Hybrid Colloids Made with Polymers
Abstract
1. Introduction
2. A Historical Background
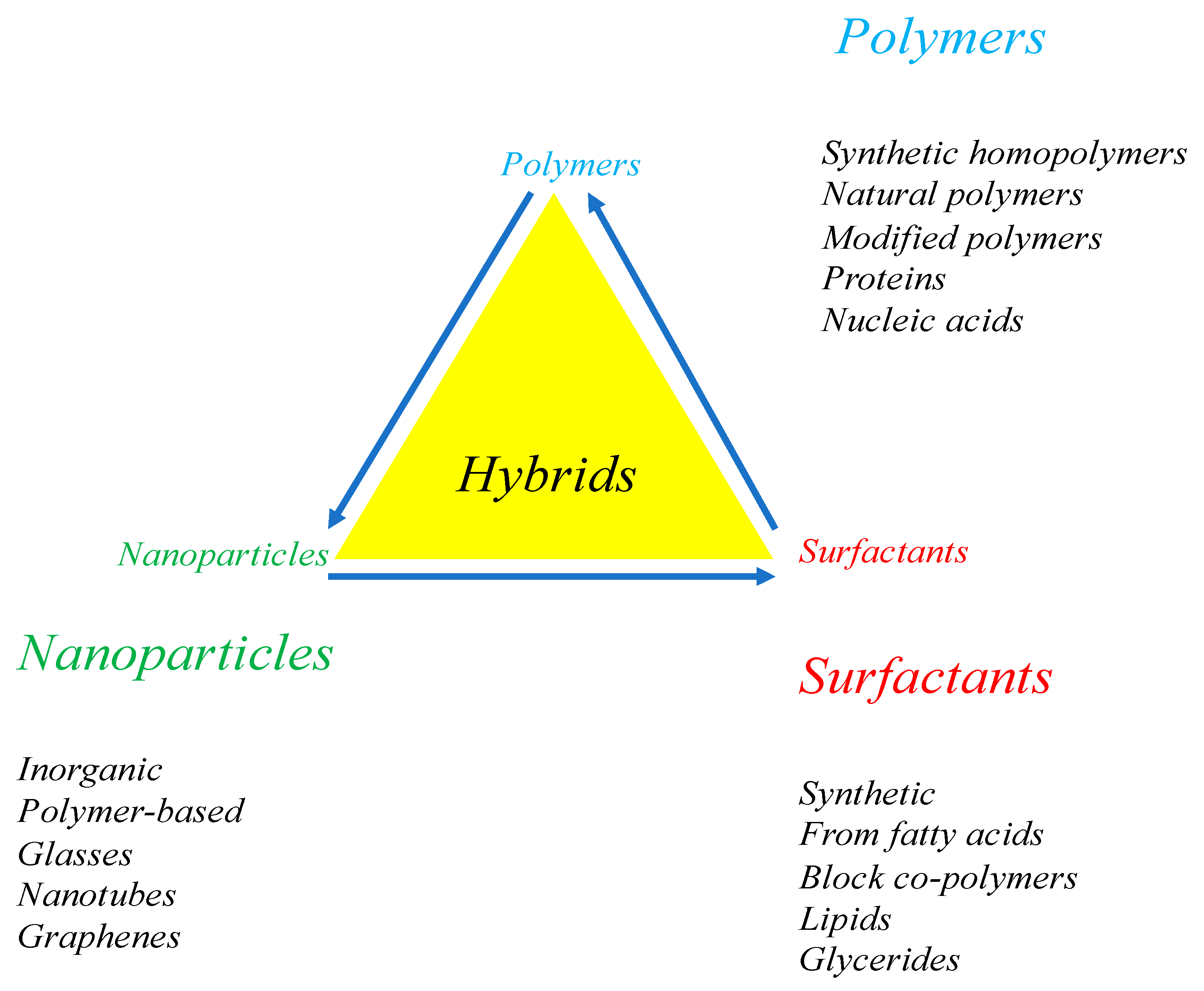
3. What Are Hybrid Colloids?
- (1)
- Single domains show diverse features, each with respect to others;
- (2)
- Their components self-organize, self-sustain, self-help, form composites, and follow the constraints dictated by thermodynamic or kinetic stability;
- (3)
- The domains in a hybrid are held together by covalent or noncovalent forces. The composites retain some properties of the parent entities, but also gain completely new ones.
4. Hybrids Containing Surfactants and Polymers
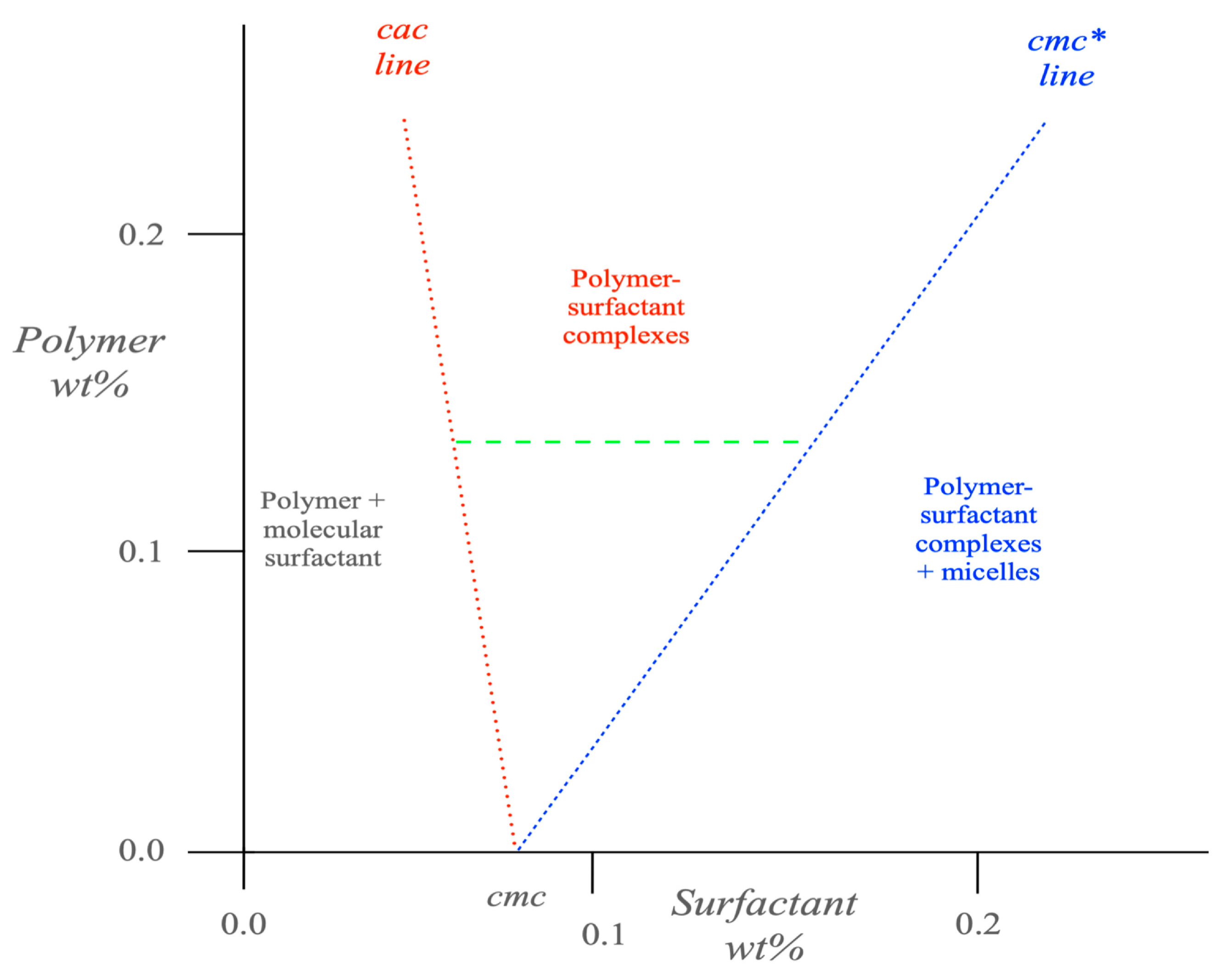
5. Polymer–Surfactant Systems
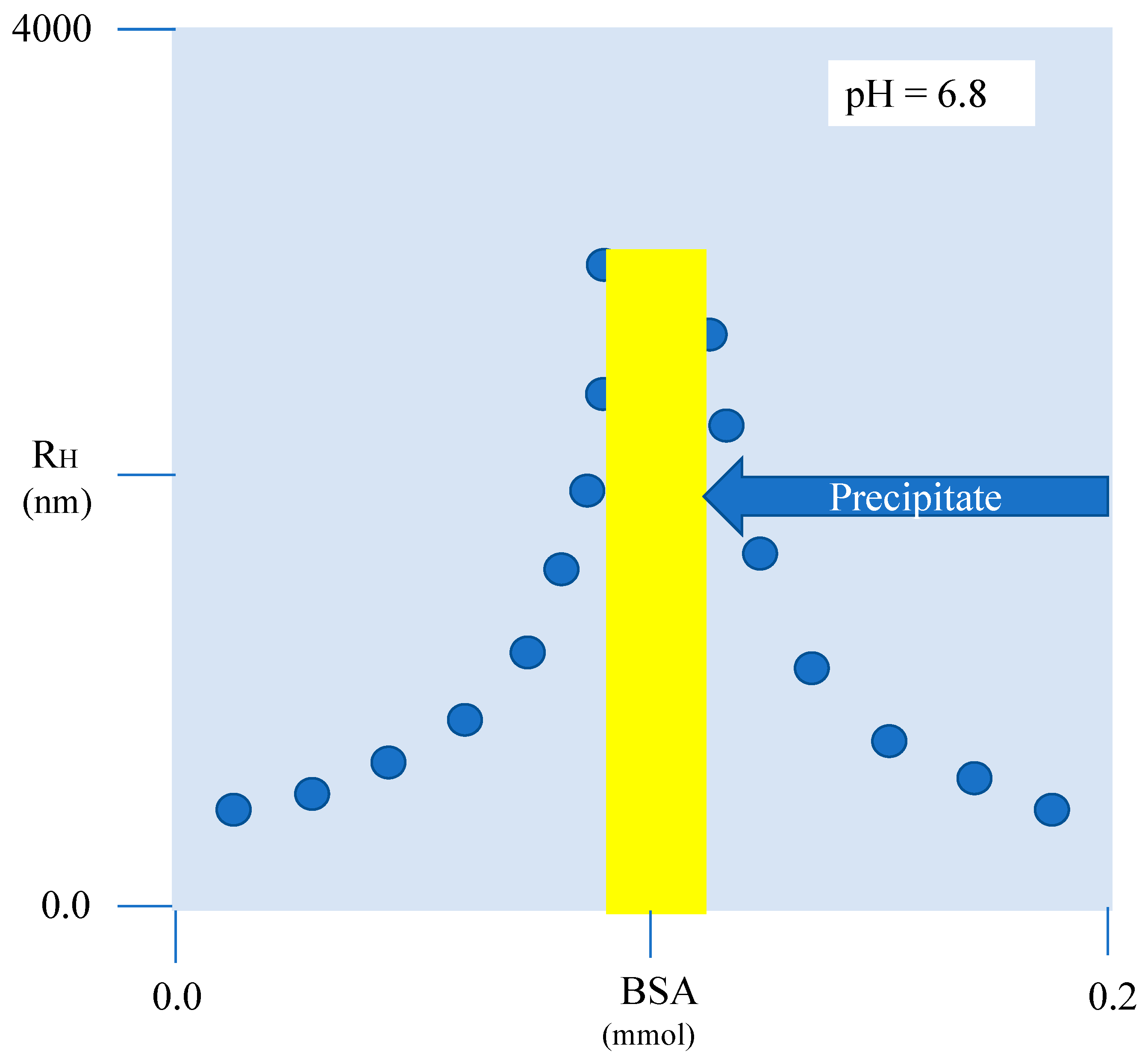
6. (Bio)Polymer Binding onto Preformed Surfactant Aggregates
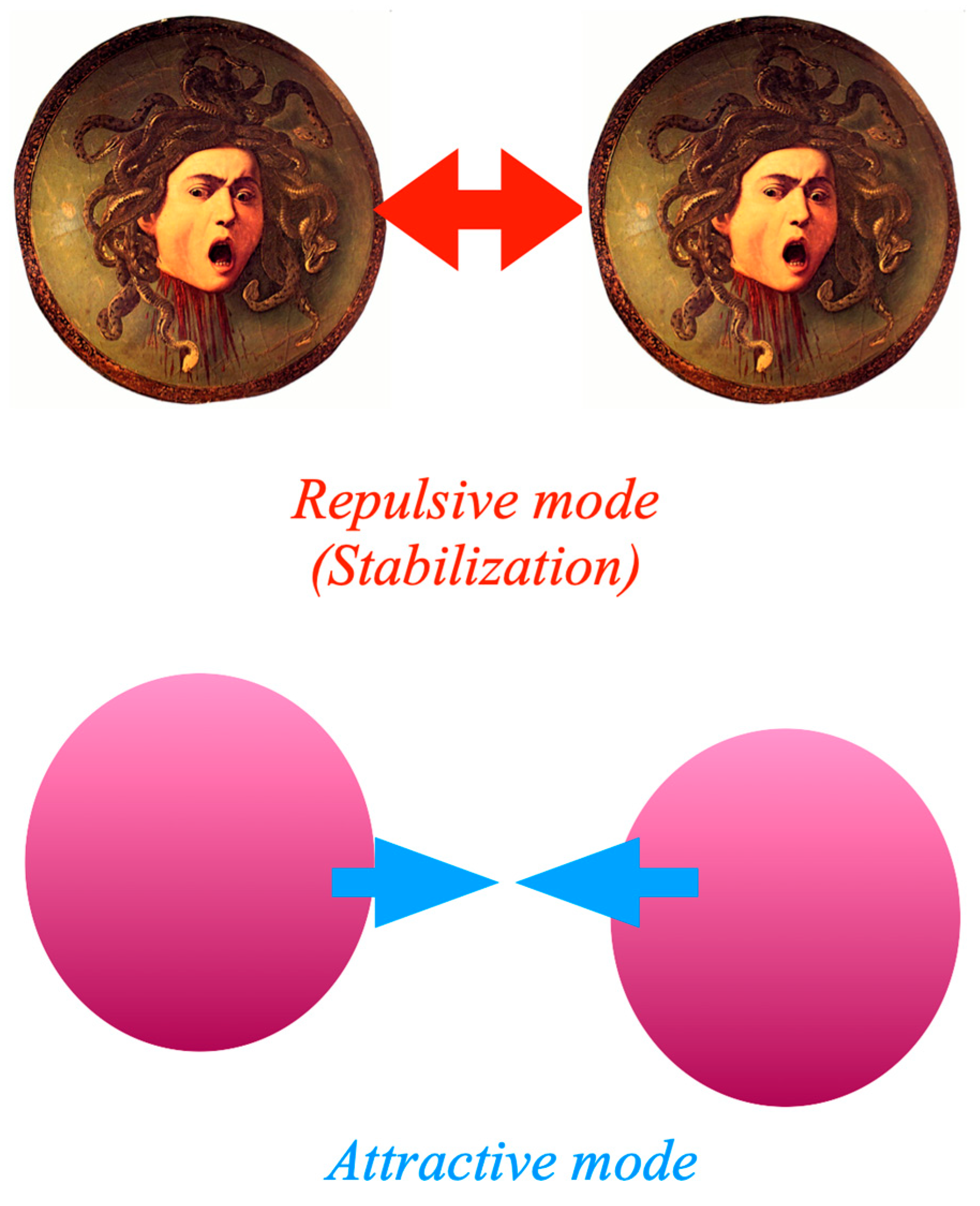
7. Hybrids Made of Nanoparticles
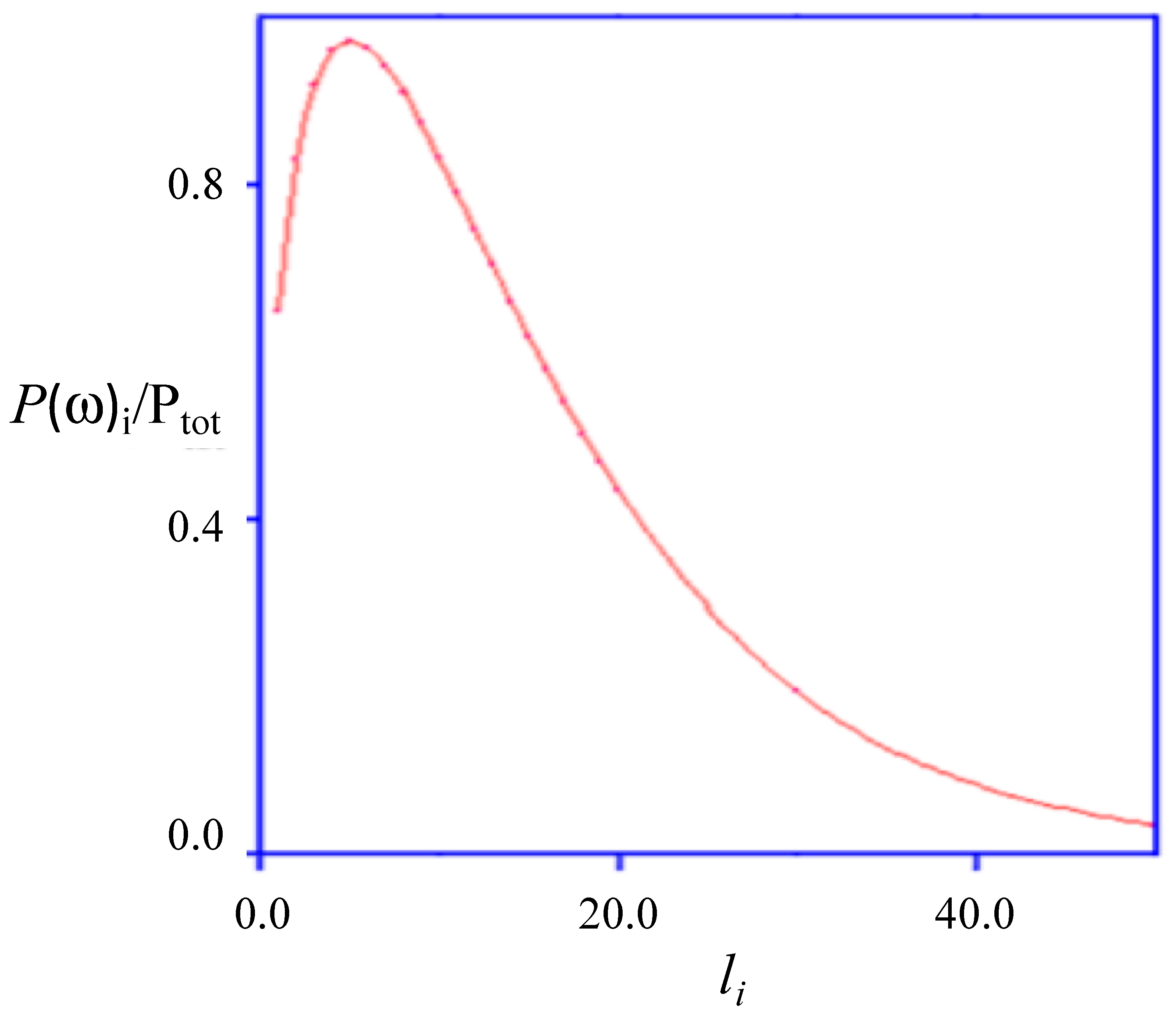
8. Polymer Wrapping
- (a)
- The polymer length is ≤NPs’ diameter, and wrapping length is lower than the extended polymer one. Different parts of the same polymer chain may wrap;
- (b)
- Polymers and NPs are size mono-disperse;
- (c)
- Polymer partition between bulk and surface states is possible, and can be substantial. The amount of surface-bound polymer is lower than Xtot, its overall mole fraction in the medium. This implies some partition with the bulk.
9. Hybrid Composites
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Zheng, X.; Grebe, V.; Pine, D.J.; Weck, M. Tunable assembly of hybrid colloids induced by regioselective depletion. Nat. Mater. 2020, 19, 1354–1361. [Google Scholar] [CrossRef]
- Kang, N.; Zhu, J.; Zhang, X.; Wang, H.; Zhang, Z. Reconfiguring Self-Assembly of Photoresponsive Hybrid Colloids. J. Am. Chem. Soc. 2022, 144, 4754–4758. [Google Scholar] [CrossRef] [PubMed]
- Romanov, S.G.; Korovin, A.V.; Regensburger, A.; Peschel, U. Hybrid Colloidal Plasmonic-Photonic Crystals. Adv. Mater. 2011, 23, 2515–2533. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, A.; Cho, E.C.; Camargo, P.H.C.; Au, L.; Ohtani, B.; Xia, Y. A Facile Synthesis of Asymmetric Hybrid Colloidal Particles. J. Am. Chem. Soc. 2009, 131, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Michel, R.; Kesselman, E.; Plostica, T.; Danino, D.; Gradzielski, M. Internalization of Silica Nanoparticles into Fluid Liposomes: Formation of Interesting Hybrid Colloids. Angew. Chem. 2014, 126, 12649–12653. [Google Scholar] [CrossRef]
- Karg, M.; Lu, Y.; Carbó-Argibay, E.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M.; Hellweg, T. Multiresponsive Hybrid Colloids Based on Gold Nanorods and Poly(NIPAM-co-allylacetic acid) Microgels: Temperature- and pH-Tunable Plasmon Resonance. Langmuir 2009, 25, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Perro, A.; Reculusa, S.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, S. Synthesis of hybrid colloidal particles: From snowman-like to raspberry-like morphologies. Colloids Surf. A Physicochem. Eng. Asp. 2006, 284–285, 78–83. [Google Scholar] [CrossRef]
- Romio, M.; La Mesa, C. Hybrid nano-composites made of ss-DNA/wrapped carbon nanotubes and titania. Colloids Surf. B Biointerfaces 2017, 152, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kalda, J. Sticky Particles in Compressible Flows: Aggregation and Richardson’s Law. Phys. Rev. Lett. 2007, 98, 064501. [Google Scholar] [CrossRef]
- Teixeira, P.; Tavares, J. Phase behaviour of pure and mixed patchy colloids—Theory and simulation. Curr. Opin. Colloid Interface Sci. 2017, 30, 16–24. [Google Scholar] [CrossRef]
- Clutton-Brock, J. Domesticated Animals from Early Times; Heinemann: London, UK, 1981. [Google Scholar]
- Posthumus, L. Hybrid Monsters in the Classical World. The Nature and Function of Hybrid Monsters in Greek Mythology, Literature and Art. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2011. [Google Scholar]
- Aldrovandi, U. AMS Historica-AlmaDL 2004–2017; I B Bellagamba, Alma Mater Studiorum: Bologna, Italy, 1602. [Google Scholar]
- Burke, J.M.; Arnold, M.L. Genetics and the Fitness of Hybrids. Annu. Rev. Genet. 2001, 35, 31–52. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology, 2nd ed.; IUPAC; the “Gold Book”; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Hauser, E.A. The history of colloid science: In memory of Wolfgang Ostwald. J. Chem. Educ. 1955, 32, 1–2. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys. Chim. URSS 1941, 14, 633. [Google Scholar] [CrossRef]
- Landau, L.D. Collected Papers of Lev Davidovich Landau; Ter-Haar, D., Ed.; Pergamon: Oxford, UK, 1965. [Google Scholar]
- Verwey, E.J.W. Theory of the Stability of Lyophobic Colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Ninham, B. On progress in forces since the DLVO theory. Adv. Colloid Interface Sci. 1999, 83, 1–17. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Acad Press: London, UK, 2007. [Google Scholar]
- de Gennes, P. Soft Matter (Nobel Lecture). Angew. Chem. Int. Ed. 1992, 31, 842–845. [Google Scholar] [CrossRef]
- Thunugunta, T.; Reddy, A.C.; Lakshmana, R.D.C. Green synthesis of nanoparticles: Current prospectus. Nanotechnol. Rev. 2015, 4, 303–323. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Singh, J.; Mohammed-Saied, W.; Kaur, R.; Badea, I. Nanoparticles in gene therapy: From design to clinical applications. Rev. Nanosci. Nanotechnol. 2013, 2, 275–299. [Google Scholar] [CrossRef]
- Li, N.; Zeng, S.; He, L.; Zhong, W. Probing Nanoparticle−Protein Interaction by Capillary Electrophoresis. Anal. Chem. 2010, 82, 7460–7466. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, K.; Kang, E.-C.; Kurumada, S.; Sunamoto, J.; Principi, T.; Winnik, F.M. Controlled Association of Amphiphilic Polymers in Water: Thermosensitive Nanoparticles Formed by Self-Assembly of Hydrophobically Modified Pullulans and Poly(N-isopropylacrylamides). Macromolecules 2000, 33, 3244–3249. [Google Scholar] [CrossRef]
- Jee, A.-Y.; Tsang, B.; Granick, S. Colloidal phase transitions. A switch for phase shifting. Nat. Mater. 2015, 14, 17–18. [Google Scholar] [CrossRef]
- Altman, R.M.; Richmond, G.L. Coming to Order: Adsorption and Structure of Nonionic Polymer at the Oil/Water Interface as Influenced by Cationic and Anionic Surfactants. Langmuir 2020, 36, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.D. Ananthapadmanabhan KP Interactions of Surfactants with Polymers and Proteins; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Gu, T.; Rupprecht, H. Hemimicelle shape and size. Colloid Polym. Sci. 1990, 268, 1148–1150. [Google Scholar] [CrossRef]
- Gasbarrone, P.; La Mesa, C. Interactions of short-chain surfactants with a nonionic polymer. Colloid Polym. Sci. 2001, 279, 1192–1199. [Google Scholar] [CrossRef]
- D’Aprano, A.; La Mesa, C.; Persi, L. Polymer−Surfactant Interactions: An Ultrasonic Relaxation Study. Langmuir 1997, 13, 5876–5880. [Google Scholar] [CrossRef]
- Deep, S.; Ahluwalia, J.C. Interaction of bovine serum albumin with anionic surfactants. Phys. Chem. Chem. Phys. 2001, 3, 4583–4591. [Google Scholar] [CrossRef]
- Leggio, C.; Galantini, L.; Pavel, N.V. About the albumin structure in solution: Cigar Expanded form versus heart Normal shape. Phys. Chem. Chem. Phys. 2008, 10, 6741–6750. [Google Scholar] [CrossRef]
- Herrington, K.L.; Kaler, E.W.; Miller, D.D.; Zasadzinski, J.A.; Chiruvolu, S. Phase behavior of aqueous mixtures of dodecyltrimethylammonium bromide (DTAB) and sodium dodecyl sulfate (SDS). J. Phys. Chem. 1993, 97, 13792–13802. [Google Scholar] [CrossRef]
- Letizia, C.; Andreozzi, P.; Scipioni, A.; La Mesa, C.; Bonincontro, A.; Spigone, E. Protein Binding onto Surfactant-Based Synthetic Vesicles. J. Phys. Chem. B 2007, 111, 898–908. [Google Scholar] [CrossRef]
- Dias, R.S.; Lindman, B.; Miguel, M.G. DNA Interaction with Catanionic Vesicles. J. Phys. Chem. B 2002, 106, 12600–12607. [Google Scholar] [CrossRef]
- Marques, E.F.; Regev, O.; Khan, A.; Miguel, M.; Lindman, B. Interactions between Catanionic Vesicles and Oppositely Charged Polyelectrolytes: Phase Behavior and Phase Structure. Macromolecules 1999, 32, 6626–6637. [Google Scholar] [CrossRef]
- Stenstam, A.; Khan, A.; Wennerström, H. Lysozyme in Catanionic Surfactant Mixtures. Langmuir 2004, 20, 7760–7765. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.; Scipioni, A.; La Mesa, C. Albumin binding onto synthetic vesicles. Soft Matter 2012, 8, 9669–9675. [Google Scholar] [CrossRef]
- Jacques, C. L’Egypte des Grands Pharaons: L’Histoire et la Légende; Success du Livres Edition; TEMPUS PERRIN: Paris, France, 2009. [Google Scholar]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface Functionalization of Nanoparticles with Polyethylene Glycol: Effects on Protein Adsorption and Cellular Uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Gevrek, T.N.; Lyskawa, J.; Szunerits, S.; Boukherroub, R.; Sanyal, R.; Woisel, P.; Sanyal, A. Bioinspired Anchorable Thiol-Reactive Polymers: Synthesis and Applications Toward Surface Functionalization of Magnetic Nanoparticles. Macromolecules 2014, 47, 5124–5134. [Google Scholar] [CrossRef]
- Sehlleier, Y.H.; Abdali, A.; Huelser, T.; Wiggers, H.; Schulz, C. Functionalization of SiO2 nanoparticles and their super-hydrophobic surface coating. Spec. Publ. R. Soc. Chem. 2012, 336, 113–120. [Google Scholar]
- Demin, A.M.; Vigorov, A.Y.; Nizova, I.A.; Uimin, M.A.; Shchegoleva, N.N.; Ermakov, A.E.; Krasnov, V.P.; Charushin, V.N. Functionalization of Fe3O4 magnetic nanoparticles with RGD peptide derivatives. Mendeleev Commun. 2014, 24, 20–22. [Google Scholar] [CrossRef]
- Vauthier, C.; Labarre, D. Modular biomimetic drug delivery systems. J. Drug Deliv. Sci. Technol. 2008, 18, 59–68. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Xing, X.; Hua, L.; Ngai, T. Depletion versus stabilization induced by polymers and nanoparticles: The state of the art. Curr. Opin. Colloid Interface Sci. 2015, 20, 54–59. [Google Scholar] [CrossRef]
- Ji, S.; Walz, J.Y. Depletion Flocculation Induced by Synergistic Effects of Nanoparticles and Polymers. J. Phys. Chem. B 2013, 117, 16602–16609. [Google Scholar] [CrossRef]
- Burger, S.; Bartsch, E. Influence of the polymer size on depletion attraction—Induced gel and glass transitions of microgel colloids. Colloids Surf. A Physicochem. Eng. Asp. 2014, 442, 6–15. [Google Scholar] [CrossRef]
- Tardani, F.; La Mesa, C. Attempts to control depletion in the surfactant-assisted stabilization of single-walled carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 123–128. [Google Scholar] [CrossRef]
- Fujigaya, T. Development of polymer-wrapping methods for functionalization of carbon materials. Polym. J. 2023, 55, 181–191. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Deshkovski, A.; Rubinstein, M. Adsorption of hydrophobic poly-electrolytes at oppositely charged surfaces. Macromolecules 2002, 34, 3421–3436. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Zhulina, E.B.; Rubinstein, M. Structure of Adsorbed Polyampholyte Layers at Charged Objects. Macromolecules 2001, 34, 627–639. [Google Scholar] [CrossRef]
- Louguet, S.; Kumar, A.C.; Guidolin, N.; Sigaud, G.; Duguet, E.; Lecommandoux, S.; Schatz, C. Control of the PEO Chain Conformation on Nanoparticles by Adsorption of PEO-block-Poly(l-lysine) Copolymers and Its Significance on Colloidal Stability and Protein Repellency. Langmuir 2011, 27, 12891–12901. [Google Scholar] [CrossRef]
- La Mesa, C.; Risuleo, G. Polymer Wrapping onto Nanoparticles Induces the Formation of Hybrid Colloids. Coatings 2023, 13, 823. [Google Scholar] [CrossRef]
- Vold, R.D.; Vold, M.J. Colloid and Interface Chemistry; Addison-Wesley: Reading, MA, USA, 1983; Chapter IV; pp. 134–148. [Google Scholar]
- Adamson, A.W. Physical Chemistry of Surfaces, 5th ed.; Wiley: New York, NY, USA, 1990; Chapter IX; pp. 421–433. [Google Scholar]
- Barisci, J.N.; Tahhan, M.; Wallace, G.G.; Badaire, S.; Vaugien, T.; Maugey, M.; Poulin, P. Properties of Carbon Nanotube Fibers Spun from DNA-Stabilized Dispersions. Adv. Funct. Mater. 2004, 14, 133–138. [Google Scholar] [CrossRef]
- Kusano, T.; Kumano, N.; Yoshimune, W.; Munekata, T.; Matsunaga, T.; Harada, M. Interplay between Interparticle Potential and Adsorption Structure in Nanoparticle Dispersions with Polymer Addition as Displayed by Small-Angle Scattering. Langmuir 2021, 37, 7503–7512. [Google Scholar] [CrossRef]
- Yagihara, S.; Hikichi, K. Cooperative Interaction on Side-Chain Motion of Poly(α-amino acid). Polym. J. 1982, 14, 233–240. [Google Scholar] [CrossRef]
- Malmsten, M.; Linse, P.; Cosgrove, T. Adsorption of PEO-PPO-PEO block copolymers at silica. Macromolecules 1992, 25, 2474–2481. [Google Scholar] [CrossRef]
- Nativ-Roth, E.; Shvartzman-Cohen, R.; Bounioux, C.; Florent, M.; Zhang, D.; Szleifer, I.; Yerushalmi-Rozen, R. Physical Adsorption of Block Copolymers to SWNT and MWNT: A Nonwrapping Mechanism. Macromolecules 2007, 40, 3676–3685. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The Size, Shape, and Hydration of Nonionic Surfactant Micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078. [Google Scholar] [CrossRef]
- Quant, C.A.; Marla, K.T.; Meredith, J.C. Osmotic pressure and chemical potential of silica nanoparticles in aqueous poly(ethyleneoxide) solution. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 129–135. [Google Scholar] [CrossRef]
- Andreozzi, P.; La Mesa, C.; Masci, G.; Suber, L. Formation and Physicochemical Characterization of Silica-Based Blackberry-like Nanoparticles Capped by Polysaccharides. J. Phys. Chem. C 2007, 111, 18004–18009. [Google Scholar] [CrossRef]
- Ghofraniha, N.; Andreozzi, P.; Russo, J.; La Mesa, C.; Sciortino, F. Assembly Kinetics in Binary Mixtures of Strongly Attractive Colloids. J. Phys. Chem. B 2009, 113, 6775–6781. [Google Scholar] [CrossRef]
- Gray, C.G.; Stiles, P.J. Nonlinear electrostatics: The Poisson–Boltzmann equation. Eur. J. Phys. 2018, 39, 053002. [Google Scholar] [CrossRef]
- Fixman, M. The Poisson–Boltzmann equation and its application to polyelectrolytes. J. Chem. Phys. 1979, 70, 4995–5005. [Google Scholar] [CrossRef]
- La Mesa, C. Surface Potentials of Mixtures Containing Oddly Charged Colloids. Coatings 2022, 12, 1715. [Google Scholar] [CrossRef]
- Dai, Q.; Bertleff-Zieschang, N.; Braunger, J.A.; Björnmalm, M.; Cortez-Jugo, C.; Caruso, F. Particle Targeting in Complex Biological Media. Adv. Health Mater. 2018, 7, 1870004. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Tardani, F.; La Mesa, C.; Poulin, P.; Maugey, M. Phase Behavior of DNA-Based Dispersions containing Carbon Nanotubes: Effects of Added Polymers and Ionic Strength on Excluded Volume. J. Phys. Chem. C 2012, 116, 9888–9894. [Google Scholar] [CrossRef]
- Tardani, F.; Pucci, C.; La Mesa, C. Confining ss-DNA/carbon nanotube complexes in ordered droplets. Soft Matter 2014, 10, 1024–1031. [Google Scholar] [CrossRef]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned—Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed]
- Schroffenegger, M.; Leitner, N.S.; Morgese, G.; Ramakrishna, S.N.; Willinger, M.; Benetti, E.M.; Reimhult, E. Polymer Topology Determines the Formation of Protein Corona on Core-Shell Nanoparticles. ACS Nano 2020, 14, 12708–12718. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Louguet, S.; Kumar, A.C.; Sigaud, G.; Duguet, E.; Lecommandoux, S.; Schatz, C. A physico-chemical investigation of poly(ethylene oxide)-block-poly(L-lysine) copolymer adsorption onto silica nanoparticles. J. Colloid Interface Sci. 2011, 359, 413–422. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Wong, M.H.; Kwak, S.; Sinclair, R.; Koman, V.B.; Strano, M.S. Rational Design Principles for the Transport and Subcellular Distribution of Nanomaterials into Plant Protoplasts. Small 2018, 14, e1802086. [Google Scholar] [CrossRef]
- Dai, J.; Dong, X.; Wang, Q.; Lou, X.; Xia, F.; Wang, S. PEG-Polymer Encapsulated Aggregation-Induced Emission Nanoparticles for Tumor Theranostics. Adv. Healthc. Mater. 2021, 10, 2101036. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Mesa, C. Hybrid Colloids Made with Polymers. Appl. Sci. 2024, 14, 5135. https://doi.org/10.3390/app14125135
La Mesa C. Hybrid Colloids Made with Polymers. Applied Sciences. 2024; 14(12):5135. https://doi.org/10.3390/app14125135
Chicago/Turabian StyleLa Mesa, Camillo. 2024. "Hybrid Colloids Made with Polymers" Applied Sciences 14, no. 12: 5135. https://doi.org/10.3390/app14125135
APA StyleLa Mesa, C. (2024). Hybrid Colloids Made with Polymers. Applied Sciences, 14(12), 5135. https://doi.org/10.3390/app14125135




