024). Acute Effects of Caffeine and Taurine on Linear and Nonlinear Measures of the Cardiovascular System in Young Adults. Applied Sciences, 14(11), 4912. https://doi.org/10.3390/app14114912







