Physical Simulation-Based Calibration for Quantitative Real-Time PCR
Abstract
:1. Introduction
2. Principle and Structure
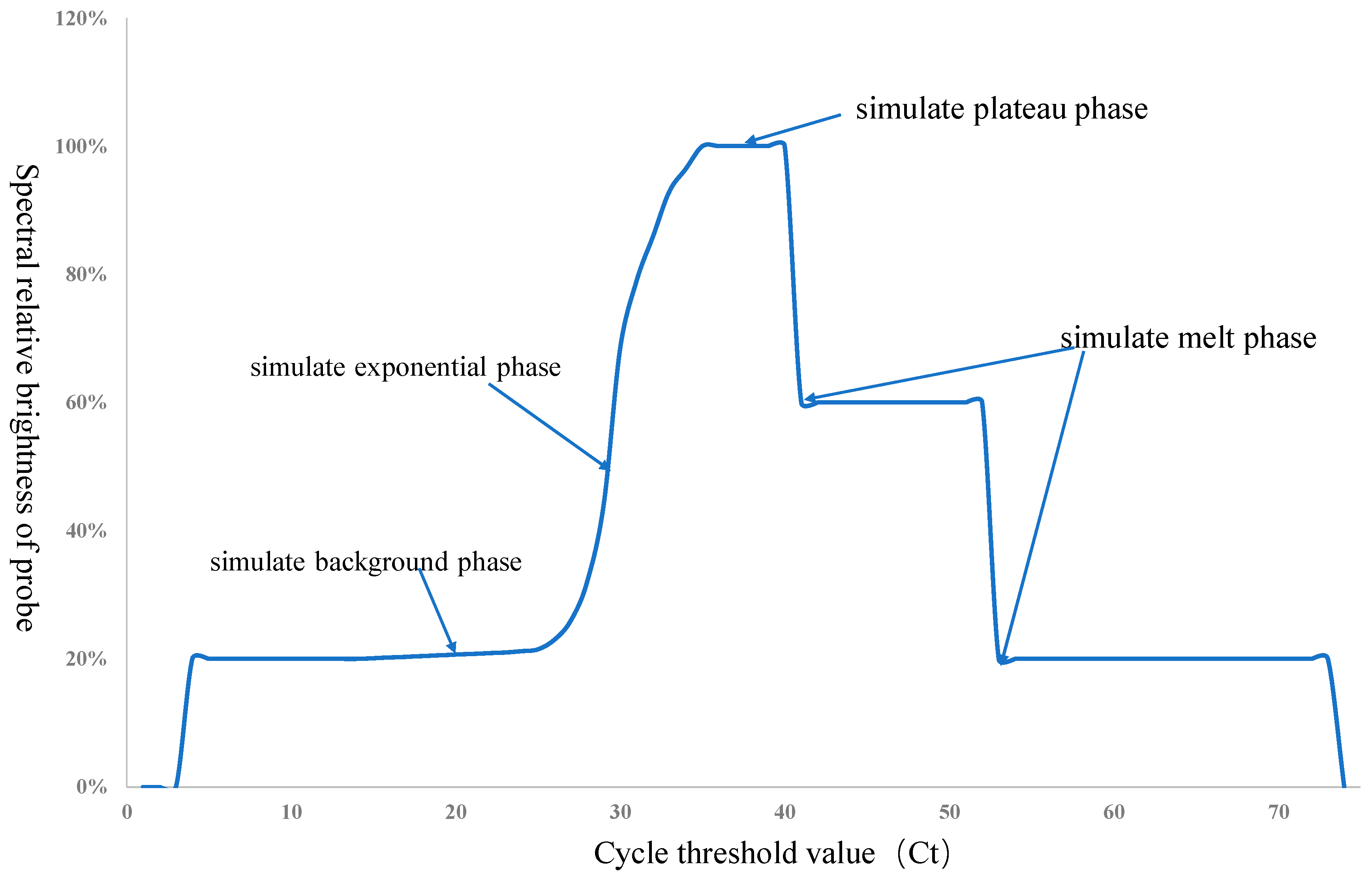
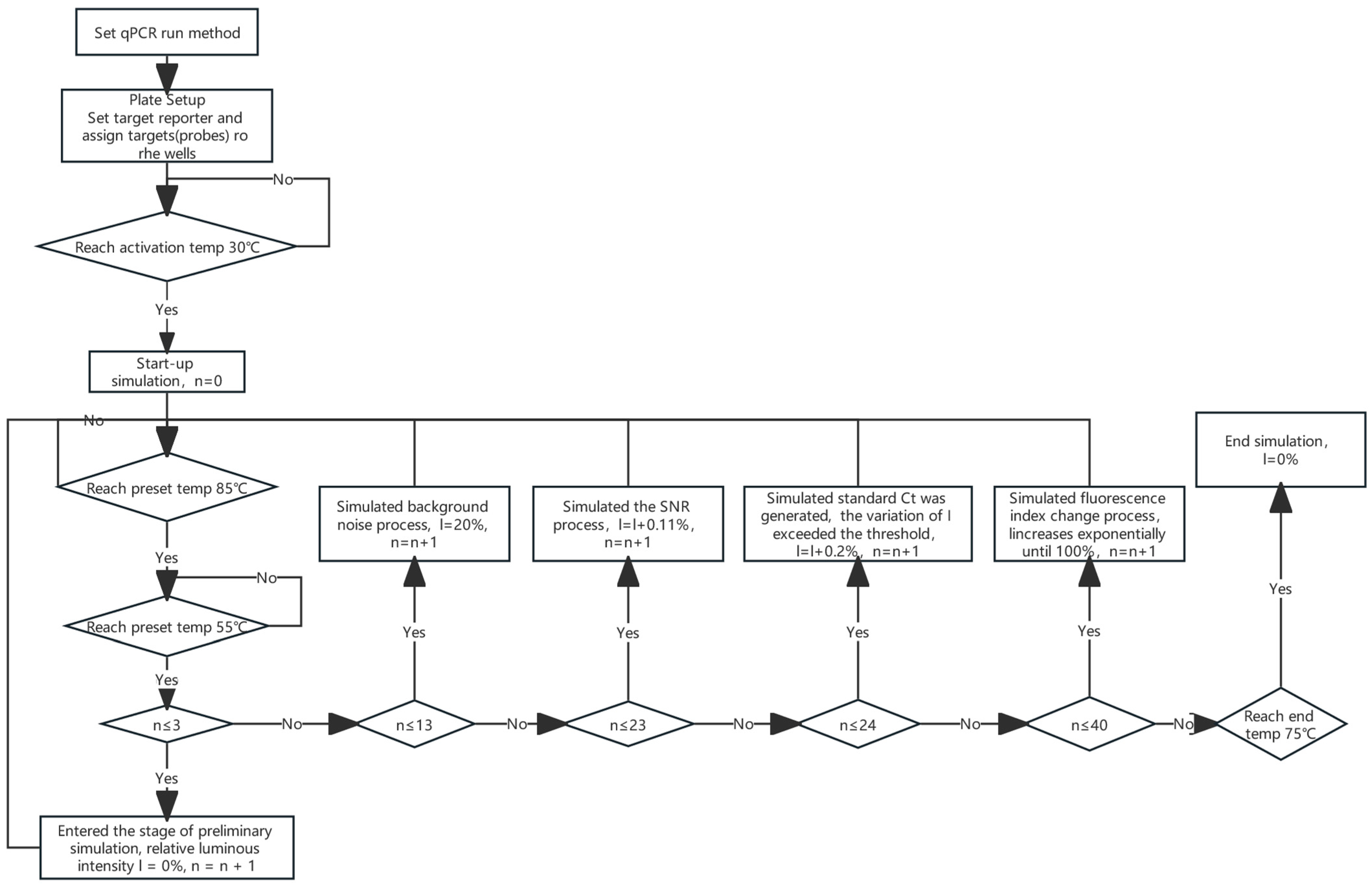
2.1. Principles of Calibration Device
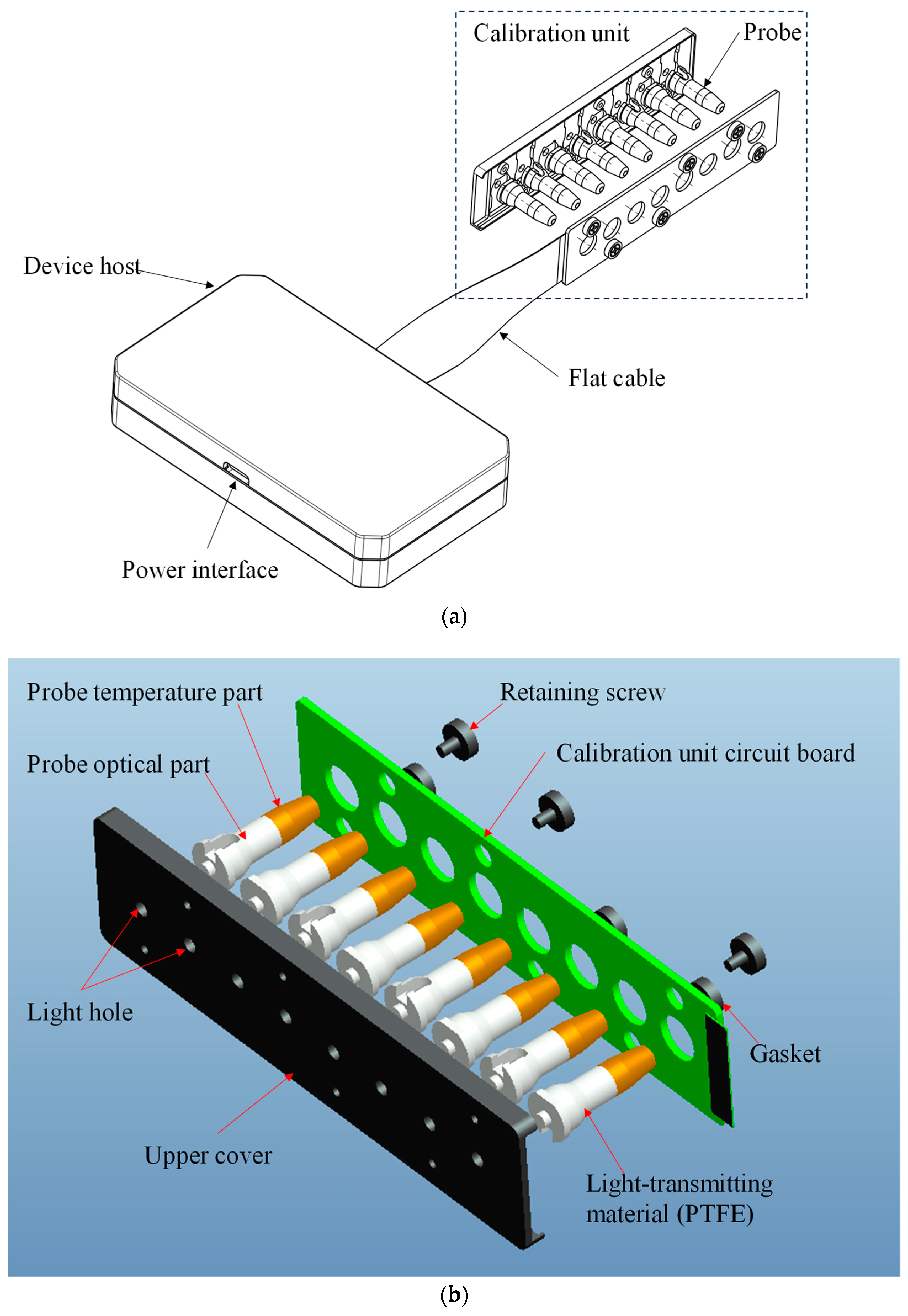
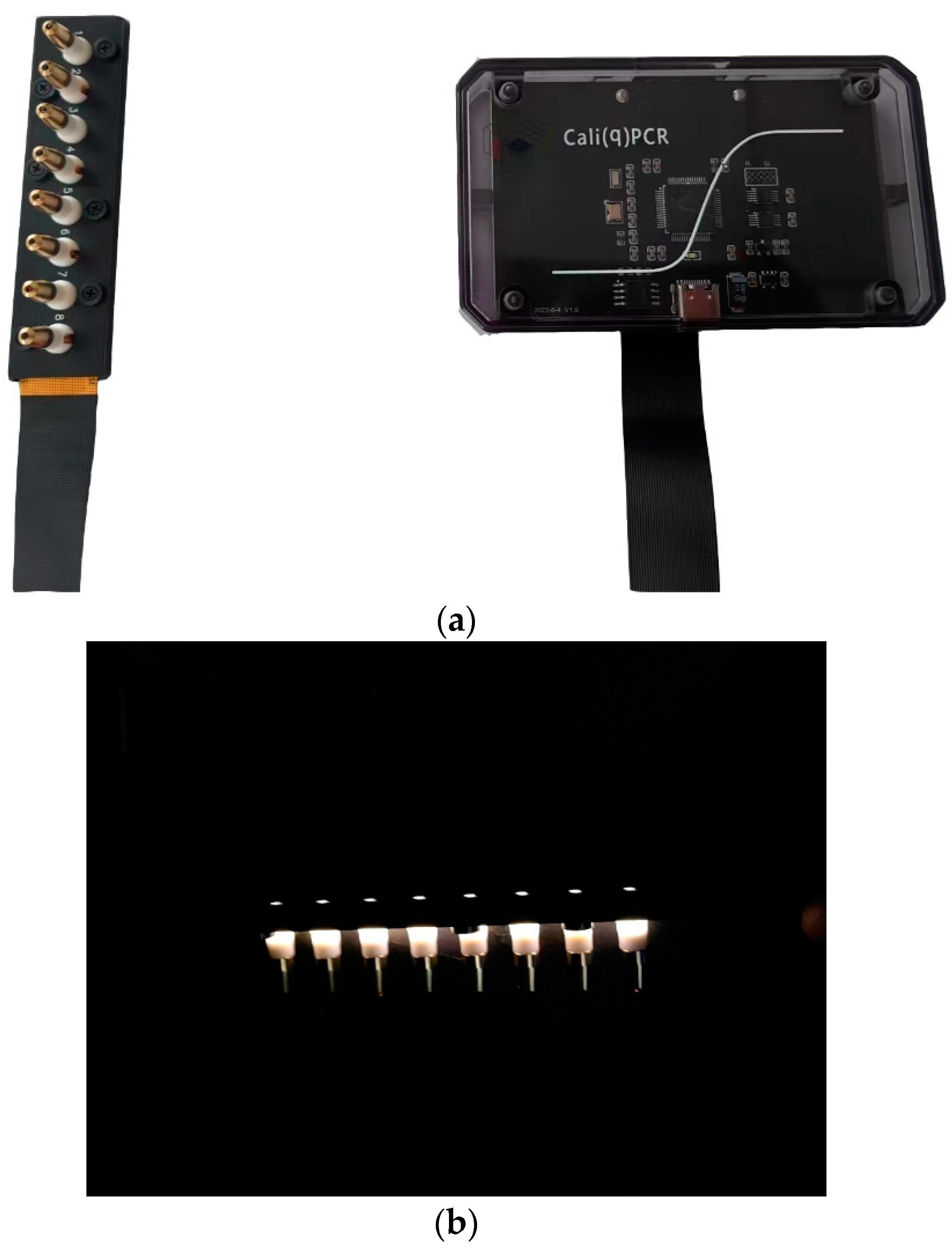
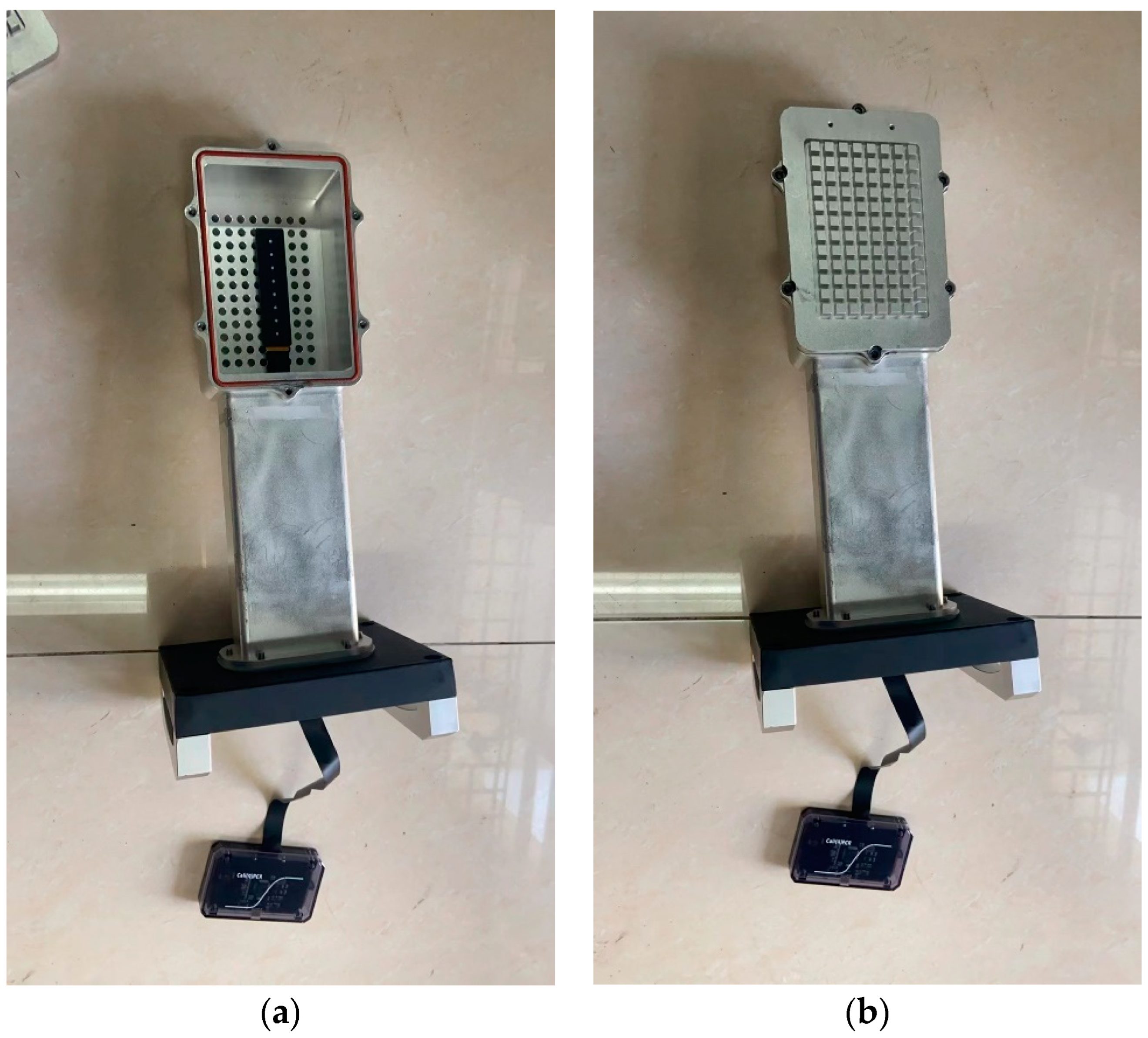
2.2. Structure of the Calibration Device
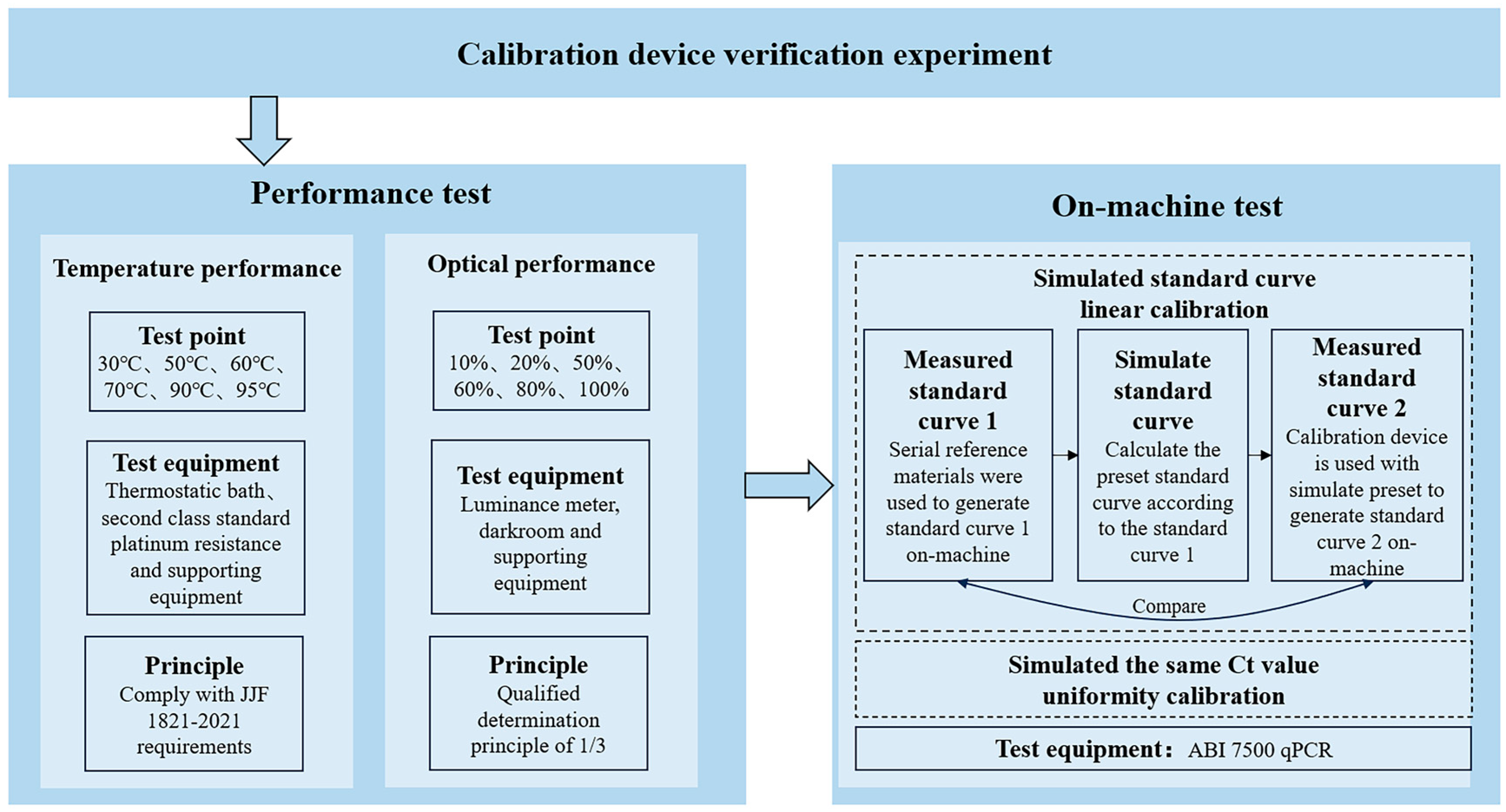
2.3. Experimental Scheme
3. Performance Test
3.1. Temperature Performance Test
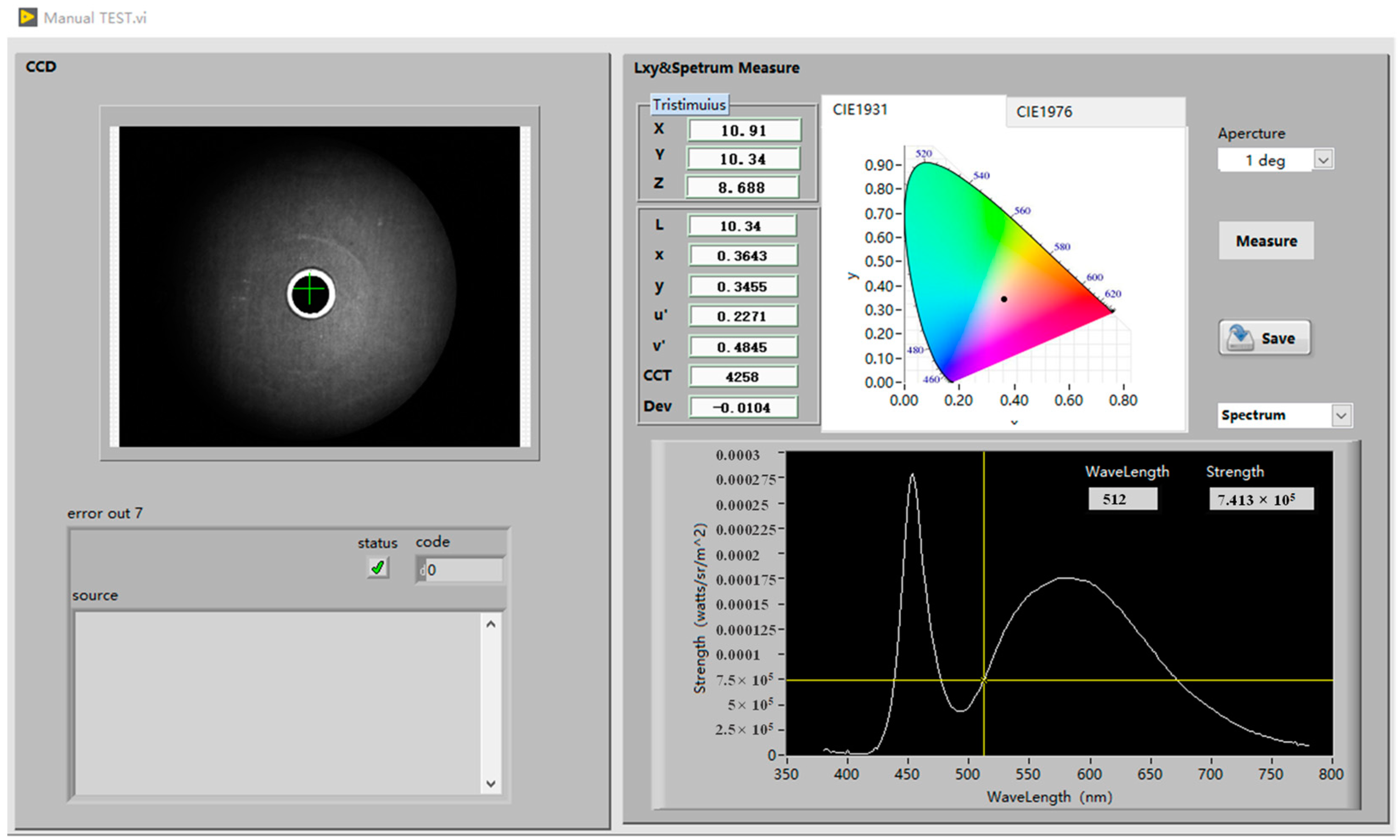
3.2. Optical Performance Test
4. On-Machine Test
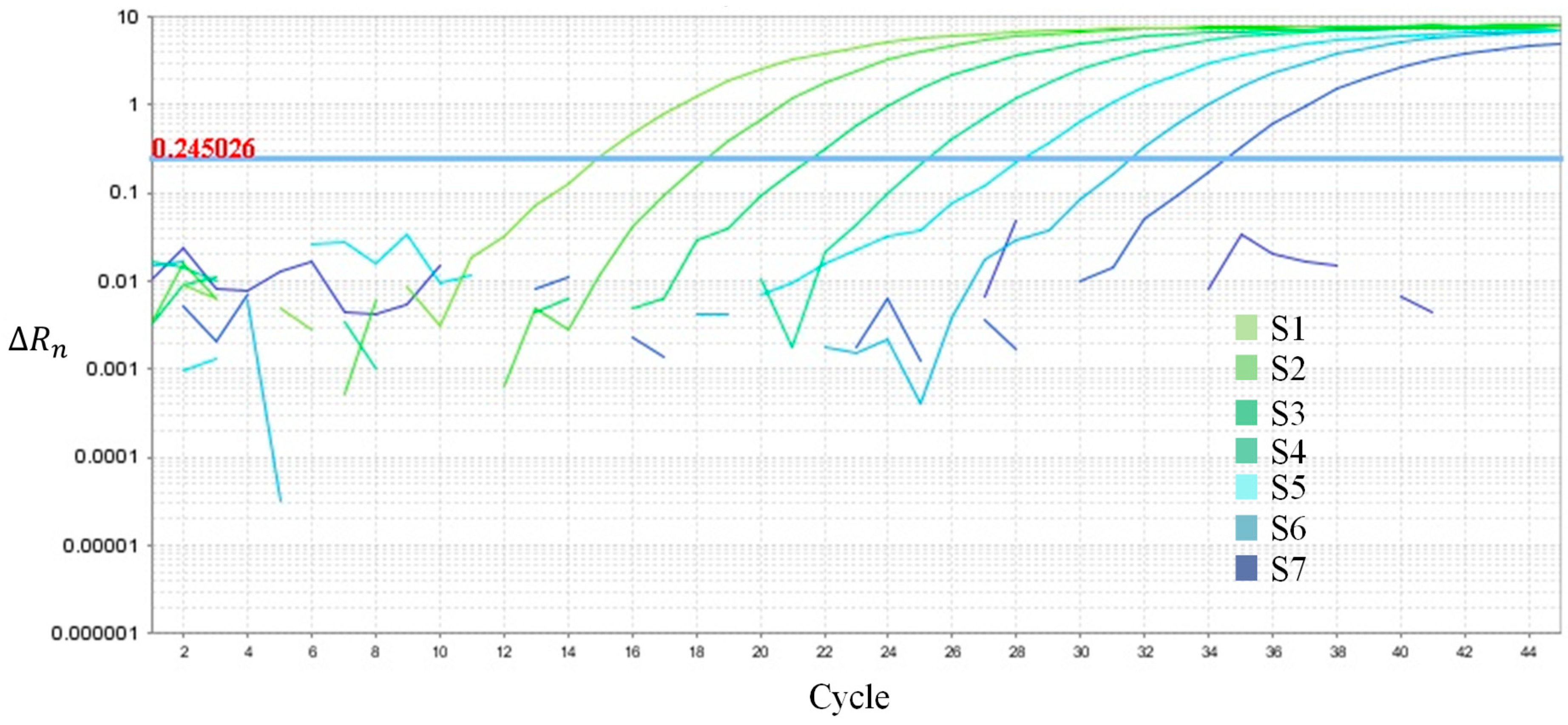
4.1. Standard Curve Simulation
4.2. Uniformity Test
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bag, S.; Ghosh, S.; Lalwani, R.; Vishnoi, P.; Gupta, S.; Dubey, D. Real-Time Polymerase Chain Reaction-Based Detection and Quantification of Hepatitis B Virus DNA. J. Vis. Exp. 2023, 15, e66249. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.R.; de Lima, T.F.; Bartolomeu-Gonçalves, G.; de Castro, I.M.; de Lima, D.G.; Borges, P.H.G.; Nakazato, G.; Kobayashi, R.K.T.; Venancio, E.J.; Tarley, C.R.T.; et al. Development of a melting-curve-based multiplex real-time PCR assay for the simultaneous detection of viruses causing respiratory infection. Microorganisms 2023, 11, 2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Du, H.; Qu, X.; Chen, Y.; Wang, J.; Wu, R. A comparative analysis of cycle threshold (Ct) values from Cobas4800 and AmpFire HPV assay for triage of women with positive hrHPV results. BMC Infect. Dis. 2023, 23, 783. [Google Scholar] [CrossRef] [PubMed]
- Shibanuma, T.; Nunomura, Y.; Oba, M.; Kawahara, F.; Mizutani, T.; Takemae, H. Development of a one-run real-time PCR detection system for pathogens associated with poultry infectious diseases. J. Veter. Med. Sci. 2023, 85, 407–411. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Taylor, D.; Linacre, A. PCR in Forensic Science: A Critical Review. Genes 2024, 15, 438. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Liu, S.; Song, Y.; Tao, X. TaqMan real-time quantitative PCR for the detection of beef tallow to assess the authenticity of edible oils. Food Control 2024, 156, 110139. [Google Scholar] [CrossRef]
- Doi, M.; Nakagawa, T.; Asano, M. A practical workflow for forensic species identification using direct sequencing of real-time PCR products. Mol. Biol. Rep. 2023, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-W.; Luo, Y.-Q.; Fan, Y.-Y.; Xian, L.-Y.; Song, Y.; Chen, X.-D.; Luo, W.-H.; Sun, D.-M.; Wei, M. Species identification of Bungarus multicinctus, Bungarus fasciatus, and Lycodon rufozonatus in Chinese medicinal crude drugs and extracts using capillary electrophoresis-based multiplex PCR. Chin. J. Anal. Chem. 2023, 51, 100272. [Google Scholar] [CrossRef]
- Acquier, M.; Zabala, A.; de Précigout, V.; Delmas, Y.; Dubois, V.; de la Faille, R.; Rubin, S.; Combe, C.; M‘Zali, F.; Kaminski, H. Performance of real-time PCR in suspected haemodialysis catheter-related bloodstream infection: A proof-of-concept study. Clin. Kidney J. 2023, 16, 494–500. [Google Scholar] [CrossRef]
- Dinç, H.; Yiğin, A.; Koyuncu, I.; Aslan, M. Investigation of the anticancer and apoptotic effect of Micromeria congesta under in vitro conditions and detection of related genes by real-time PCR. In Veterinary Research Forum; Faculty of Veterinary Medicine, Urmia University: Urmia, Iran, 2022; Volume 13. [Google Scholar]
- Artika, I.M.; Dewi, Y.P.; Nainggolan, I.M.; Siregar, J.E.; Antonjaya, U. Real-time polymerase chain reaction: Current techniques, applications, and role in COVID-19 diagnosis. Genes 2022, 13, 2387. [Google Scholar] [CrossRef]
- Nagelkerke, E.; Hetebrij, W.A.; Koelewijn, J.M.; Kooij, J.; van der Drift, A.M.R.; van der Beek, R.F.; de Jonge, E.F.; Lodder, W.J. PCR standard curve quantification in an extensive wastewater surveillance program: Results from the Dutch SARS-CoV-2 wastewater surveillance. Front. Public Health 2023, 11, 1141494. [Google Scholar] [CrossRef]
- Sahoo, S.; Mandal, S.; Das, P.; Bhattacharya, S.; Chandy, M. An analysis of the standard curve parameters of cytomegalovirus, BK virus and hepatitis B virus quantitative polymerase chain reaction from a clinical virology laboratory in eastern India. Indian J. Med. Microbiol. 2022, 40, 81–85. [Google Scholar] [CrossRef]
- Ling, W.; Zhou, W.; Cui, J.; Shen, Z.; Wei, Q.; Chu, X. Experimental study on the heating/cooling and temperature uniformity performance of the microchannel temperature control device for nucleic acid PCR amplification reaction of COVID-19. Appl. Therm. Eng. 2023, 226, 120342. [Google Scholar] [CrossRef]
- Liu, H.; Fang, Y.; Su, X.; Wang, Y.; Ji, M.; Xing, H.; Gao, Y.; Zhang, Y.; He, N. Temperature control algorithm for polymerase chain reaction (PCR) instrumentation based upon improved hybrid fuzzy proportional integral derivative (PID) control. Instrum. Sci. Technol. 2023, 51, 109–131. [Google Scholar] [CrossRef]
- Xi, B.; Huang, S.; An, Y.; Gong, X.; Yang, J.; Zeng, J.; Ge, S.; Zhang, D. Sophisticated and precise: Design and implementation of a real-time optical detection system for ultra-fast PCR. RSC Adv. 2023, 13, 19770–19781. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hansel, D.E.; Curlin, M.E.; Townes, J.M.; Messer, W.B.; Fan, G.; Qin, X. Bimodal distribution pattern associated with the PCR cycle threshold (Ct) and implications in COVID-19 infections. Sci. Rep. 2022, 12, 14544. [Google Scholar] [CrossRef]
- Markewitz, R.; Dargvainiene, J.; Junker, R.; Wandinger, K.P. Cycle threshold of SARS-CoV-2 RT-PCR as a driver of retesting. Sci. Rep. 2024, 14, 2423. [Google Scholar] [CrossRef]
- Abdulrahman, A.; I Mallah, S.; Alawadhi, A.; Perna, S.; Janahi, E.M.; AlQahtani, M.M. Association between RT-PCR Ct values and COVID-19 new daily cases: A multicenter cross-sectional study. Le Infez. Med. 2021, 29, 416. [Google Scholar]
- Schmidt, P.J.; Acosta, N.; Chik, A.H.S.; D’aoust, P.M.; Delatolla, R.; Dhiyebi, H.A.; Glier, M.B.; Hubert, C.R.J.; Kopetzky, J.; Mangat, C.S.; et al. Realizing the value in “non-standard” parts of the qPCR standard curve by integrating fundamentals of quantitative microbiology. Front. Microbiol. 2023, 14, 1048661. [Google Scholar] [CrossRef]
- Sala, E.; Shah, I.S.; Manissero, D.; Juanola-Falgarona, M.; Quirke, A.M.; Rao, S.N. Systematic review on the correlation between SARS-CoV-2 real-time PCR cycle threshold values and epidemiological trends. Infect. Dis. Ther. 2023, 12, 749–775. [Google Scholar] [CrossRef]
- Jeon, B.R. Variation in PCR efficiencies between quantification standards and clinical specimens using different real-time quantitative PCR interpretation methods. Int. J. Biol. Biomed. Eng. 2020, 14, 39–42. [Google Scholar]
- Olveira, J.G.; Souto, S.; Bandín, I.; Dopazo, C.P. Development and validation of a SYBR green real time PCR protocol for detection and quantification of nervous necrosis virus (NNV) using different standards. Animals 2021, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hughes, C.; Ding, R.; Snopkowski, C.; Salinas, T.; Schwartz, J.; Dadhania, D.; Suthanthiran, M. Development of a Bak gene based standard curve for absolute quantification of BK virus in real time quantitative PCR assay and noninvasive diagnosis of BK virus nephropathy in kidney allograft recipients. J. Immunol. Methods 2022, 509, 113341. [Google Scholar] [CrossRef] [PubMed]
- Bonacorsi, S.; Visseaux, B.; Bouzid, D.; Pareja, J.; Rao, S.N.; Manissero, D.; Hansen, G.; Vila, J. Systematic review on the correlation of quantitative PCR cycle threshold values of gastrointestinal pathogens with patient clinical presentation and outcomes. Front. Med. 2021, 8, 711809. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Vila, J.; Hansen, G.; Manissero, D.; Pareja, J.; Rao, S.N.; Visseaux, B. Systematic review on the association between respiratory virus real-time PCR cycle threshold values and clinical presentation or outcomes. J. Antimicrob. Chemother. 2021, 76 (Suppl. 3), iii33–iii49. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Behrens, S.F. Effect of target gene sequence evenness and dominance on real-time PCR quantification of artificial sulfate-reducing microbial communities. PLoS ONE 2024, 19, e0299930. [Google Scholar] [CrossRef] [PubMed]
- Leta, D.; Gutema, G.; Hagos, G.G.; Diriba, R.; Bulti, G.; Sura, T.; Ayana, D.; Chala, D.; Lenjiso, B.; Bulti, J.; et al. Effect of heat inactivation and bulk lysis on real-time reverse transcription PCR detection of the SARS-COV-2: An experimental study. BMC Res. Notes 2022, 15, 295. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Singh, P.; Sengupta, S.; Muthukrishnan, A.B.; Jayaraman, G. DNA-Aptamer-Based qPCR Using Light-Up Dyes for the Detection of Nucleic Acids. ACS Omega 2023, 8, 47277–47282. [Google Scholar] [CrossRef]
- Goyal, S.; Singh, P.; Sengupta, S.; Muthukrishnan, A.B.; Jayaraman, G. Performance evaluation of QuantStudio 1 plus real-time PCR instrument for clinical laboratory analysis: A proof-of-concept study. Pract. Lab. Med. 2023, 36, e00330. [Google Scholar]
- JJF 1821-2020; Calibration Specification of Temperature Calibration Device for Polymerase China Reaction Analyzers. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2020. Available online: http://jjg.spc.org.cn/resmea/view/stdonline (accessed on 25 April 2024).
- Li, X.; Nguyen, L.V.; Hill, K.; Ebendorff-Heidepriem, H.; Schartner, E.P.; Zhao, Y.; Zhou, X.; Zhang, Y.; Warren-Smith, S.C. All-fiber all-optical quantitative polymerase chain reaction (qPCR). Sens. Actuators B Chem. 2020, 323, 128681. [Google Scholar] [CrossRef]
- Span, M.; Verblakt, M.; Hendrikx, T. Measurement uncertainty in calibration and compliancy testing of PCR and qPCR thermal cyclers. In Proceedings of the 18th International Congress of Metrology, Paris, France, 19–21 September 2017; EDP Sciences: Les Ulis, France, 2017. [Google Scholar]
- Gaňová, M.; Wang, X.; Yan, Z.; Zhang, H.; Lednický, T.; Korabečná, M.; Neužil, P. Temperature non-uniformity detection on dPCR chips and temperature sensor calibration. RSC Adv. 2022, 12, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-Y.; Chao, L.-C.; Hsu, C.-N.; Huang, K.-C.; Tsai, Y.-H.; Shieh, D.-B.; Yang, C.-C. Development of Non-contact Composite Temperature Sensing (CTS) for photothermal Real-time quantitative PCR Device. In Proceedings of the 2020 IEEE Sensors Applications Symposium (SAS), Kuala Lumpur, Malaysia, 9–11 March 2020. [Google Scholar]
- Luque-Perez, E.; Mazzara, M.; Weber, T.P.; Foti, N.; Grazioli, E.; Munaro, B.; Pinski, G.; Bellocchi, G.; Eede, G.V.D.; Savini, C. Testing the robustness of validated methods for quantitative detection of GMOs across qPCR instruments. Food Anal. Methods 2013, 6, 343–360. [Google Scholar] [CrossRef]
- Willis, J.R.; Sivaganesan, M.; Haugland, R.A.; Kralj, J.; Servetas, S.; Hunter, M.E.; Jackson, S.A.; Shanks, O.C. Performance of NIST SRM® 2917 with 13 recreational water quality monitoring qPCR assays. Water Res. 2022, 212, 118114. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gong, P.; Zhang, J.; Shan, Y.; Han, X.; Zhang, L. Use of qPCR for the analysis of population heterogeneity and dynamics during Lactobacillus delbrueckii spp. bulgaricus batch culture. Artif. Cells Nanomed. Biotechnol. 2021, 49, 1–10. [Google Scholar] [PubMed]
- Josefsen, M.H.; Löfström, C.; Hansen, T.; Reynisson, E.; Hoorfar, J. Instrumentation and fluorescent chemistries used in qPCR. In Quantitative Real-Time PCR in Applied Microbiology; Caister Academic Press: Wymondham, UK, 2012; pp. 27–52. [Google Scholar]
- Sivaganesan, M.; Willis, J.R.; Karim, M.; Babatola, A.; Catoe, D.; Boehm, A.B.; Wilder, M.; Green, H.; Lobos, A.; Harwood, V.J.; et al. Interlaboratory performance and quantitative PCR data acceptance metrics for NIST SRM® 2917. Water Res. 2022, 225, 119162. [Google Scholar] [CrossRef] [PubMed]
- Beinhauerova, M.; Beinhauerova, M.; McCallum, S.; Sellal, E.; Ricchi, M.; O’Brien, R.; Blanchard, B.; Slana, I.; Babak, V.; Kralik, P. Development of a reference standard for the detection and quantification of Mycobacterium avium subsp. paratuberculosis by quantitative PCR. Sci. Rep. 2021, 11, 11622. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Barnewall, R.J.; Marsh, I.B.; Szentirmay, A.N.; Quinn, J.C.; van Houdt, R.; Gunst, Q.D.; Hoff, M.J.B.v.D. Efficiency correction is required for accurate quantitative PCR analysis and reporting. Clin. Chem. 2021, 67, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Grgicak, C.M.; Urban, Z.M.; Cotton, R.W. Investigation of reproducibility and error associated with qPCR methods using Quantifiler® Duo DNA quantification kit. J. Forensic Sci. 2010, 55, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Span, M.; Verblakt, M.; Hendrikx, T. Comparison of temperature dynamics of various thermal cycler calibration methods. In Proceedings of the 19th International Congress of Metrology (CIM2019), Paris, France, 24–26 September 2019; EDP Sciences: Les Ulis, France, 2019; p. 19003. [Google Scholar]
- Lima, Â.; Sousa, L.G.; Cerca, N. Accurate absolute quantification of bacterial populations in mixed cultures by qPCR. In PCR: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 105–115. [Google Scholar]
- Morinha, F.; Magalhães, P.; Blanco, G. Standard guidelines for the publication of telomere qPCR results in evolutionary ecology. Mol. Ecol. Resour. 2020, 20, 635–648. [Google Scholar] [CrossRef]
- Dawson, P.; Buyukyavuz, A.; Ionita, C.; Northcutt, J. Effects of DNA extraction methods on the real time PCR quantification of Campylobacter jejuni, Campylobacter coli, and Campylobacter lari in chicken feces and ceca contents. Poult. Sci. 2023, 102, 102369. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Wang, Y.; Sun, A.; Teplý, T.; Korabečná, M.; Zhang, H.; Neuzil, P. Smallest dual-color qPCR device. Sens. Actuators B Chem. 2023, 394, 134299. [Google Scholar] [CrossRef]
- Ramezani, A. CtNorm: Real time PCR cycle of threshold (Ct) normalization algorithm. J. Microbiol. Methods 2021, 187, 106267. [Google Scholar] [CrossRef] [PubMed]
- Just, V.M.; Welzel, F.; Jacobs, H.; Gau, G.; Hauschultz, M.T.; Friedo, M.H.; Foitzik, A.H. Development of a Thermal Cycler for a Low-Cost Real-Time PCR Application. Key Eng. Mater. 2023, 968, 73–79. [Google Scholar] [CrossRef]
- Chen, Z.; Halford, N.G.; Liu, C. Real-time quantitative PCR: Primer design, reference gene selection, calculations and statistics. Metabolites 2023, 13, 806. [Google Scholar] [CrossRef]




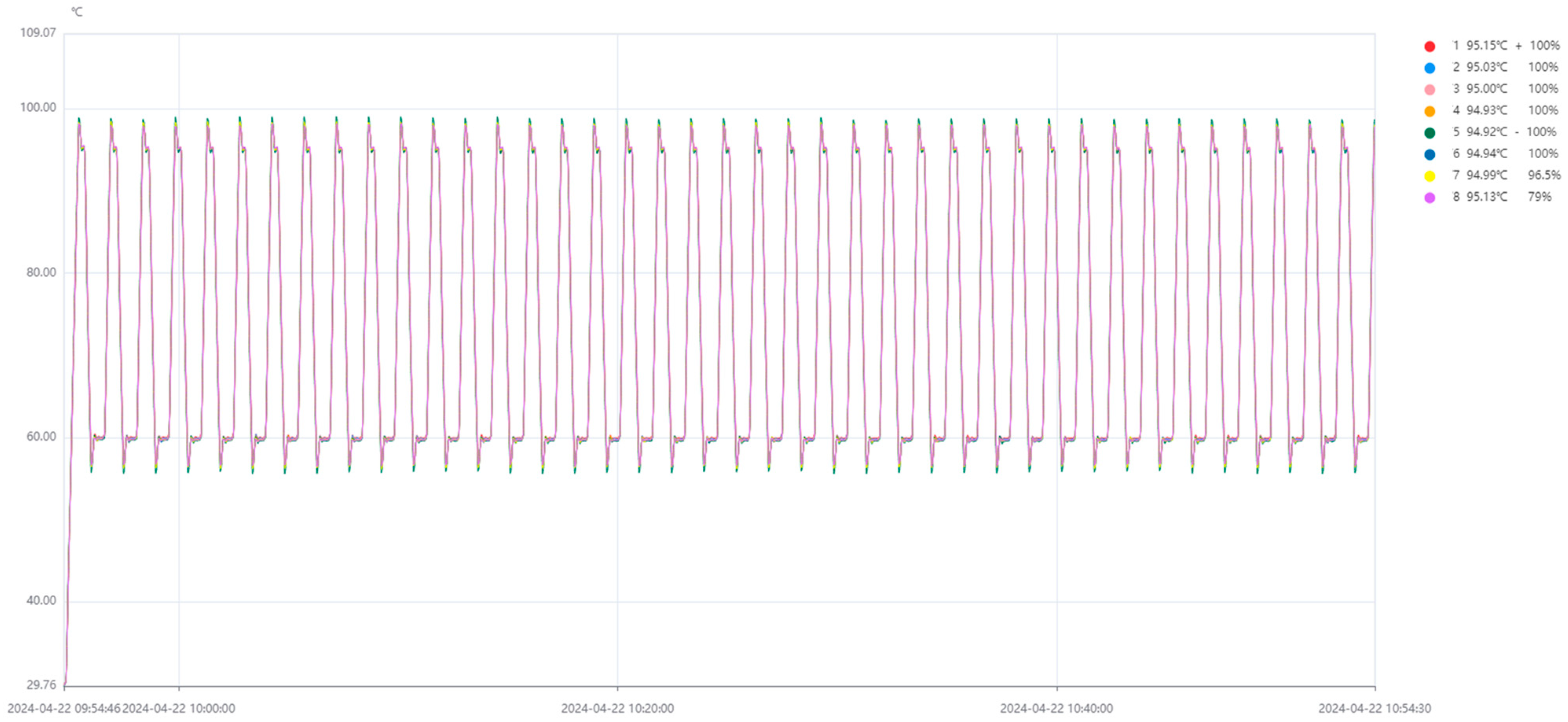

| Device Name | Model | Manufacturing Number | Manufacturer | Range | Performance |
|---|---|---|---|---|---|
| Platinum resistance Thermometer | WZPB-2 | 11427 | DAFANG (Kunming, China) | 0–419.5 °C | second class |
| Digital multimeter | 2001 | 4055379 | KEITHLEY (Washington, DC, USA) | 100 Ω–100 MΩ | 0.0032 + 0.0004 (90 days) |
| Thermostatic water bath | CJTH-95A | 5713 | WEILI (Huzhou, China) | +15~95 °C | fluctuation ± 0.01 °C/30 min uniformity < 0.01 °C |
| Thermostatic oil bath | CJTH-300A | 5208 | WEILI (Huzhou, China) | 90–300 °C | fluctuation ± 0.01 °C/30 min uniformity < 0.01 °C |
| Isothermal block | CaliTemp-10 | 86025001 | Thermo-leader (Beijing, China) | −40–300 °C | Uniformity: ≤0.03 °C; Fluctuation: ≤0.02 °C/10 min |
| No. | Temperature Error/°C | Measurement Uncertainty U, k = 2 | |||||
|---|---|---|---|---|---|---|---|
| 30 | 50 | 60 | 70 | 90 | 95 | ||
| 1 | +0.02 | +0.01 | +0.01 | +0.01 | +0.01 | +0.01 | 0.04 |
| 2 | +0.01 | +0.01 | +0.03 | +0.01 | +0.02 | +0.01 | 0.04 |
| 3 | +0.01 | +0.03 | +0.01 | +0.02 | +0.02 | +0.02 | 0.04 |
| 4 | +0.02 | +0.02 | +0.01 | +0.01 | +0.01 | +0.02 | 0.04 |
| 5 | +0.01 | +0.02 | +0.01 | +0.01 | +0.02 | +0.02 | 0.04 |
| 6 | +0.02 | +0.01 | +0.01 | +0.01 | +0.02 | +0.01 | 0.04 |
| 7 | +0.02 | +0.01 | +0.01 | +0.01 | +0.02 | +0.01 | 0.04 |
| 8 | +0.01 | +0.02 | +0.02 | +0.03 | +0.02 | +0.02 | 0.04 |
| No. | Measured Luminance Values/cd/m2 | r | Measurement Uncertainty , k = 2 | |||||
|---|---|---|---|---|---|---|---|---|
| 100% | 80% | 60% | 50% | 20% | 10% | |||
| 1 | 9.560 | 7.580 | 5.658 | 4.707 | 1.937 | 1.0030 | 0.9999 | 5.3% |
| 2 | 9.568 | 7.572 | 5.607 | 4.649 | 1.900 | 0.9518 | 0.9998 | 5.3% |
| 3 | 9.707 | 7.733 | 5.765 | 4.790 | 1.944 | 0.9817 | 0.99995 | 5.3% |
| 4 | 9.587 | 7.640 | 5.714 | 4.765 | 2.048 | 0.9668 | 0.99985 | 5.3% |
| 5 | 9.754 | 7.766 | 5.792 | 4.815 | 1.984 | 1.0210 | 0.99995 | 5.3% |
| 6 | 9.818 | 7.821 | 5.838 | 4.860 | 1.975 | 0.9292 | 0.99995 | 5.3% |
| 7 | 9.750 | 7.755 | 5.772 | 4.813 | 1.979 | 1.0080 | 0.9999 | 5.3% |
| 8 | 9.749 | 7.752 | 5.793 | 4.814 | 2.019 | 0.9979 | 0.9999 | 5.3% |
| Name | Sample Type | Concentration |
|---|---|---|
| S1 | Standard | 1.04 × 107 copies/uL |
| S2 | Standard | 1.06 × 106 copies/uL |
| S3 | Standard | 1.06 × 105 copies/uL |
| S4 | Standard | 1.07 × 104 copies/uL |
| S5 | Standard | 1.12 × 103 copies/uL |
| S6 | Standard | 1.10 × 102 copies/uL |
| S7 | Standard | 1.11 × 101 copies/uL |
| NTC | Negative control | 0 |
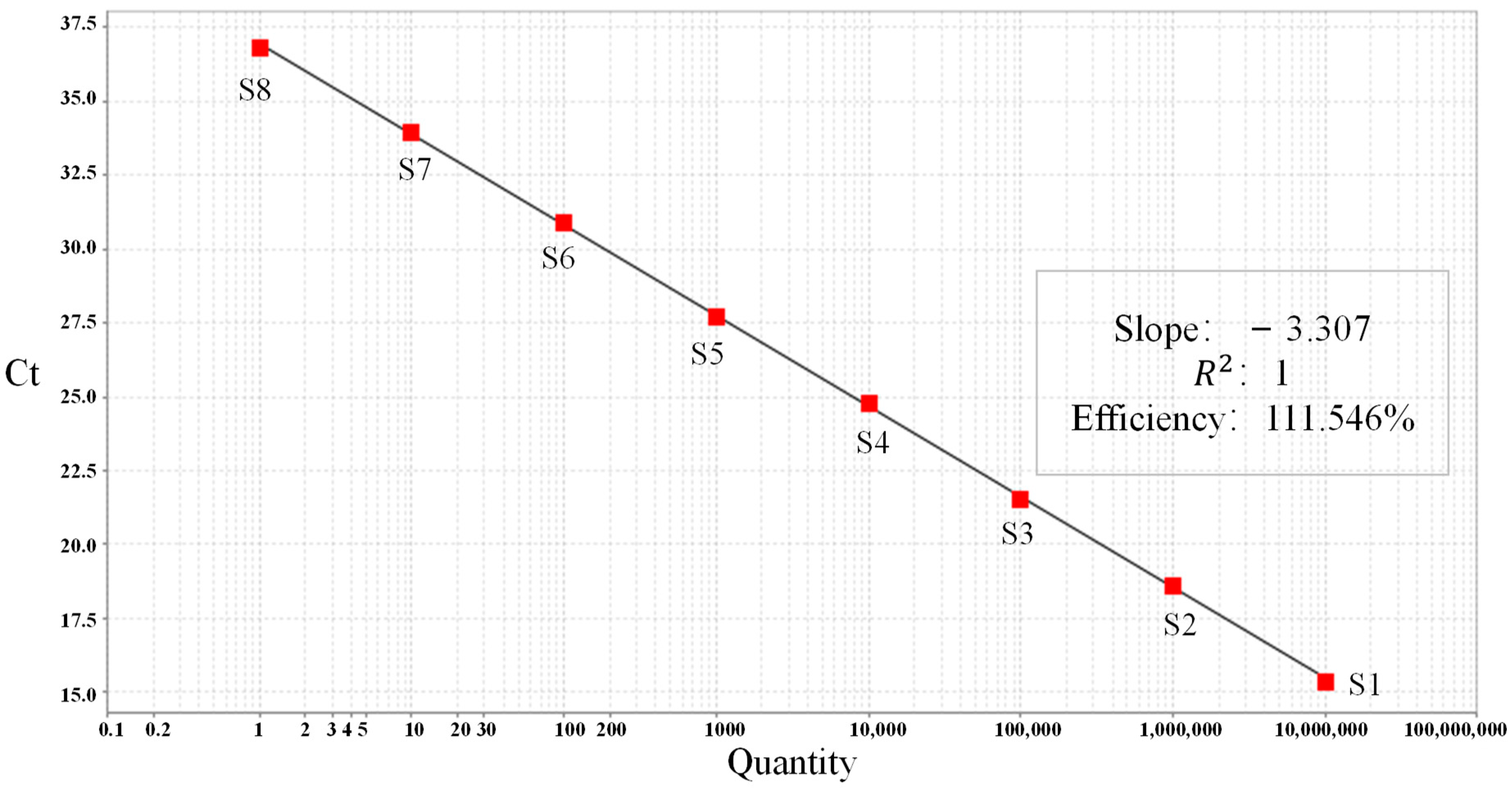
| Name | Sample Type | Concentration | Logarithm of Concentration | Measured Ct Value |
|---|---|---|---|---|
| S1 | Standard | 1.04 × 107 copies/uL | 7 | 14.934 |
| S2 | Standard | 1.06 × 106 copies/uL | 6 | 18.284 |
| S3 | Standard | 1.06 × 105 copies/uL | 5 | 21.440 |
| S4 | Standard | 1.07 × 104 copies/uL | 4 | 25.243 |
| S5 | Standard | 1.12 × 103 copies/uL | 3 | 28.333 |
| S6 | Standard | 1.10 × 102 copies/uL | 2 | 31.497 |
| S7 | Standard | 1.11 × 101 copies/uL | 1 | 34.488 |
| NTC | Negative control | 0 | / | undetermined |
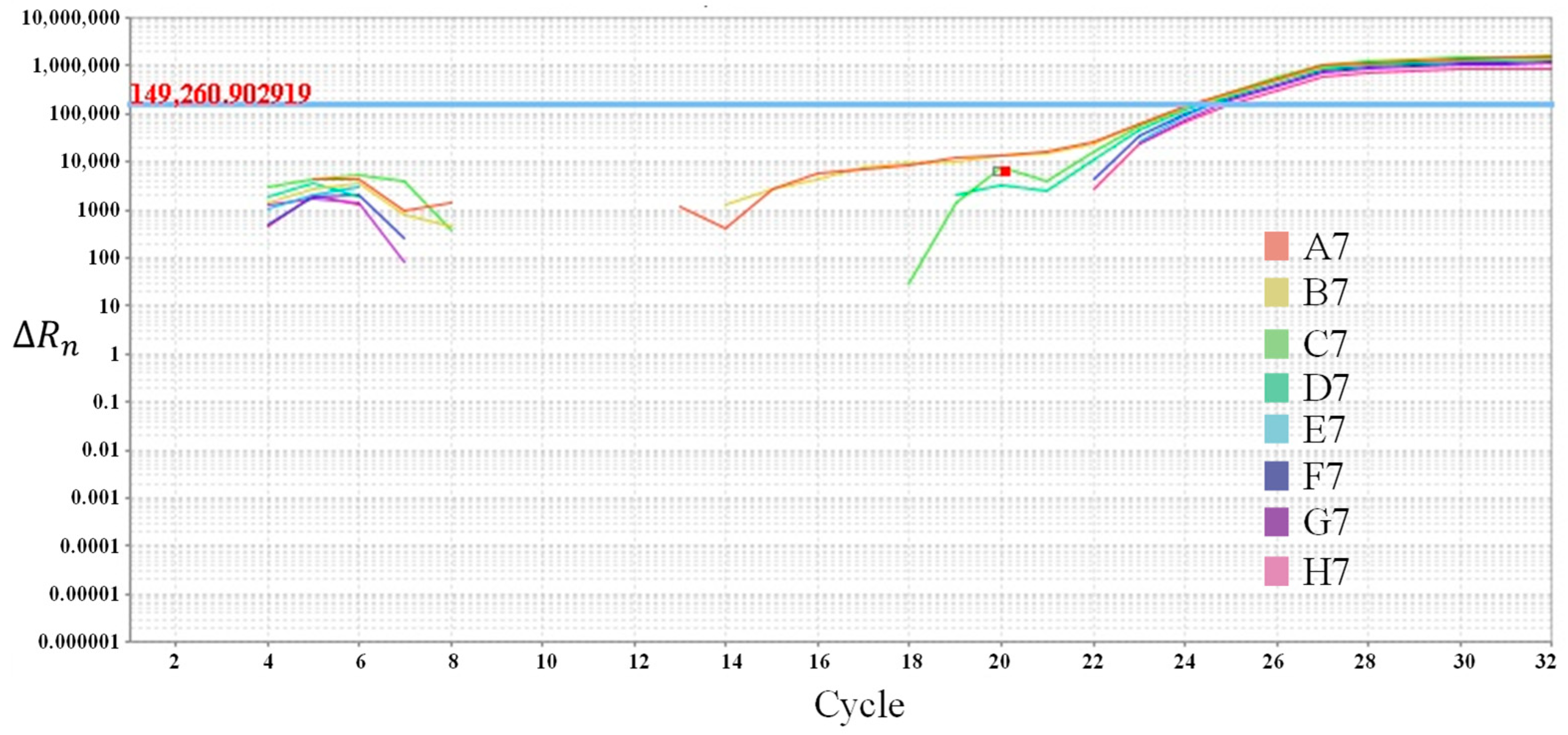
| No. | Well’s Position | Simulated Ct Value | Simulated Sample Concentrations | Simulated Sample Logarithm | Simulated Sample Type |
|---|---|---|---|---|---|
| 1 | A7 | 15 | 1.0 × 107 copies/uL | 7 | S1 |
| 2 | B7 | 18 | 1.0 × 106 copies/uL | 6 | S2 |
| 3 | C7 | 21 | 1.0 × 105 copies/uL | 5 | S3 |
| 4 | D7 | 24 | 1.0 × 104 copies/uL | 4 | S4 |
| 5 | E7 | 27 | 1.0 × 103 copies/uL | 3 | S5 |
| 6 | F7 | 30 | 1.0 × 102 copies/uL | 2 | S6 |
| 7 | G7 | 33 | 1.0 × 101 copies/uL | 1 | S7 |
| 8 | H7 | 36 | 1.0 × 100 copies/uL | 0 | S8 |
| Probe Position | Pre-Set Simulated Ct Value | Experimentally Measured Ct Value |
|---|---|---|
| A7 | 15 | 15.369 |
| B7 | 18 | 18.592 |
| C7 | 21 | 21.509 |
| D7 | 24 | 24.795 |
| E7 | 27 | 27.676 |
| F7 | 30 | 30.882 |
| G7 | 33 | 33.973 |
| H7 | 36 | 36.831 |
| Probe Position | A7 | B7 | C7 | D7 | E7 | F7 | G7 | H7 |
|---|---|---|---|---|---|---|---|---|
| Measured Ct value | 24.18 | 24.33 | 24.22 | 24.45 | 24.57 | 24.67 | 24.78 | 24.82 |
| Average value | 24.50 | |||||||
| Uniformity of Ct | 0.64 | |||||||
| Precision of Ct | 1.01% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Liu, X.; Xiao, X. Physical Simulation-Based Calibration for Quantitative Real-Time PCR. Appl. Sci. 2024, 14, 5031. https://doi.org/10.3390/app14125031
Zhu T, Liu X, Xiao X. Physical Simulation-Based Calibration for Quantitative Real-Time PCR. Applied Sciences. 2024; 14(12):5031. https://doi.org/10.3390/app14125031
Chicago/Turabian StyleZhu, Tianyu, Xin Liu, and Xinqing Xiao. 2024. "Physical Simulation-Based Calibration for Quantitative Real-Time PCR" Applied Sciences 14, no. 12: 5031. https://doi.org/10.3390/app14125031
APA StyleZhu, T., Liu, X., & Xiao, X. (2024). Physical Simulation-Based Calibration for Quantitative Real-Time PCR. Applied Sciences, 14(12), 5031. https://doi.org/10.3390/app14125031







