Abstract
Solid-State fermentation (SSF) is a valuable process used for the enhancement of the nutritional profile of agro-industrial by-products. The main objective of the present study concerns the exploitation of a mixture consisting of Cottonseed Cake (CSC) and Lathyrus clymenum pericarp (LCP) at a ratio of 80–20% w/w, which was utilized as substrate for the initiated by Pleurotus ostreatus SSF process. The final goal is the improvement of their nutritional value and the parallel reduction in their gossypol content. The obtained results revealed a statistically significant increase (p < 0.05) in protein content by 34.91%, while 1,3-1,6 β-glucans exceeded a 5-fold statistically significant increment (p < 0.05) at Day 11. Furthermore, lignin was reduced significantly (−26.71%) at Day 11. Free gossypol’s presence was lowered by 12.45%, while SSF presented a profound effect concerning the total gossypol level since the latter underwent a statistically significant reduction (p < 0.05) that exceeded 9-fold at Day 11. The study herein highlights SSF’s efficiency as a potential means to reduce free and total gossypol content with a parallel upgrade of its nutritional value. The fermentation outcome reveals its potential as a feed supplement and contributes to the reduction in the environmental footprint within the framework of a circular economy.
1. Introduction
Cotton (Gossypium) is a globally cultivated annual non-edible crop. Its cultivation is expansive, covering an area that exceeds 80 countries globally and accounts for 2.3% of the world’s arable land [1,2]. Cotton is classified in the Gossypium genus, which belongs to the Malvaceae family. It is distinguished into two main types, namely, the cultivated and the wild, which are divided into the species G. hirsutum, G. herbaceum, G. barbadense, and G. arboretum, comprising the four most common varieties that are cultivated worldwide [1,3]. Cotton bud forms the predominant part of the plant and is considered the most valuable since it is used for the development of many products, such as textiles, edible oils, and animal feedstuffs, while it is also useful for the pharmaceutical industries [1,4,5]. Cotton fiber constitutes the major product derived from the cultivation of the respective plant and is characterized as a valuable fiber source, initiating a growing global interest in the production of increased volumes of cotton. This endeavor generates vast amounts of by-products that are inadequately disposed of, burdening the nearby environment and posing a significant environmental threat.
Legume crops belong to the Fabaceae family, which includes the Lathyrus species. They are widely known for their rich nutritional value since their seeds contain a plethora of nutrients, proteins, minerals, and vitamins [6]. The Lathyrus genus includes 200 species and subspecies that are primarily cultivated in temperate regions [7]. Among them, various species, such as Lathyrus clymenum (LC), are being utilized for human and animal nutrition, thus reaping their nutritional benefits [8]. LC is an annual plant that is mainly cultivated in the Mediterranean basin countries and more specifically in the Aegean islands of Santorini, Anafi, and Karpathos [9]. The predominant by-product of LC cultivation is the pericarp, which is composed of a complex mixture of polysaccharides such as cellulose, hemicellulose, and lignin. Despite the fact that Lathyrus clymenum pericarp (LCP) forms an energy source for ruminants, it is not considered a suitable option for exploitation as animal feed due to its inadequate nutritional profile [9,10].
Cottonseed Cake (CSC) constitutes one of the most significant by-products that is generated during cottonseed’s oil extraction in order to be harnessed in various applications, such as the production of biodiesel. CSC is highlighted for its rich nutritional profile and specifically for its crude protein content, which accounts the 34.3–48.9% of CSC [11]. Primarily, CSC was used in animal nutrition as a cheap feedstuff of high nutritional value. Soon, its wide utilization caused serious concerns for animal health because of the presence of a toxic compound called gossypol. In specific, the administration of CSC as a feedstuff with a high gossypol content led to various adverse effects in animals, including respiratory problems, weight loss, gastrointestinal problems, etc. [12].
Gossypol (C30H30O8) is a toxic compound that is classified into the phenols category. It is generated by the pigment glands that are located in many parts of the plant, such as stems, leaves, flower buds, and seeds. Gossypol displays the potential to act as a pest resistance agent for the respective plant and is simultaneously capable of exerting a toxic effect on vertebrates. Furthermore, pigment glands produce additional phenolic pigments, in lower concentrations as compared to gossypol, without provoking any important toxicological implications. Naturally, gossypol exists as two enantiomers, namely, (−) and (+) gossypol. Among them, because of its biological activity, the molecule of (−)-gossypol is removed at a lower rate, justifying its enhanced toxic profile as compared to the respective (+)-enantiomer [13,14,15,16]. Within organisms, gossypol exists in two types: Free Gossypol (FG) and Bound Gossypol (BG), both forming Total Gossypol (TG). BG is created through the formation of covalent bonds among gossypol and the free epsilon-amino groups of the amino acids lysine and arginine [16] in terms of browning or the Maillard reaction [13]. It must be noted that this particular reaction results in the depletion of amino acids’ availability, especially lysine’s, which is absorbed by animals [17]. Nevertheless, the volume of total gossypol is affected by a plethora of factors, such as weather and cultivated cotton variety [16]. Specifically, concerning the weather, it is observed that gossypol’s production is increased with the elevated amounts of rainfall, while it is negatively correlated with the temperature. Additionally, storage conditions decrease gossypol’s concentration, which is reduced upon a long period of storage [16]. Finally, it must be noted that according to the UN Food Agriculture Organization (FAO), the permitted concentration of FG must not exceed 0.045% when it is used for human nutrition, whereas according to FSSAI guidelines, the maximum presence of TG and FG is 0.6% and 1.2%, respectively [2]. From the abovementioned limitations emerge the necessity of applying specific strategies to monitor gossypol’s presence. For this purpose, the application of the Solid-State Fermentation (SSF) procedure can be considered an ideal candidate that exhibits the potential to act as a suitable, valuable, and environmentally acceptable method to address the issue of gossypol toxicity.
SSF is an eco-friendly and sustainable bioprocess that displays the ability to serve as a potential gossypol degradation agent. Moreover, the application of this procedure can be effectively employed to upgrade the nutritional value of agro-industrial by-products with the participation of suitable microorganisms such as fungi. Fungi are characterized as potent means because of their ability to deal with challenging conditions, anti-nutritional factors, and toxicity issues raised during the exploitation of the original raw material [12]. On the other hand, Pleurotus species are capable of colonizing the generated lignocellulosic by-products by secreting lignin-modifying enzymes targeting lignin’s degradation in order to upgrade their nutritional profile. Additionally, their diverse nature may exemplify their potential to break down a plethora of organic environmental compounds such as gossypol [18].
The main objective of the present study concerns the application of the SSF procedure initiated by Pleurotus ostreatus on CSC and its mixture with LCP in a ratio of 80–20% w/w, aiming to upgrade their nutritional value and simultaneously reduce their content with the extremely toxic gossypol. The final goal is their transformation into a high-added-value proteinaceous feedstuff.
2. Materials and Methods
2.1. Substrates Preparation, Inoculation, and Determination of Physicochemical Parameters
The P. ostreatus strain was utilized to achieve a successful performance of SSF on the investigated substrates composed of CSC and LCP mixtures 80–20% w/w ratio. In addition, the growth of P. ostreatus requires a high moisture content on the examined substrate of approximately 60–70%, which was achieved with a daily addition of tap water. All substrates were weighed to a final weight of 300 g, placed into glass test vessels, and sterilized at 121 °C for 15 min. The commercial name of the employed P. ostreatus strain is “White 2000 P67 LOTTO 1551 MN 01827” (Fungi SEM, La Rioja, Spain), and was obtained in a solid form.
Subsequently, the substrates underwent an inoculation process in a vertical laminar flow chamber with the addition of 5% w/w P. ostreatus strain to their surfaces. The glass test vessels were capped by the respective caps that were not sealed, in order to permit access to the atmospheric air as well as to preserve the moisture content intact in the examined substrates. Then, the inoculated substrates were placed in an incubator at 25 °C in the dark for 10 days.
The investigated substrates were subjected to freeze-drying to preserve their nutritional and enzyme content. The samples were subsequently collected in order to evaluate several physicochemical attributes, namely, proteins, total soluble sugars (TSS), reducing soluble sugars (RSS), fibers (crude fiber substances, cellulose, lignin, and ash), and β-glucan’s presence. Moisture levels and ash content were determined by applying AOAC methods [19]. Proteins were assessed by performing the Kjeldahl method, which is AOAC’s official method for the evaluation of total nitrogen [19]. The total protein content of the examined substrates was calculated by multiplying the total nitrogen content (determined by the Kjeldahl method) by a factor of 6.25. Total soluble sugars (TSS) were estimated by performing the phenol-sulfuric acid method described by Dubois et al. [20], whereas reducing soluble sugars (RSS) was determined by applying the 3,5-dinitrosalicylic acid (DNS) method developed by Miller [21]. These sugar evaluations were conducted on aqueous extracts of the samples and the results were expressed as g/100 g of glucose. The presence of crude fiber substance (CFS) was determined according to the Weende method (AOAC Official Method 978.10) [19]. Cellulose and lignin content were assessed through the acid-detergent fiber (ADF) method (AOAC Official Method 973.18) [19].
Finally, the content of bioactive compounds of 1,3-1,6 β-glucans was determined according to the MEGAZYME enzymatic assay kit (β-Glucan Assay Kit Yeast & Mushroom, Megazyme Product code: K-YBGL). All experiments were implemented in triplicates, and the respective results are expressed as g/100 g of their dry weight.
2.2. Gossypol’s Extraction
2.2.1. Preparation of Solvents and Reagents
The complexing reagent was prepared by adding 4 mL of 3-amino-l-propanol and 20 mL of glacial acetic acid. Subsequently, the prepared mixture was cooled and diluted with the addition of 200 mL of N,N,dimethylformamide (DMF). The mobile phase was prepared by mixing methanol:water to an 87:13 v/v volume by adding 0.1% phosphoric acid of 7.8 pH value. Gossypol’s standard solution was prepared with the addition of 25 mg from a standard solution of gossypol and acetic acid in 25 mL of complexing reagent.
2.2.2. Extraction of Total Gossypol (TG)
The investigated samples were milled to a particle size of 2 mm, an amount of 0.2 g was weighed, placed in a conical flask of 200 mL volume, and 25 mL of complexing reagent was added. The conical flask was placed in a water bath at 95–100 °C for a period of 30 min and then was allowed to reach room temperature. Then, 100 mL of the mobile phase reagent was added and the sample was subsequently filtered twice through Whatman No. 2 filters and a 0.45 μm Millipore Millex-FGS filter, providing the sample used for the HPLC analysis.
2.2.3. Extraction of Free Gossypol (FG)
One gram of each sample was placed in a 250 mL volume conical flask, equipped with a screw cap, and 50 mL of 70% v/v acetone in water was added, and the resulting mixture was stirred for 60 min. Then, the sample was filtered through Whatman No. 2 filters with the first 5 mL being discarded, then 20 mL of the filtrate was separated into a 100 mL volumetric flask, and 20 mL of the complexing reagent was added. The resulting mixture was transferred into a water bath at 95 °C–100 °C for 30 min, cooled to room temperature, and diluted by adding 100 mL of the mobile phase reagent. The resulting mixture was filtrated through a 0.45 μm Millipore Millex-FGS filter and subjected to HPLC analysis.
2.2.4. HPLC Analysis
All High-Performance Liquid Chromatography (HPLC) analyses were performed using an Agilent 1200 series instrument equipped with a 4.6 mm × 250 mm, 5 μm Zorbax Eclipse XBD-C18 reverse-phase column. The column was eluted with an isocratic mobile phase that consisted of methanol:water to an 87:13 v/v volume by adding 0.1% phosphoric acid of 7.8 pH value with a flow rate of 1 mL/min, and the investigated samples were injected using a manual injector (Rheodyne). A loop of 20 μL Gossypol was detected at 254 nm using an Agilent 1200 Series Diode Array detector (Model G1315B) (Santa Clara, CA, USA). The obtained data and the integration procedure were processed using the Chemstation Rev. B.02.01-SR1 software 260. The calibration curve was prepared with the addition of the following volumes of gossypol standard solution: 0.2, 0.5, 1.0, 2.0, 3.0, and 5.0 mL into 100 volumetric flasks by adding complexing reagent in order to adjust a total volume of 20 mL, and then they were placed in a water bath with a stable temperature of 95 °C for 30 min. Subsequently, samples were cooled, and their final volume was adjusted to 100 mL by adding the mobile phase solution. Ten ml of the respective samples were filtered using a 0.45 μm Millipore Millex-FGS filter (Merck, Boston, MA, USA) and then injected into the HPLC system. The total program lasted for 10 min. Total and Free gossypol were evaluated according to the equation that was obtained by the development of the standard curve. The obtained concentrations that were within the linear part were accepted, whereas the samples that displayed a concentration that was outside of the standard curve’s linear part were diluted appropriately in order to be transferred into the linear part.
2.3. Statistical Analysis
All experiments were carried out in triplicate and the obtained results are expressed as means ± standard deviation (±S.D.). Kolmogorov–Smirnov and Shapiro–Wilk tests were applied for the assessment of data normality. The differences among the examined groups were determined by performing independent and paired t-test (p < 0.05 was considered significant).
3. Results
In the present study, we exploited the feasibility of two substrates, both containing CSC as the predominant constituent, for the application of the SSF process. In specific, the investigated two substrates contained either 100% w/w of CSC or a CSC mixture with 20% w/w LCP. According to the results obtained, the SSF procedure was not successful when performed using 100% w/w CSC as substrate. This can be rationalized considering that the employed microorganism (P. ostreatus) requires an energy source in order to perform the biological processes. Therefore, for the successful implementation of SSF, it is essential to include a proportion of 20% w/w of LCP, which acts as a natural external nitrogen source, facilitating the growth of P. ostreatus and initiating the SSF process.
Figure 1 is a representative image of the substrates on which the SSF process was applied. More specifically, the substrate on the left side is composed of 100% w/w of CSC, while the substrate on the right is a CSC mixture with 20% w/w LCP. According to Figure 1, SSF was not successfully applied to the 100% w/w CSC substrate, whereas the latter procedure was successfully performed in the mixture by the addition of LCP.

Figure 1.
Representative figure of the examined substrates at the end of the process.
Table 1 contains the outcome of the following physicochemical parameter assessments: moisture, total and reducing soluble sugars, ash, proteins, and 1,3-1,6 β-glucans concentration on the first (Day 0) and last day (Day 11) of fermentation. Results indicated that moisture recorded a statistically significant increase (p < 0.05) by 15.83% during the SSF process, while the number of soluble sugars ranged from 2.53% to 4.39% between Days 0 and 11, presenting a statistically significant increment (p < 0.05) at Day 11. A similar pattern was observed for the reducing soluble sugars since they underwent a statistically significant increase (p < 0.05) by 56.67% at the end of the SSF process. Ash concentration was increased at Day 11, affording a statistically significant (p < 0.05) approximately two-fold increment. Proteins displayed similar behavior since their content ranged from 17.67% to 23.84% on Days 0 and 11, recording a statistically significant increase (p < 0.05) of 34.91%. Finally, the concentration of 1,3-1,6 β-glucans underwent a statistically significant increment (p < 0.05), which exceeded 5-fold.

Table 1.
SSF’s impact on the Physicochemical Profile of the investigated sample.
Table 2 demonstrates SSF’s impact on fibers’ profile at the examined substrate. In particular, crude fiber substances were elevated by 7.57% at Day 11, whereas cellulose content remained almost intact presenting a slight increase by 4.10% at the end of the process. On the other hand, lignin content underwent a statistically significant reduction (p < 0.05) by 26.71% at the end of the SSF procedure.

Table 2.
Assessment of the Fiber Substances’ concentration on the investigated sample.
Table 3 presents the impact of the SSF process initiated by P. ostreatus on gossypol’s profile between Days 0 and 11. In specific, the amount of TG displayed a statistically significant reduction by approximately 9-fold at Day 11, whereas FG’s content was slightly decreased at the end of the process, ranging from 2.73 to 2.39 ppm.

Table 3.
SSF’s impact on gossypol’s profile at the investigated substrate.
Figure 2 displays the concentration of TG on Days 0 and 11 of the SSF process. In the abovementioned chromatogram, the blue peak with a Retention Time of 10.102 represents the concentration of TG at the beginning of the procedure (Day 0). The orange peak with a Retention Time of 10.125 depicts the concentration of TG at the end of the SSF process (Day 11). It is evident that the concentration of TG in the fermentation outcome has been reduced, highlighting the effectiveness of the SSF process as a potent means for the degradation of gossypol.
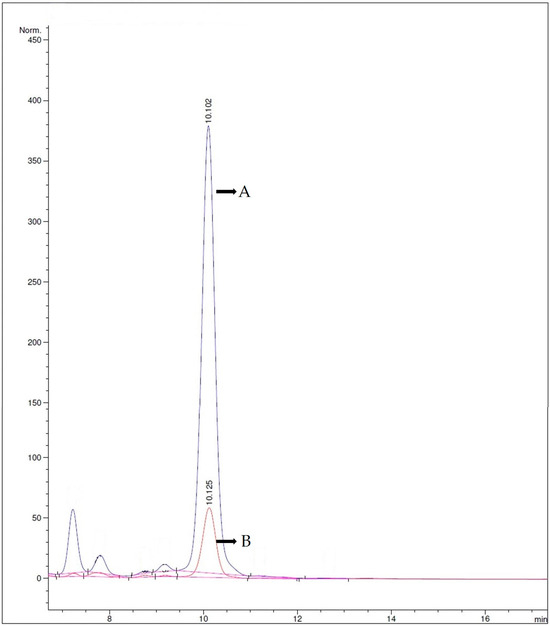
Figure 2.
Representative chromatogram of Total Gossypol (R.T. ≈ 10.1) at Day 0 (chrom. A) and at the end of the SSF procedure (Day 11) (chrom. B).
4. Discussion
The main objective of the present study concerned the application of a biotechnological procedure suitable to utilize CSC, targeting the reduction of gossypol. According to some preliminary experiments that we performed, and in accordance with the existing literature, it was shown that the addition of LCP, which is a by-product rich in nitrogen, promotes the conditions that facilitate mycelium’s colonization and, therefore, the application of the SSF process. Therefore, according to the latter, a substrate was prepared by adding LCP at a ratio of 20% w/w since the specific addition displayed the best results. The final goal concerned the evaluation of SSF’s technique on a substrate consisting of a CSC-LCP (80–20% w/w) mixture, focusing on the reduction of its gossypol content and the parallel enhancement of its nutritional value in order to be transformed into a suitable material that could potentially be used as a feed supplement in animals’ diets.
The nutritional value of the examined substrate was improved after employing the SSF procedure, which is attested by the evaluation of its physicochemical parameters, especially for its proteins and 1,3-1,6 β-glucans content.
Moisture is a very important attribute that affects SSF’s efficiency, since it is closely associated with nutrient availability [22]. In specific, a reasonable presence of moisture exerts a beneficial effect on SSF by promoting fungal growth, nutrient transfer, and enzymatic activity, whereas a high moisture content is capable of mitigating the substrate’s porosity, limiting oxygen’s transfer, and facilitating possible contamination, since the employed substrate forms an ideal environment for the growth of unfavorable microorganisms. On the other hand, a low moisture content is an aggravating factor for the successful implementation of the SSF process since accessibility and solubility are confined. Hence, the employed microorganism is not able to absorb the required amounts of the necessary nutrients, resulting in poor fungal growth [23]. It must be noted however that at the end of the SSF procedure, moisture content was found to be increased. This can be rationalized considering that during SSF implementation, the water balance is affected by several factors such as the presence of required water, which is essential for starch’s hydrolysis, the production of metabolic water, the absorption of intracellular water through biomass synthesis, and the evaporation of water assisted by the metabolic heat [24]. According to Fan et al. [25], a moisture content of 60% is considered the optimum value for a successful SSF application. Our results are in line with previous literature reports, since herein the moisture content reached 68% at the end of the SSF procedure.
One of the most common strategies adopted for the efficient utilization of lignocellulosic materials includes the application of SSF by employing suitable microorganisms, namely, white-rot fungi. The latter is capable of consuming the available nutrients included in the respective materials, targeting the production of new organic compounds that can be transferred to the whole biomass through fungal activity, thus enhancing their nutritional value [26]. P. ostreatus displays the capability to act as a lignocellulosic degradation agent since it is composed of extracellular enzymes that can modify the lignocellulosic materials. In the present work, the amount of Crude Fiber Substances (CFS) was increased during the process implementation. A possible explanation that justifies the increased level of CFS is connected with the parallel increase in cellulose content [10]. Furthermore, CFS’s elevated presence could probably be rationalized by microbial activity. In specific, during the SSF procedure, the employed microorganisms display the ability to convert into assimilable forms the substrate’s molecules in order to fulfill their biological requirements. Peptidoglycan, which is included among these assimilable molecules, is a polysaccharide that is considered one of the main components of the bacterial wall. At the end of the SSF procedure, the respective microorganism is not separated from the biomass, affording a possible increase in CFS content as a consequence of the respective molecules’ conversion into peptidoglycan via the biological action of involved microorganism [27,28].
The observed reduction in cellulose content is presumably justified considering the different ratios of fiber fractions that are present in the initial raw materials. Additionally, cellulose’s reduction is possibly associated with the nutritional composition of the studied substrate since the latter displays the potential to provide the participating microorganism with the required nutrients such as N, P, and K, leading to an extensive degradation [29].
The enzymatic activity of P. ostreatus is responsible for the reduction of the unwanted lignin content, probably due to the action of lignin-modifying enzymes [30]. Furthermore, according to Ritota and Manzi [30], Pleurotus spp. can be characterized as selective degraders since they degrade lignin more efficiently as compared to cellulose. TSS and RSS constitute an energy source for the employed microorganism. They observed that the content at Day 11 was possibly correlated to the excessed secretion of enzymes that are used to facilitate the degradation process [31].
According to Rajesh et al. [32], the elevated ash content during the SSF process is presumably connected with the enzymatic activity of P. ostreatus, which facilitates the reduction of organic matter on the investigated substrate. Moreover, it must be noted that the increased ash presence is of high importance since it can be considered a marker indicating a significant mineral content [33].
The nutritional value of mushrooms is highlighted by the presence of various bioactive compounds, including 1,3-1,6 β-glucans, which are well known for their health-beneficial effects on both humans and animals. Herein, the concentration of 1,3-1,6 β-glucans increased considerably at the end of the procedure; an outcome connected with P. ostreatus’ biochemical activity that transforms a substrate’s nutrients into organic compounds.
The protein content of the investigated substrate constitutes the most significant attribute. SSF has the potential to act as a valuable procedure since its efficacy can be attested by the bioconversion of the examined substrate into protein-enriched material. Considering our results, protein content increases during the SSF procedure, presumably as a result of the excessed nitrogen assimilation in terms of the aerobic fermentation initiated by P. ostreatus, which is the employed microorganism [34]. An additional reason justifying the increased protein profile concerns the secretion of various proteinaceous extracellular enzymes that display the ability to facilitate degradation during the procedure [35]. According to Fanchini Terrasan and Carmona [36], the elevated protein concentration could possibly be connected with the fungal growth and colonization of the employed microorganism [37]
As has already been pointed out, CSC is a by-product with serious constraints and obstacles with respect to it being exploited as a feed supplement, especially for non-ruminants, all connected to gossypol’s presence. This has initiated the implementation of various research endeavors toward the development of effective practices for the reduction of the contained FG and TG in order to be suitable for utilization in the animal sector. For this purpose, various strategies have been considered, among which the SSF presents an intriguing case, since it was determined as one of the most efficient. Du et al. [17] reported that the performance of the SSF process on cottonseed hulls initiated by the Pleurotus species reduced the presence of FG. Grewal et al. [12] stated that the fermentation by P. chrysosporium, T. reesei, A. niger, and their consortium displayed a significant reduction of gossypol in the CSC, which could be presumably explained by the interaction between the secreted enzymes of the used consortium. The latter enzymes could be a potent means for the degradation and transformation of highly polymerized carbohydrates and proteins in CSC. Additionally, according to Zhang et al. [38], the application of the SSF process on cottonseed meal by B. coagulans S17 revealed a significant reduction in FG’s presence. Wang et al. [39] described that L. agilis WWK129, which was firstly isolated from the rumen contents, could be characterized as a potential gossypol degradation agent since the latter decreased gossypol’s levels in cottonseed meal up to 80% by applying anaerobic fermentation for 5 days. Mageshwaran et al. [40] reported that the performance of the SSF procedure initiated by a mixed fungal culture consisting of C. tropicalis and S. cerevisiae on CSC displayed a reduced gossypol profile, including FG and BG, with a parallel upgrade of the latter’s physicochemical properties.
In our study, the amount of unwanted FG and TG was reduced as a consequence of SSF utilization, since P. ostreatus secretes various specific enzymes (cellulolytic, amylases, proteases, lipolytic) that are capable of releasing BG and breaking down the FG, resulting in the reduction of both FG and TG [40,41]. Another possible explanation that could justify the observed reduction in the FG level is connected with microbial activity, since the utilized microorganism has the potential to degrade FG or convert FG into BG [42]. Additionally, it has been reported that this conversion of FG to BG facilitates the reduction in overall toxicity, probably due to the microbial processes that are carried out during SSF. The observed significant reduction in the TG level at the end of the procedure can be rationalized considering that FG is consumed by P. ostreatus since it constitutes a carbon source that supports its biochemical activities [43]. It must be noted however that several literature reports indicate that BG does not have a direct impact on animals’ health since it is not assimilable by the animals through the digestive tract. Nevertheless, Soares Neto et al. [44] stated that although BG is considered a non-toxic compound, its presence poses a significant threat, especially in high concentrations since during the digestion period where it can be transformed into its free form. Therefore, our study is of high significance since our results reveal a significant reduction in TG levels, exceeding 9-fold at the end of the process, which also results in BG’s depletion.
5. Conclusions
The present study highlights the significance of the SSF procedure as a sustainable, environmentally friendly, and efficient process revealing the latter’s potential to deplete the presence of the anti-nutritional factors of the CSC-LCP (80–20% w/w) mixture. Notably, SSF’s application enhanced the nutritional value of the examined substrate since proteins were found to be significantly increased by 34.91%, whereas the amount of their 1,3-1,6 β-glucans exceeded the 5-fold increment. Additionally, the SSF procedure resulted in the reduction of the substrate’s anti-nutritional factors such as lignin and FG by 26.71% and 12.45%, respectively, while the amount of TG was also reduced significantly (9-fold). Overall, the SSF procedure can be considered a viable tool for the bioconversion of CSC-LCP (80–20% w/w) by-products mixture into a novel proteinaceous animal feed supplement, evading the toxicity constraints that are caused by the gossypol’s presence with a parallel contribution to the reduction in the environmental footprint in terms of a circular economy.
Author Contributions
Conceptualization, C.E., G.M., S.A.H. and D.A.; methodology, C.E., I.L., E.K., G.S., G.M., S.A.H. and D.A.; software, C.E., G.M., S.A.H. and D.A.; validation, C.E., I.L., E.K., G.S., G.M., S.A.H. and D.A.; formal analysis, C.E., I.L., E.K., G.S., G.M., S.A.H. and D.A.; investigation, C.E., I.L., E.K., G.S., G.M., S.A.H. and D.A.; data curation, C.E., I.L., E.K., G.S., G.M., S.A.H. and D.A.; writing—original draft preparation, C.E., I.L., E.K. and G.S.; writing—review and editing, G.M., S.A.H. and D.A.; supervision, S.A.H. and D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Egbuta, M.A.; McIntosh, S.; Waters, D.L.; Vancov, T.; Liu, L. Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant. Molecules 2017, 22, 93. [Google Scholar] [CrossRef] [PubMed]
- Kadam, D.M.; Kasara, A.; Parab, S.S.; Mahawar, M.K.; Kumar, M.; Arude, V. Optimization of Process Parameters for Degossypolisation of De-Oiled Cottonseed Cake by Response Surface Methodology (RSM). Food Humanit. 2023, 1, 210–218. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, D.; Zhang, X.; Lin, Z. Isolation, Characterization and Mapping of Genes Differentially Expressed during Fibre Development between Gossypium hirsutum and G. Barbadense by cDNA-SRAP. J. Genet. 2013, 92, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ezuruike, U.F.; Prieto, J.M. The Use of Plants in the Traditional Management of Diabetes in Nigeria: Pharmacological and Toxicological Considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef]
- Rogers, G.M.; Poore, M.H.; Paschal, J.C. Feeding Cotton Products to Cattle. Vet. Clin. Food Anim. Pract. 2002, 18, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Protein Isolates from Two Mediterranean Legumes: Lathyrus clymenum and Lathyrus annuus. Chemical Composition, Functional Properties and Protein Characterisation. Food Chem. 2010, 122, 533–538. [Google Scholar] [CrossRef]
- Zawieja, B.; Rybiński, W.; Nowosad, K.; Bocianowski, J. Assessment of Lathyrus Species Accession Variability Using Visual and Statistical Methods. Pak. J. Bot. 2018, 50, 2277–2284. [Google Scholar]
- Tamburino, R.; Guida, V.; Pacifico, S.; Rocco, M.; Zarelli, A.; Parente, A.; Di Maro, A. Nutritional Values and Radical Scavenging Capacities of Grass Pea (“Lathyrus sativus” L.) Seeds in Valle Agricola District, Italy. Aust. J. Crop Sci. 2012, 6, 149–156. [Google Scholar]
- Ralli, P.; Koutis, K.; Drosou, I.; Alexandris, C. The in Situ Conservation of ‘Arakas’ (Lathyrus clymenum L.) in Thera Island throughout the Centuries. Landraces 2020, 20, 34. [Google Scholar]
- Eliopoulos, C.; Markou, G.; Chorianopoulos, N.; Haroutounian, S.A.; Arapoglou, D. Transformation of Mixtures of Olive Mill Stone Waste and Oat Bran or Lathyrus clymenum Pericarps into High Added Value Products Using Solid State Fermentation. Waste Manag. 2022, 149, 168–176. [Google Scholar] [CrossRef]
- Gadelha, I.; Rangel, A.H.d.N.; Silva, A.; Soto-Blanco, B. Effects of Gossypol on Animal Reproduction. Acta Vet. Bras. 2011, 5, 129–135. [Google Scholar]
- Grewal, J.; Tiwari, R.; Khare, S. Secretome Analysis and Bioprospecting of Lignocellulolytic Fungal Consortium for Valorization of Waste Cottonseed Cake by Hydrolase Production and Simultaneous Gossypol Degradation. Waste Biomass Valorization 2020, 11, 2533–2548. [Google Scholar] [CrossRef]
- Soto-Blanco, B. Gossipol e Fatores Antinutricionais Da Soja. In Toxicologia Aplicada à Medicina Veterinária; Manole: São Paulo, Brazil, 2008; pp. 531–545. [Google Scholar]
- Hron, R.; Kim, H.; Calhoun, M.; Fisher, G. Determination of (+)-,(−)-, and Total Gossypol in Cottonseed by High-Performance Liquid Chromatography. J. Am. Oil Chem. Soc. 1999, 76, 1351–1355. [Google Scholar] [CrossRef]
- Lordelo, M.; Davis, A.; Calhoun, M.; Dowd, M.; Dale, N. Relative Toxicity of Gossypol Enantiomers in Broilers. Poult. Sci. 2005, 84, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, I.C.N.; Fonseca, N.B.S.; Oloris, S.C.S.; Melo, M.M.; Soto-Blanco, B. Gossypol Toxicity from Cottonseed Products. Sci. World J. 2014, 2014, 231635. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Zhou, Y.; Zhang, J.; Han, S.; Liu, X.; Yuan, C.; Ndayisenga, F.; Shi, J.; Zhang, B. Optimized Strategy Valorizing Unautoclaved Cottonseed Hull as Ruminant Alternative Feeds via Solid-State Fermentation: Detoxifying Polyphenols, Restraining Hazardous Microflora and Antibiotic-Resistance Gene Hosts. Environ. Technol. Innov. 2022, 28, 102937. [Google Scholar] [CrossRef]
- Rajarathnam, S.; Shashirekha, M.; Bano, Z. Biodegradation of Gossypol by the White Oyster Mushroom, Pleurotus florida, during Culturing on Rice Straw Growth Substrate, Supplemented with Cottonseed Powder. World J. Microbiol. Biotechnol. 2001, 17, 221–227. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 14th ed.; Association of Official Analytical Chemists: Washigton, DC, USA, 1995. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Abdullah, J.J.; Greetham, D.; Pensupa, N.; Tucker, G.A.; Du, C. Optimizing Cellulase Production from Municipal Solid Waste (MSW) Using Solid State Fermentation (SSF). J. Fundam. Renew. Energy Appl. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal Solid-State Fermentation and Various Methods of Enhancement in Cellulase Production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Nagel, F.J.; Tramper, J.; Bakker, M.S.; Rinzema, A. Model for On-line Moisture-content Control during Solid-state Fermentation. Biotechnol. Bioeng. 2001, 72, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Pandey, A.; Mohan, R.; Soccol, C. Use of Various Coffee Industry Residues for the Cultivation of Pleurotus Ostreatus in Solid State Fermentation. Acta Biotechnol. 2000, 20, 41–52. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic Residues: Biodegradation and Bioconversion by Fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Eze, S.; Ibe, O. Effect of Fermentation on the Nutritive Value of B. eurycoma “Achi”. J. Chem. 2005, 30, 1–5. [Google Scholar]
- Peace, O.; Aladesanmi, A. Effect of Fermentation on Some Chemical and Nutritive Properties of Berlandier Nettle Spurge (Jatropha cathartica) and Physic Nut (Jatropha curcas) Seeds. Pak. J. Nutr. 2008, 7, 292–296. [Google Scholar]
- Anele, U.Y.; Anike, F.N.; Davis-Mitchell, A.; Isikhuemhen, O.S. Solid-State Fermentation with Pleurotus ostreatus Improves the Nutritive Value of Corn Stover-Kudzu Biomass. Folia Microbiol. 2021, 66, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food by-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Kremmyda, A.; Haroutounian, S.A.; Arapoglou, D. Enrichment of Pistachio Shell with Olive Mill Waste or Lathyrus clymenum Pericarp Mixtures via Solid State Fermentation with Pleurotus ostreatus. Fermentation 2022, 8, 59. [Google Scholar] [CrossRef]
- Rajesh, N.; Imelda-Joseph; Paul Raj, R. Value Addition of Vegetable Wastes by Solid-State Fermentation Using Aspergillus niger for Use in Aquafeed Industry. Waste Manag. 2010, 30, 2223–2227. [Google Scholar] [CrossRef]
- Okpako, C.; Ntui, V.; Osuagwu, A.; Obasi, F. Proximate Composition and Cyanide Content of Cassava Peels Fermented with Aspergillus niger and Lactobacillus rhamnosus. J. Food Agric. Environ. 2008, 6, 251. [Google Scholar]
- Akinfemi, A.; Adu, O.; Doherty, F. Conversion of Sorghum Stover into Animal Feed with White-Rot Fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Afr. J. Biotechnol. 2010, 9, 1706–1712. [Google Scholar] [CrossRef]
- Oseni, O.; Akindahunsi, A. Some Phytochemical Properties and Effect of Fermentation on the Seed of Jatropha curcas L. Am. J. Food Technol. 2011, 6, 158–165. [Google Scholar] [CrossRef]
- Fanchini Terrasan, C.R.; Carmona, E. Solid-State Fermentation of Brewer’s Spent Grain for Xylanolytic Enzymes Production by Penicillium janczewskii and Analyses of the Fermented Substrate. Biosci. J. 2015, 31, 1826–1836. [Google Scholar] [CrossRef]
- Darwish, G.A.; Bakr, A.; Abdallah, M. Nutritional Value Upgrading of Maize Stalk by Using Pleurotus ostreatus and Saccharomyces cerevisiae in Solid State Fermentation. Ann. Agric. Sci. 2012, 57, 47–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, D.; Liu, L.; Chang, Z.; Peng, N. Effective Gossypol Removal from Cottonseed Meal through Optimized Solid-State Fermentation by Bacillus coagulans. Microb. Cell Factories 2022, 21, 252. [Google Scholar] [CrossRef]
- Wang, W.-K.; Li, W.-J.; Wu, Q.-C.; Wang, Y.-L.; Li, S.-L.; Yang, H.-J. Isolation and Identification of a Rumen Lactobacillus Bacteria and Its Degradation Potential of Gossypol in Cottonseed Meal during Solid-State Fermentation. Microorganisms 2021, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Mageshwaran, V.; Satankar, V.; Paul, S. Solid-State Fermentation for Gossypol Detoxification and Nutritive Enrichment of Cottonseed Cake: A Scale-Up of Batch Fermentation Process. BioResources 2024, 19, 1107. [Google Scholar] [CrossRef]
- Shaikh, A.; Kathe, A.; Mageshwaran, V. Reduction of Gossypol and Increase in Crude Protein Level of Cottonseed Cake Using Mixed Culture Fermentation. Asia-Pac. J. Sci. Technol. 2014, 19, 67–73. [Google Scholar]
- Wang, X.; Tang, J.; Yao, X.; Wu, Y.; Sun, H.; Xu, Y. Effect of Bacillus Cereus Br on Bacterial Community and Gossypol Content during Fermentation in Cottonseed Meal. Afr. J. Microbiol. Res. 2012, 6, 6537–6544. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Punia, S.; Grasso, S.; Arrutia, F.; Choudhary, J.; Singh, S.; Verma, P.; Mahapatra, A.; Patil, S. Cottonseed: A Sustainable Contributor to Global Protein Requirements. Trends Food Sci. Technol. 2021, 111, 100–113. [Google Scholar] [CrossRef]
- Soares Neto, C.B.; Conceicao, A.A.; Gomes, T.G.; de Aquino Ribeiro, J.A.; Campanha, R.B.; Barroso, P.A.V.; Machado, A.E.V.; Mendonca, S.; De Siqueira, F.G.; Miller, R.N.G. A Comparison of Physical, Chemical, Biological and Combined Treatments for Detoxification of Free Gossypol in Crushed Whole Cottonseed. Waste Biomass Valorization 2021, 12, 3965–3975. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).