A Comprehensive Review on Mine Tailings as a Raw Material in the Alkali Activation Process
Abstract
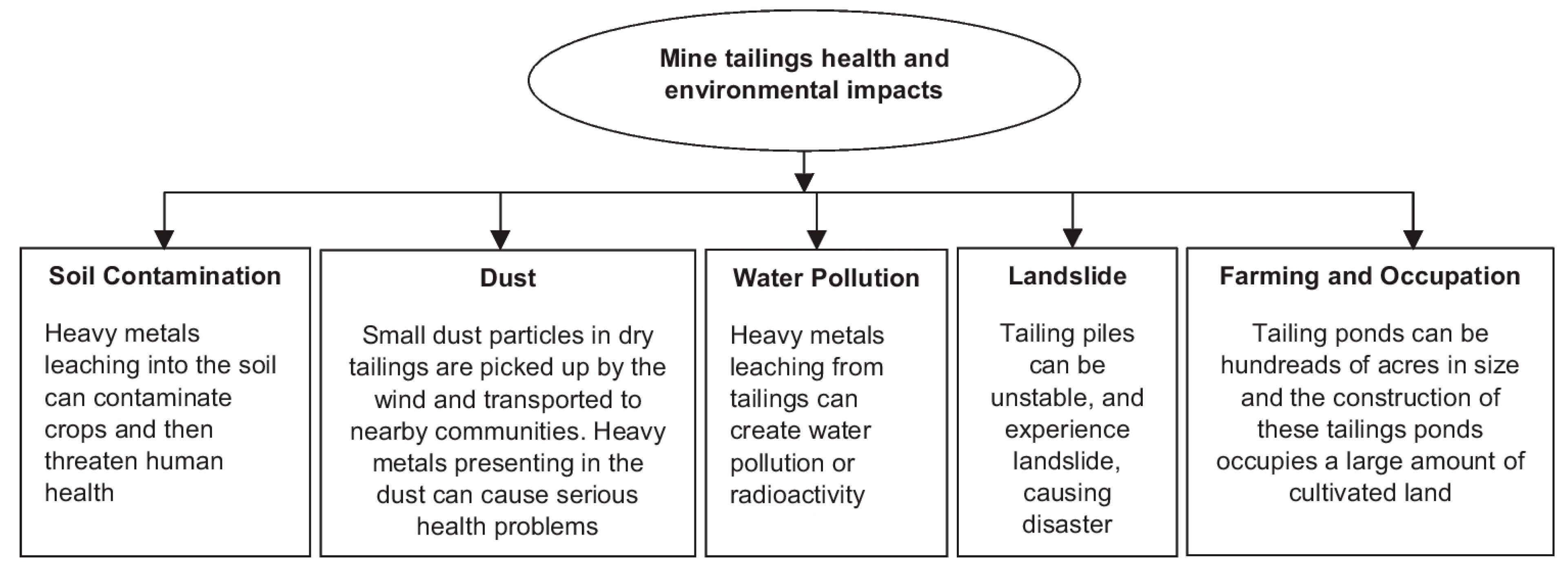
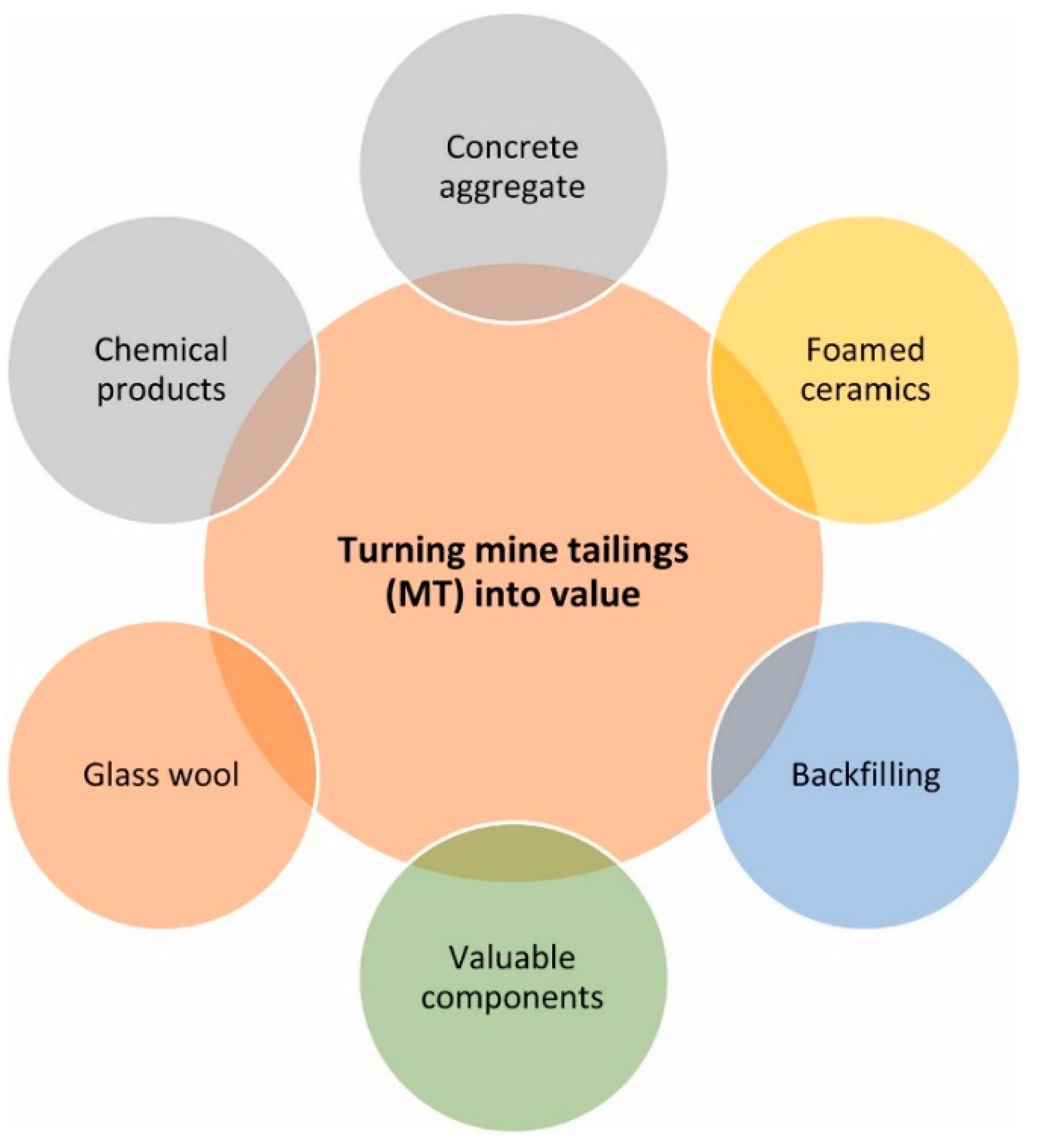
:1. Introduction
2. Mineralogy of Mine Tailings
3. Alkali Activation
3.1. Alkali-Activated Mine Tailings
3.2. Mine Tailings as a Filler Material in Alkali Activation
4. Pre-Treatment Methods
4.1. Mechanical Activation
4.2. Heat Treatment
4.3. Alkaline Fusion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Ma, T.; Zhang, J.; Shi, B. The Role of Construction Industry and Construction Policy on Sustainable Rural Development in China. Environ. Sci. Pollut. Res. 2023, 30, 7942–7955. [Google Scholar] [CrossRef] [PubMed]
- Najafi, E.K.; Tavares, P.; Miranda, T.; Manaviparast, H.R.; Cristelo, N. Ophthalmic Glass Lens Waste as an Alternative Soluble Silica Source for Alkali-Activation Reactions. Constr. Build. Mater. 2023, 392, 131854. [Google Scholar] [CrossRef]
- Manaviparast, H.R.; Pinheiro, J.; Najafi, E.K.; Abreu, C.; Araújo, N.; Cristelo, N.; Miranda, T. Mechanical Behavior of a Mine Tailing Stabilized with a Sustainable Binder. Appl. Sci. 2023, 13, 4103. [Google Scholar] [CrossRef]
- Shengo, L.M. Review of Practices in the Management of Mineral Wastes: The Case of Waste Rocks and Mine Tailings. Water Air Soil Pollut. 2021, 232, 273. [Google Scholar] [CrossRef]
- Maijin, R. Mineral Characterization Study of Gold Deposit in Tawau, Sabah Focusing on Mineralogy and Geochemistry. Ph.D. Thesis, Universiti Sains Malaysia, Gelugor, Malaysia, 2022. [Google Scholar]
- Yao, G.; Liu, Q.; Wang, J.; Wu, P.; Lyu, X. Effect of Mechanical Grinding on Pozzolanic Activity and Hydration Properties of Siliceous Gold Ore Tailings. J. Clean. Prod. 2019, 217, 12–21. [Google Scholar] [CrossRef]
- Kiventerä, J.; Perumal, P.; Yliniemi, J.; Illikainen, M. Mine Tailings as a Raw Material in Alkali Activation: A Review. Int. J. Miner. Metall. Mater. 2020, 27, 1009–1020. [Google Scholar] [CrossRef]
- Kalisz, S.; Kibort, K.; Mioduska, J.; Lieder, M.; Małachowska, A. Waste Management in the Mining Industry of Metals Ores, Coal, Oil and Natural Gas—A Review. J. Environ. Manag. 2022, 304, 114239. [Google Scholar] [CrossRef] [PubMed]
- Barati, S.; Tabatabaie Shourijeh, P.; Samani, N.; Asadi, S. Stabilization of Iron Ore Tailings with Cement and Bentonite: A Case Study on Golgohar Mine. Bull. Eng. Geol. Environ. 2020, 79, 4151–4166. [Google Scholar] [CrossRef]
- Shalash, I. Introduction to Geology & Minerals; Atlantic International University: Honolulu, HI, USA, 2019. [Google Scholar]
- Maruthupandian, S.; Chaliasou, A.; Kanellopoulos, A. Recycling Mine Tailings as Precursors for Cementitious Binders—Methods, Challenges and Future Outlook. Constr. Build. Mater. 2021, 312, 125333. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A Review of Recent Strategies for Acid Mine Drainage Prevention and Mine Tailings Recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Queiroz, H.M.; Ruiz, F.; Deng, Y.; de Souza Júnior, V.S.; Ferreira, A.D.; Otero, X.L.; Ferreira, T.O. Mine Tailings in a Redox-Active Environment: Iron Geochemistry and Potential Environmental Consequences. Sci. Total Environ. 2022, 807, 151050. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Won, S.; Ha, M.G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for Environmental Remediation of Toxic Metals and Metalloids: A Review on Soils, Sediments, and Mine Tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and Health Risk Assessment of Heavy Metals in China’s Lead–Zinc Mine Tailings: A Meta–Analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef] [PubMed]
- Manaviparast, H.R.; Araújo, N.; Cristelo, N.; Miranda, T. Study on Guardrail Post Behavior Located on Organic Soil Using Simplified Experimental and Numerical Methods. Soils Rocks 2022, 45, e2022077921. [Google Scholar] [CrossRef]
- Mora-Sánchez, F.J.; Gómez-Álvarez, A.; Encinas-Romero, M.A.; Villalba-Atondo, A.I.; Valenzuela-García, J.L.; Jara-Marini, M.E.; Encinas-Soto, K.K. Prediction of Acid Mine Drainage Generation from Mine Waste at an Abandoned Mining Site in a Semi-arid Environment. Water Air Soil Pollut. 2024, 235, 140. [Google Scholar] [CrossRef]
- Oboni, F.; Oboni, C. Tailings Dam Management for the Twenty-First Century; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Yilmaz, E.; Koohestani, B.; Cao, S. Recent Practices in Mine Tailings’ Recycling and Reuse. In Managing Mining and Minerals Processing Wastes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 271–304. [Google Scholar]
- Behera, S.K.; Mishra, D.P.; Singh, P.; Mishra, K.; Mandal, S.K.; Ghosh, C.N.; Mandal, P.K. Utilization of Mill Tailings, Fly Ash and Slag as Mine Paste Backfill Material: Review and Future Perspective. Constr. Build. Mater. 2021, 309, 125120. [Google Scholar] [CrossRef]
- Mashifana, T.; Sithole, T. Clean Production of Sustainable Backfill Material from Waste Gold Tailings and Slag. J. Clean. Prod. 2021, 308, 127357. [Google Scholar] [CrossRef]
- Al-Bakri, A.Y.; Ahmed, H.M.; Hefni, M.A. Eco-Sustainable Recycling of Cement Kiln Dust (CKD) and Copper Tailings (CT) in the Cemented Paste Backfill. Sustainability 2023, 15, 3229. [Google Scholar] [CrossRef]
- Liu, L.; Xin, J.; Huan, C.; Qi, C.; Zhou, W.; Song, K.I. Pore and Strength Characteristics of Cemented Paste Backfill Using Sulphide Tailings: Effect of Sulphur Content. Constr. Build. Mater. 2020, 237, 117452. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Rathnaweera, T.D.; Perera, M.S.A. Recent Advances in Alkaline Cement Binders: A Review. J. Clean. Prod. 2019, 227, 70–87. [Google Scholar] [CrossRef]
- Tekle, B.H.; Holschemacher, K. Alkali Activated Cement Mixture at Ambient Curing: Strength, Workability, and Setting Time. Struct. Concr. 2022, 23, 2496–2509. [Google Scholar] [CrossRef]
- Siddique, S.; Jang, J.G. Assessment of Molybdenum Mine Tailings as Filler in Cement Mortar. J. Build. Eng. 2020, 31, 101322. [Google Scholar] [CrossRef]
- dos Santos, C.P.; Bruschi, G.J.; Mattos, J.R.G.; Consoli, N.C. Stabilization of Gold Mining Tailings with Alkali-Activated Carbide Lime and Sugarcane Bagasse Ash. Transp. Geotech. 2022, 32, 100704. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Gu, X.; Nehdi, M.L.; Marani, A.; Zhang, L. Utilization of Iron Ore Tailings with High Volume in Green Concrete. J. Build. Eng. 2023, 72, 106585. [Google Scholar] [CrossRef]
- Behera, S.K.; Ghosh, C.N.; Mishra, K.; Mishra, D.P.; Singh, P.; Mandal, P.K.; Sethi, M.K. Utilization of Lead–Zinc Mill Tailings and Slag as Paste Backfill Materials. Environ. Earth Sci. 2020, 79, 389. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.; de Carvalho, J.M.F.; Ribeiro, J.C.L. Application of Eco-Friendly Alternative Activators in Alkali-Activated Materials: A Review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.D.; Vieira, C.M.F. Reaction Mechanisms of Alkali-Activated Materials. Rev. IBRACON Estrut. Mater. 2021, 14, e14309. [Google Scholar] [CrossRef]
- Parthiban, D.; Vijayan, D.S.; Koda, E.; Vaverkova, M.D.; Piechowicz, K.; Osinski, P.; Van Duc, B. Role of Industrial Based Precursors in the Stabilization of Weak Soils with Geopolymer—A Review. Case Stud. Constr. Mater. 2022, 16, e00886. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Geng, Z.; Xu, X.; Yu, W.; Chen, J. Structure, Fractality, Mechanics and Durability of Calcium Silicate Hydrates. Fract. Fract. 2021, 5, 47. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of Source Materials, Additives on the Mechanical Properties and Durability of Fly Ash and Fly Ash-Slag Geopolymer Mortar: A Review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Zhang, Y.; Gu, G.; Hu, J. Micro-assessment of Heavy Metal Immobilization within Alkali-Activated Copper Tailings-Slag Geopolymer. Cem. Concr. Compos. 2024, 149, 105510. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Cui, M.; Wang, J.; Lyu, X. Preparation of Eco-Friendly One-Part Geopolymers from Gold Mine Tailings by Alkaline Hydrothermal Activation. J. Clean. Prod. 2021, 298, 126806. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, M.; Li, X.; Wang, J.; Wang, Z.; Li, L.; Lyu, X. Alkali-Hydrothermal Activation of Mine Tailings to Prepare One-Part Geopolymer: Activation Mechanism, Workability, Strength, and Hydration Reaction. Ceram. Int. 2022, 48, 30407–30417. [Google Scholar] [CrossRef]
- Mashifana, T.; Sebothoma, J.; Sithole, T. Alkaline Activation of Basic Oxygen Furnace Slag Modified Gold Mine Tailings for Building Material. Adv. Civ. Eng. 2021, 2021, 9984494. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Falah, M.; Illikainen, M. Assessment of Mine Tailings as Precursors for Alkali-Activated Materials for On-Site Applications. Constr. Build. Mater. 2020, 246, 118470. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Alaba, P.A.; Rashedi, A. Selected Performance of Alkali-Activated Mine Tailings as Cementitious Composites: A Review. J. Build. Eng. 2022, 50, 104154. [Google Scholar] [CrossRef]
- Xiaolong, Z.; Shiyu, Z.; Hui, L.; Yingliang, Z. Disposal of Mine Tailings via Geopolymerization. J. Clean. Prod. 2021, 284, 124756. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine Tailings-based Geopolymers: Properties, Applications and Industrial Prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Guedes, J.P.C.; Silvani, C.; Carvalho, J.V.D.A.; Wagner, A.C.; Silva, J.P.D.S.; Consoli, N.C. Mechanical Behaviour of Fibre-Reinforced Cemented Iron Ore Tailings Across the Compaction Curve. Geotech. Geol. Eng. 2024, 1–12. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Zhou, H.; He, K.; Luo, C.; Liu, Z.; Tang, X. Review on the Development and Utilization of Ionic Rare Earth Ore. Minerals 2022, 12, 554. [Google Scholar] [CrossRef]
- Seigneur, N.; De Windt, L.; Déjeant, A.; Lagneau, V.; Descostes, M. Long-term Evolution of Uranium Mobility within Sulfated Mill Tailings in Arid Regions: A Reactive Transport Study. Minerals 2021, 11, 1201. [Google Scholar] [CrossRef]
- Mahabub, M.S.; Alahi, F.; Al Imran, M. Unlocking the Potential of Microbes: Biocementation Technology for Mine Tailings Restoration—A Comprehensive Review. Environ. Sci. Pollut. Res. 2023, 30, 91676–91709. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.S.; Rissoli, A.L.C.; Cacciari, P.P.; da Fonseca, A.J.P.V.; Scheuermann Filho, H.C.; Wagner, A.C.; Consoli, N.C. Triaxial Testing Response of Compacted Iron Ore Tailings Considering a Broad Spectrum of Confining Pressures. Soils Found. 2024, 64, 101438. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Huang, X.; Jiao, B.; Li, D. Enhancement Experiment on Cementitious Activity of Copper-Mine Tailings in a Geopolymer System. Fibers 2017, 5, 47. [Google Scholar] [CrossRef]
- Manjarrez, L.; Nikvar-Hassani, A.; Shadnia, R.; Zhang, L. Experimental Study of Geopolymer Binder Synthesized with Copper Mine Tailings and Low-Calcium Copper Slag. J. Mater. Civ. Eng. 2019, 31, 04019156. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Thobakgale, R.; Ngoejana, P.C.; Ramugondo, L.; Matidza, M.; Nemapate, N. Physico-Chemical and Mineralogical Characterization of Musina Mine Copper and New Union Gold Mine Tailings: Implications for Fabrication of Beneficial Geopolymeric Construction Materials. J. Afr. Earth Sci. 2018, 137, 218–228. [Google Scholar] [CrossRef]
- Falayi, T. A Comparison Between Fly Ash- and Basic Oxygen Furnace Slag-Modified Gold Mine Tailings Geopolymers. Int. J. Energy Environ. Eng. 2020, 11, 207–217. [Google Scholar] [CrossRef]
- Demir, F.; Derun, E.M. Modelling and Optimization of Gold Mine Tailings Based Geopolymer by Using Response Surface Method and Its Application in Pb2+ Removal. J. Clean. Prod. 2019, 237, 117766. [Google Scholar] [CrossRef]
- Kiventerä, J.; Sreenivasan, H.; Cheeseman, C.; Kinnunen, P.; Illikainen, M. Immobilization of Sulfates and Heavy Metals in Gold Mine Tailings by Sodium Silicate and Hydrated Lime. J. Environ. Chem. Eng. 2018, 6, 6530–6536. [Google Scholar] [CrossRef]
- Niu, H.; Abdulkareem, M.; Sreenivasan, H.; Kantola, A.M.; Havukainen, J.; Horttanainen, M.; Illikainen, M. Recycling Mica and Carbonate-Rich Mine Tailings in Alkali-Activated Composites: A Synergy with Metakaolin. Miner. Eng. 2020, 157, 106535. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Shin, W.; Jo, S.; Jun, H. Psychrophilic Methanogenesis of Food Waste in a Bio-Electrochemical Anaerobic Digester with Rotating Impeller Electrode. J. Clean. Prod. 2018, 188, 556–567. [Google Scholar] [CrossRef]
- dos Santos, L.F.; de Carvalho, J.M.F.; Peixoto, R.A.F.; Brigolini, G.J. Iron Ore Tailing-Based Geopolymer Containing Glass Wool Residue: A Study of Mechanical and Microstructural Properties. Constr. Build. Mater. 2019, 220, 375–385. [Google Scholar]
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D. Utilization of Iron Ore Mine Tailings for the Production of Geopolymer Bricks. Int. J. Min. Reclam. Environ. 2016, 30, 92–114. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of Geopolymers from Vanadium Tailings by Mechanical Activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Perumal, P.; Piekkari, K.; Sreenivasan, H.; Kinnunen, P.; Illikainen, M. One-Part Geopolymers from Mining Residues–Effect of Thermal Treatment on Three Different Tailings. Miner. Eng. 2019, 144, 106026. [Google Scholar] [CrossRef]
- Zhao, S.; Xia, M.; Yu, L.; Huang, X.; Jiao, B.; Li, D. Optimization for the Preparation of Composite Geopolymer Using Response Surface Methodology and Its Application in Lead-Zinc Tailings Solidification. Constr. Build. Mater. 2021, 266, 120969. [Google Scholar] [CrossRef]
- McDonald, J.E.D.; Roache, S.C.; Kawatra, S.K. Repurposing Mine Tailings: Cold Bonding of Siliceous Iron Ore Tailings. Miner. Metall. Process. 2016, 33, 47–52. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Properties of an Aged Geopolymer Synthesized from Calcined Ore-Dressing Tailing of Bauxite and Slag. Cem. Concr. Res. 2017, 100, 23–31. [Google Scholar] [CrossRef]
- Song, M.; Jiaping, L.; Qian, J.; Jianzhong, L.; Liang, S. Experimental Study on Utilization of Quartz Mill Tailings as a Filler to Prepare Geopolymer. Miner. Process. Extr. Metall. Rev. 2016, 37, 311–322. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X.; Castro-Gomes, J. Preparation Conditions for the Synthesis of Alkali-Activated Binders Using Tungsten Mining Waste. J. Mater. Civ. Eng. 2017, 29, 04017181. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; Leon-Patino, C.A.; Ma, Y.; Yin, W. Consolidation of Mine Tailings through Geopolymerization at Ambient Temperature. J. Am. Ceram. Soc. 2019, 102, 2451–2461. [Google Scholar] [CrossRef]
- Povarennykh, A.S. Crystal Chemical Classification of Minerals; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Silicate Minerals—Potential Source of Potash—A Review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Bai, S. Wettability Alteration Mechanisms in Carbonate Rocks: An Experimental and Molecular-Scale Computational Study. Ph.D. Thesis, University of Wyoming, Laramie, Wyoming, 2020. [Google Scholar]
- El Aref, M.; Abd El-Rahman, Y.; Zoheir, B.; Surour, A.; Helmy, H.M.; Abdelnasser, A.; El Aref, M. Mineral Resources in Egypt (I): Metallic Ores. In The Geology of Egypt; Springer International Publishing: Cham, Switzerland, 2019; pp. 521–587. [Google Scholar]
- Kasap, T.; Yilmaz, E.; Sari, M. Physico-Chemical and Micro-Structural Behavior of Cemented Mine Backfill: Effect of pH in Dam Tailings. J. Environ. Manag. 2022, 314, 115034. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Yin, Z.; Dong, J.; Xu, L.; Ma, R.; Li, S. Pretreatment of Refractory Gold Ore by Curing with Concentrated Sulfuric Acid. Min. Metall. Explor. 2024, 41, 1079–1087. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Li, S. Silicic Protective Surface Films for Pyrite Oxidation Suppression to Control Acid Mine Drainage at the Source. Environ. Sci. Pollut. Res. 2019, 26, 25725–25732. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, B. Biochar as a Potential Strategy for Remediation of Contaminated Mining Soils: Mechanisms, Applications, and Future Perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Plante, B.; Schudel, G.; Benzaazoua, M. Generation of Acid Mine Drainage. In Hard Rock Mine Reclamation: From Prediction to Management of Acid Mine Drainage; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–20. [Google Scholar]
- Firnia, D.; Rohmawati, I. Chemical Properties of Acid Soil after Amelioration of Dolomite, Coal, and Microbes. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 978, Issue 1; p. 012010. [Google Scholar]
- Xiu-ting, S.; Mei-rong, L.; Jun-tao, X.; Chen-chu, L.; Guang-hui, Y.; Ying-Chang, C. The Complex Effect of Organic Acids on the Dissolution of Feldspar at High Temperature. Environ. Earth Sci. 2021, 80, 244. [Google Scholar] [CrossRef]
- Zhou, Y.; Short, M.D.; Li, J.; Fan, R.; Qian, G. Non-carbonate Geochemical Options for Long-term Sustainable Acid and Metalliferous Drainage Control At-source. Environ. Earth Sci. 2019, 78, 157. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Du, S.; Zhang, Z.; Lu, W.; Su, P.; Zhao, Y. A Review: The Formation, Prevention, and Remediation of Acid Mine Drainage. Environ. Sci. Pollut. Res. 2023, 30, 111871–111890. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable Resolutions for Environmental Threat of the Acid Mine Drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef]
- Kanagaraj, B.; Lubloy, E.; Anand, N.; Hlavicka, V.; Kiran, T. Investigation of Physical, Chemical, Mechanical, and Micro-Structural Properties of Cement-less Concrete–State-of-the-Art Review. Constr. Build. Mater. 2023, 365, 130020. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Ralli, Z.G.; Pantazopoulou, S.J. State of the Art on Geopolymer Concrete. Int. J. Struct. Integr. 2021, 12, 511–533. [Google Scholar] [CrossRef]
- Kamseu, E.; Alzari, V.; Nuvoli, D.; Sanna, D.; Lancellotti, I.; Mariani, A.; Leonelli, C. Dependence of the Geopolymerization Process and End-products to the Nature of Solid Precursors: Challenge of the Sustainability. J. Clean. Prod. 2021, 278, 123587. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly Ash-based Eco-friendly Geopolymer Concrete: A Critical Review of the Long-term Durability Properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-term Durability Properties of Geopolymer Concrete: An In-depth Review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Xing, J.; Zhao, Y.; Qiu, J.; Sun, X. Microstructural and Mechanical Properties of Alkali Activated Materials from Two Types of Blast Furnace Slags. Materials 2019, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Portela Farenzena, H.; Bruschi, G.J.; Schmitt Medina, G.; de Sousa Silva, J.P.; Lotero, A.; Consoli, N.C. Iron Ore Tailings Stabilization with Alternative Alkali-activated Cement for Dry Stacking: Mechanical and Microstructural Insights. Can. Geotech. J. 2024, 61, 4. [Google Scholar] [CrossRef]
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to Improve Sustainability of Alkali-activated Materials: A Review of Side-stream Based Activators. J. Clean. Prod. 2021, 286, 125558. [Google Scholar] [CrossRef]
- Zhang, P.; Muhammad, F.; Yu, L.; Xia, M.; Lin, H.; Huang, X.; Li, D. Self-cementation solidification of heavy metals in lead-zinc smelting slag through alkali-activated materials. Constr. Build. Mater. 2020, 249, 118756. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Waste-Derived Activators for Alkali-Activated Materials: A Review. Cem. Concr. Compos. 2021, 118, 103980. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, C.; Li, Y.; Yang, C. Solidification/Stabilization of Gold Ore Tailings Powder Using Sustainable Waste-Based Composite Geopolymer. Eng. Geol. 2022, 309, 106793. [Google Scholar] [CrossRef]
- Martins, N.P.; Srivastava, S.; Simão, F.V.; Niu, H.; Perumal, P.; Snellings, R.; Habert, G. Exploring the Potential for Utilization of Medium and Highly Sulfidic Mine Tailings in Construction Materials: A Review. Sustainability 2021, 13, 12150. [Google Scholar] [CrossRef]
- Tuomivaara, S. Granulation and Alkali Activation of MSWI Bottom Ash. Master’s Thesis, University of Oulu, Oulu, Finland, 2021. Available online: https://oulurepo.oulu.fi/handle/10024/18879 (accessed on 10 June 2024).
- Zhao, X.; Liu, W.; Cai, Z.; Fu, J.; Duan, J.; Zhao, D.; Feng, Y. Reductive Immobilization of Uranium by Stabilized Zero-Valent Iron Nanoparticles: Effects of Stabilizers, Water Chemistry, and Long-Term Stability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125315. [Google Scholar] [CrossRef]
- Illikainen, M.; Kiventerä, J. Mine Tailings as Precursors for Alkali-Activated Materials and Ettringite Binders. In Industrial Waste: Characterization, Modification and Applications of Residues; De Gruyter: Berlin, Germany, 2021; p. 345. [Google Scholar]
- Seney, C.S.; Kiefer, A.M.; Aljic, S. Determination of Mercury Solvation during Cyanidation of Artisanal & Small-Scale Gold Mining Tailings via Inductively Coupled Plasma Optical Emission Spectroscopy in Comparison to Direct Mercury Analysis. Int. J. Environ. Anal. Chem. 2021, 101, 2058–2068. [Google Scholar]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A Review of the Effects of Alkali and Alkaline Earth Metal Species on Biomass Gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Qaidi, S.M.; Tayeh, B.A.; Zeyad, A.M.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Recycling of Mine Tailings for Geopolymers Production: A Systematic Review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Simonsen, A.M.T.; Solismaa, S.; Hansen, H.K.; Jensen, P.E. Evaluation of Mine Tailings’ Potential as Supplementary Cementitious Materials Based on Chemical, Mineralogical, and Physical Characteristics. Waste Manag. 2020, 102, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Asadizadeh, M.; Clements, C.; Hedayat, A.; Tunstall, L.; Gonzalez, J.A.V.; Alvarado, J.W.V.; Neira, M.T. The Effect of Class F Fly Ash on the Geopolymerization and Compressive Strength of Lightweight Aggregates Made from Alkali-Activated Mine Tailings. Constr. Build. Mater. 2023, 395, 132275. [Google Scholar] [CrossRef]
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef]
- Cheah, C.B.; Tan, L.E.; Ramli, M. Recent Advances in Slag-Based Binder and Chemical Activators Derived from Industrial By-Products—A Review. Constr. Build. Mater. 2021, 272, 121657. [Google Scholar] [CrossRef]
- Ng, W.S.; Liu, Y.; Wang, Q.; Chen, M. The Fate of the Arsenic Species in the Pressure Oxidation of Refractory Gold Ores: Practical and Modelling Aspects. Miner. Process. Extr. Metall. Rev. 2023, 44, 155–187. [Google Scholar] [CrossRef]
- Kiventerä, J.; Yliniemi, J.; Golek, L.; Deja, J.; Ferreira, V.; Illikainen, M. Utilization of Sulphidic Mine Tailings in Alkali-Activated Materials. MATEC Web Conf. 2019, 274, 01001. [Google Scholar] [CrossRef]
- Cristelo, N.; Coelho, J.; Oliveira, M.; Cesar Consoli, N.; Palomo, Á.; Fernández-Jiménez, A. Recycling and Application of Mine Tailings in Alkali-Activated Cements and Mortars—Strength Development and Environmental Assessment. Appl. Sci. 2020, 10, 2084. [Google Scholar] [CrossRef]
- Kehinde, O.; Hughes, D.; Amalu, E.H. Mineral Wastes. In Green Materials in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2024; pp. 181–199. [Google Scholar]
- Han, R.; Guo, X.; Guan, J.; Yao, X.; Hao, Y. Activation Mechanism of Coal Gangue and Its Impact on the Properties of Geopolymers: A Review. Polymers 2022, 14, 3861. [Google Scholar] [CrossRef]
- Tian, Q.; Bai, Y.; Pan, Y.; Chen, C.; Yao, S.; Sasaki, K.; Zhang, H. Application of Geopolymer in Stabilization/Solidification of Hazardous Pollutants: A Review. Molecules 2022, 27, 4570. [Google Scholar] [CrossRef]
- Perumal, P.; Kiventerä, J.; Illikainen, M. Influence of Alkali Source on Properties of Alkali-Activated Silicate Tailings. Mater. Chem. Phys. 2021, 271, 124932. [Google Scholar] [CrossRef]
- Sari, M.; Yilmaz, E.; Kasap, T. Long-term Ageing Characteristics of Cemented Paste Backfill: Usability of Sand as a Partial Substitute of Hazardous Tailings. J. Clean. Prod. 2023, 401, 136723. [Google Scholar] [CrossRef]
- Qing, L.; Shaokang, S.; Zhen, J.; Junxiang, W.; Xianjun, L. Effect of CaO on Hydration Properties of One-Part Alkali-Activated Material Prepared from Tailings through Alkaline Hydrothermal Activation. Constr. Build. Mater. 2021, 308, 124931. [Google Scholar] [CrossRef]
- Castillo, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. Simple Compressive Strength Results of Sodium-Hydroxide-and Sodium-Silicate-Activated Copper Flotation Tailing Geopolymers. Appl. Sci. 2022, 12, 5876. [Google Scholar] [CrossRef]
- Watari, T.; Northey, S.; Giurco, D.; Hata, S.; Yokoi, R.; Nansai, K.; Nakajima, K. Global Copper Cycles and Greenhouse Gas Emissions in a 1.5 °C World. Resour. Conserv. Recycl. 2022, 179, 106118. [Google Scholar] [CrossRef]
- Esmaeili, J.; Aslani, H. Use of Copper Mine Tailing in Concrete: Strength Characteristics and Durability Performance. J. Mater. Cycles Waste Manag. 2019, 21, 729–741. [Google Scholar] [CrossRef]
- Xu, W.; Li, Q.; Liu, B. Coupled Effect of Curing Temperature and Age on Compressive Behavior, Microstructure, and Ultrasonic Properties of Cemented Tailings Backfill. Constr. Build. Mater. 2020, 237, 117738. [Google Scholar] [CrossRef]
- Sedira, N. Novel Waste-Based Alkali-Activated Binders by Combining Mining and Other Mineral Waste. Ph.D. Dissertation, Universidade da Beira Interior, Covilhã, Portugal, 2021. [Google Scholar]
- Yang, Y.; Zhang, J.; Fu, Y.; Hou, D.; Dong, B. Alkaline Hydrothermal Activation of Molybdenum Tailings to Prepare One-Part Geopolymer: Activation Mechanism and Strength. J. Mater. Res. Technol. 2023, 25, 3789–3802. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.; Song, S.; Rao, F.; Xia, L. Effects of Curing Temperature on the Compressive Strength and Microstructure of Copper Tailing-Based Geopolymers. Chemosphere 2020, 253, 126754. [Google Scholar] [CrossRef]
- Helser, J.; Perumal, P.; Cappuyns, V. Valorizing (Cleaned) Sulfidic Mine Waste as a Resource for Construction Materials. J. Environ. Manag. 2022, 319, 115742. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, Z.; Yilmaz, E.; Han, J.; Qiu, J.; Dong, C. Effectiveness of Alkali-Activated Slag as Alternative Binder on Workability and Early Age Compressive Strength of Cemented Paste Backfills. Constr. Build. Mater. 2019, 218, 689–700. [Google Scholar] [CrossRef]
- Falah, M.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Effects of Activator Properties and Curing Conditions on Alkali-Activation of Low-Alumina Mine Tailings. Waste Biomass Valorization 2020, 11, 5027–5039. [Google Scholar] [CrossRef]
- Sephton, M.G.; Webb, J.A.; McKnight, S. Applications of Portland Cement Blended with Fly Ash and Acid Mine Drainage Treatment Sludge to Control Acid Mine Drainage Generation from Waste Rocks. Appl. Geochem. 2019, 103, 1–14. [Google Scholar] [CrossRef]
- Allah, P. Exploring the Use of Mineral Carbonation and Alkali Activation for the Valorization of Mg-rich Mine Tailings. Master’s Thesis, University of Oulu, Oulu, Finland, 2022. [Google Scholar]
- Luukkanen, S.; Tanhua, A.; Zhang, Z.; Canales, R.M.; Auranen, I. Towards Waterless Operations from Mine to Mill. Miner. Eng. 2022, 187, 107793. [Google Scholar] [CrossRef]
- Mucsi, G. A Review on Mechanical Activation and Mechanical Alloying in Stirred Media Mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into Activated Carbon from Different Kinds of Chemical Activating Agents: A Review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Zhang, Y.; Chen, T. Thermal Stability of a Silica-Rich Vanadium Tailing Based Geopolymer. Constr. Build. Mater. 2013, 38, 43–47. [Google Scholar] [CrossRef]
- Abboud, J.; Mazumder, J. Developing of Nano Sized Fibrous Eutectic Silicon in Hypereutectic Al–Si Alloy by Laser Remelting. Sci. Rep. 2020, 10, 12090. [Google Scholar] [CrossRef] [PubMed]
- El-Seidy, E.; Chougan, M.; Sambucci, M.; Al-Kheetan, M.J.; Valente, M.; Ghaffar, S.H. Lightweight Alkali-Activated Materials and Ordinary Portland Cement Composites Using Recycled Polyvinyl Chloride and Waste Glass Aggregates to Fully Replace Natural Sand. Constr. Build. Mater. 2023, 368, 130399. [Google Scholar] [CrossRef]
- LV, C.; Ding, J.; Liu, Y.; Fu, G.Y.; Qian, P.; Ye, S.F. Characterization of a Copper Mine Tailing and Comprehensive Recovery of Cu and S from the Tailing. J. Residuals Sci. Technol. 2017, 14, 125. [Google Scholar]
- Kalinkina, E.V.; Gurevich, B.I.; Kalinkin, A.M. Alkali-Activated Binder Based on Milled Antigorite. Minerals 2018, 8, 503. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Yliniemi, J.; Illikainen, M. Mechanical Transformation of Phyllite Mineralogy toward Its Use as Alkali-Activated Binder Precursor. Miner. Eng. 2020, 145, 106093. [Google Scholar] [CrossRef]
- Niu, H.; Kinnunen, P.; Sreenivasan, H.; Adesanya, E.; Illikainen, M. Structural Collapse in Phlogopite Mica-Rich Mine Tailings Induced by Mechanochemical Treatment and Implications to Alkali Activation Potential. Miner. Eng. 2020, 151, 106331. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Hao, M.; Li, B.; Liu, S. Microscopic Spreading Characteristics of Non-Polar Oil Droplet on Low Rank Coal Surface: Effects of Surfactant Pre-Treatment and Oxygen-Containing Groups. J. Mol. Liq. 2021, 325, 115232. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.C.; Zunino, F.; Marsh, A.T.; Maier, M.; Wang, B.; Martirena-Hernández, F. Clay Calcination Technology: State-of-the-Art Review by the RILEM TC 282-CCL. Mater. Struct. 2022, 55, 3. [Google Scholar] [CrossRef]
- Xu, W.; Shen, K.; Cao, Z.; Liu, F.; Zhang, Y.; Zhang, T.; Ouyang, S. Crystallization and Thermal Stability Effects on Tailings Glass-Ceramics by Various Heat Treating Processes. Mater. Chem. Phys. 2021, 263, 124334. [Google Scholar] [CrossRef]
- Fernando, P.T.; João, C.G.; Said, J. Durability and Environmental Performance of Alkali-Activated Tungsten Mine Waste Mud Mortars. J. Mater. Civ. Eng. 2010, 22, 897–904. [Google Scholar] [CrossRef]
- Aydın, S.; Kızıltepe, C.Ç. Valorization of Boron Mine Tailings in Alkali-Activated Mortars. J. Mater. Civ. Eng. 2019, 31, 04019224. [Google Scholar] [CrossRef]
- Ionescu, C.; Höck, V.; Simon, V. Effect of the Temperature and the Heating Time on the Composition of an Illite-Rich Clay: An XRPD Study. Babes-Bolyai Phys 2011, 56, 70. [Google Scholar]
- Feng, D.; Provis, J.L.; van Deventer, J.S. Thermal Activation of Albite for the Synthesis of One-Part Mix Geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Haddaji, Y.; Majdoubi, H.; Mansouri, S.; Tamraoui, Y.; Manoun, B.; Oumam, M.; Hannache, H. Effect of Synthetic Fibers on the Properties of Geopolymers Based on Non-Heat Treated Phosphate Mine Tailing. Mater. Chem. Phys. 2021, 260, 124147. [Google Scholar] [CrossRef]
- da Silva, M.R.C.; Malacarne, C.S.; Longhi, M.A.; Kirchheim, A.P. Valorization of Kaolin Mining Waste from the Amazon Region (Brazil) for Low-Carbon Cement Production. Case Stud. Constr. Mater. 2021, 15, e00756. [Google Scholar] [CrossRef]
- Solouki, A.; Viscomi, G.; Lamperti, R.; Tataranni, P. Quarry Waste as Precursors in Geopolymers for Civil Engineering Applications: A Decade in Review. Materials 2020, 13, 3146. [Google Scholar] [CrossRef]
- Bao, J.; Ning, P.; Wang, F.; Sun, X.; Wang, C.; Song, X.; Li, K. Thermal Modification of Copper Slag via Phase Transformation for Simultaneous Removal of SO2 and NOx from Acid-Making Tail Gas. Chem. Eng. J. 2021, 425, 131646. [Google Scholar] [CrossRef]
- Moukannaa, S.; Nazari, A.; Bagheri, A.; Loutou, M.; Sanjayan, J.G.; Hakkou, R. Alkaline Fused Phosphate Mine Tailings for Geopolymer Mortar Synthesis: Thermal Stability, Mechanical and Microstructural Properties. J. Non-Cryst. Solids 2019, 511, 76–85. [Google Scholar] [CrossRef]
- Ray, A.R.; Mishra, S. Hydro Metallurgical Technique as Better Option for the Recovery of Rare Earths from Mine Tailings and Industrial Wastes. Sustain. Chem. Pharm. 2023, 36, 101311. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Mbey, J.A.; Elimbi, A.; Diffo, B.K.; Njopwouo, D. Synthesis of Volcanic Ash-Based Geopolymer Mortars by Fusion Method: Effects of Adding Metakaolin to Fused Volcanic Ash. Ceram. Int. 2013, 39, 1613–1621. [Google Scholar] [CrossRef]
- Zhu, P.; Xia, B.; Li, H.; Liu, H.; Qian, G. A Novel Approach to Recycle Waste Serpentine Tailing for Mg/Al Layered Double Hydroxide Used as Adsorption Material. Environ. Eng. Sci. 2021, 38, 99–106. [Google Scholar] [CrossRef]
- Moukannaa, S.; Aboulayt, A.; Hakkou, R.; Benzaazoua, M.; Ohenoja, K.; Palomo, A.; Fernández-Jimenez, A. Fusion of Phosphate By-Products and Glass Waste for Preparation of Alkali-Activated Binders. Compos. Part B Eng. 2022, 242, 110044. [Google Scholar] [CrossRef]
- Vizureanu, P.; Nergis, D.D.B.; Sandu, A.V.; Nergis, D.P.B.; Baltatu, M.S. The Physical and Mechanical Characteristics of Geopolymers Using Mine Tailings as Precursors. In Advances in Geopolymer-Zeolite Composites-Synthesis and Characterization; IntechOpen: London, UK, 2021. [Google Scholar]
| Mine Tailings | Country | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | Na2O | K2O | P2O5 | V2O3 | V2O5 | SO2 | TiO2 | SO3 | ZnO | MnO | CuO | BaO | PbO | LOIa | Others | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper | China | 58.5 | 10.8 | 13.3 | 5.4 | 1.9 | 2.8 | 4.0 | - | - | - | - | - | 0.6 | - | - | - | - | - | - | - | [48] |
| USA | 55.8 | 3.07 | 14.11 | 2.27 | 1.77 | 3.02 | 3.88 | 0.19 | - | - | 2.26 | 0.50 | - | - | 0.067 | - | - | - | - | - | [49] | |
| Africa | 65.48 | 8.07 | 12.08 | 5.91 | 2.85 | 1.23 | 0.55 | 0.12 | - | 0.04 | - | 0.88 | - | - | 0.07 | 0.09 | - | - | 3.52 | 0.07 | [50] | |
| Gold | Africa | 74.6 | 7.03 | 6.98 | 0.54 | 5.16 | 0.28 | 1.26 | 0.09 | - | 0.03 | - | 0.45 | 3.05 | 0.03 | 0.06 | 0.02 | 0.08 | 0.03 | - | 0.42 | [51] |
| Turkey | 89.22 | 0.84 | 6.2 | - | 0.29 | - | 1.24 | - | - | - | - | - | 0.09 | - | - | - | - | - | - | - | [52] | |
| Finland | 49.9 | 9.2 | 10.4 | 11.8 | 6.8 | - | 1.2 | - | - | - | - | 1.2 | - | - | - | - | - | - | 13.6 | - | [53] | |
| Phosphate | Finland | 32.98 | 7.98 | 7.08 | 12.92 | 17.28 | - | 5.53 | 0.96 | - | - | - | 0.27 | - | - | 0.12 | - | - | - | 14.01 | 0.88 | [54] |
| Morrocco | 22.8 | 0.85 | 2.5 | 34.3 | 4.1 | 0.9 | 0.4 | 14.0 | - | - | - | - | - | - | - | - | - | - | 19 | - | [55] | |
| Iron | Brazil | 40.0 | 48.9 | 8.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6 | 1.8 | [56] |
| Australia | 57.30 | 25.12 | 9.57 | 0.03 | 0.07 | 0.05 | 0.04 | - | - | - | - | 0.61 | 0.16 | - | - | - | - | - | 6.67 | - | [57] | |
| Vanadium | China | 61.92 | 4.13 | 7.35 | 6.52 | 1.24 | 2.68 | 1.25 | - | - | 0.42 | - | 0.46 | 7.15 | - | - | - | - | - | 6.93 | - | [58] |
| Kaolinite | Finland | 72.55 | 1.90 | 16.44 | 0.05 | 0.83 | 0.08 | 3.06 | - | - | - | - | - | - | - | - | - | - | - | 4.42 | 0.74 | [59] |
| Lead–zinc | China | 62.09 | 2.73 | 4.35 | 20.45 | 0.56 | - | 0.55 | - | - | - | - | 0.2 | 3.63 | - | - | - | - | - | - | - | [60] |
| Hematite | USA | 68.77 | 28.13 | 0.87 | 0.28 | 0.74 | 0.03 | 0.04 | - | - | - | - | 0.05 | - | - | - | 0.001 | 0.02 | - | - | 0.07 | [61] |
| Lithium | Finland | 78.45 | 0.51 | 12.56 | 0.30 | 0.15 | 4.44 | 2.81 | - | - | - | - | - | - | - | - | - | - | - | 0.2 | 0.58 | [56] |
| Bauxite | China | 32.25 | 8.66 | 37.39 | 3.16 | 0.85 | 0.86 | - | - | - | - | - | 2.31 | - | - | - | - | - | - | 13.74 | - | [62] |
| Quartz | China | 97.48 | 0.30 | 1.11 | 0.17 | 0.22 | 0.04 | 0.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | [63] |
| Tungsten | Portugal | 47.85 | 9.97 | 21.07 | 0.85 | 1.01 | 1.32 | 4.12 | - | - | - | - | 0.66 | 8.73 | - | - | - | - | - | - | - | [64] |
| Zinc | China | 38.4 | 11.8 | 12.65 | 29.8 | - | - | 1.65 | 0.84 | - | - | - | 0.59 | 0.52 | 2.96 | 0.62 | 0.21 | - | 0.23 | - | 0.12 | [65] |
| Sphalerite | China | 6.22 | 0.18 | 2.06 | 33.65 | 9.94 | 0.32 | 0.24 | 0.27 | - | - | - | 0.09 | 1.43 | 1.75 | - | - | 0.31 | 0.15 | 43.65 | - | [65] |
| Mine Tailings | Procedure Parameters | Main Elements | Mineralogy | Final Properties | Ref. |
|---|---|---|---|---|---|
| Metal, coal, uranium, and potash tailings | Liquid toxic waste mixed with a geopolymer precursor; curing at 60 °C | - | - | High immobilization of heavy metals; high mechanical performance | [106,107,108] |
| Kaltails tailings | Fly ash as a co-binder | Si, Fe, Al, Ca, Mg, Na, and SO3 | - | Maximum mine tailing content of 70 wt%; stronger hardpan achieved with greater fly ash content | [109,110] |
| Copper tailings | Fly ash as a co-binder; curing at 90 °C; pressure used in preparation stage | - | Quartz, albite, potassium aluminum silicate, and gypsum | Improved mechanical strength with higher co-binder content and elevated curing temperature | [111,112,113,114,115] |
| Molybdenum tailings | Metakaolin as a co-binder; curing at room temperature | - | Calcite, andradite, quartz, dolomite, and calcium aluminum silicate | The improved strength of the final material with metakaolin | [116,117,118] |
| Gold tailings | Blast furnace slag and metakaolin as co-binders; curing at room temperature | - | Dolomite, quartz, chlinoclore, muscovite, albite, and gypsum | Metakaolin used for adjusting the scarce Al content in the mixture; the effect of co-binder content on the mechanical properties of the final material | [119] |
| Gold tailings | Slag, gypsum, cement, lime, and silica fume as co-binders | - | Quartz, albite, mica, sanidine, and microcline | Appropriate material to utilize for paste backfill technology | [120,121] |
| Sulfidic mine waste rock | Fly ash as a co-binder | - | Chlorite, quartz, illite, and pyrite | Suitable to utilize sulfidic mining waste rock as aggregate in alkali activation | [122] |
| Mine Tailings | Procedure Parameters | Mineralogy | Mineralogical Changes | Ref. |
|---|---|---|---|---|
| Vanadium tailings | 60 min grinding; 10:1 weight ratio of grinding ball to powder | Quartz, feldspar, diopside, mullite, and hematite | Decreased amount of crystalline quartz; higher mechanical strength of alkali-activated material obtained with higher leaching of Al and Si and higher amorphous content | [127] |
| Copper tailings | 1–4 h grinding; 10:1 weight ratio of grinding ball to powder | Quartz, dolomite, chlorite, and albite | Decreased content of albite (lower crystalline silica content) | [130] |
| Antigorite waste | 600 s grinding; 6:1 weight ratio of grinding ball to powder | Mg and Si minerals | Cementitious properties obtained in milled mine tailings | [131] |
| Phyllite waste | 9–15 min grinding | Clay, quartz, and albite | Reduction in chamosite and muscovite; decreased albite content | [132] |
| Mine Tailings | Main Minerals | Calcination Temperatures | Mineralogy Changes | Other Comments | Ref. |
|---|---|---|---|---|---|
| Tungsten tailings | Muscovite and quartz | 950 °C | High thermal resistance for muscovite | Increased amount of amorphous phase; thermal behavior similar to that of phyllosilicate minerals | [137] |
| Boron tailings | Colemanite (Ca2B6O11·5H2O), hydroboracite (CaMgB6O8(OH)6·3H2O), quartz, illite, and calcite | 600, 700, and 800 °C | Colemanite and hydroboracite turned into amorphous phase at 600 °C; illite and calcite decomposed at 800 °C | Illite had similar properties to muscovite | [138] |
| Phosphate tailings | Quartz, calcite, fluorapatite, dolomite, mullite, and feldspar | 750 °C (2 h) | Quartz, fluorapatite, and mullite remained constant; dolomite and calcite content was reduced; feldspar content was eliminated | High mechanical strength achieved with 50 wt% phosphate tailing in the mixture | [141] |
| Kaolinite tailings | Muscovite, kaolinite, and quartz | 750°C | Kaolinite turned into amorphous metakaolin | Possible recrystallization of amorphous phase beyond optimum temperature | [142] |
| Copper tailings | Quartz, albite, chlorite, and dolomite | 400–600 °C | Crystalline chlorite was dissolved | - | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaviparast, H.R.; Miranda, T.; Pereira, E.; Cristelo, N. A Comprehensive Review on Mine Tailings as a Raw Material in the Alkali Activation Process. Appl. Sci. 2024, 14, 5127. https://doi.org/10.3390/app14125127
Manaviparast HR, Miranda T, Pereira E, Cristelo N. A Comprehensive Review on Mine Tailings as a Raw Material in the Alkali Activation Process. Applied Sciences. 2024; 14(12):5127. https://doi.org/10.3390/app14125127
Chicago/Turabian StyleManaviparast, Hamid Reza, Tiago Miranda, Eduardo Pereira, and Nuno Cristelo. 2024. "A Comprehensive Review on Mine Tailings as a Raw Material in the Alkali Activation Process" Applied Sciences 14, no. 12: 5127. https://doi.org/10.3390/app14125127





