Microbial Biopolymers: From Production to Environmental Applications—A Review
Abstract
:1. Introduction
2. Microbial Polymers
2.1. Bacterial Cellulose
2.2. Microbial Hyaluronic Acid
2.3. Xanthan Gum
2.4. Microbial β-Glucan
2.5. Alginate
2.6. Poly (D/L-γ-Glutamic Acid) (γ-PGA)
2.7. Pullulan
2.8. Chitosan
2.9. Polyhydroxyalkanoates (PHA) and Polyhydroxybutyrate (PHB)
2.10. Polylactide (PLA)
3. Characteristics of Microbial Biopolymers
- (i)
- (ii)
- Microbial biopolymers such as proteins or polypeptides act as catalysts in numerous biochemical reactions. This helps to decline the activation energy when used in the chemical reaction. So, these catalysts enable microorganisms to breakdown nutrients and produce essential nutrients for cellular pathways [239].
- (iii)
- They are storage factories that reserve energy and permit microorganisms to acclimate extra energy in case of metabolic demand [240].
- (iv)
- (v)
- Microbial biopolymers act as intermediaries for transmission between microorganisms and their environment. They assist in receiving and sending signals to organize their behaviors and their response to alerting environments [242].
4. Factors Influencing the Production of Microbial Biopolymers
4.1. Intrinsic Factors
4.2. Extrinsic Factors
5. Role of Microbial Biopolymers in Wastewater Treatment Processes
6. Applications of Microbial Biopolymers
6.1. Microbial Biopolymers as Adsorbents
6.2. Microbial Biopolymers as Coagulants and Flocculants
6.3. Microbial Biopolymers as Filters in Membrane Processes
6.4. Bioremediation and Soil Stabilization
7. Challenges and Future Perspectives of Microbial Biopolymers in Environmental Applications
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roa, K.; Oyarce, E.; Boulett, A.; Alsamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Sivakami, M.S.; Gomathi, T.; Venkatesan, J.; Jeong, H.S.; Kim, S.K.; Sudha, P.N. Preparation and characterization of nano chitosan for treatment wastewaters. Int. J. Biol. Macromol. 2013, 57, 204–212. [Google Scholar] [CrossRef]
- FAO. Background Notes on Sustainable, Productive and Resilient Agro-Food Systems: Value Chains, Human Capital, and the 2030 Agenda; FAO: Rome, Italy, 2019; pp. 1–70. [Google Scholar]
- FAO. Extent of food losses and waste. In Global Food Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2014; pp. 1–37. [Google Scholar]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Fradinho, J.; Allegue, L.D.; Ventura, M.; Melero, J.A.; Reis, M.A.M.; Puyol, D. Up-scale challenges on biopolymer production from waste streams by Purple Phototrophic Bacteria mixed cultures: A critical review. Bioresour. Technol. 2021, 327, 124820. [Google Scholar] [CrossRef]
- Das, S.; Das, P.; Lal, B. Development of Microbial Biopolymers: The Eco-friendly Sustainable Products for Environmental Applications. In Novel Polymeric Materials for Environmental Applications; World Scientific: Singapore, 2022; pp. 1–20. [Google Scholar]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef]
- Horue, M.; Berti, I.R.; Cacicedo, M.L.; Castro, G.R. Microbial production and recovery of hybrid biopolymers from wastes for industrial applications—A review. Bioresour. Technol. 2021, 340, 125671. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Ghosh, S.; Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Pathak, S.K.; Edinur, H.A.; Pati, S.; Ray, R.R. Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials. Polymers 2021, 13, 1242. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Faria, M.; Cunha, C.; Gomes, M.; Mendonça, I.; Kaufmann, M.; Ferreira, A. Cordeiro, Bacterial cellulose biopolymers: The sustainable solution to water-polluting microplastics. Water Res. 2022, 222, 118952. [Google Scholar] [CrossRef]
- Fatima, A.; Ortiz-Albo, P.; Neves, L.A.; Nascimento, F.X.; Crespo, J.G. Biosynthesis and characterization of bacterial cellulose membranes presenting relevant characteristics for air/gas filtration. J. Membr. Sci. 2023, 674, 121509. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Collins, C.; Saha, S.K.; Major, I.; Murray, P. Sustainable production and pharmaceutical applications of β-glucan from microbial sources. Microbiol. Res. 2023, 274, 127424. [Google Scholar] [CrossRef] [PubMed]
- Utama, G.L.; Dio, C.; Sulistiyo, J.; Chye, F.Y.; Lembong, E.; Cahyana, Y.; Verma, D.K.; Thakur, M.; Patel, A.R.; Singh, S. Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi J Biol Sci. 2021, 28, 6765–6773. [Google Scholar] [PubMed]
- Volpi, N.; Maccari, F. Purification and characterization of hyaluronic acid from the mollusc bivalve Mytilus galloprovincialis. Biochimie 2003, 85, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [PubMed]
- Mohapatra, S.; Pattnaik, S.; Maity, S.; Sharma, S.; Akhtar, J.; Pati, S.; Samantaray, D.P.; Varma, A. Comparative analysis of PHAs production by Bacillus megaterium OUAT 016 under submerged and solid-state fermentation. Saudi J Biol Sci. 2020, 27, 1242–1250. [Google Scholar] [CrossRef]
- Ramya, R.; Devi, S.; Manikandan, A.; Kannan, V.R. Standardization of biopolymer production from seaweed associative bacteria. Int. J. Biol. Macromol. 2017, 102, 550–564. [Google Scholar]
- Parati, M.; Khalil, I.; Tchuenbou-Magaia, F.; Adamus, G.; Mendrek, B.; Hill, R.; Radecka, I. Building a circular economy around poly(D/L-γ-glutamic acid)- a smart microbial biopolymer. Biotechnol. Adv. 2022, 6, 108049. [Google Scholar] [CrossRef]
- Kedia, G.; Hill, D.; Hill, R.; Radecka, I. Production of Poly-γ-Glutamic Acid by Bacillus subtilis and Bacillus licheniformis with Different Growth Media. J. Nanosci. Nanotechnol. 2010, 10, 5926–5934. [Google Scholar] [CrossRef]
- Rosalam, S.; England, R. Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzyme Microb. Technol. 2006, 39, 197–207. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Gao, M.-J.; Zhu, L.; Zhan, X.-B. High production of xanthan gum by a glycerol-tolerant strain Xanthomonas campestris WXLB-006. Prep. Biochem. Biotechnol. 2017, 47, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Tenorio, F.; Giraldo-Estrada, C. Characterization and chemical modification of pullulan produced from a submerged culture of Aureobasidium pullulans ATCC 15233. Polym. Test. 2022, 114, 107686. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Mir, S.A.; Khanday, F.A. Pullulan production by Aureobasidium pullulans MTCC 1991 from apple pomace and its characterization. Food Biosci. 2023, 51, 102254. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, K.; Zhao, S.; Wang, W.; Ru, X.; Song, J.; Cong, H.; Yang, Q. Screening and identification of a strain of Aureobasidium pullulans and its application in potato starch industrial waste. Environ. Res. 2022, 214, 113947. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Fanelli, F.; Sisti, M. Role of melanin in the black yeast fungi Aureobasidium pullulans and Zalaria obscura in promoting tolerance to environmental stresses and to antimicrobial compounds. Fungal Biol. 2022, 126, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B.C.; Schrezenmeir, J.; Knutsen, S.H. Effects of orally administered yeast-derived beta-glucans: A review. Mol. Nutr. Food Res. 2014, 58, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Abdeshahian, P.; Ascencio, J.J.; Philippini, R.R.; Antunes, F.A.F.; Santos, J.C.D.; da Silva, S.S. Utilization of sugarcane straw for production of β-glucan biopolymer by Lasiodiplodia theobromae CCT 3966 in batch fermentation process. Bioresour. Technol. 2020, 314, 123716. [Google Scholar] [CrossRef]
- Kayanna, N.; Suppavorasatit, I.; Bankeeree, W.; Lotrakul, P.; Punnapayak, H.; Prasongsuk, S. Production of prebiotic aubasidan-like β-glucan from Aureobasidium thailandense NRRL 58543 and its potential as a functional food additive in gummy jelly. LWT 2022, 163, 113617. [Google Scholar] [CrossRef]
- Almeida, R.R.; Pinto, N.A.R.; Soares, I.C.; Ferreira, L.B.C.; Lima, L.L.; Leitão, A.A.; Guimarães, L.G.D.L. Production and physicochemical properties of fungal chitosans with efficacy to inhibit mycelial growth activity of pathogenic fungi. Carbohydr. Res. 2023, 525, 108762. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.R.R.; de Araújo, M.B.; da Costa, D.P.; de Lima, M.A.B.; de Almeida, J.W.L.; de Medeiros, E.V. Agroindustrial waste as ecofriendly and low-cost alternative to production of chitosan from Mucorales fungi and antagonist effect against Fusarium solani (Mart.) Sacco and Scytalidium lignicola Pesante. Int. J. Biol. Macromol. 2020, 161, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Logesh, A.R.; Thillaimaharani, K.A.; Sharmila, K.; Kalaiselvam, M.; Raffi, S.M. Production of chitosan from endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Furuike, T.; Osaka, I.; Fujimori, H.; Kawasaki, H.; Arakawa, R.; Tokura, S.; Stevens, W.F.; Kurozumi, S.; Takamori, Y.; et al. Laboratory scale production of 13C labeled chitosan by fungi Absidia coerulea and Gongronella butleri grown in solid substrate and submerged fermentation. Carbohydr. Polym. 2011, 84, 743–750. [Google Scholar] [CrossRef]
- Sugiono, S.; Ferdiansyah, D. Biorefinery Sequential Extraction of Alginate by Conventional and Hydrothermal Fucoidan from the Brown Alga, Sargassum cristaefolium. Biosci. Biotechnol. Res. Commun. 2019, 12, 894–903. [Google Scholar] [CrossRef]
- Torabi, P.; Hamdami, N.; Keramat, J. Microwave-assisted extraction of sodium alginate from brown macroalgae Nizimuddinia zanardini, optimization and physicochemical properties. Sep. Sci. Technol. 2022, 57, 872–885. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; Assis, D.D.J.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Microalgae as source of polyhydroxyalkanoates (PHAs)—A review. Int. J. Biol. Macromol. 2019, 131, 536–547. [Google Scholar] [CrossRef]
- Mourão, M.M.; Gradíssimo, D.G.; Santos, A.V.; Schneider, M.P.C.; Faustino, S.M.M.; Vasconcelos, V.; Xavier, L.P. Optimization of Polyhydroxybutyrate Production by Amazonian Microalga Stigeoclonium sp. B23. Biomolecules 2020, 10, 1628. [Google Scholar] [CrossRef]
- Cassuriaga, A.P.A.; Freitas, B.C.B.; Morais, M.G.; Costa, J.A.V. Innovative polyhydroxybutyrate production by Chlorella fusca grown with pentoses. Bioresour. Technol. 2018, 265, 456–463. [Google Scholar] [CrossRef]
- Kumari, P.; Kiran, B.R.; Mohan, S.V. Polyhydroxybutyrate production by Chlorella sorokiniana SVMIICT8 under Nutrient-deprived mixotrophy. Bioresour. Technol. 2022, 354, 127135. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Sayin, S.; Kohlhaas, T.; Veziroglu, S.; Okudan, E.; Naz, M.; Schröder, S.; Saygili, E.I.; Açil, Y.; Faupel, F.; Wiltfang, J.; et al. Marine Algae-PLA composites as de novo alternative to porcine derived collagen membranes. Mater. Today Chem. 2020, 17, 100276. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Wang, H.Y. Highly effective removal of microplastics by microalgae Scenedesmus abundans. Chem. Eng. J. 2022, 435, 135079. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Kathryn, M.S.; Blair, N.E.; Levinson, W.; Louise, M.E.-W. Contribution of Fungal Macromolecules to Soil Carbon Sequestration. In Soil Carbon; Alfred, E.H., McSweeney, K., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 155–161. [Google Scholar]
- Ebinesar, A.; More, V.S.; Ramya, D.L.; Amrutha, G.R.; More, S.S. Fungal Chitosan: The Importance and Beneficiation of this Biopolymer in Industrial and Agricultural Process. In Microbial Polymers: Applications and Ecological Perspectives; Vaishnav, A., Choudhary, D.K., Eds.; Springer: Singapore, 2021; pp. 311–340. [Google Scholar]
- Alsharari, S.F.; Tayel, A.A.; Moussa, S.H. Soil emendation with nano-fungal chitosan for heavy metals biosorption. Int. J. Biol. Macromol. 2018, 118, 2265–2268. [Google Scholar] [CrossRef]
- Singh, G.; Kumari, A.; Mittal, A.; Goel, V.; Yadav, A.; Aggarwal, N.K. Cost Effective Production of Poly-β-Hydroxybutyrate by Bacillus subtilis NG05 Using Sugar Industry Waste Water. J Polym Environ. 2013, 21, 441–449. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Pattanasupong, A. Evaluation of Biodegradabilities of Biosynthetic Polyhydroxyalkanoates in Thailand Seawater and Toxicity Assessment of Environmental Safety Levels. Polymers 2022, 14, 428. [Google Scholar] [CrossRef]
- Namboodiri, M.M.T.; Paul, T.; Medisetti, R.M.N.; Pakshirajan, K.; Narayanasamy, S.; Pugazhenthi, G. Solid state fermentation of rice straw using Penicillium citrinum for chitosan production and application as nanobiosorbent. Bioresour. Technol. Rep. 2022, 18, 101005. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, A.; Paul, D.; Chakraborty, S.; Choudhury, P. Utilization of fleshing waste of leather processing for the growth of zygomycetes: A new substrate for economical production of bio-polymer chitosan. J. Environ. Manag. 2023, 343, 118141. [Google Scholar] [CrossRef]
- Farooq, U.; Ullah, M.W.; Yang, Q.; Aziz, A.; Xu, J.; Zhou, L.; Wang, S. High-density phage particles immobilization in surface-modified bacterial cellulose for ultra-sensitive and selective electrochemical detection of Staphylococcus aureus. Biosens. Bioelectron. 2020, 157, 112163. [Google Scholar] [CrossRef] [PubMed]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Mihalache, N.; Botez, A.; Matei, C.; Berger, D.; Damian, C.M.; Ionita, V. Box-Behnken experimental design for chromium(VI) ions removal by bacterial cellulose-magnetite composites. Int. J. Biol. Macromol. 2016, 91, 1062–1072. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Cheese whey: A cost-effective alternative for hyaluronic acid production by Streptococcus zooepidemicus. Food Chem. 2016, 198, 54–61. [Google Scholar] [CrossRef]
- Nasr, S.; Soudi, M.R.; Haghighi, M. Xanthan Production by a Native Strain of X. campestris and Evaluation of Application in EOR. Pak. J. Biol. Sci. 2007, 10, 3010–3013. [Google Scholar] [CrossRef]
- Sampaio, I.C.F.; Crugeira, P.J.L.; Soares, L.G.P.; Santos, J.N.D.; de Almeida, P.F.; Pinheiro, A.L.B.; Silveira, L. Composition of Xanthan gum produced by Xanthomonas campestris using produced water from a carbonated oil field through Raman spectroscopy. J. Photochem. Photobiol. B Biol. 2020, 213, 112052. [Google Scholar] [CrossRef]
- Jayasekara, L.A.C.B.; Poonsawad, A.; Watchaputi, K.; Wattanachaisaereekul, S.; Soontorngun, N. Media optimization of antimicrobial activity production and beta-glucan content of endophytic fungi Xylaria sp. BCC 1067. Biotechnol. Rep. 2022, 35, e00742. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Abara, P.; Castillo, T.; Ponce, B.; Urtuvia, V.; Peña, C.; Díaz-Barrera, A. Continuous Bioproduction of Alginate Bacterial under Nitrogen Fixation and Nonfixation Conditions. Fermentation 2023, 9, 426. [Google Scholar] [CrossRef]
- Moral, Ç.K.; Yıldız, M. Alginate Production from Alternative Carbon Sources and Use of Polymer Based Adsorbent in Heavy Metal Removal. Int. J. Polym. Sci. 2016, 7109825. [Google Scholar] [CrossRef]
- Mohanraj, R.; Gnanamangai, B.M.; Ramesh, K.; Priya, P.; Srisunmathi, R.; Poornima, S.; Ponmurugan, P.; Robinson, J.P. Optimized production of gamma poly glutamic acid (γ-PGA) using sago. Biocatal. Agric. Biotechnol. 2019, 22, 101413. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Singhal, R.S. Flocculation Properties of Poly(γ-Glutamic Acid) Produced from Bacillus subtilis Isolate. Food Bioproc. Tech. 2011, 4, 745–752. [Google Scholar] [CrossRef]
- Lončarević, B.; Lješević, M.; Marković, M.; Anđelković, I.; Gojgić-Cvijović, G.; Jakovljević, D.; Beškoski, V. Microbial levan and pullulan as potential protective agents for reducing adverse effects of copper on Daphnia magna and Vibrio fischeri. Ecotoxicol. Environ. Saf. 2019, 181, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Radulović, M.D.; Cvetković, O.G.; Nikolić, S.D.; Dordević, D.S.; Jakovljević, D.M.; Vrvić, M.M. Simultaneous production of pullulan and biosorption of metals by Aureobasidium pullulans strain CH-1 on peat hydrolysate. Bioresour. Technol. 2008, 99, 6673–6677. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Alkarkhi, A.F.M.; Jason, O.C.; Easa, A.M.; Norulaini, N.A.N. Production of the lactic acid from mango peel waste—Factorial experiment. J. King Saud Univ. Sci. 2013, 25, 39–45. [Google Scholar] [CrossRef]
- Nakano, S.; Ugwu, C.U.; Tokiwa, Y. Efficient production of d-(−)-lactic acid from broken rice by Lactobacillus delbrueckii using Ca(OH)2 as a neutralizing agent. Bioresour. Technol. 2012, 104, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Khattak, W.A.; Khan, T.; Ul-Islam, M.; Ullah, M.W.; Khan, S.; Wahid, F.; Park, J.K. Production, characterization and biological features of bacterial cellulose from scum obtained during preparation of sugarcane jaggery (gur). J. Food Sci. Technol. 2015, 52, 8343–8349. [Google Scholar] [CrossRef] [PubMed]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. J. Food Sci. Technol. 2015, 16, 14832–14849. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Ovchinnikova, E.V.; Gladysheva, E.K.; Kashcheyeva, E.I.; Pavlov, I.N.; Sakovich, G.V. A technology for pilot production of bacterial cellulose from oat hulls. Chem. Eng. J. 2020, 383, 123128. [Google Scholar] [CrossRef]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.R.; Tischer, C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Mater. Sci. Eng. R Rep. 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, Y.; Zhang, Y.; Sun, S.; Ullah, M.W.; Xu, W. Biotransformation of nylon-6,6 hydrolysate to bacterial cellulose. Green Chem. 2021, 23, 7805–7815. [Google Scholar] [CrossRef]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.F.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresour. Technol. 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yang, X.Y.; Xiong, L.; Guo, H.J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Nguyen, T.V.L.; Nguyen, T.T.D.; Nguyen, N.N. Effects of different hydrocolloids on the production of bacterial cellulose by Acetobacter xylinum using Hestrin–Schramm medium under anaerobic condition. Bioresour. Technol. Rep. 2022, 17, 100878. [Google Scholar] [CrossRef]
- Sharma, P.; Mittal, M.; Yadav, A.; Aggarwal, N.K. Bacterial cellulose: Nano-biomaterial for biodegradable face masks—A greener approach towards environment, Environmental Nanotechnology. Monit. Manag. 2023, 19, 100759. [Google Scholar] [CrossRef] [PubMed]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Wang, T.; Sun, B.; Tang, K.; Shen, W.; Chen, C.; Sun, D. Sustainable bacterial cellulose derived composites for high-efficiency hydrogen evolution reaction. Int. J. Biol. Macromol. 2023, 242, 125173. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef] [PubMed]
- Galdino, C.J.S.; Maia, A.D.; Meira, H.M.; Souza, T.C.; Amorim, J.D.P.; Almeida, F.C.G.; Costa, A.F.S.; Sarubbo, L.A. Use of a bacterial cellulose filter for the removal of oil from wastewater. Process Biochem. 2020, 91, 288–296. [Google Scholar] [CrossRef]
- Cazón, P.; Velázquez, G.; Vázquez, M. Characterization of bacterial cellulose films combined with chitosan and polyvinyl alcohol: Evaluation of mechanical and barrier properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Cielecka, I.; Szustak, M.; Kalinowska, H.; Gendaszewska-Darmach, E.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Glycerol-plasticized bacterial nanocellulose-based composites with enhanced flexibility and liquid sorption capacity. Cellulose 2019, 26, 5409–5426. [Google Scholar] [CrossRef]
- Chiaoprakobkij, N.; Seetabhawang, S.; Sanchavanakit, N.; Phisalaphong, M. Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J. Biomater. Sci. Polym. Ed. 2019, 30, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.A.; Silva, W.E.; Belian, M.F.; Lins, L.S.G.; Galembeck, A. Bacterial cellulose membranes for environmental water remediation and industrial wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3997–4008. [Google Scholar] [CrossRef]
- Jasim, A.; Ullah, M.W.; Shi, Z.; Lin, X.; Yang, G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr. Polym. 2017, 163, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kotsiri, Z.; Vidic, J.; Vantarakis, A. Applications of biosensors for bacteria and virus detection in food and water–A systematic review. J Environ Sci. 2022, 111, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ieamviteevanich, P.; Daneshvar, E.; Eshaq, G.; Puro, L.; Mongkolthanaruk, W.; Pinitsoontorn, S.; Bhatnagar, A. Synthesis and Characterization of a Magnetic Carbon Nanofiber Derived from Bacterial Cellulose for the Removal of Diclofenac from Water. ACS Omega 2022, 7, 7572–7584. [Google Scholar] [CrossRef]
- Lv, P.; Yao, Y.; Li, D.; Zhou, H.; Naeem, M.A.; Feng, Q.; Huang, J.; Cai, Y.; Wei, Q. Self-assembly of nitrogen-doped carbon dots anchored on bacterial cellulose and their application in iron ion detection. Carbohydr. Polym. 2017, 172, 93–101. [Google Scholar] [CrossRef]
- Rana, A.K.; Scarpa, F.; Thakur, V.K. Cellulose/polyaniline hybrid nanocomposites: Design, fabrication, and emerging multidimensional applications. Ind. Crops Prod. 2022, 187, 115356. [Google Scholar] [CrossRef]
- Ray, S.; Panjikar, S.; Anand, R. Design of Protein-Based Biosensors for Selective Detection of Benzene Groups of Pollutants. ACS Sens. 2018, 3, 1632–1638. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Bacterial cellulose/polyaniline nanocomposite aerogels as novel bioadsorbents for removal of hexavalent chromium: Experimental and simulation study. J. Clean. Prod. 2021, 278, 123817. [Google Scholar] [CrossRef]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Opin. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose hydrogel and its derivatives: A review of application in heavy metal adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Wu, G.T.; Kam, J.; Bloom, J.D. Hyaluronic Acid Basics and Rheology. Clin. Plast. Surg. 2022, 30, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.J.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef]
- Sze, J.H.; Brownlie, J.C.; Love, C.A. Biotechnological production of hyaluronic acid: A mini review. 3 Biotech 2016, 6, 67. [Google Scholar] [CrossRef]
- Marinelli, L.; Cacciatore, I.; Eusepi, P.; Di Biase, G.; Morroni, G.; Cirioni, O.; Giacometti, A.; Di Stefano, A. Viscoelastic behaviour of hyaluronic acid formulations containing carvacrol prodrugs with antibacterial properties. Int. J. Pharm. 2020, 582, 119306. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Casas, A.; Toubarro, D.; Barros, A.N.; Teixeira, J.A. Microbial Hyaluronic Acid Production: A Review. Molecules 2023, 28, 2084. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.F.; Blank, L.M.; McLaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.M.B.; Macedo, A.C.; Eguchi, S.Y.; Santana, M.H.A. Microbial production of hyaluronic acid from agricultural resource derivatives. Bioresour. Technol. 2010, 101, 6506–6509. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Factories 2010, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Liu, C.S.; Tian, Y.; Wang, J.; Xin, S.; Sheng, X. An eco-friendly photo-responsive hyaluronic acid-based supramolecular polysaccharide hybrid hydrogels for plant growth regulation and heavy metal ions adsorption. Int. J. Biol. Macromol. 2023, 242, 125194. [Google Scholar] [CrossRef] [PubMed]
- Taşdelen, B.; Çifçi, D.İ.; Meriç, S. Preparation and characterization of chitosan/hyaluronic acid/itaconic acid hydrogel composite to remove manganese in aqueous solution. Desalination Water Treat. 2021, 209, 204–211. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Pastrana, L.; Piñeiro, C.; Teixeira, J.A.; Pérez-Martín, R.I.; Amado, I.R. Production of Hyaluronic Acid by Streptococcus zooepidemicus on Protein Substrates Obtained from Scyliorhinus canicula Discards. Mar. Drugs 2015, 13, 6537–6549. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Microbial production of hyaluronic acid from agro-industrial by-products: Molasses and corn steep liquor. Biochem. Eng. J. 2017, 117, 181–187. [Google Scholar] [CrossRef]
- Um, I.C.; Fang, D.; Hsiao, B.S.; Okamoto, A.; Chu, B. Electro-Spinning and Electro-Blowing of Hyaluronic Acid. Biomacromolecules 2004, 5, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Um, I.C.; Fang, D.; Okamoto, A.; Hsiao, B.S.; Chu, B. Formation of water-resistant hyaluronic acid nanofibers by blowing-assisted electro-spinning and non-toxic post treatments. Polymer 2005, 46, 4853–4867. [Google Scholar] [CrossRef]
- Nsengiyumva, E.M.; Alexandridis, P. Xanthan gum in aqueous solutions: Fundamentals and applications. Int. J. Biol. Macromol. 2022, 216, 583–604. [Google Scholar] [CrossRef] [PubMed]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Bhat, I.M.; Wani, S.M.; Mir, S.A.; Masoodi, F.A. Advances in xanthan gum production, modifications and its applications. Biocatal. Agric. Biotechnol. 2022, 42, 102328. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of xanthan gum for whole cell immobilization and its impact in bioremediation—A review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.; Taha, M.R.; Rahman, N.A.; Taib, A.M. Performance of soil stabilized with biopolymer materials—Xanthan gum and guar gum. Phys. Chem. Earth Parts A/B/C 2022, 128, 103276. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Wang, Y.; Quan, Z.; Zong, L.; Wang, A. Synthesis and application of eco-friendly superabsorbent composites based on xanthan gum and semi-coke. Int. J. Biol. Macromol. 2021, 179, 230–238. [Google Scholar] [CrossRef]
- Feng, D.; Liang, B.; He, X.; Yi, F.; Xue, J.; Wan, Y.; Xue, Q. Mechanical properties of dredged soil reinforced by xanthan gum and fibers. J. Rock Mech. Geotech. Eng. 2023, 15, 2147–2157. [Google Scholar] [CrossRef]
- de Souza, C.K.; Ghosh, T.; Lukhmana, N.; Tahiliani, S.; Priyadarshi, R.; Hoffmann, T.G.; Purohit, S.D.; Han, S.S. Pullulan as a sustainable biopolymer for versatile applications: A review, Mater. Today Commun. 2023, 36, 106477. [Google Scholar] [CrossRef]
- Keykhosravi, A.; Vanani, M.B.; Aghayari, C. TiO2 nanoparticle-induced Xanthan Gum Polymer for EOR: Assessing the underlying mechanisms in oil-wet carbonates. J. Pet. Sci. Eng. 2021, 204, 108756. [Google Scholar] [CrossRef]
- Ko, M.S.; Jeon, Y.J.; Kim, K.W. Novel application of xanthan gum-based biopolymer for heavy metal immobilization in soil. J. Environ. Chem. 2022, 10, 108240. [Google Scholar] [CrossRef]
- Njuguna, D.G.; Schönherr, H. Smart and regeneratable Xanthan gum hydrogel adsorbents for selective removal of cationic dyes. J. Environ. Chem. Eng. 2022, 10, 107620. [Google Scholar] [CrossRef]
- Gohari, R.M.; Safarnia, M.; Koohi, A.D.; Salehi, M.B. Adsorptive removal of cationic dye by synthesized sustainable xanthan gum-g p(AMPS-co-AAm) hydrogel from aqueous media: Optimization by RSM-CCD model. Chem. Eng. Res. Des. 2022, 188, 714–728. [Google Scholar] [CrossRef]
- Taktak, F.F.; Özyaranlar, E. Semi-interpenetrating network based on xanthan gum-cl-2-(N-morpholinoethyl methacrylate)/titanium oxide for the single and binary removal of cationic dyes from water. Int. J. Biol. Macromol. 2022, 221, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Pirahmadi, P.; Shakeri, S.E.; Khoshbakhti, E.; Sharafkhani, S.; Fakhri, V.; Saeidi, A.; McClements, D.J.; Chen, W.H.; Chia, H.; et al. A novel environmentally friendly nanocomposite aerogel based on the semi-interpenetrating network of polyacrylic acid into Xanthan gum containing hydroxyapatite for efficient removal of methylene blue from wastewater. Int. J. Biol. Macromol. 2022, 201, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yan, L.; Xu, M.; Tang, L. Photo-degradable salecan/xanthan gum ionic gel induced by iron (III) coordination for organic dye decontamination. Int. J. Biol. Macromol. 2023, 238, 124132. [Google Scholar] [CrossRef]
- Lara, G.; Yakoubi, S.; Villacorta, C.M.; Uemura, K.; Kobayashi, I.; Takahashi, C.; Nakajima, M.; Neves, M.A. Spray technology applications of xanthan gum-based edible coatings for fresh-cut lotus root (Nelumbo nucifera). Food Res. Int. 2020, 137, 109723. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Tan, K.B.; Lin, J.; Chen, M.; Zhu, Y. Polyvinyl alcohol/xanthan gum composite film with excellent food packaging, storage and biodegradation capability as potential environmentally-friendly alternative to commercial plastic bag. Int. J. Biol. Macromol. 2022, 212, 402–411. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, Y.; Zhuang, Y.; Tan, K.B.; Chen, J. Effects of grape seed extract on the properties of pullulan polysaccharide/xanthan gum active films for apple preservation. Int. J. Biol. Macromol. 2023, 241, 124617. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Santos, J.C.D.; da Silva, S.S.; Chandel, A.K. Fermentative Production of Beta-Glucan: Properties and Potential Applications, Bioprocessing for Biomolecules Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 303–320. [Google Scholar]
- Chavanke, S.N.; Penna, S.; Dalvi, S.G. β-Glucan and its nanocomposites in sustainable agriculture and environment: An overview of mechanisms and applications. Environ. Sci. Pollut. Res. 2022, 29, 80062–80087. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Preparation of β-d-glucan nanoparticles and its antifungal activity. Int. J. Biol. Macromol. 2014, 70, 440–443. [Google Scholar] [CrossRef]
- Anusuya, S.; Sathiyabama, M. Foliar application of β-d-glucan nanoparticles to control rhizome rot disease of turmeric. Int. J. Biol. Macromol. 2015, 72, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V. Effects of β-glucan on some environmental toxins: An overview. Biomed. Pap. 2014, 158, 001–004. [Google Scholar] [CrossRef]
- Li, L.; Zhu, B.; Yao, Z.; Jiang, J. Directed preparation, structure–activity relationship and applications of alginate oligosaccharides with specific structures: A systematic review. Food Res. Int. 2023, 170, 112990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, X.; Liu, J.; Wang, M.; Zhao, T.; Kang, L.; Zhong, S.; Cui, X. Fabrication of modified alginate-based biocomposite hydrogel microspheres for efficient removal of heavy metal ions from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129736. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ahmadi, S.J.; Lacroix, M. Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohydr. Polym. 2020, 240, 116211. [Google Scholar] [CrossRef]
- Duc, T.H.; Vu, T.K.; Dang, C.T.; Nguyen, V.H.; La, D.D.; Kim, G.M.; Chang, S.W.; Bui, X.T.; Dang, T.D.; Nguyen, D.D. Synthesis and application of hydrogel calcium alginate microparticles as a biomaterial to remove heavy metals from aqueous media. Environ. Technol. Innov. 2021, 22, 101400. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, J.; Wang, B.; Wang, H.; He, Q.; Song, J.; Zhang, H.; Tang, M.; Zhou, L.; Gao, Y.; et al. Efficient heavy metal removal from water by alginate-based porous nanocomposite hydrogels: The enhanced removal mechanism and influencing factor insight. J. Hazard. Mater. 2021, 418, 126358. [Google Scholar] [CrossRef]
- Cai, R.; Chen, Y.; Hu, J.; Xiong, J.; Lu, J.; Liu, J.; Tan, X.; Liu, W.; Zhou, Y.; Chen, Y. A self-supported sodium alginate composite hydrogel membrane and its performance in filtering heavy metal ions. Carbohydr. Polym. 2023, 300, 120278. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.A.; Dar, A.A.; Banday, J.A. Rheological, morphological and swelling properties of dysprosium-based composite hydrogel beads of alginate and chitosan: A promising material for the effective cationic and anionic dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 131046. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Jeyanthi, J. Dynamic modelling of Alginate-Cobalt ferrite nanocomposite for removal of binary dyes from textile effluent. J. Environ. Chem. Eng. 2021, 9, 104924. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Modibane, K.D.; Kang, M.; Hato, M.J. Sequestration of methylene blue dye using sodium alginate poly(acrylic acid)@ZnO hydrogel nanocomposite: Kinetic, Isotherm, and Thermodynamic Investigations. Int. J. Biol. Macromol. 2020, 162, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.M. Novel synthesis of ultrafiltration membranes by crosslinking metal (II)-Alginate hydrogels with hexamethylene 1,6-Diisocyanate in inert solvent: Application for remediation of wastewater by removal of toxic pollutants. Chem. Eng. J. 2023, 452, 139093. [Google Scholar] [CrossRef]
- Thirumavalavan, M. Functionalized chitosan and sodium alginate for the effective removal of recalcitrant organic pollutants. Int. J. Biol. Macromol. 2023, 243, 125276. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Ahuja, V.; Chandel, N.; Gurav, R.; Bhatia, R.K.; Govarthanan, M.; Tyagi, V.K.; Kumar, V.; Pugazendhi, A.; Banu, J.R.; et al. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar]
- Zhang, H.; Guan, G.; Lou, T.; Wang, X. High performance, cost-effective and ecofriendly flocculant synthesized by grafting carboxymethyl cellulose and alginate with itaconic acid. Int. J. Biol. Macromol. 2023, 231, 123305. [Google Scholar] [CrossRef]
- Lu, T.; Xiang, T.; Huang, X.L.; Li, C.; Zhao, W.F.; Zhang, Q.; Zhao, C.S. Post-crosslinking towards stimuli-responsive sodium alginate beads for the removal of dye and heavy metals. Carbohydr. Polym. 2015, 133, 587–595. [Google Scholar] [CrossRef]
- da Cunha, A.B.M.; Leal, D.A.; Santos, L.R.L.; Riegel-Vidotti, I.C.; Marino, C.E.B. pH-sensitive microcapsules based on biopolymers for active corrosion protection of carbon steel at different pH. Surf. Coat. Technol. 2020, 402, 126338. [Google Scholar] [CrossRef]
- Gopishetty, V.; Tokarev, I.; Minko, S. Biocompatible stimuli-responsive hydrogel porous membranes via phase separation of a polyvinyl alcohol and Na-alginate intermolecular complex. J. Mater. Chem. 2012, 22, 19482–19487. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Wang, L.L.; Liu, Y.M.; Liu, H.M.; Shi, Q.S.; Peng, R.Q.; Xie, X.B. The role of structural evolution in the complexation and flocculation of heavy metals by the microbial product poly-γ-glutamic acid. Chemosphere 2022, 308, 136441. [Google Scholar] [CrossRef]
- SLuo, G.; Chien, C.C.; Sheu, Y.T.; Verpoort, F.; Chen, S.C.; Kao, C.M. Enhanced bioremediation of trichloroethene-contaminated groundwater using modified γ-PGA for continuous substrate supplement and pH control: Batch and pilot-scale studies. J. Clean. Prod. 2021, 278, 123736. [Google Scholar]
- Luo, S.G.; Chen, S.C.; Cao, W.Z.; Lin, W.H.; Sheu, Y.T.; Kao, C.M. Application of γ-PGA as the primary carbon source to bioremediate a TCE-polluted aquifer: A pilot-scale study. Chemosphere 2019, 237, 124449. [Google Scholar] [CrossRef]
- Fernandes, A.R.A.C.; Sganzerla, W.G.; Granado, N.P.A.; Campos, V. Implication of organic solvents in the precipitation of γ-polyglutamic acid for application as a sustainable flocculating agent. Biocatal. Agric. Biotechnol. 2023, 50, 102698. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kawase, Y. Adsorption capacities of poly-γ-glutamic acid and its sodium salt for cesium removal from radioactive wastewaters. J. Environ. Radioact. 2016, 165, 151–158. [Google Scholar] [CrossRef]
- Campos, V.; Fernandes, A.R.A.C.; Medeiros, T.A.M.; Andrade, E.L. Physicochemical characterization and evaluation of PGA bioflocculant in coagulation-flocculation and sedimentation processes. J. Environ. Chem. Eng. 2016, 4, 3753–3760. [Google Scholar] [CrossRef]
- Abdelnaby, T.; Li, Z.; Cao, W.; Xue, C. The effect of gamma-poly glutamic acid as a cryoprotectant on crayfish physicochemical and texture properties during frozen storage. LWT 2023, 184, 109960. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chiu, J.Y. A novel γ-PGA composite gellan membrane containing glycerol for guided bone regeneration. Mater. Sci. Eng. C. 2021, 118, 111404. [Google Scholar] [CrossRef]
- Zhang, K.; Li, F.; Wu, Y.; Feng, L.; Zhang, L. Construction of ionic thermo-responsive PNIPAM/γ-PGA/PEG hydrogel as a draw agent for enhanced forward-osmosis desalination. Desalination 2020, 495, 114667. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.M.; Chang, Y.H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (γ-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021, 171, 457–464. [Google Scholar] [CrossRef]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Sivamani, S.; Sivakumar, N.; Maran, J.P.; Hosseini-Bandegharaei, A. Ecofriendly biopolymers and composites: Preparation and their applications in water-treatment. Biotechnol. Adv. 2021, 52, 107815. [Google Scholar] [CrossRef]
- Kachhawa, D.K.; Bhattacharjee, P.; Singhal, R.S. Studies on downstream processing of pullulan. Carbohydr. Polym. 2003, 52, 25–28. [Google Scholar] [CrossRef]
- Cruz-Santos, M.M.; Antunes, F.A.F.; Arruda, G.L.; Shibukawa, V.P.; Prado, C.A.; Ortiz-Silos, N.; Castro-Alonso, M.J.; Marcelino, P.R.F.; Santos, J.C. Production and applications of pullulan from lignocellulosic biomass: Challenges and perspectives. Bioresour. Technol. 2023, 385, 129460. [Google Scholar] [CrossRef]
- Kizildag, N. Pullulan Films with PCMs: Recyclable Bio-Based Films with Thermal Management Functionality. Coatings 2023, 13, 414. [Google Scholar] [CrossRef]
- Milenković, I.; Radotić, K.; Despotović, J.; Lončarević, B.; Lješević, M.; Spasić, S.Z.; Nikolić, A.; Beškoski, V.P. Toxicity investigation of CeO2 nanoparticles coated with glucose and exopolysaccharides levan and pullulan on the bacterium Vibrio fischeri and aquatic organisms Daphnia magna and Danio rerio. Aquat. Toxicol. 2021, 236, 105867. [Google Scholar] [CrossRef]
- Song, W.; Yang, Y.; Liang, X.; Liu, F.; Gadd, G.M. Influence of metals and metalloids on the composition and fluorescence quenching of the extracellular polymeric substances produced by the polymorphic fungus Aureobasidium pullulans. Appl. Microbiol. Biotechnol. 2020, 104, 7155–7164. [Google Scholar] [CrossRef]
- Wu, L.; Shi, M.; Guo, R.; Dong, W. Development of a novel pullulan/polydopamine composite hydrogel adsorbent for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129632. [Google Scholar] [CrossRef]
- Richa, A. Roy Choudhury, Synthesis of a novel gellan-pullulan nanogel and its application in adsorption of cationic dye from aqueous medium. Carbohydr. Polym. 2020, 227, 115291. [Google Scholar] [CrossRef]
- Su, T.; Wu, L.; Zuo, G.; Pan, X.; Shi, M.; Zhang, C.; Qi, X.; Dong, W. Incorporation of dumbbell-shaped and Y-shaped cross-linkers in adjustable pullulan/polydopamine hydrogels for selective adsorption of cationic dyes. Environ. Res. 2020, 182, 109010. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, C.; Li, J.; Pan, X.; Zhai, X.; Jiao, Y.; Li, Y.; Dong, W.; Qi, X. Highly efficient removal of antibiotic from biomedical wastewater using Fenton-like catalyst magnetic pullulan hydrogels. Carbohydr. Polym. 2021, 262, 117951. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Cheng, S.; Zhang, C.; Jiao, Y.; Lin, X.; Dong, W.; Qi, X. Mussel-inspired magnetic pullulan hydrogels for enhancing catalytic degradation of antibiotics from biomedical wastewater. Chem. Eng. J. 2021, 409, 128203. [Google Scholar] [CrossRef]
- Santos, S.M.; Carbajo, J.M.; Quintana, E.; Ibarra, D.; Gomez, N.; Ladero, M.; Eugenio, M.E.; Villar, J.C. Characterization of purified bacterial cellulose focused on its use on paper restoration. Carbohydr. Polym. 2015, 116, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Kumar, U.S.U.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liang, Q.; Rashid, A.; Qayum, A.; Chi, Z.; Ren, X.; Ma, H. Ultrasound-assisted development and characterization of novel polyphenol-loaded pullulan/trehalose composite films for fruit preservation. Ultrason Sonochem. 2023, 92, 106242. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Chai, Y.; Guo, C.; Luo, C.; Chen, D.; Cheng, X.; Wang, F.; Huang, C. Chitosan–pullulan films enriched with Artemisia annua essential oil: Characterization and application in grape preservation. Int. J. Biol. Macromol. 2023, 243, 125216. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, Y.J.; Tu, Q.; Jian, Y.Y.; Xie, D.M.; Bai, T.; Li, S.S.; Liu, Y.T.; Li, C.; Wang, C.X.; et al. Preparation and characterization of carboxymethyl chitosan/pullulan composite film incorporated with eugenol and its application in the preservation of chilled meat. Meat Sci. 2023, 198, 109085. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and characterization of levan/pullulan/chitosan edible films enriched with ε-polylysine for active food packaging. Food Chem. 2022, 388, 132989. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, L.; Yi, Y.; Li, B.; Sun, R.; Deng, H. Preservation mechanism and flavor variation of postharvest button mushroom (Agaricus Bisporus) coated compounds of protocatechuic acid-CaCl2-NaCl-pullulan. LWT 2022, 169, 114020. [Google Scholar] [CrossRef]
- Rasweefali, M.K.; Sabu, S.; Azad, K.S.M.; Rahman, M.K.R.; Sunooj, K.V.; Sasidharan, A.; Anoop, K.K. Influence of deproteinization and demineralization process sequences on the physicochemical and structural characteristics of chitin isolated from Deep-sea mud shrimp (Solenocera hextii). Adv. Biomark. Sci. Technol. 2022, 4, 12–27. [Google Scholar] [CrossRef]
- Crognale, S.; Russo, C.; Petruccioli, M.; D’Annibale, A. Chitosan Production by Fungi: Current State of Knowledge. Future Oppor. Constraints Ferment. 2022, 8, 76. [Google Scholar]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Fungal chitosan: Prospects and challenges. In Handbook of Chitin and Chitosan: Volume 1: Preparation and Properties; Elsevier: Amsterdam, The Netherlands, 2020; pp. 419–452. [Google Scholar]
- Wang, L.; Xin, M.; Li, M.; Liu, W.; Mao, Y. Effect of the structure of chitosan quaternary phosphonium salt and chitosan quaternary ammonium salt on the antibacterial and antibiofilm activity. Int. J. Biol. Macromol. 2023, 242, 124877. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Taghavi, E.; Foong, S.Y.; Rajaei, A.; Amiri, H.; de Tender, C.; Peng, W.; Lam, S.S.; Aghbashlo, M.; Rastegari, H.; et al. Comparison of shrimp waste-derived chitosan produced through conventional and microwave-assisted extraction processes: Physicochemical properties and antibacterial activity assessment. Int. J. Biol. Macromol. 2023, 242, 124841. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.J.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the antibacterial property of chitosan nanoparticles against Escherichia coli and Salmonella typhimurium and their application as vegetable wash disinfectant. Food Control. 2018, 86, 294–301. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Pérez, A.S.; Romero, J.; Fraceto, L.F. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, J.; Chen, F.; Fan, N.; Wang, X.; Xiao, Z.; Wang, Z. Meta-analysis of chitosan-mediated effects on plant defense against oxidative stress. Sci. Total Environ. 2022, 851, 158212. [Google Scholar] [CrossRef] [PubMed]
- Ingle, P.U.; Shende, S.S.; Shingote, P.R.; Mishra, S.S.; Sarda, V.; Wasule, D.L.; Rajput, V.D.; Minkina, T.; Rai, M.; Sushkova, S.; et al. Chitosan nanoparticles (ChNPs): A versatile growth promoter in modern agricultural production. Heliyon 2022, 8, e11893. [Google Scholar] [CrossRef] [PubMed]
- Lakkaboyana, S.K.; Soontarapa, K.; Vinaykumar; Marella, R.K.; Kannan, K. Preparation of novel chitosan polymeric nanocomposite as an efficient material for the removal of Acid Blue 25 from aqueous environment. Int. J. Biol. Macromol. 2021, 168, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.F.; Gulshan, F.; Asrafuzzaman, F.N.U.; Das, H.; Rashid, R.; Hoque, S.M. Synthesis of mesoporous silica and chitosan-coated magnetite nanoparticles for heavy metal adsorption from wastewater. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100801. [Google Scholar] [CrossRef]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Cyclic desorption based efficacy of polyvinyl alcohol-chitosan variant resins for multi heavy-metal removal. Int. J. Biol. Macromol. 2023, 242, 124812. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, G.; Wen, J.; Li, X.; Zhu, J.; Wu, Z. Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: Performance, interaction and mechanism. Chemosphere 2023, 318, 137869. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-based hollow nanofiber membranes with polyvinylpyrrolidone and polyvinyl alcohol for efficient removal and filtration of organic dyes and heavy metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Pandian, U.K.; Vani, C.; Bharani, R.S.A.; Kavisri, M.; Meivelu, M. Chitosan nanocomposite as an effective carrier of potential herbicidal metabolites for noteworthy phytotoxic effect against major aquatic invasive weed water hyacinth (Eichhornia crassipes). Int. J. Biol. Macromol. 2023, 226, 1597–1610. [Google Scholar] [CrossRef]
- Al-Manhel, A.J.; Al-Hilphy, A.R.S.; Niamah, A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018, 17, 186–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, B.; Shen, B.; Jia, L.; Liao, J.; Zhu, W. Preparation of biochar@chitosan-polyethyleneimine for the efficient removal of uranium from water environment. Carbohydr. Polym. 2023, 312, 120834. [Google Scholar] [CrossRef] [PubMed]
- Maneechote, W.; Cheirsilp, B.; Angelidaki, I.; Suyotha, W.; Boonsawang, P. Chitosan-coated oleaginous microalgae-fungal pellets for improved bioremediation of non-sterile secondary effluent and application in carbon dioxide sequestration in bubble column photobioreactors. Bioresour. Technol. 2023, 372, 128675. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Sun, S.; Tang, F.; Chen, H.; Chen, S.; Zhao, C.; Li, L. A novel chitosan-biochar immobilized microorganism strategy to enhance bioremediation of crude oil in soil. Chemosphere 2023, 313, 137367. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, J.C.; Ma, Y.M.; Chen, G.Q. Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 2020, 58, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Fuoco, M.; Canosa, A.; Papini, M.P.; Majone, M. Use of poly-β-hydroxy-butyrate as a slow-release electron donor for the microbial reductive dechlorination of TCE. Water Sci. Technol. 2008, 57, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bergsma, S.; Colpa, D.I.; Euverink, G.J.W.; Krooneman, J. Polyhydroxyalkanoates (PHAs) synthesis and degradation by microbes and applications towards a circular economy. J. Environ. Manag. 2023, 341, 118033. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.X.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHAs with different chemical structures under controlled composting conditions. Polymer Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef]
- Dhania, S.; Bernela, M.; Rani, R.; Parsad, M.; Kumar, R.; Thakur, R. Polyhydroxybutyrate (PHB) in nanoparticulate form improves physical and biological performance of scaffolds. Int. J. Biol. Macromol. 2023, 236, 123875. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Seko, N. Biodegradable dual-layer Polyhydroxyalkanoate (pha)/Polycaprolactone (pcl) mulch film for agriculture: Preparation and characterization. Energy Nexus 2022, 8, 100137. [Google Scholar] [CrossRef]
- Kelwick, R.J.R.; Webb, A.J.; Wang, Y.; Heliot, A.; Allan, F.; Emery, A.M.; Templeton, M.R.; Freemont, P.S. AL-PHA beads: Bioplastic-based protease biosensors for global health applications. Mater. Today 2021, 47, 25–37. [Google Scholar] [CrossRef]
- Amanat, N.; Matturro, B.; Rossi, M.M.; Valentino, F.; Villano, M.; Papini, M.P. Assessment of Long-Term Fermentability of PHA-Based Materials from Pure and Mixed Microbial Cultures for Potential Environmental Applications. Water 2021, 13, 897. [Google Scholar] [CrossRef]
- Palmeiro-Sánchez, T.; Oliveira, C.S.S.; Gouveia, A.R.; Noronha, J.P.; Ramos, A.M.; Mosquera-Corral, A.; Reis, M.A.M. NaCl presence and purification affect the properties of mixed culture PHAs. Eur. Polym. J. 2016, 85, 256–265. [Google Scholar] [CrossRef]
- Yang, M.; Zou, Y.; Wang, X.; Liu, X.; Wan, C.; Harder, M.; Yan, Q.; Nan, J.; Ntaikou, I.; Antonopoulou, G.; et al. Synthesis of intracellular polyhydroxyalkanoates (PHA) from mixed phenolic substrates in an acclimated consortium and the mechanisms of toxicity. J. Environ. Chem. Eng. 2022, 10, 107944. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and Opportunities for Customizing Polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235–249. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. Valorization of municipal organic waste into purified lactic acid. Bioresour. Technol. 2021, 342, 125933. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Othman, I.; Rambabu, K.; Bharath, G.; Taher, H.; Hasan, S.W.; Banat, F. Polymerization of lactic acid produced from food waste by metal oxide-assisted dark fermentation. Environ. Technol. Innov. 2021, 24, 101862. [Google Scholar] [CrossRef]
- Menezes, O.; Roberts, T.; Motta, G.; Patrenos, M.H.; McCurdy, W.; Alotaibi, A.; Vanderpool, M.; Vaseghi, M.; Beheshti, A.; Davami, K. Performance of additively manufactured polylactic acid (PLA) in prolonged marine environments. Polym. Degrad. Stab. 2022, 199, 109903. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and the response of bacterial community in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Wang, Y.; Lin, J.; Ke, Y.; Zhou, C.; Wang, J.; Wen, J.; Gan, F.; Wang, L.; Ma, C. Polylactic acid/multi-wall carbon nanotubes composite fibrous membrane and their applications in oil-water separation. Surf. Interfaces 2023, 39, 102908. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Binod, P.; Pandey, A.; R, R.; Sirohi, R. Remodeling agro-industrial and food wastes into value-added bioactives and biopolymers. Ind. Crops. Prod. 2020, 154, 112621. [Google Scholar] [CrossRef]
- Gautam, K.; Vishvakarma, R.; Sharma, P.; Singh, A.; Gaur, V.K.; Varjani, S.; Srivastava, J.K. Production of biopolymers from food waste: Constrains and perspectives. Bioresour. Technol. 2022, 361, 127650. [Google Scholar] [CrossRef] [PubMed]
- Klai, N.; Yadav, B.; El Hachimi, O.; Pandey, A.; Sellamuthu, B.; Tyagi, R.D. Agro-Industrial Waste Valorization for Biopolymer Production and Life-Cycle Assessment Toward Circular Bioeconomy. In Biomass, Biofuels, Biochemicals: Circular Bioeconomy-Current Developments and Future Outlook; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–555. [Google Scholar]
- da Silva, J.A.; Cardoso, L.G.; de Jesus Assis, D.; Gomes, G.V.P.; Oliveira, M.B.P.P.; de Souza, C.O.; Druzian, J.I. Xanthan Gum Production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from Lignocellulosic Agroindustrial Wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Sajjad, W.; Khan, T.; Wahid, F. Production of bacterial cellulose from industrial wastes: A review. Cellulose 2019, 26, 2895–2911. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Schnepp, Z. Biopolymers as a Flexible Resource for Nanochemistry. Angew. Chem. Int. Ed. 2013, 52, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Van Loosdrecht, M.C.M.; Pot, M.A.; Heijnen, J.J. Importance of bacterial storage polymers in bioprocesses. Water Sci. Technol. 1997, 35, 41–47. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial Extracellular Polysaccharides Involved in Biofilm Formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [PubMed]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Rathour, R.; Medhi, K.; Tyagi, B.; Thakur, I.S. Microbial-Derived Natural Bioproducts for a Sustainable Environment: A Bioprospective for Waste to Wealth. In Refining Biomass Residues for Sustainable Energy and Bioproducts: Technology, Advances, Life Cycle Assessment, and Economics; Academic Press: Cambridge, MA, USA, 2020; pp. 51–85. [Google Scholar]
- Alipour, A.; Bahrami, A.; Saebnoori, E. Investigation in effect of different culture medium on the anti-corrosive performance of bacterial biopolymer. J. Taiwan Inst. Chem. Eng. 2017, 77, 64–72. [Google Scholar] [CrossRef]
- Corsino, S.F.; Di Trapani, D.; Torregrossa, N.; Piazzese, D. Preliminary evaluation of biopolymers production by mixed microbial culture from citrus wastewater in a MBR system using respirometric techniques. J. Water Process. Eng. 2021, 41, 102003. [Google Scholar] [CrossRef]
- Aramvash, A.; Gholami-Banadkuki, N.; Moazzeni-Zavareh, F. An Environmentally Friendly and Efficient Method for Extraction of PHB Biopolymer with Non-Halogenated Solvents. J. Microbiol. Biotechnol. 2015, 25, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Aramvash, A.; Zavareh, F.M.; Banadkuki, N.G. Comparison of different solvents for extraction of polyhydroxybutyrate from Cupriavidus necator. Eng. Life Sci. 2018, 18, 20–28. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P.; Roy, I. A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem. Eng. J. 2019, 150, 107283. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.E.R.-S.; Martín-Hernández, E.; Briones, R.; Martín, M. Process design and scale-up study for the production of polyol-based biopolymers from sawdust. Sustain. Prod. Consum. 2021, 27, 462–470. [Google Scholar] [CrossRef]
- Blunt, W.; Levin, D.B.; Cicek, N. Bioreactor Operating Strategies for Improved Polyhydroxyalkanoate (PHA) Productivity. Polymers 2018, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Anand, P.B.; Naik, R.N.M.; Gunashekaran, S. Effect of aging on the biopolymer composites: Mechanisms, modes and characterization. Polym. Compos. 2022, 43, 4115–4125. [Google Scholar] [CrossRef]
- Deroiné, M.; Le Duigou, A.; Corre, Y.M.; Le Gac, P.Y.; Davies, P.; César, G.; Bruzaud, S. Accelerated ageing and lifetime prediction of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in distilled water. Polym. Test. 2014, 39, 70–78. [Google Scholar] [CrossRef]
- Siviello, C.; Greco, F.; Larobina, D. Analysis of the aging effects on the viscoelasticity of alginate gels. Soft Matter 2016, 12, 8726–8735. [Google Scholar] [CrossRef] [PubMed]
- Leceta, I.; Peñalba, M.; Arana, P.; Guerrero, P.; De La Caba, K. Ageing of chitosan films: Effect of storage time on structure and optical, barrier and mechanical properties. Eur. Polym. J. 2015, 66, 170–179. [Google Scholar] [CrossRef]
- Cui, L.; Imre, B.; Tátraaljai, D.; Pukánszky, B. Physical ageing of Poly(Lactic acid): Factors and consequences for practice. Polymer 2020, 186, 122014. [Google Scholar] [CrossRef]
- Samer, M. (Ed.) Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar]
- Alsamman, M.T.; Sánchez, J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arab. J. Chem. 2021, 14, 103455. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J Ind Eng Chem. 2019, 79, 29–40. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Lim, H.N.; Sreeraj, T.R.; Amalraj, A.; Gopi, S. Advantages of Biopolymers over Synthetic Polymers: Social, Economic, and Environmental Aspects. In Biopolymers and Their Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 351–372. [Google Scholar]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Pal, A. Application of biopolymers as a new age sustainable material for surfactant adsorption: A brief review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100145. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.D.A.; Cadaval, T.R.S.A.; Breslin, C.B. Recent Developments in Chitosan-Based Adsorbents for the Removal of Pollutants from Aqueous Environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Benavente, M.; Moreno, L.; Martinez, J. Sorption of heavy metals from gold mining wastewater using chitosan. J. Taiwan Inst. Chem. Eng. 2011, 42, 976–988. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Godiya, C.B.; Xiao, Y.; Lu, X. Amine functionalized sodium alginate hydrogel for efficient and rapid removal of methyl blue in water. Int. J. Biol. Macromol. 2020, 144, 671–681. [Google Scholar] [CrossRef]
- Yi, X.; Sun, F.; Han, Z.; Han, F.; He, J.; Ou, M.; Gu, J.; Xu, X. Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicol. Environ. Saf. 2018, 158, 309–318. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Li, X.Y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Bio-Based Polymeric Flocculants and Adsorbents for Wastewater Treatment. Sustainability 2023, 15, 9844. [Google Scholar] [CrossRef]
- Rahman, A.S.A.; Fizal, A.N.S.; Khalil, N.A.; Yahaya, A.N.A.; Hossain, M.S.; Zulkifli, M. Fabrication and Characterization of Magnetic Cellulose–Chitosan–Alginate Composite Hydrogel Bead Bio-Sorbent. Polymers 2023, 15, 2494. [Google Scholar] [CrossRef]
- Qalyoubi, L.; Al-Othman, A.; Al-Asheh, S. Recent progress and challenges on adsorptive membranes for the removal of pollutants from wastewater. Part I: Fundamentals and classification of membranes. Case Stud. Therm. Eng. 2021, 3, 100086. [Google Scholar]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, M.; Wu, T.; Guo, L.; Han, W. Covalent Crosslinking Cellulose/Graphene Aerogels with High Elasticity and Adsorbability for Heavy Metal Ions Adsorption. Polymers 2023, 15, 2434. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Nguyen, T.-M.-C.; Hoang, G.-H.; Lam, H.-H.; Phan, H.-P.; Tran, T.-K.-A. Thiol-Surface-Engineered Cellulose Nanocrystals in Favor of Copper Ion Uptake. Polymers 2023, 15, 2562. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Yao, Y.; Su, Z.; Hu, S. Construction of composite chitosan-glucose hydrogel for adsorption of Co2+ ions. Int. J. Biol. Macromol. 2019, 139, 213–220. [Google Scholar] [CrossRef]
- Pavithra, S.; Thandapani, G.; Sugashini, S.; Sudha, P.N.; Alkhamis, H.H.; Alrefaei, A.F.; Almutairi, M.H. Batch adsorption studies on surface tailored chitosan/orange peel hydrogel composite for the removal of Cr(VI) and Cu(II) ions from synthetic wastewater. Chemosphere 2021, 271, 129415. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Yuan, H.; Zhang, L.; Mao, S.; Liu, X.; Alharbi, N.S.; Rohani, S.; Lu, J. Fabrication of xanthate-modified chitosan/poly(N-isopropylacrylamide) composite hydrogel for the selective adsorption of Cu(II), Pb(II) and Ni(II) metal ions. Chem. Eng. Res. Des. 2018, 139, 197–210. [Google Scholar] [CrossRef]
- Yang, L.; Bao, L.; Dong, T.; Xie, H.; Wang, X.; Wang, H.; Wu, J.; Hao, C. Adsorption properties of cellulose/guar gum/biochar composite hydrogel for Cu2+, Co2+ and methylene blue. Int. J. Biol. Macromol. 2023, 242, 125021. [Google Scholar] [CrossRef]
- Ding, W.; Liang, H.; Zhang, H.; Sun, H.; Geng, Z.; Xu, C. A cellulose/bentonite grafted polyacrylic acid hydrogel for highly-efficient removal of Cd(II). J. Water Process. Eng. 2023, 51, 103414. [Google Scholar] [CrossRef]
- Tao, X.; Wang, S.; Li, Z.; Zhou, S. Green synthesis of network nanostructured calcium alginate hydrogel and its removal performance of Cd2+ and Cu2+ ions. Mater. Chem. Phys. 2021, 258, 123931. [Google Scholar] [CrossRef]
- Lan, S.; Wu, X.; Li, L.; Li, M.; Guo, F.; Gan, S. Synthesis and characterization of hyaluronic acid-supported magnetic microspheres for copper ions removal. Colloids Surf. A Physicochem. Eng. Asp. 2013, 425, 42–50. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Application of Xanthan gum/ n-acetyl cysteine modified mica bionanocomposite as an adsorbent for the removal of toxic heavy metals. Groundw. Sustain. Dev. 2018, 7, 101–108. [Google Scholar] [CrossRef]
- Jafarigol, E.; Ghotli, R.A.; Hajipour, A.; Pahlevani, H.; Salehi, M.B. Tough dual-network GAMAAX hydrogel for the efficient removal of cadmium and nickle ions in wastewater treatment applications. J. Ind. Eng. Chem. 2021, 94, 352–360. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Zhang, M.; Tong, X.; Jiang, N.; Pan, W.; Xiong, W.; Li, Y.; Xu, J.; Shen, J.; et al. Efficient decontamination of heavy metals from aqueous solution using pullulan/polydopamine hydrogels. Int. J. Biol. Macromol. 2020, 145, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Alraddadi, H.M.; Fagieh, T.M.; Bakhsh, E.M.; Akhtar, K.; Khan, S.B.; Khan, S.A.; Bahaidarah, E.A.; Homdi, T.A. Adsorptive removal of heavy metals and organic dyes by sodium alginate/coffee waste composite hydrogel. Int. J. Biol. Macromol. 2023, 247, 125708. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, H.; Wang, Y.; Piao, C.; Cai, D.; Wang, Y.; Liu, J.; Cheng, Z. Preparation and characterization of a novel porous whey protein concentrate/pullulan gel induced by heating for Cu2+ absorption. Food Chem. 2020, 322, 126772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-K.; Li, T.-T.; Ren, H.-T.; Peng, H.-K.; Shiu, B.-C.; Lin, J.-H.; Lou, C.-W. Construction of a novel hydrogel composite with polylactic acid nonwoven fibers: Rapid and effective removal of Pb(II) and Ni(II). Text. Res. J. 2023, 93, 266–279. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, Z.; Wan, X.; Chen, H.; Zhong, J.; Zhang, Y.F. Efficient selective recycle of acid blue 93 by NaOH activated acrolein/chitosan adsorbent via size-matching effect. Carbohydr. Polym. 2023, 301, 120314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jeyabalan, J.; Priyan, V.V.; Patra, C.C.; Narayanasamy, S. Fabrication of a novel bio-polymer adsorbent with high adsorptive capacity towards organic dyes. Ind. Crops Prod. 2023, 203, 117166. [Google Scholar] [CrossRef]
- Liu, A.; He, S.; Zhang, J.; Liu, J.; Shao, W. Preparation and characterization of novel cellulose based adsorbent with ultra-high methylene blue adsorption performance. Mater. Chem. Phys. 2023, 296, 127261. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, Y.; Tang, S.; Yang, J.; Chen, Y.; Chen, S.; Li, P.; Yang, Z. Construction of chitosan/polyacrylate/graphene oxide composite physical hydrogel by semi-dissolution/acidification/sol-gel transition method and its simultaneous cationic and anionic dye adsorption properties. Carbohydr. Polym. 2020, 229, 115431. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Yu, Y.; Chen, J.; Shi, X.; Zhou, J.; Deng, H.; Du, Y. Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: Chitosan and cellulose. Polym. Chem. 2017, 8, 2913–2921. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, J.A.; Naim, A.A.; Ibrahim, W.A.W. New crosslinked-chitosan graft poly(N-vinyl-2-pyrrolidone) for the removal of Cu(II) ions from aqueous solutions. Int. J. Biol. Macromol. 2018, 107, 891–897. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wan, X.; Xie, L.; Chen, H.; Qu, G.; Zhang, H.; Zhang, Y.F.; Zhao, S. Selective adsorption and separation of methylene blue by facily preparable xanthan gum/amantadine composites. Int. J. Biol. Macromol. 2023, 241, 124640. [Google Scholar] [CrossRef]
- Tanzifi, M.; Esmizadeh, E.; Bazgir, H.; Nazari, A.; Vahidifar, A. Adsorption of Methylene Blue Dye from Aqueous Solution Using Polyaniline/Xanthan Gum Nanocomposite: Kinetic and Isotherm Studies. J. Polym. Compos. 2019, 7, 17–26. [Google Scholar]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Asmarandei, I.; Harabagiu, V.; Ghimici, L.; Ascenzi, P.; Fundueanu, G. Removal of anionic dyes from aqueous solutions by an ion-exchanger based on pullulan microspheres. Carbohydr. Polym. 2013, 91, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Isik, B.; Ugraskan, V. Methyl orange dye sequestration using polyaniline nanotube-filled sodium alginate bio-composite microbeads. Mater. Chem. Phys. 2023, 307, 128083. [Google Scholar] [CrossRef]
- Kahya, N.; Kaygusuz, H.; Erim, F.B. Aqueous Removal of Sodium Dodecyl Benzene Sulfonate (SDBS) by Crosslinked Chitosan Films. J. Polym. Environ. 2018, 26, 2166–2172. [Google Scholar] [CrossRef]
- Das, D.; Pal, A. Adsolubilization phenomenon perceived in chitosan beads leading to a fast and enhanced malachite green removal. Chem. Eng. J. 2016, 290, 371–380. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Chinga-Carrasco, G.; Paso, K.; Stenius, P. Reduction of water wettability of nanofibrillated cellulose by adsorption of cationic surfactants. Cellulose 2011, 18, 257–270. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016. [Google Scholar]
- Cruz, D.; Pimentel, M.; Russo, A.; Cabral, W. Charge Neutralization Mechanism Efficiency in Water with High Color Turbidity Ratio Using Aluminium Sulfate and Flocculation Index. Water 2020, 12, 572. [Google Scholar] [CrossRef]
- Aghyani, R.; Bidhendi, G.N.; Mehrdadi, N.; Amiri, M.J. Comparative study of Poly Aluminum Ferric and Poly Aluminum Chloride Performance for Turbidity Removal from River Water. Environ. Energy Econ. Res. 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Akinnawo, S.O.; Ayadi, P.O.; Oluwalope, M.T. Chemical coagulation and biological techniques for wastewater treatment. Ovidius Univ. Ann. Chem. 2023, 34, 14–21. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Unuabonah, E.I.; Amuda, O.S.; Kolawole, O.M. Polysaccharides as a Green and Sustainable Resources for Water and Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Nurudeen, A.O.; Unuabonah, E.I.; Omotayo, S.A.; Olatunji, M.K. Tapping into Microbial Polysaccharides for Water and Wastewater Purifications. In Polysaccharides as a Green and Sustainable Resources for Water and Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2017; pp. 91–110. [Google Scholar]
- Salehizadeh, H.; Yan, N.; Farnood, R. Recent advances in polysaccharide bio-based flocculants. Biotechnol. Adv. 2018, 36, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Grenda, K.; Gamelas, J.A.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Evaluation of Anionic and Cationic Pulp-Based Flocculants With Diverse Lignin Contents for Application in Effluent Treatment From the Textile Industry: Flocculation Monitoring. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Ghimici, L.; Nichifor, M. Dextran derivatives application as flocculants. Carbohydr. Polym. 2018, 190, 162–174. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a novel dextran-based flocculant on treatment of dye wastewater: Effect of kaolin particles. Sci. Total Environ. 2018, 640–641, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Rodríguez, D.; Lizardi-Mendoza, J.; López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Capacity of ‘nopal’ pectin as a dual coagulant-flocculant agent for heavy metals removal. Chem. Eng. J. 2017, 323, 19–28. [Google Scholar] [CrossRef]
- Mao, Y.; Millett, R.; Lee, C.S.; Yakubov, G.; Harding, S.E.; Binner, E. Investigating the influence of pectin content and structure on its functionality in bio-flocculant extracted from okra. Carbohydr. Polym. 2020, 241, 116414. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Y.; Chen, Y. Removal of Oil and Cr(VI) from Wastewater Using Modified Pectin Flocculants. J. Environ. Eng. 2014, 140, 04013004. [Google Scholar] [CrossRef]
- Hasan, A.; Fatehi, P. Flocculation of kaolin particles with cationic lignin polymers. Sci. Rep. 2019, 9, 2672. [Google Scholar] [CrossRef]
- Jumadi, J.; Kamari, A.; Hargreaves, J.S.J.; Yusof, N. A review of nano-based materials used as flocculants for water treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3571–3594. [Google Scholar] [CrossRef]
- Ahsan, A.; Ismail, A.F. Nanotechnology in Water and Wastewater Treatment, 1st ed.; Elsevier: New York, NY, USA, 2019. [Google Scholar]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Xu, Y.; Wang, J.; Chu, R.; Yin, Z.; Mo, F.; Zhu, L. A review on flocculation as an efficient method to harvest energy microalgae: Mechanisms, performances, influencing factors and perspectives. Renew. Sust. Energ. Rev. 2020, 131, 110005. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, M.; Zhu, C.; Xu, Y.; Zheng, H.; Xiao, X.; Wu, H.; Xia, T.; You, Z. UV-Initiated Graft Copolymerization of Cationic Chitosan-Based Flocculants for Treatment of Zinc Phosphate-Contaminated Wastewater. Ind. Eng. Chem. Res. 2016, 55, 10025–10035. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, L.; Ni, C. Preparation of modified alginate nanoflocculant and adsorbing properties for Pb2+ in wastewater. Russ. J. Appl. Chem. 2017, 90, 641–647. [Google Scholar] [CrossRef]
- Feng, L.; Zhong, K.; Zhou, W.; Liu, J.; Liu, B.; Wang, W.; Zheng, H. Synthesis of a chitosan-based flocculant CS-g-P(AM-IA-AATPAC) and evaluation of its performance on Ni2+ removal: Role of chelating-coordination and flocculation. J. Environ. Chem. Eng. 2023, 11, 109138. [Google Scholar] [CrossRef]
- Ghimici, L.; Nafureanu, M.M.; Constantin, M. Cationic Pullulan Derivatives Based Flocculants for Removal of Some Metal Oxides from Simulated Wastewater. Int. J. Mol. Sci. 2023, 24, 4383. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, H.; Wang, Y.; Chen, X.; Zhao, C.; An, Y.; Tang, X. A novel carboxyl-rich chitosan-based polymer and its application for clay flocculation and cationic dye removal. Sci. Total Environ. 2018, 640–641, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sudirgo, M.M.; Surya, R.A.; Kristianto, H.; Prasetyo, S.; Sugih, A.K. Application of xanthan gum as coagulant-aid for decolorization of synthetic Congo red wastewater. Heliyon 2023, 9, e15011. [Google Scholar] [CrossRef]
- Ghimici, L.; Constantin, M. Removal of the commercial pesticides Novadim Progress, Bordeaux mixture and Karate Zeon by pullulan derivatives based flocculants. J. Environ. Manag. 2018, 218, 31–38. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Rasteiro, M.G. Environmentally friendly cellulose-based polyelectrolytes in wastewater treatment. Water Sci. Technol. 2017, 76, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Zhang, G.; Liu, W.; Xu, J.; Tian, Y.; Wang, Y.; Xie, X.; Peng, Z.; Li, A.; et al. Efficient treatment of the starch wastewater by enhanced flocculation–coagulation of environmentally benign materials. Sep. Purif. Technol. 2023, 307, 122788. [Google Scholar] [CrossRef]
- Jiang, X.; Lou, C.; Hua, F.; Deng, H.; Tian, X. Cellulose nanocrystals-based flocculants for high-speed and high-efficiency decolorization of colored effluents. J. Clean. Prod. 2020, 251, 119749. [Google Scholar] [CrossRef]
- Liu, C.; Gao, B.; Wang, S.; Guo, K.; Shen, X.; Yue, Q.; Xu, X. Synthesis, characterization and flocculation performance of a novel sodium alginate-based flocculant. Carbohydr. Polym. 2020, 248, 116790. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Sarkar, A.; Panda, A.B.; Pal, S. Evaluation of the Flocculation Characteristics of Polyacrylamide Grafted Xanthan Gum/Silica Hybrid Nanocomposite. Ind. Eng. Chem. Res. 2013, 52, 9731–9740. [Google Scholar] [CrossRef]
- Adhikary, P.; Singh, R.P. Synthesis characterization, and flocculation characteristics of hydrolyzed and unhydrolyzed polyacrylamide grafted xanthan gum. J. Appl. Polym. Sci. 2004, 94, 1411–1419. [Google Scholar] [CrossRef]
- Ghimici, L.; Constantin, M. Novel thermosensitive flocculanting agent based on pullulan. J. Hazard. Mater. 2011, 192, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhong, H.; Wang, L.; Elbashier, M.M.A. Bacillus amyloliquefaciens (IAE635) and their metabolites could purify pollutants, Vibrio spp. and coliform bacteria in coastal aquaculture wastewater. Int. J. Agric. Biol. Eng. 2021, 14, 205–210. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yang, H.; Xie, X.; Zhu, Y.; Xu, G.; Hu, X.; Jin, Z.; Hu, Y.; Hai, Z.; et al. Treatment of potato starch wastewater by dual natural flocculants of chitosan and poly-glutamic acid. J. Clean. Prod. 2020, 264, 121641. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Lian, L.; Wang, X.; Zhang, H.; Gao, W.; Lou, D. Flocculation performance of alginate grafted polysilicate aluminum calcium in drinking water treatment. Process Saf. Environ. Prot. 2021, 155, 287–294. [Google Scholar] [CrossRef]
- Morantes, D.; Muñoz, E.; Kam, D.; Shoseyov, O. Highly Charged Cellulose Nanocrystals Applied as A Water Treatment Flocculant. Nanomaterials 2019, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Suopajärvi, T.; Liimatainen, H.; Hormi, O.; Niinimäki, J. Coagulation–flocculation treatment of municipal wastewater based on anionized nanocelluloses. Chem. Eng. J. 2013, 231, 59–67. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Yang, X.; Liu, H.; Zhang, X.; Yao, J. An Eco-friendly One-Step Synthesis of Dicarboxyl Cellulose for Potential Application in Flocculation. Ind. Eng. Chem. Res. 2015, 54, 2825–2829. [Google Scholar] [CrossRef]
- Gough, C.R.; Callaway, K.; Spencer, E.; Leisy, K.; Jiang, G.; Yang, S.; Hu, X. Biopolymer-Based Filtration Materials. ACS Omega 2021, 6, 11804–11812. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.D.L.M.D.; Junior, C.J.G.D.S.; Amorim, J.D.P.D.; Durval, I.J.B.; Costa, A.F.D.S.; Sarubbo, L.A. Oily Wastewater Treatment: Methods, Challenges, and Trends. Processes 2022, 10, 743. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Sun, W.; He, S.; Zhang, Y.; Wang, X. Ceramic membrane fouling mechanisms and control for water treatment. Front. Environ. Sci. Eng. 2023, 17, 126. [Google Scholar] [CrossRef]
- Mpala, T.J.; Richards, H.; Etale, A.; Mahlangu, O.T.; Nthunya, L.N. Carbon nanotubes and silver nanoparticles modification of PVDF membranes for improved seawater desalination in direct contact membrane distillation. Front. Membr. Sci Technol. 2023, 2, 1165678. [Google Scholar] [CrossRef]
- Baig, N.; Matin, A.; Faizan, M.; Anand, D.; Ahmad, I.; Khan, S.A. Antifouling low-pressure highly permeable single step produced loose nanofiltration polysulfone membrane for efficient Erichrome Black T/divalent salts fractionation. J. Environ. Chem. Eng. 2022, 10, 108166. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.D.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Ehsan, M.; Razzaq, H.; Razzaque, S.; Kanwal, M.; Hussain, I. Engineering nanocomposite membranes of sodium alginate-graphene oxide for efficient separation of oil-water and antifouling performance. J. Environ. Chem. Eng. 2023, 11, 109185. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Cui, Y.; Chen, N. Removal of Copper Ions from Wastewater: A Review. Int. J. Environ. Res. Public Health 2023, 20, 3885. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.H.; Mishra, A.K.; Gallucci, F.; Kim, J.H.; Ulbricht, M.; Coclite, A.M.; Hosseini, S.S. Advances in surface modification and functionalization for tailoring the characteristics of thin films and membranes via chemical vapor deposition techniques. J. Appl. Polym. Sci. 2023, 140, e53720. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Yuan, X.; Ding, W.; Li, Y.; Wang, J. Separation of oil-water emulsion and adsorption of Cu(II) on a chitosan-cellulose acetate-TiO2 based membrane. Chemosphere 2019, 235, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.; Oh, T.H. Polymer Nanocomposite Membrane for Wastewater Treatment: A Critical Review. Polymers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Khamis, F.; Hegab, H.M.; Banat, F.; Arafat, H.A.; Hasan, S.W. Development of sustainable pH-responsive adsorptive modified mangrove-based polylactic acid ultrafiltration membrane for the removal of heavy metals from aqueous solution. Chem. Eng. J. 2023, 474, 145471. [Google Scholar] [CrossRef]
- Ghorbani, M.; Vakili, M.H.; Ameri, E. Fabrication and evaluation of a biopolymer-based nanocomposite membrane for oily wastewater treatment. Mater. Today Commun. 2021, 28, 102560. [Google Scholar] [CrossRef]
- Vinodhini, P.A.; Sudha, P.N. Removal of heavy metal chromium from tannery effluent using ultrafiltration membrane. Text Cloth Sustain. 2016, 2, 5. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Fu, J.; Wang, A.; Ding, M.; Zhang, L.; Han, L.; Gao, L. Hyaluronic acid-modified nanofiltration membrane for ultrahigh water permeance and efficient rejection of PFASs. Process Saf. Environ. Prot. 2022, 166, 214–221. [Google Scholar] [CrossRef]
- El-Sayed, M.M.H.; Elsayed, R.E.; Attia, A.; Farghal, H.H.; Azzam, R.A.; Madkour, T.M. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Ampawan, S.; Phreecha, N.; Chantarak, S.; Chinpa, W. Selective separation of dyes by green composite membrane based on polylactide with carboxylated cellulose microfiber from empty fruit bunch. Int. J. Biol. Macromol. 2023, 225, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Gul, B.Y.; Esmaeili, M.S.; Pekgenc, E.; Teber, O.O.; Tuncay, G.; Karimi, H.; Parvaz, S.; Maleki, A.; Vatanpour, V. Modification of PVDF membranes by incorporation Fe3O4@Xanthan gum to improve anti-fouling, anti-bacterial, and separation performance. J. Environ. Chem. Eng. 2022, 10, 107784. [Google Scholar] [CrossRef]
- Chai, P.V.; Sailenra, K.R.; Chan, W.J.; Liew, J.T.; Teng, W.T.; Wong, Y.T. Green Synthesis of Polyvinylidene Fluoride using Xanthan Gum Biopolymer and Dimethyl Sulfoxide Green Solvent. J. Appl. Membr. Sci. Technol. 2023, 27, 1–11. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Wang, Y.; Li, S.; Tian, S. Ethylenediamine-oxidized sodium alginate hydrogel cross-linked graphene oxide nanofiltration membrane with self-healing property for efficient dye separation. J. Membr. Sci. 2023, 670, 121366. [Google Scholar] [CrossRef]
- Zhijiang, C.; Ping, X.; Cong, Z.; Tingting, Z.; Jie, G.; Kongyin, Z. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 2018, 25, 5123–5137. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Yu, J.; Zhang, L.; Li, S.; Li, H. Alginate-based nanofibrous membrane with robust photo-Fenton self-cleaning property for efficient crude oil/water emulsion separation. Sep. Purif. Technol. 2022, 287, 120569. [Google Scholar] [CrossRef]
- Cheng, X.; Li, T.; Yan, L.; Jiao, Y.; Zhang, Y.; Wang, K.; Cheng, Z.; Ma, J.; Shao, L. Biodegradable electrospinning superhydrophilic nanofiber membranes for ultrafast oil-water separation. Sci. Adv. 2023, 9, eadh8195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.S.; Deng, J.; Lei, B.; He, A.; Zhang, X.; Ma, L.; Li, S.; Zhao, C. Toward 3D graphene oxide gels based adsorbents for high-efficient water treatment via the promotion of biopolymers. J. Hazard. Mater. 2013, 263, 467–478. [Google Scholar] [CrossRef]
- Nassar, L.; Wadi, V.S.; Hegab, H.M.; Khalil, H.; Banat, F.; Naddeo, V.; Hasan, S.W. Sustainable and green polylactic acid-based membrane embedded with self-assembled positively charged f-MWCNTs/GO nanohybrids for the removal of nutrients from wastewater. NPJ Clean Water 2022, 5, 57. [Google Scholar] [CrossRef]
- Kimura, K.; Toshima, S.; Amy, G.; Watanabe, Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004, 245, 71–78. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment; Murillo-Tovar, M.A., Saldarriaga-Noreña, H., Saeid, A., Eds.; IntechOpen: Rijeka, Yugoslavia, 2020. [Google Scholar]
- Wang, M.; Chen, S.; Jia, X.; Chen, L. Concept and types of bioremediation. In Handbook of Bioremediation: Physiological. Molecular and Biotechnological Interventions; Academic Press: Cambridge, MA, USA, 2021; pp. 3–8. [Google Scholar]
- Ayangbenro, A.S.; Babalola, O.O. Metal(loid) Bioremediation: Strategies Employed by Microbial Polymers. Sustainability 2018, 10, 3028. [Google Scholar] [CrossRef]
- Gutnick, D.L.; Bach, H. Engineering bacterial biopolymers for the biosorption of heavy metals; new products and novel formulations. Appl. Microbiol. Biotechnol. 2000, 54, 451–460. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Dhawi, F. How Can We Stabilize Soil Using Microbial Communities and Mitigate Desertification? Sustainability 2023, 15, 863. [Google Scholar] [CrossRef]
- Mishra, S.; Huang, Y.; Li, J.; Wu, X.; Zhou, Z.; Lei, Q.; Bhatt, P.; Chen, S. Biofilm-mediated bioremediation is a powerful tool for the removal of environmental pollutants. Chemosphere 2022, 294, 133609. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B. Source identification of total petroleum hydrocarbons and polycyclic aromatic hydrocarbons in PM10 and street dust of a hot spot for petrochemical production: Asaluyeh County, Iran. Sustain. Cities Soc. 2019, 45, 214–230. [Google Scholar] [CrossRef]
- Miri, S.; Davoodi, S.M.; Robert, T.; Brar, S.K.; Martel, R.; Rouissi, T. Enzymatic biodegradation of highly p-xylene contaminated soil using cold-active enzymes: A soil column study. J. Hazard. Mater. 2022, 423, 127099. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Caldeira, J.B.; Branco, R.; Morais, P.V. Enhancing the Strength of Mine Residue Soil by Bioremediation Combined with Biopolymers. Appl. Sci. 2023, 13, 10550. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Prasidhi, A.K.; Cho, G.C. Effects of Xanthan gum biopolymer on soil strengthening. Constr Build Mater. 2015, 74, 65–72. [Google Scholar] [CrossRef]
- Latifi, N.; Horpibulsuk, S.; Meehan, C.L.; Majid, M.Z.A.; Tahir, M.M.; Mohamad, E.T. Improvement of Problematic Soils with Biopolymer—An Environmentally Friendly Soil Stabilizer. J. Mater. Civ. Eng. 2017, 29, 4016204. [Google Scholar] [CrossRef]
- Kwon, Y.-M.; Ham, S.-M.; Kwon, T.-H.; Cho, G.-C.; Chang, I. Surface-erosion behaviour of biopolymer-treated soils assessed by EFA. Geotech. Lett. 2020, 10, 106–112. [Google Scholar] [CrossRef]
- Qureshi, M.U.; Chang, I.; Al-Sadarani, K. Strength and durability characteristics of biopolymer-treated desert sand. Geomech. Eng. 2017, 12, 785–801. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Wiszniewski, M.; Skutnik, Z. Effects of Xanthan Gum Biopolymer on the Permeability, Odometer, Unconfined Compressive and Triaxial Shear Behavior of a Sand. Soil Mech. Found. Eng. 2017, 54, 356–361. [Google Scholar] [CrossRef]
- Ayeldeen, M.K.; Negm, A.M.; El Sawwaf, M.A. Evaluating the physical characteristics of biopolymer/soil mixtures. Arab. J. Geosci. 2016, 9, 371. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Coagulation of soil suspensions containing nonionic or anionic surfactants using chitosan, polyacrylamide, and polyaluminium chloride. Chemosphere 2009, 75, 1307–1314. [Google Scholar] [CrossRef]
- Zou, H.; Pan, G.; Chen, H.; Yuan, X. Removal of cyanobacterial blooms in Taihu Lake using local soils II. Effective removal of Microcystis aeruginosa using local soils and sediments modified by chitosan. Environ. Pollut. 2006, 141, 201–205. [Google Scholar] [CrossRef]
- Zinchenko, A.; Sakai, T.; Morikawa, K.; Nakano, M. Efficient stabilization of soil, sand, and clay by a polymer network of biomass-derived chitosan and carboxymethyl cellulose. J. Environ. Chem. Eng. 2022, 10, 107084. [Google Scholar] [CrossRef]
- Jamshidi, M.; Mokhberi, M.; Vakili, A.H.; Nasehi, A. Effect of chitosan bio-polymer stabilization on the mechanical and dynamic characteristics of marl soils. Transp. Geotech. 2023, 42, 101110. [Google Scholar] [CrossRef]
- Ilman, B.; Balkis, A.P. Sustainable biopolymer stabilized earthen: Utilization of chitosan biopolymer on mechanical, durability, and microstructural properties. J. Build. Eng. 2023, 76, 107220399. [Google Scholar] [CrossRef]
- Bhatia, S.K. Microbial Biopolymers: Trends in Synthesis, Modification, and Applications. Polymers 2023, 15, 1364. [Google Scholar] [CrossRef]
- Jose, A.A.; Hazeena, S.H.; Lakshmi, N.M.; Arun, K.B.; Madhavan, A.; Sirohi, R.; Tarafdar, A.; Sindhu, R.; Awasthi, M.K.; Pandey, A.; et al. Bacterial biopolymers: From production to applications in biomedicine. Sustain. Chem. Pharm. 2022, 25, 100582. [Google Scholar] [CrossRef]
- Alloul, A.; Ganigué, R.; Spiller, M.; Meerburg, F.; Cagnetta, C.; Rabaey, K.; Vlaeminck, S.E. Capture–Ferment–Upgrade: A Three-Step Approach for the Valorization of Sewage Organics as Commodities. Environ. Sci. Technol. 2018, 52, 6729–6742. [Google Scholar] [CrossRef]
- Söderholm, P. The green economy transition: The challenges of technological change for sustainability. Sustain. Earth 2020, 3, 6. [Google Scholar] [CrossRef]
- Rodila, D.; Ray, N.; Gorgan, D. Conceptual model for environmental science applications on parallel and distributed infrastructures. Environ. Syst. Res. 2015, 4, 23. [Google Scholar] [CrossRef]
- Delacámara, G.; O’Higgins, T.G.; Lago, M.; Langhans, S. Ecosystem-Based Management: Moving from Concept to Practice. In Ecosystem-Based Management, Ecosystem Services and Aquatic Biodiversity: Theory, Tools and Applications; O’Higgins, T.G., Lago, M., DeWitt, T.H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 39–60. [Google Scholar]
- Bretschger, L.; Pittel, K. Twenty Key Challenges in Environmental and Resource Economics. Environ Resour Econ. 2020, 77, 725–750. [Google Scholar] [CrossRef]
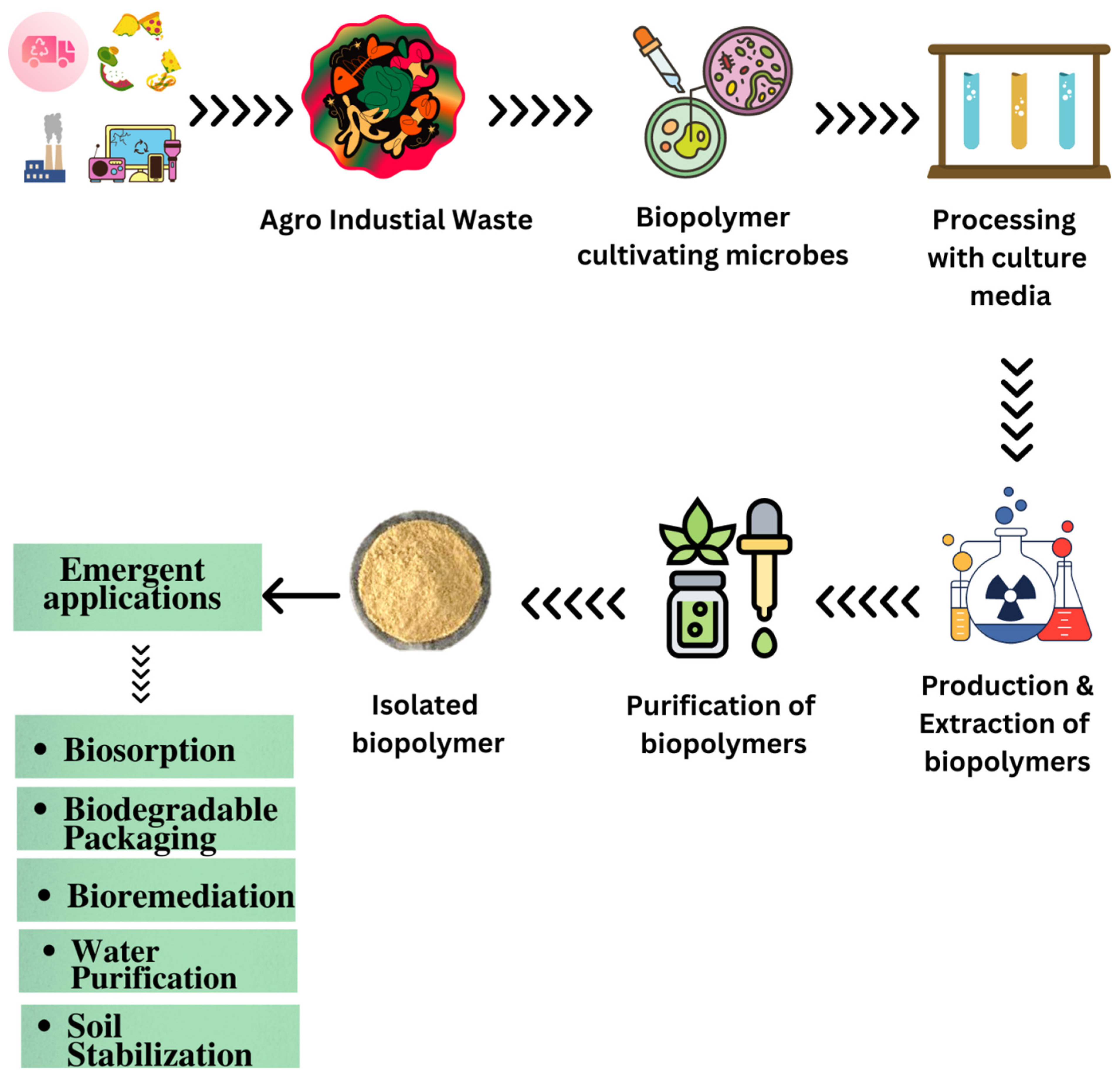
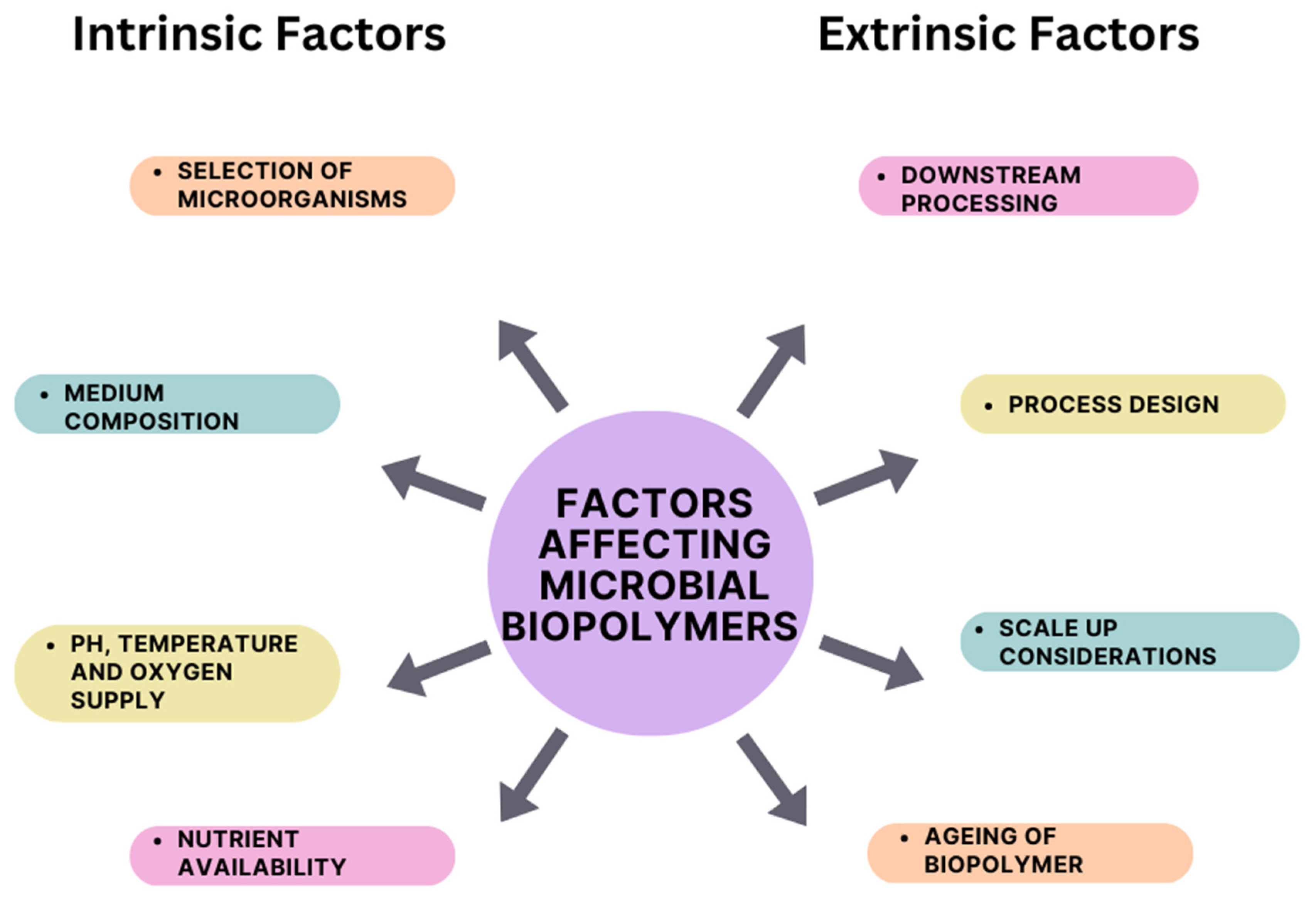
| Biopolymer | Category | Bacterial Strain | References |
|---|---|---|---|
| Alginate | Polysaccharide | Laminaria digitata spp., Macrocystis pyrifera | [13] |
| Pseudomonas spp. | [14] | ||
| Bacterial Cellulose | Polysaccharide | Komagataeibacter saccharivorans | [15] |
| Komagataeibacter spp. FXV3, Komagataeibacter spp. NFXK3, and K. intermedius LMG 18909 | [16] | ||
| β-glucan | Polysaccharide | Bacillus subtilis | [17] |
| Xanthomonas campestris, Bacillus natto | [18] | ||
| Hyaluronic acid | Polysaccharide | Mytilus galloprovincialis | [19] |
| Streptococcus zooepidemicus | [20] | ||
| Polyhydroxyalkanoates | Polyester | Bacillus megaterium OUAT 016 | [21] |
| Bacillus cereus RBL6 and Pseudomonas pseudoalcaligens RBL7 | [22] | ||
| Poly (γ-glutamic acid) | Polyamide | B. megaterium WH320 | [23] |
| B. licheniformis 9945a and B. subtilis natto | [24] | ||
| Xanthan gum | Polysaccharide | Xanthomonas campestris bacterium | [25] |
| Xanthomonas campestris WXLB-006 | [26] |
| Biopolymer | Category | Fungal Strain | References |
|---|---|---|---|
| Pullulan | Polysaccharide | Aureobasidium pullulans ATCC 15233 | [27] |
| Aureobasidium pullulans MTCC 1991 | [28] | ||
| Aureobasidium pullulans HIT-LCY3 | [29] | ||
| Aureobasidium pullulans ATCC 15233 | [30] | ||
| β-glucan | Polysaccharide | Saccharomyces cerevisiae | [31] |
| Lasiodiplodia theobromae CCT 3966 | [32] | ||
| Aureobasidium thailandense NRRL 58543 | [33] | ||
| Saccharomyces cerevisiae, Aspergillus oryzae | [18] | ||
| Chitosan | Polysaccharide | Rhizopus stolonifera | [34] |
| Mucorales | [35] | ||
| Roccella montagnei. | [36] | ||
| Absidia coerulea and Gongronella butleri | [37] |
| Biopolymer | Category | Algae Species | References |
|---|---|---|---|
| Alginate | Polysaccharide | Sargassum cristaefolium | [38] |
| Nizimuddinia zanardini | [39] | ||
| Polyhydroxyalkanoates | Polyester | Synechococcus subsalsus | [40] |
| Chlorella minutissima | [40] | ||
| Spirulina spp. | [41] | ||
| Polyhydroxybutyrate | Polyester | Stigeoclonium spp. B23 | [42] |
| Chlorella fusca | [43] | ||
| Chlorella spp. | [44] | ||
| Microcystis spp. | [45] | ||
| Polylactide | Polyester | Corallina elongata | [46] |
| Scenedesmus abundans | [47] |
| Biopolymer | Microbial Strains | Carbon /Nitrogen Source | Nutrient Used | Duration of Fermentation | Type of Fermentation | Fermentation Conditions (Temperature, pH, etc.) | Maximum Yield (g/L) | Environmental Application | References |
|---|---|---|---|---|---|---|---|---|---|
| Polyhydroxybutyrate | Bacillus subtilis NG05 | Sugarcane Molasses (Maltose) | Beef extract, Peptone | 18 h | Batch | pH = 7, 30 °C | 4.94 | Waste utilization and development of Bioplastic | [52] |
| Ralstonia eutropha ATCC 17,699 | Glycerol | NaH2PO4, (NH4)2SO4, MgSO4·7H2O, K2HPO4 | 62 h | Fed-batch | pH = 10, 60 °C | 68 | Potential as biodegradable material | [53] | |
| Chitosan | Penicillium citrinum | Rice Straw | Potato dextrose | 10 days | Solid State | pH = 6.5–7.5, 30 °C | 7 | Heavy metal removal | [54] |
| Rhizopus oryzae, Mucor sp. (MTCC 3340), Absidia coerulea (MTCC 1335) | Tannery fleshing waste | Yeast extract | 70 h 140 h 200 h | Submerged | pH = 8.5, 25 °C | 1.53 2.74 2.05 | Utilization of leather flesh waste | [55] | |
| Bacterial Cellulose | Gluconacetobacter xylinus | Glucose | Yeast extract, Peptone | 10 days | Static | pH = 5; 30 °C | - | Detection of Staphylococcus aureus | [56] |
| Gluconacetobacter sp. | Apple waste | 2% Fructose | 7 days | Static | pH = 6.4; 28 °C | - | Removal of chromium ions | [57] | |
| Gluconacetobacter xylinus | Orange peel | Yeast/Peptone extract | 8 days | Static | pH = 4.75; 30 °C | 6.13 | Waste reduction | [58] | |
| Hyaluronic Acid | Streptococcus zooepidemicus | Cheese whey | Yeast extract | 2 days | Batch | pH = 6.7; 37 °C | 3.37 | 70 % cost reduction as compared to synthetic media | [59] |
| Xanthan Gum | Xanthomonas campestris | Wastewater, Malt | Yeast broth | 72 h | Batch | pH = 7; 28 °C | 15.56 | Improved oil recovery | [60] |
| Xanthomonas campestris | Wastewater, Malt | Yeast broth | 120 h | Batch | pH = 7; 28 °C | - | Enhanced oil recovery | [61] | |
| β-glucan | Lasiodiplodia theobromae CCT 3966 | Sugarcane straw | Yeast extract, malt extract, peptone | 72 h | Batch | pH = 7; 30 °C | 3.28 | Waste utilization: conversion of cellulose to glucose | [32] |
| Xylaria sp. BCC 1067 | Glucose | Malt extract, peptone | 28 days | Batch | pH = 7; 25 °C | 0.115 | Antifungal activity | [62] | |
| Alginate | Azotobacter vinelandii | Sucrose | K2HPO4·3H2O, CaSO4·2H2O, NaCl, MgSO4·7H2O, Na2MoO4·2H2O, FeSO4·7H2O | 20 h | Batch | pH = 7; 30 °C | 3.06 | Nitrogen fixation | [63] |
| Azotobacter vinelandii ATCC 9046 | Sugar beet molasses/maltose/starch | Yeast extract, | 72 h | Batch | pH = 7.2; 30 °C | 5.44 | Adsorbent for heavy metals | [64] | |
| γ-PGA | Bacillus subtilis | Sago and Soyabean | Yeast Extract | 5 days | Batch | pH = 6.5; 37 °C | 35.32 | Act as a Bio flocculant | [65] |
| Bacillus subtilis | Glucose | Beef extract, Yeast extract, Peptone | 2 days | Batch | pH = 7, 37 °C | 25.38 | Act as a Bio flocculant | [66] | |
| Pullulan | Aureobasidium pullulans | Potato starch | Yeast extract, peptone | 5 days | Batch | pH = 6; 26 °C | 23.47 | Could be used as a potential purifying agent and as a flocculant for heavy metal reduction | [29] |
| Aureobasidium pullulans | Sucrose | Barley malt extract | 170 h | Batch | pH = 6; 26 °C | 50 | Biosorbent of heavy metals; Protective agent | [67,68] | |
| Polylactide | Indigenous microorganism | Mango Peel Waste | - | 6 days | Anaerobic submerged | pH = 10, 35 °C | 17.48 | Cost-effective biodegradable material | [69] |
| Lactobacillus delbrueckii | Broken rice | Yeast Extract, Peptone | 50 h | Solid State | pH = 6, 40 °C | 79 | Cost-effective Biodegradable material | [70] |
| Polymer | Pollutant | Adsorption Capacity (mg⋅g−1) | References |
|---|---|---|---|
| Inorganic contaminants | |||
| Carboxylated cellulose nanocrystal/sodium alginate hydrogel | Pb2+ | 335.3 | [278] |
| Cellulose/PVA/Graphene Composite aerogel | Cu2+ Cd2+ Cr3+ Co2+ Zn2+ Pb2+ | 80.12 102.23 123.02 62.38 69.55 57.16 | [279] |
| Thioglycolic Acid-Esterified Cellulose Nanocrystals | Cu2+ | 4.244 | [280] |
| Chitosan-glucose hydrogel | Co2+ | 202 | [281] |
| Chitosan/orange peel hydrogel | Cu2+ Cr6+ | 116.6 107.5 | [282] |
| Xanthate-modified chitosan/poly (N-isopropylacrylamide) | Cu2+ Pb2+ Ni2+ | 115.1 172.0 66.9 | [283] |
| Cellulose/guar gum/biochar | Cu2+ Co2+ | 805.45 772.52 | [284] |
| Cellulose/bentonite grafted polyacrylic acid hydrogel | Cd2+ | 242.53 | [285] |
| Alginate/polyethyleneimine | Cu2+ Pb2+ | 322.6 344.8 | [269] |
| 3D network nanostructured sodium alginate | Cd2+ Cu2+ | 9.54 13.38 | [286] |
| Hyaluronic acid-supported magnetic microspheres | Cu2+ | 12.2 | [287] |
| Xanthan gum/n-acetyl cysteine modified mica bionanocomposite | Pb2+ Cu2+ Ni2+ | 530.54 177.2 51.48 | [288] |
| Poly (Acrylamide-co-Acrylic Acid)/Xanthan Gum hydrogel | Cd2+ Ni2+ | 312.15 185.0 | [289] |
| Pullulan/polydopamine hydrogels | Cu2+ | 100.9 | [290] |
| Sodium alginate/coffee waste | Pb2+ | 984.4 | [291] |
| Whey protein concentrate/pullulan hydrogel | Cu2+ | 81.6 | [292] |
| Polylactic acid hydrogel | Pb2+ Ni2+ | 416.07 243.10 | [293] |
| Organic contaminants | |||
| Sodium hydroxide activated acrolein/chitosan | Acid blue 93 | 2500 | [294] |
| Carboxymethyl cellulose/chitosan/triethylenetetramine | Direct blue Congo red | 534.25 519.53 | [295] |
| Cellulose/guar gum/biochar | Methylene blue | 598.28 | [284] |
| Cellulose/Poly(acrylic acid) | Methylene blue | 1492.99 | [296] |
| Chitosan/polyacrylate/graphene oxide | Methylene blue FY3 | 296.5 280.3 | [297] |
| Chitosan/cellulose | Congo red | 380 | [298] |
| Chitosan/poly(n-vinyl-2-pyrrolidone) | Orange G | 63.7 | [299] |
| Xanthan gum/amantadine composites | Methylene blue | 565 | [300] |
| Polyaniline/Xanthan Gum Nanocomposite | Methylene Blue | 22.52 | [301] |
| Xanthan gum/polyacrylic acid/graphene oxide | Methyl violet Methylene Blue | 1052.63 793.65 | [302] |
| Pullulan-graft-poly(3-acrylamidopropyl trimethylammonium chloride) microspheres | Azocarmine B | 113.63 | [303] |
| Sodium alginate-polyaniline nanotube | Methyl orange | 370.4 | [304] |
| Sodium alginate/coffee waste | Acridine orange | 805.3 | [291] |
| Crosslinked chitosan films | SDBS | 714 | [305] |
| Nanofibrillated cellulose | Cationic surfactants | 462.28 | [306] |
| Hydrogel chitosan beads | SDS | 1300 | [307] |
| Polymer | Pollutant | Removal Rates (%) | References |
|---|---|---|---|
| Inorganic contaminants | |||
| Chitosan/Poly(acrylamide-acryloyloxyethyl) trimethylammonium chloride | Zn2+ | 99.3 | [329] |
| Sodium alginate/triethylenetetramine nanoflocculant | Pb2+ | 97 | [330] |
| Chitosan/acrylamide/itaconic acid/3-acrylamide propyl trimethylammonium chloride | Ni2+ | 86.3 | [331] |
| Cationic Pullulan Derivatives | FeO TiO2 | 95 75 | [332] |
| Organic contaminants | |||
| Chitosan/poly (acrylamide-itaconic acid) | Crystal violet | 81.6 | [333] |
| Polyaluminium chloride/xanthan gum | Congo red | 93.81 | [334] |
| Cationic pullulan derivatives | Novadim Bordeaux mixture Karate Zeon | 90 98 80 | [335] |
| Cationic cellulose/bentonite | Methylene Blue Duasyn Direct Red Acid Black 2 Crystal Violet Basic Green 1 | 99 95 100 100 99 | [336] |
| Carboxymethyl cellulose/itaconic acid/sodium alginate | Crystal violet | 92.2 | [337] |
| Cellulose nanocrystals | Reactive blue 19 | 80 | [338] |
| Sodium alginate/methacryloxyethyltrimethyl ammonium chloride | Dissolved Organic Carbon (DOC) | 35.42 | [339] |
| Other contaminants (Water Quality Parameters) | |||
| Sodium alginate/methacryloxyethyltrimethyl ammonium chloride | UV254 | 32.42 | [339] |
| Xanthan Gum/Polyacrylamide /SiO2 | Turbidity Total Solids (TS) Total Dissolved Solids (TDS) Total Suspended (TSS) | 93.95 76.97 48.21 93.75 | [340] |
| xanthan gum/polyacrylamide | Turbidity | 52.63 | [341] |
| Pullulan/p(N-isopropylacrylamide) | Turbidity | 88 | [332,342] |
| Poly-γ-glutamic acid | Turbidity | 86.6 | [343] |
| Chitosan/Poly(acrylamide-acryloyloxyethyl) trimethylammonium chloride | Total Phosphorus (TP) | 98.8 | [329] |
| Chitosan/poly-glutamic acid | COD Total Nitrogen (TN) TP Turbidity | 44.8 53.4 28.1 98.3 | [344] |
| Bentonite/chitosan/poly-glutamic acid | COD TN TP Turbidity | 86.2 80.3 52.3 98.2 | [285] |
| Sodium alginate/methacryloxyethyltrimethyl ammonium chloride | Turbidity | 95.96 | [339] |
| Sodium alginate/polysilicate aluminum calcium | Turbidity | 97.2 | [345] |
| Chitosan/Poly(acrylamide-acryloyloxyethyl) trimethylammonium chloride | COD | 72.5 | [329] |
| Cellulose Nanocrystals | Turbidity | 99.7 | [346] |
| Dicarboxyl cellulose | Turbidity COD | 80 60 | [347] |
| Dicarboxyl cellulose | Turbidity | 99.5 | [348] |
| Polymer | Pollutant | Removal Rates (%) | References |
|---|---|---|---|
| Inorganic contaminants | |||
| Polylactic acid/amino-activated carbon/modified mangrove particles pH-responsive adsorptive membrane | Cu2+ Pb2+ Ni2+ | 99.95 100 99.95 | [361] |
| Cellulose acetate/chitosan/TiO2 | Cu2+ | 97.0 | [359] |
| PLA/PHB/Polybutylene succinate/polypropylene carbonate/silica nanoparticles | Mn2+ | 14.17 | [362] |
| Cellulose acetate/nanochitosan/PEG | Cr6+ | 95 | [363] |
| Hyaluronic acid/polyamide | Na2SO4 | 94.90 | [364] |
| Organic contaminants | |||
| Cellulose acetate/PLA/polyurethane | Methylene Blue Congo Red | 45 60 | [365] |
| Cellulose microfiber/PLA/poly(butylene adipate-co-terephthalate)/maleic anhydride | Methylene Blue | 97.2 | [366] |
| Fe3O4/Xanthan gum/polyvinylidene fluoride | Reactive Black 5 Reactive Red 120 | 84.8 73.8 | [367] |
| Xanthan Gum/polyvinylidene fluoride/dimethyl sulfoxide | Congo red | 91.25 | [368] |
| Cellulose microfiber/PLA/poly(butylene adipate-co-terephthalate) | Methylene Blue | 58.7 | [366,369] |
| Sodium alginate hydrogel/graphene oxide | MB Direct Red (DR80) Congo Red (CR) Crystal Violet (CV) | 100 98.80 100 100 | [369] |
| Chitosan/bacterial cellulose/carboxyl multi-walled carbon nanotubes | Direct Orange S, Procion Red mx-5B Stilbene Yellow Methylene Blue | 99.7 97.8 62.8 27.5 | [370] |
| Hyaluronic acid/polyamide | Perfluorohexane sulfonic acid (PFHxS) | 93.40 | [364] |
| Other contaminants | |||
| Sodium alginate/tannic acid/β-FeOOH | Oil | 99.64 | [371] |
| Chitosan/bacterial cellulose/carboxyl multi-walled carbon nanotubes | Oil | 97.67 | [370] |
| Pristine PLA | Oil | 93.9 | [372] |
| PLA/polyethylene oxide | Oil | 99.6 | [373] |
| Cellulose acetate/chitosan/TiO2 | Oil | 99.4 97.0 | [359] |
| PLA/multi-walled carbon nanotubes/graphene oxide | Ammonium-nitrogen Phosphate | 90.1 71.3 | [374] |
| PLA/PHB/Polybutylene succinate/polypropylene carbonate/silica nanoparticles | Oil Total Dissolved Solids (TDS) Turbidity | 98.6 22.56 11.33 89.15 | [362] |
| Cellulose acetate | Sulfamethoxazole Primidone Carbamazepine Phenacetine 17β-Estradiol | 82 85 85 10 29 | [375] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Tellili, N.; Kacem, I.; Rouissi, T. Microbial Biopolymers: From Production to Environmental Applications—A Review. Appl. Sci. 2024, 14, 5081. https://doi.org/10.3390/app14125081
Sharma M, Tellili N, Kacem I, Rouissi T. Microbial Biopolymers: From Production to Environmental Applications—A Review. Applied Sciences. 2024; 14(12):5081. https://doi.org/10.3390/app14125081
Chicago/Turabian StyleSharma, Mohit, Nihed Tellili, Imen Kacem, and Tarek Rouissi. 2024. "Microbial Biopolymers: From Production to Environmental Applications—A Review" Applied Sciences 14, no. 12: 5081. https://doi.org/10.3390/app14125081







