Abstract
Osteoarthritis (OA) is a degenerative joint disease that mainly occurs due to the cellular inflammatory response and the destruction of joint cartilage. Natural eggshell membrane (NEM), a byproduct of egg processing, might be a promising knee OA treatment because of its anti-inflammatory properties and resemblance to synovial membrane components. Therefore, we aimed to study the anti-inflammatory effects of NEM in OA, utilizing both in vitro experiments with primary chondrocytes and in vivo studies with a surgical rat model of knee OA. In vitro studies showed that NEM treatment improved cell viability in chondrocytes exposed to interleukin-1α by upregulating chondrogenic genes and inhibiting enzymes that degrade the extracellular matrix (ECM). Furthermore, the anti-inflammatory effects of NEM were observed in chondrocytes induced by lipopolysaccharide. Administering NEM orally for 56 days after OA surgery resulted in enhanced joint swelling reduction and improved mobility in animal models, as well as an increase in bone density and cartilage compressive strength in a concentration-dependent manner. It inhibited inflammatory markers (5-lipoxygenase and prostaglandin E2) and extracellular matrix (ECM)-degrading enzymes (MMP-2 and MMP-9) in both the cartilage and synovium. Simultaneously, there was an upregulation in the expression of chondrogenic genes (Sox9, aggrecan, and Col-2). The histopathological and immunohistochemical analyses demonstrated that NEM’s anti-inflammatory, anti-apoptotic, and chondrogenic properties contributed to the mitigation of joint degradation and synovial inflammation. Therefore, NEM is a potential alternative or functional food agent that addresses both anti-inflammatory and chondroprotective aspects in OA.
1. Introduction
Osteoarthritis (OA) is the most prevalent articular disease among the elderly [1]. It involves alterations in joint structure and function, primarily resulting from a degenerative inflammatory process occurring in the articular cartilage (AC) [2,3]. OA affects nearly 70% of individuals at some point in their lives, leading to significant economic and social consequences for both patients and healthcare systems [4,5]. Moreover, OA is characterized by the degradation of AC [6] and is the most prevalent clinical syndrome associated with joint pain, frequently causing various degrees of functional disability and a diminished quality of life [7]. The extracellular matrix (ECM) of AC is primarily composed of collagen and proteoglycans (PGs), including aggrecan and glycosaminoglycans (GAGs) [8,9,10,11]. As an effect of OA, AC components, particularly PGs, vanish, resulting in tissue destruction and hypocellularity and ultimately loss of joint function [12]. Notably, proinflammatory cytokines promote the breakdown of cartilage and hinder the production of new matrix components in the articular tissue [13]. Cytokines are also known to trigger an increase in prostaglandin E2 (PGE2) and the release of nitric oxide from chondrocytes [14,15]. Both PGE2 and nitric oxide are reported to play roles in the process of cartilage matrix turnover [16,17]. Hence, developing disease-modifying OA drugs is necessary [18]. Presently, the main clinical strategy for managing OA is based on the use of nonsteroidal anti-inflammatory drugs (NSAIDs), analgesics, and hyaluronan. Although these treatments provide symptom relief, they do not induce a significant disease-modifying effect [19]. In certain cases, NSAIDs can potentially have harmful effects as they impede the synthesis of PG, a substance that plays a crucial role in preserving cartilage function [19]. Therefore, we should explore the development of alternative agents aimed at preventing cartilage degradation or promoting its effective repair. Thus, numerous efforts have been undertaken to investigate potent cartilage-preserving agents derived from natural sources and combinations with medicinal foods, with the goal of achieving more powerful anti-arthritic treatments.
Natural eggshell membrane (NEM) is a naturally occurring byproduct of egg processing, often overlooked and discarded as industrial waste, thereby adding to the environmental burden [20]. However, NEM contains an abundance of fibrous proteins, collagen, hyaluronic acid, chondroitin sulfate, and glucosamine [21]; thus, it holds the promise of being a functional bioactive compound in the field of health science. In rodent studies, oral supplementation of eggshell membrane (ESM) is generally considered safe, as it has demonstrated both anti-inflammatory and anti-obesity effects [22,23]. Moreover, NEM contains bioactive glycosaminoglycans, including dermatan sulfate, chondroitin sulfate, and hyaluronic acid [24], making it similar to the components found in human joints, particularly in the synovial membrane. Due to its composition, NEM has the potential to enhance joint health, alleviate pain, and reduce the stiffness often associated with OA. It achieves this by supplementing crucial anabolic factors that are often lacking in the typical Western diet and by mitigating inflammation through immunomodulatory effects [25].
Thus, the purpose of this study was to explore the anti-OA effect of NEM and its underlying mechanisms in primary chondrocytes and a knee OA rodent surgery model.
2. Materials and Methods
2.1. Preparation of NEM
We acquired NEM, provided by ESM Technologies, from Seongdong Corporation, Seoul, Korea. The NEM samples were stored in the herbarium of the Medical Research Center for Herbal Convergence on Liver Disease at Daegu Haany University in Gyeongsan, Korea. Fresh eggshells were subjected to heat treatment (75–95 °C for 5–10 min) to facilitate separation of the eggshell membrane. The separated membrane was then dried and ground. Subsequently, the powdered eggshell membrane underwent sterilization (95–105 °C for 5–10 min). Partial enzymatic hydrolysis using pepsin, trypsin, and papain was employed to generate hydrolyzed and dried eggshell membranes [26,27]. These components were blended and dried, followed by a final grinding process to produce the raw NEM. NEM powder dissolves well in distilled water up to a concentration of 20 mg/mL. After dissolution, the solution was filtered through a syringe filter with a pore size of 0.22 µm for use in the experiments. We refrigerated the NEM stock solutions at 4 °C until use.
2.2. Animals and Housing
All laboratory animals were preapproved by the Institutional Animal Care and Use Committee at Daegu Haany University, Gyeongsan, Korea, prior to the initiation of the experiments, with the approval numbers DHU2021-068 and DHU2021-072 for the in vivo and in vitro studies, respectively. In our study, we used a total of 30 rats for the in vitro experiments to isolate chondrocytes. For the in vivo experiments, we used a total of 60 rats, with 10 rats assigned to each group: (1) Sham: sham-operated vehicle (distilled water)-administered group, (2) OA: OA-operated vehicle-administered group, (3) OA + diclofenac sodium (DS): OA-operated DS 3 mg/kg intraperitoneally treated group, (4) OA + NEM100: OA-operated NEM 100 mg/kg administered group, (5) OA + NEM50: OA-operated NEM 50 mg/kg administered group, and (6) OA + NEM25: OA-operated NEM 25 mg/kg administered group. We obtained 6-week-old male Sprague–Dawley rats, which were specific-pathogen-free and free of viral antibodies, from Orient Bio Inc., Seongnam, Korea. Rats were housed in a polycarbonate cage in a facility maintained at 20–25 °C and 45–55% humidity, with a 12 h light/dark cycle. The rats were provided with standard rodent chow (Cat. No. 38057; Purinafeed, Seoul, Republic of Korea) and water ad libitum. Following a 2-week acclimatization period, anesthesia was induced in the rats using a mixture of 70% N2O and 28.5% O2, with 2–3% isoflurane for induction and 1–1.5% isoflurane for maintenance, for either knee joint sampling or surgical induction of the OA model. At the end of the study, all animals were euthanized using CO2 gas.
2.3. Preparation and Treatment of Normal Rat Articular Chondrocytes for In Vitro Study
We isolated chondrocytes from the AC of a total of 30 Sprague–Dawley rats (6-week-old males; OrientBio, Seoul, Republic of Korea), following the methods previously described [10]. Briefly, the knee joint cartilage was rinsed with phosphate-buffered saline and then finely minced into approximately 1–3 mm3 pieces. The cartilage tissue was initially digested for 0.5 h using 0.2% pronase (Sigma-Aldrich, St. Louis, MO, USA), followed by a 4 h digestion with 0.1% collagenase (Sigma-Adrich, USA) at 37 °C. After isolating individual cells through brief centrifugation, we cultured them in Dulbecco’s modified Eagle’s medium supplemented with an antibiotic–antimycotic solution (containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.25 mg/mL amphotericin B; Life Technologies, Carlsbad, CA, USA). The medium was refreshed every 2 days, and the cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 using a CO2 incubator. For this experiment, only cells within the first five passages were used. Following that, the cells were seeded onto a 24-well plate at different densities: 2 × 104 cells/well for assessing cell viability and for measuring 5-lipoxygenase (5-LPO) activity and PGE2 levels; 5 × 104 cells/well for assessing MMP levels; and 5 × 106 cells/well for gene expression analysis. The cells were cultured overnight before being treated with NEM in five different concentrations (0.0001, 0.001, 0.01, 0.1, and 1 mg/mL) in a culture medium containing 2% fetal bovine serum for a duration of 24 h. We used LPS (50 μg/mL; Sigma-Aldrich, USA) to elicit inflammatory responses in primary cultured articular chondrocytes from rats, and recombinant human interleukin-1 alpha (rhIL-1α, 5 ng/mL; eBioscience, San Diego, CA, USA) to induce degradation and cytotoxicity, in line with previously established methods [11,28]. For the biochemical analysis of 5-LPO and PGE2, cell culture supernatants were collected.
2.4. Assessment of Cell Viability
We incubated the cells in a CO2 incubator at 37 °C with NEM samples at concentrations of 0.001, 0.01, 0.1, 1, and 10 mg/mL for 24 h. Recombinant human IL-1α at 5 ng/mL was added one hour after initiating treatment with the test substance, either in its presence or absence. Following the treatment period, 100 μL of 2.5 mg/mL dimethylthiazol-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, USA) was added, and the cells were further incubated at 37 °C for 4 h. Subsequently, the medium containing MTT was discarded, and MTT formazan was solubilized using 1 mL of dimethyl sulfoxide (DMSO). The absorbance and optical density were then measured at 570 nm, using 650 nm as the reference wavelength, with a microplate reader (Sunrise; Tecan, Zurich, Switzerland).
2.5. Osteoarthritis Rodent Animal Model and Treatment for In Vivo Study
The surgical procedure was executed as follows: The OA treatment group underwent an open surgical procedure, which entailed the transection of the anterior cruciate ligament and a partial medial meniscectomy. For this procedure, an incision was made on the medial aspect of the joint capsule, anterior to the medial collateral ligament. In the postoperative phase, the incision was sutured in two layers, starting with the peripheral tissues, followed by the skin closure with interrupted sutures, utilizing dissolvable 3-0 catgut (D78532, Braun, Melsungen. AG, Germany). This surgical intervention triggered the onset of OA pathogenesis, denoting it as the surgically induced side. The right knee joint, which remained nonoperated and intact, served as the contralateral control. Conversely, in the second group, the rats were subjected to a sham operation, wherein a similar incision was made in the joint capsule; however, the transection of the anterior cruciate ligament and partial medial meniscectomy were not performed, adhering to our previously established protocols [29,30,31].
Randomization was utilized to assign the rats into distinct treatment groups, aiming to minimize selection bias and ensure group comparability. On the 13th day post-surgery, a total of 60 rats were selected for the study, with 10 rats assigned to each group based on body weight and knee deviations: sham, OA, OA + diclofenac sodium (DS), OA + NEM100, OA + NEM50, and OA + NEM25. To preserve the integrity of NEM and DS (Wako Pure chemical Industries, Ltd., Tokyo, Japan) against light and moisture exposure, these were stored at −20 °C. Two weeks after the OA surgery, NEM was administered orally to the rats via gastric gavage, employing a stainless-steel gavage needle attached to a 5 mL syringe, with NEM dissolved in distilled water at concentrations of 5, 10, or 20 mg/mL. The administration volume was set at 5 mL/kg, corresponding to doses of 25, 50, and 100 mg/kg/day, administered once daily for 56 days. DS was dissolved in physiological saline to a concentration of 0.4 mg/mL and administered subcutaneously on the dorsal back skin, using a 23-gauge needle attached to a 5 mL syringe, once daily for 56 days at a volume of 5 mL/kg (equating to a 2 mg/kg dose). For the sham and OA groups, only distilled water was administered orally at a volume of 5 mL/kg. Body weight and knee thickness measurements were conducted weekly, starting from the day before OA surgery and continuing until sacrifice (24 h post the final 56th administration). Three days prior to euthanasia, bromodeoxyuridine (BrdU) (Sigma-Aldrich) was administered intraperitoneally at a dose of 50 mg/kg in saline.
2.6. Assessment of 5-LPO and MMP Activities, PGE2 Level Using Assay Kits
These evaluations were performed on supernatants from chondrocyte cultures and homogenized joint tissue samples. After measuring each knee’s maximum extension angle, we meticulously isolated sections of the femoral and tibial articular cartilage (AC) and the synovial membrane (SM). Tissue homogenates were prepared using a bead beater (Model Taco™Pre, GeneResearch Biotechnology Corp., Taichung, Taiwan), an ultrasonic cell disruptor (Model KS-750, Madell Technology Corp., Ontario, CA, USA), and a radioimmunoprecipitation assay buffer (Sigma-Aldrich, USA). The supernatants obtained were then centrifuged at 16,582× g and 4 °C in a cryocentrifuge (Labocene 1236 MGR, Gyrozen, Daejeon, Korea) for subsequent analysis of PGE2 levels, 5-LPO, MMP-2, and MMP-9 activities. All tissue homogenates were stored at −150 °C in an ultra-deep freezer (Model MDF-1156, Sanyo, Tokyo, Japan) pending further analysis. For the quantification of PGE2 levels and the activities of 5-LPO and MMPs, we employed commercial assay kits: the PGE2 assay kit (SKGE004B, Parameter™, R&D Systems, Minneapolis, MN, USA), the Lipoxygenase Inhibitor Screening Assay kit (760700, Cayman Chemical, Ann Arbor, MI, USA), and MMP ELISA kits (MBS494797, MBS722532, Mybiosource, San Diego, CA, USA), analyzed with a microplate reader (Sunrise; Tecan, Männedorf, Switzerland). Results were obtained following the protocols provided with the assay kits.
2.7. Real-Time Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
We evaluated the gene expression levels of Col-2, aggrecan, and Sox9 in cultured chondrocytes and tissue samples derived from the femoral and tibial articular cartilage (AC), synovial membrane (SM), and infrapatellar fat pad, in accordance with established protocols [29]. RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and its concentration and integrity were assessed with the CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA). Contaminating DNA was removed by treatment with recombinant DNase I (DNA-free DNA Removal Kit; Cat No. AM1906, Thermo Fisher Scientific Inc., Rockford, IL, USA). Reverse transcription of RNA to cDNA was performed using the High-Capacity cDNA Reverse Transcription Kit (Cat. No. 4368813.7, Thermo Fisher Scientific Inc., Rockford, IL, USA), adhering to the manufacturer’s protocol. The resulting cDNA served as the template for subsequent PCR amplification, which was carried out under the following conditions: an initial denaturation at 94 °C for 2 min, followed by 35 cycles of 15 s at 94 °C, 30 s at 60 °C, and 1 min at 68 °C, with a final extension at 72 °C for 5 min. This analysis utilized the ABI Step One Plus Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Gene expression levels were quantified relative to a vehicle control and normalized to β-actin mRNA, using the comparative threshold cycle (Ct) method. Table 1 lists the sequences of the PCR oligonucleotide primers.

Table 1.
Oligonucleotides for real-time RT-PCR used in this study.
2.8. Measurement of Knee Thickness and Maximum Extension
Using an electronic digital caliper (CD-15CPS, Mitutoyo, Tokyo, Japan), we measured the thickness of the individual hind knees. These measurements were carried out on the left side, which had been subjected to OA surgery, initially on the 13th postoperative day, on the day of the first treatment administration, and subsequently, at weekly intervals. To account for any potential variability introduced by surrounding tissues, knee thickness was also assessed post-mortem, following joint capsule exposure during euthanasia. For a comprehensive analysis, knees from the operated region extending from the coxofemoral to the ankle region were dissected with care to preserve the articular capsule’s integrity. Post-dissection, the maximum extension angle of each knee was evaluated, in accordance with established protocols [31]. To ensure consistency and reduce measurement bias, a single veterinarian performed all surgical and extension angle measurement procedures.
2.9. Assessment of Focal Bone Mineral Density (BMD) and Cartilage Stiffness in OA Models
The average focal BMD of the entire knee joint, including the femoral and tibial AC, was quantitatively measured in both OA-operated and sham-operated rats using live dual-energy X-ray absorptiometry (DEXA; InAlyzer, Medikors, Seongnam, Republic of Korea). BMD measurements for each experimental group are reported in grams per square centimeter (g/cm2). Additionally, to determine the focal stiffness of the cartilage on the articular surfaces of the femur and tibia, we utilized a computerized testing machine (SV-H1000, Japan Instrumentation System Co., Tokyo, Japan), measuring the force in Newtons for each group separately.
2.10. Histopathological Analysis
Knee joint components were dissected, ensuring the preservation of the joint capsules, and subsequently fixed in 10% neutral buffered formalin. The samples were then decalcified in a solution containing 24.4% formic acid and 0.5N sodium hydroxide for a duration of 5 days, with the decalcifying solution being replaced daily. Following decalcification, each knee joint was longitudinally sectioned and embedded in paraffin. Sections of 3–4 μm thickness were prepared and stained with hematoxylin and eosin (H&E) for general histopathological examination and with safranin O (SO) to highlight cartilaginous tissues. Histological evaluations were conducted on various regions of the knee joint, including the femoral and tibial articular cartilage and the synovial membrane-lining epithelium, focusing on the presence and quantity of inflammatory cells. The severity of OA was assessed using the modified Mankin scoring system, which evaluates four categories—cartilage surface integrity, hypocellularity, cell clustering, and staining intensity—with scores ranging from 0 to 3 points for each category (semiquantitative scores; maximum score = 12). A higher score indicated a more severe OA progression. All histological analyses were conducted by the same experienced histopathologist using a computer-assisted automated image analyzer (iSolution FL version 9.1, IMT i-Solution Inc., Vancouver, BC, Canada).
2.11. Immunohistochemistry Analysis
Immunoreactivities for the cell proliferation marker Bromodeoxyuridine (BrdU) (ab8152, Abcam, Cambridge, UK; dilution 1:100) were assessed using a purified primary antibody in conjunction with an avidin–biotin–peroxidase complex and a peroxidase substrate kit (Vector Labs, Burlingame, CA, USA) on femoral and tibial articular cartilage and synovial membrane tissues. Furthermore, immunoreactivity for the apoptotic marker cleaved Poly (ADP-ribose) polymerase (PARP) (9545, Cell Signaling Technology Inc., Danvers, MA, USA; dilution 1:100) and the proinflammatory cytokines Tumor Necrosis Factor-alpha (TNF-α) (sc-52746, Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:200) and Cyclooxygenase-2 (COX-2) (160126, Cayman; dilution 1:200) was evaluated. The procedure began with the quenching of endogenous peroxidase activity by incubating sections in methanol containing 0.3% H2O2 for 30 min. To prevent non-specific antibody binding, sections were treated with normal horse serum as a blocking solution for 1 h in a humidified chamber. Epitope retrieval was achieved through pretreatment with trypsin (Sigma-Aldrich, St. Louis, MO, USA) and 2N hydrochloric acid (HCl). Primary antibodies were applied overnight at 4 °C in a humidified chamber, followed by incubation with a biotinylated universal secondary antibody and avidin–biotin–peroxidase complex reagents for 1 h at room temperature. Development of the signal was conducted using the peroxidase substrate kit for 3 min at room temperature. Between each step, sections were washed thrice with 0.01 M phosphate-buffered saline. This analysis was performed blindly by a histopathologist. Positive immunoreactivity, defined as over 20% staining for each antibody (BrdU, PARP, TNF-α, and COX-2), was quantified in cells/mm2 in both femoral and tibial articular cartilage along with synovial membrane tissues.
2.12. Statistical Analyses
Results are expressed as the mean ± standard deviation, based on six independent in vitro experiments and in vivo experiments with sample sizes of 10. To evaluate the normality of the distribution and homogeneity of variances, we employed the Kolmogorov–Smirnov and Levene tests, respectively. For data adhering to a normal distribution, one-way Analysis of Variance (ANOVA) was applied. Two-way ANOVA was utilized to assess kinetic changes in body weight and knee thickness across different time points, considering time as a repeated measure. Multiple comparison post hoc analyses were conducted using the Tukey’s Honestly Significant Difference (HSD) test for datasets with equal variances and Dunnett’s T3 test for those with unequal variances. Statistical analyses were conducted using SPSS 18.0 for Windows (IBM-SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Chondroprotective Effect of NEM In Vitro
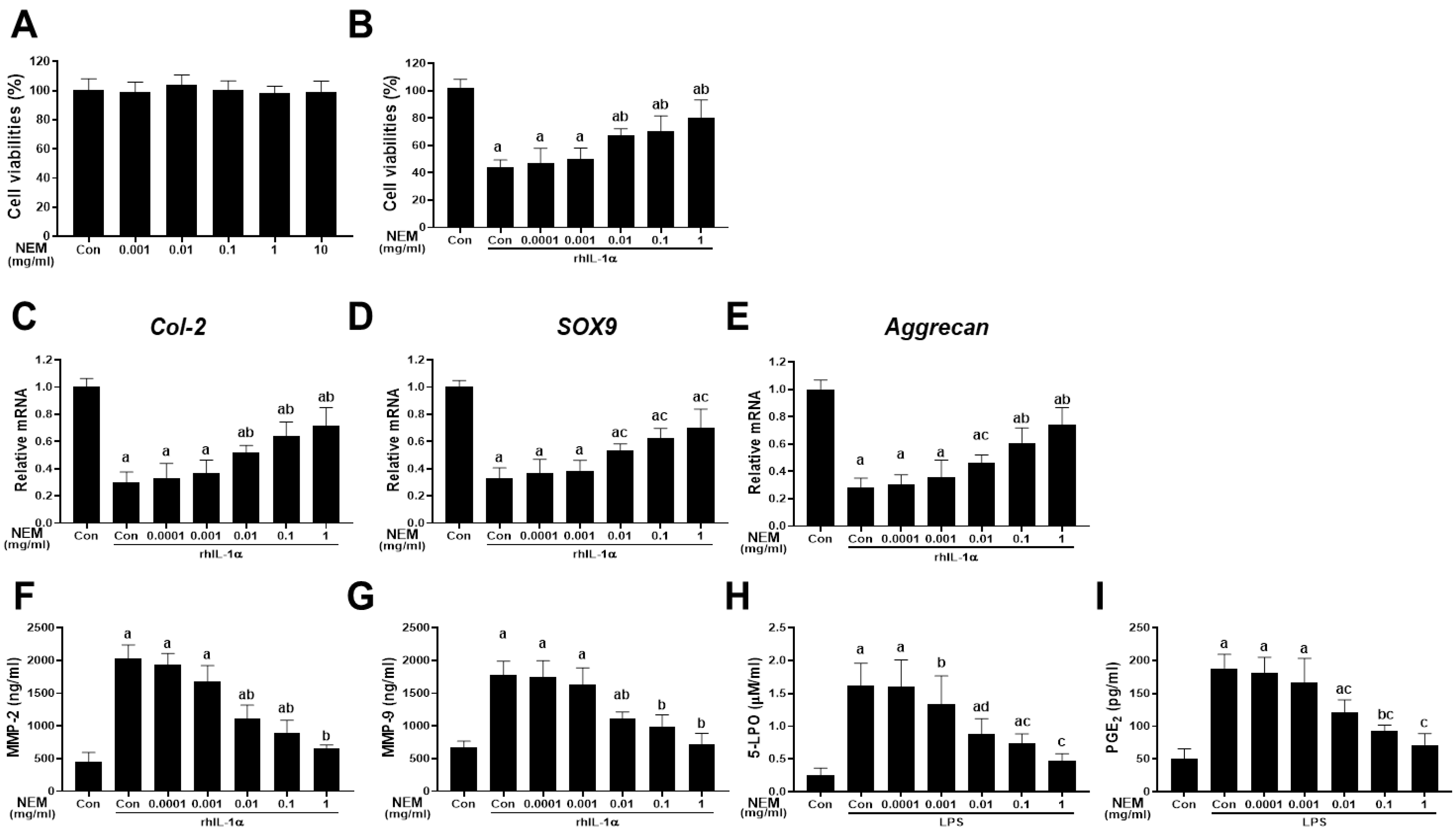
Figure 1 illustrates the isolation and 24 h incubation of primary chondrocytes from rat knee joints with NEM at concentrations ranging from 0.0001 to 1 mg/mL in distilled water. Across all tested concentrations (0.001, 0.01, 0.1, 1, and 10 mg/mL) of NEM, there was no significant change in the cell viability of NEM-treated primary cultured rat articular chondrocytes compared with the vehicle-treated normal controls (Figure 1A). In the rhIL-1α-induced cell injury model, cell viability decreased in the vehicle-treated rhIL-1α model control compared with that in the normal control (Figure 1B). However, pretreatment with NEM at 0.01, 0.1, and 1 mg/mL suppressed the cell viability reduction induced by rhIL-1α treatment. In addition, the model control group treated with rhIL-1α showed decreased expression of chondrogenic genes such as Col-2, SOX9, and aggrecan (Figure 1C–E), and increased MMP2 and MMP9 levels compared with the normal control group (Figure 1F,G). However, we noted significantly decreased levels of MMP-2 and MMP-9 and increased expression levels of the three genes in the 0.01, 0.1, and 1 mg/mL NEM treatment groups compared with in the rhIL-1α-treated model control group. In the LPS-induced cell inflammation model, 5-LPO activity and PGE2 content significantly increased after LPS treatment compared with the normal control (Figure 1H,I). Nonetheless, pretreatment with NEM at concentrations of 0.01, 0.1, and 1 mg/mL effectively reduced these increases compared with the LPS model control group.
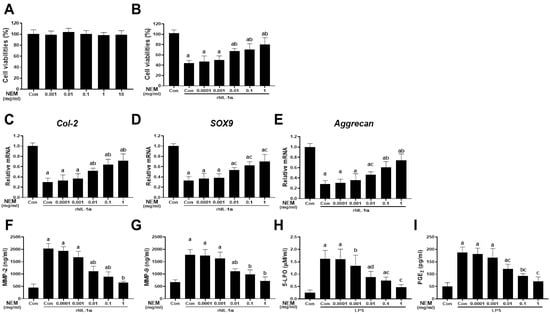
Figure 1.
Cytoprotective effects in vitro (A) Cell viability in primary articular chondrocytes. (B) Cell viability in rhIL-1α-exposed chondrocytes. (C–E) Relative expression levels of chondrogenic genes (Col-2, aggrecan, and Sox9) when exposed to rhIL-1α. (F,G) MMP-2 and MMP-9 levels upon exposure to rhIL-1α. (H,I) 5-lipoxygenase (5-LPO) activity and prostaglandin E2 (PGE2) level in LPS-exposed chondrocytes. Values are expressed as mean ± SD in six independent experiments after NEM treatment at the indicated doses. a p < 0.01 and b p < 0.05 as compared with the control; c p < 0.01 and d p < 0.05 as compared with rhIL-1α- or LPS-treated control.
3.2. Effect of NEM on Knee Thickness Changes In Vivo
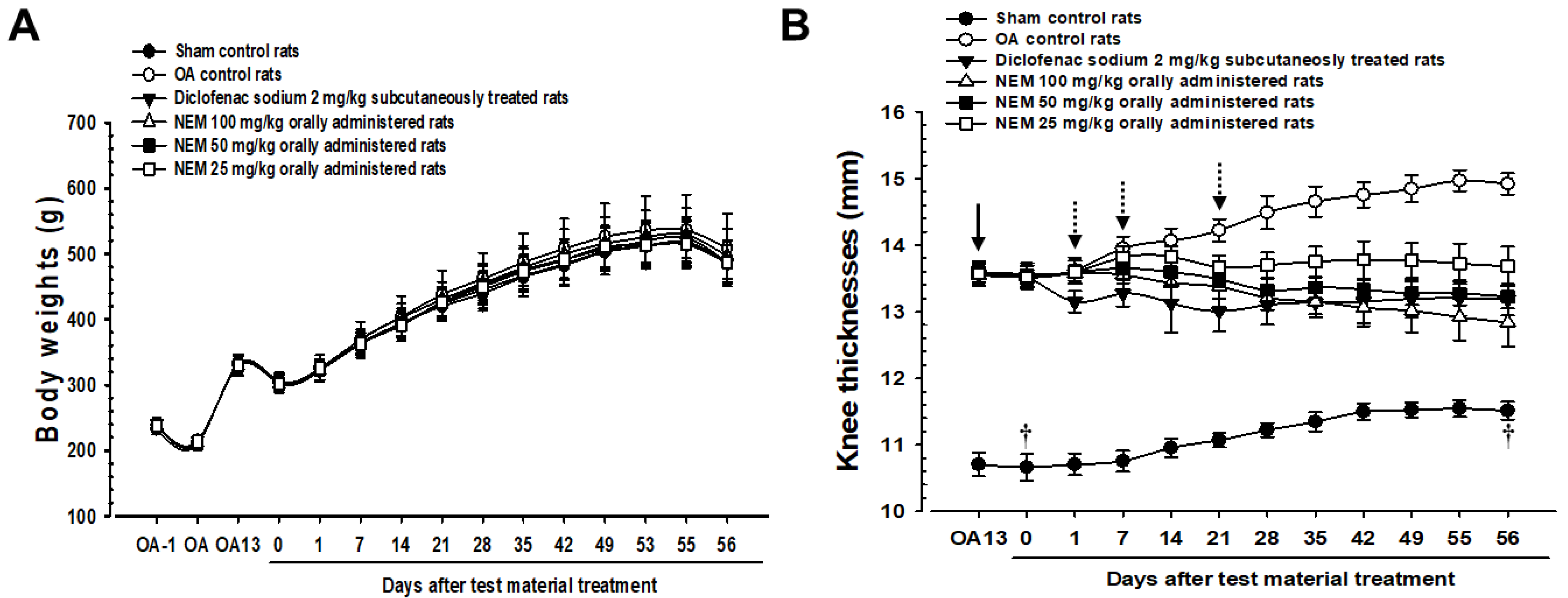
As mentioned, the sham and OA model groups received distilled water orally, and the other OA models received 2 mg/kg of DS subcutaneously or 100, 50, or 25 mg/kg of NEM (NEM100, NEM50, and NEM25, respectively) orally. Figure 2 illustrates the changes in body weight and knee thickness when measured weekly for 8 weeks postoperatively. Body weight did not significantly change among the groups (Figure 2A). Meanwhile, knee thickness was significantly increased in OA group rats compared with in sham rats (Figure 2B). However, the knee thickness of rats subcutaneously administered with DS decreased from day 1 after initial treatment compared to the OA group. Furthermore, oral administration of NEM at doses of 100 and 50 mg/kg showed a significant decrease in knee thickness from day 7, while the initial oral administration of NEM at 25 mg/kg showed significance from day 21.
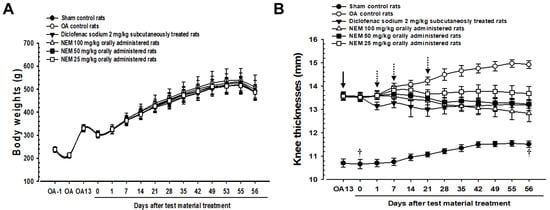
Figure 2.
Changes in body weight and knee thickness in vivo. (A) Body weight changes. (B) Knee thickness changes. Values are expressed as mean ± SD in 10 rats. After 13 days from OA surgery, knee thickness significantly increased in the OA model (p < 0.01) compared with that in the sham (arrow). However, it significantly decreased (p < 0.01 or p < 0.05) in rats from day 1 after the initial subcutaneous treatment of diclofenac sodium (2 mg/kg), from day 7 after the initial oral administration of NEM at 100 and 50 mg/kg, and from day 21 after the initial oral administration of NEM at 25 mg/kg (dotted arrows). OA13 means at day 13 after OA surgery, 0 means at the start of administration, 14 means at postoperative day 14, and 56 means day 56 after the initiation of test material treatment and at sacrifice. All animals were overnight fasted before the first test material treatment and the sacrifice (†).
3.3. Effect of NEM on Knee Thickness and Maximum Extension Angle after Capsule Exposure
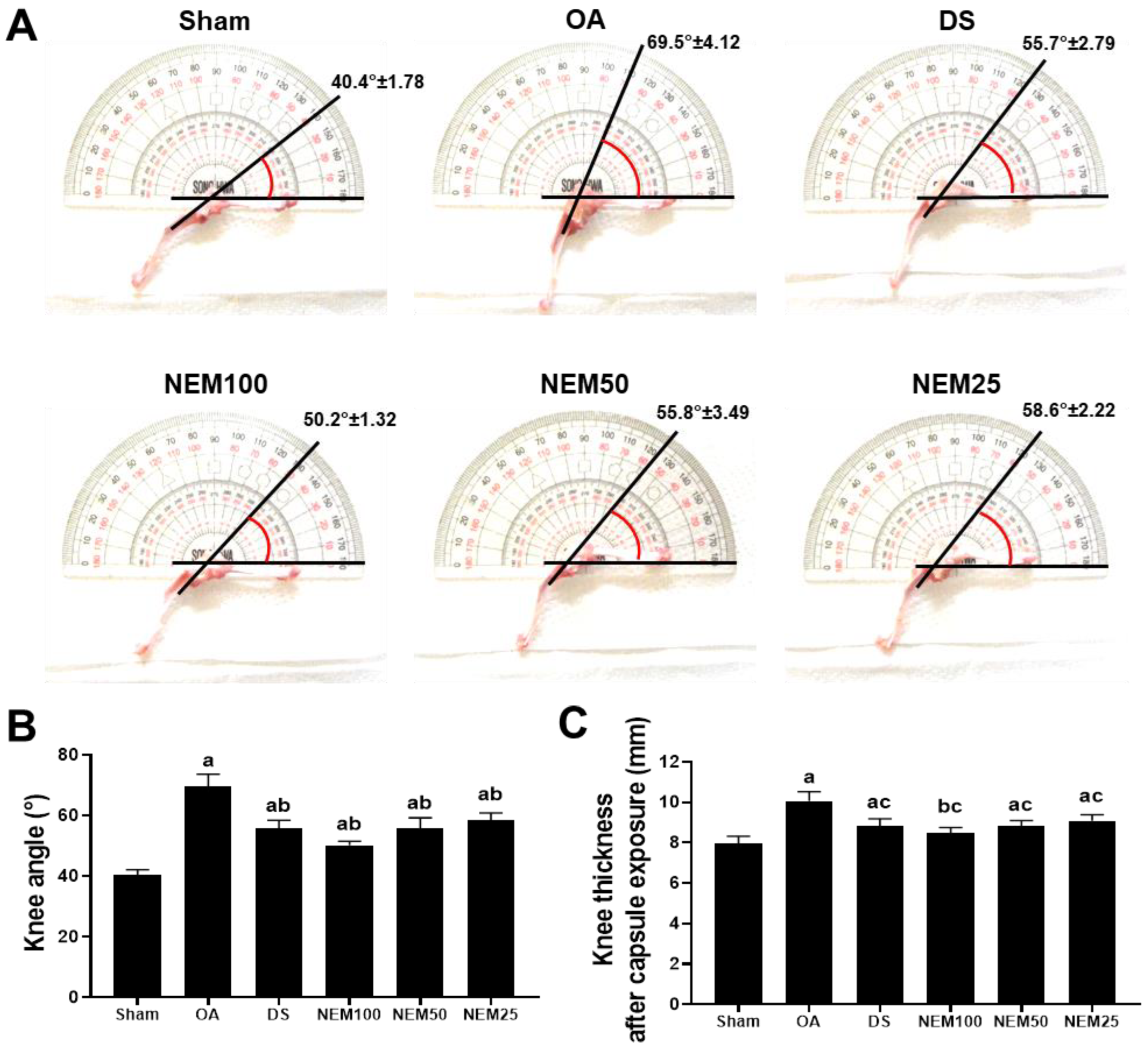
To assess knee joint stiffness reduction by NEM, we measured the thickness of the knee joint capsule exposed to the surrounding tissue and then evaluated the maximum extension angle of the joint after all treatments (Figure 3). The OA group showed a significantly increased maximum knee extension angle compared with the sham, but the DS and NEM treatments significantly inhibited the maximum knee extension angle (Figure 3A,B). In assessing joint capsule thickness, NEM significantly inhibited the increase in capsule thickness induced by OA surgery in a concentration-dependent manner, similar to DS (Figure 3C).
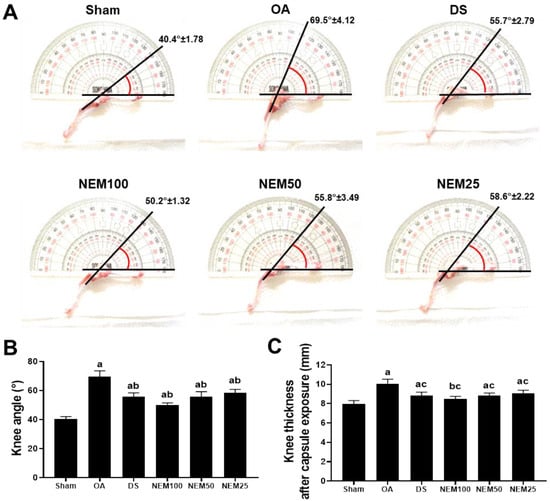
Figure 3.
Knee thickness after capsule exposure and maximum extension angle in vivo. (A) Representative images of knee maximum extension angles. The line indicates a guide for measurement. (B) Maximal joint extension angle. Values are expressed as the mean ± SD of 10 rats. a p < 0.01 as compared with sham by DT3; b p < 0.01 as compared with OA by DT3. (C) Knee thickness after capsule exposure. Values are expressed as the mean ± SD of 10 rats. a p < 0.01 and b p < 0.05 as compared with sham by THSD; c p < 0.01 as compared with OA by THSD.
3.4. Effect of NEM on Bone Mineral Deposition and Compressive Strength of AC
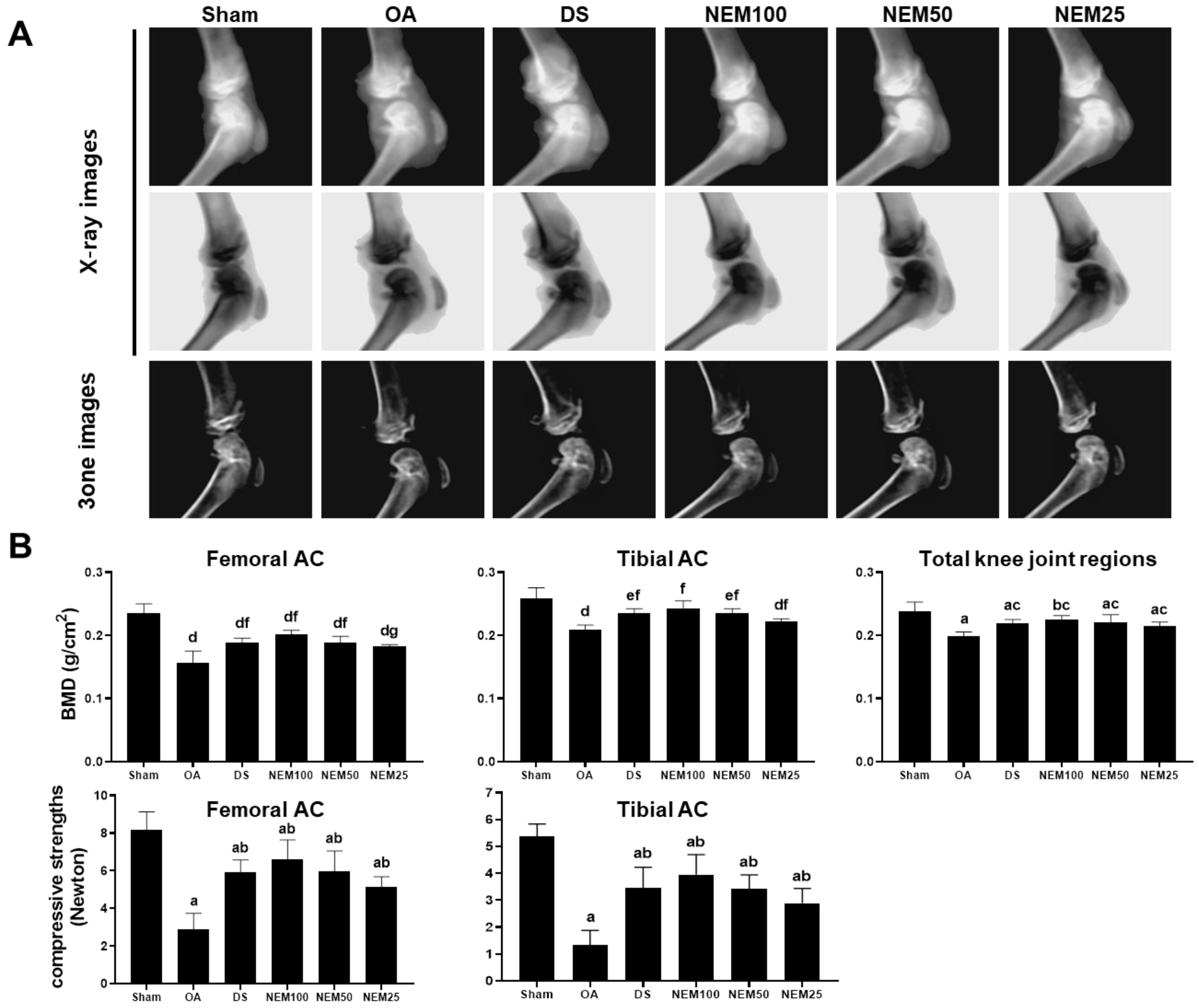
We assessed the BMD in the AC using live dual-energy X-ray absorptiometry (DEXA) images and then measured the AC’s comprehensive strength. In the OA group, DEXA image analysis revealed noticeable edematous changes in the knee joint and erosive signs in the femoral and tibial AC (Figure 4A). Meanwhile, focal BMD was significantly decreased in the entire knee joint, femur, and tibia joint surface areas (Figure 4B). However, these signs were alleviated in the DS, NEM100, and NEM50 groups. Given that BMD affects bone strength, we additionally evaluated the compressive strength of both types of AC. The compressive strength of the femoral and tibial AC was decreased in the OA model group compared with that in the sham group but was significantly increased in the DS and NEM treatment groups compared with the OA group (Figure 4B). In particular, BMD and compressive strength were higher in the AC of the NEM100 group than in the DS group.
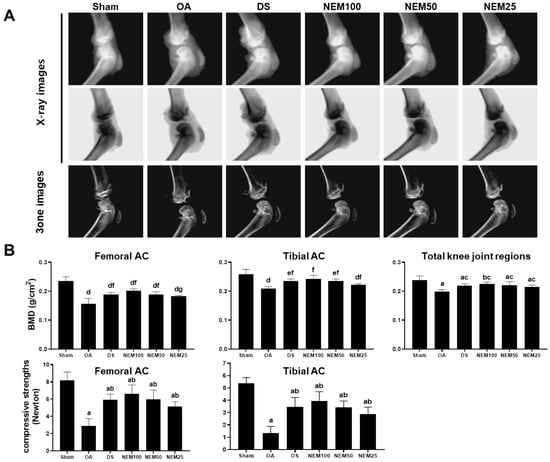
Figure 4.
Bone mineral density (BMD) and compressive strength in vivo. (A) DEXA images of the knee joints. (B) BMD and compressive strength in the femoral and tibial AC. Values are expressed as means ± SD in 10 rats. a p < 0.01 and b p < 0.05 as compared with sham by THSD; c p < 0.01 as compared with OA by THSD; d p < 0.01 and e p < 0.05 as compared with sham by DT3; and f p < 0.01 and g p < 0.05 as compared with OA by DT3.
3.5. Effect of NEM on Inflammation and Extracellular Matrix Degradation of Joint Tissue In Vivo
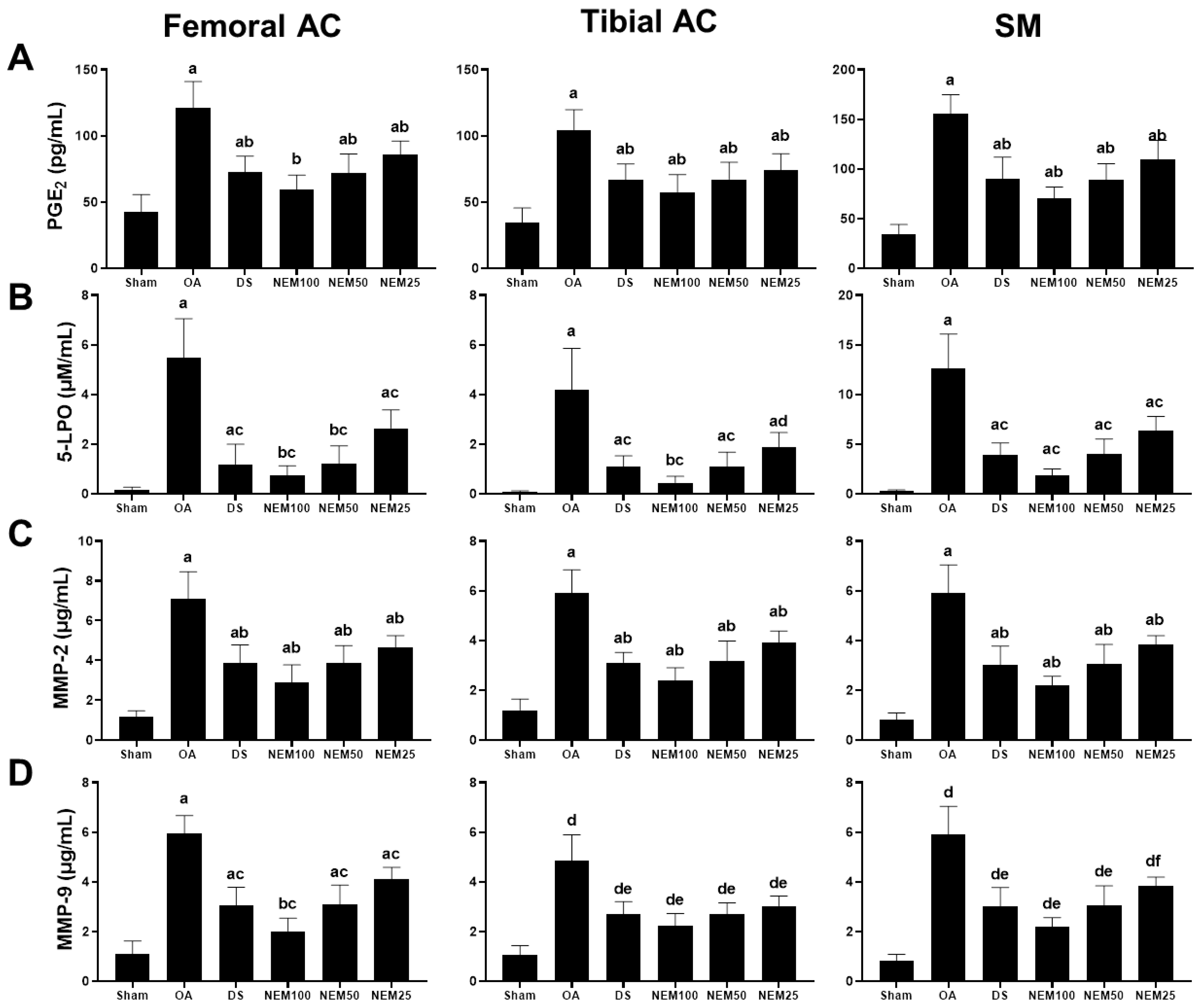
The levels of the inflammatory mediators, PGE2 and 5-LPO, and the degradation enzymes, MMP-2 and MMP-9, were assessed in the femoral and tibial AC and SM (Figure 5). The 5-LPO activity and PGE2, MMP-2, and MMP-9 levels in the femoral and tibial AC and SM were found to be increased in the OA group compared with in the sham group. However, they were significantly reduced in the DS and NEM groups compared with the OA group. In particular, NEM100 showed a greater inhibitory effect on these mediators and enzymes than DS.
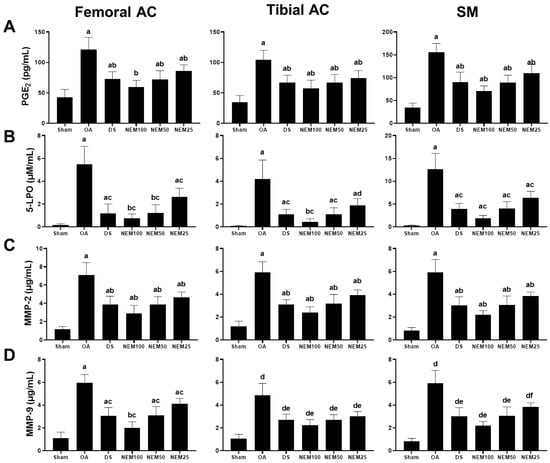
Figure 5.
Anti-inflammatory and anti-extracellular matrix (ECM) degradation activities in in vivo PGE2 and 5-LPO, MMP-2, and MMP-9 activities in the femoral and tibial AC and the SM. Values are expressed as means ± SD in 10 rats. (A) a p < 0.01 as compared with sham by THSD; b p < 0.01 as compared with OA by THSD. (B) a p < 0.01 and b p < 0.05 as compared with sham by DT3; c p < 0.01 and d p < 0.05 as compared with OA by DT3. (C) a p < 0.01 as compared with sham by DT3; b p < 0.01 as compared with OA by DT3. (D) a p < 0.01 and b p < 0.05 as compared with sham by THSD; c p < 0.01 as compared with OA by THSD; d p < 0.01 as compared with sham by DT3; e p < 0.01 and f p < 0.05 as compared with OA by DT3.
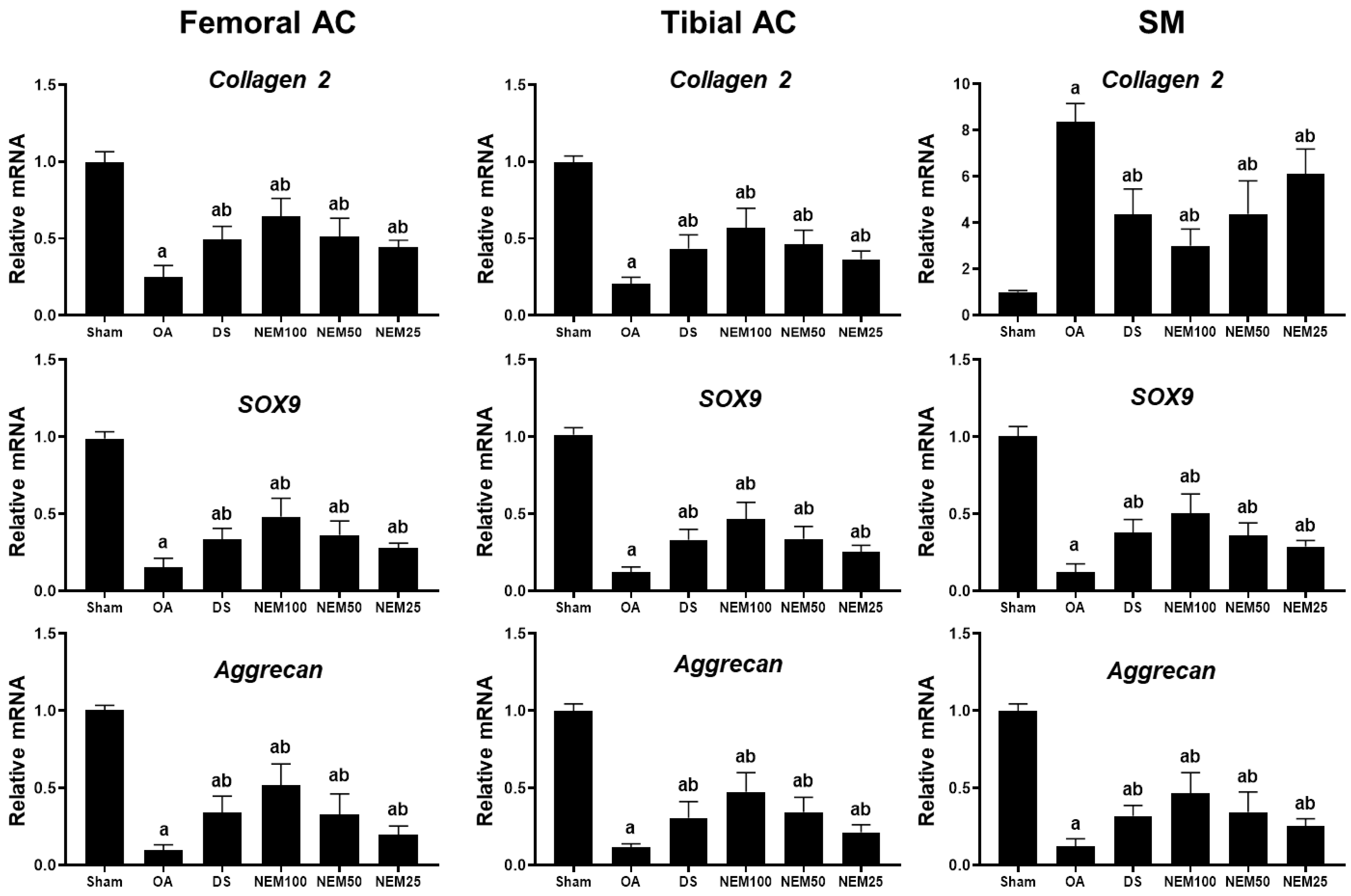
3.6. Effect of NEM on Cartilage Composition-Related Gene Expression in Joint Tissue In Vivo
We measured the mRNA expression levels of Col-2 and aggrecan, key components of the cartilage ECM, and SOX9, a transcription factor for cartilage differentiation, in the femoral and tibial AC and SM. The OA group showed decreased expression levels of Col 2, aggrecan, and SOX9 in the femoral and tibial AC compared with the sham group (Figure 6). However, their expression levels were significantly increased in the DS, NEM100, and NEM50 groups compared with in the OA group. Regarding the SM, the expression of aggrecan and Sox9 were decreased, whereas Col-2 expression was significantly increased in the OA group. However, their expression was significantly reversed by the administration of DS, NEM100, and NEM50.
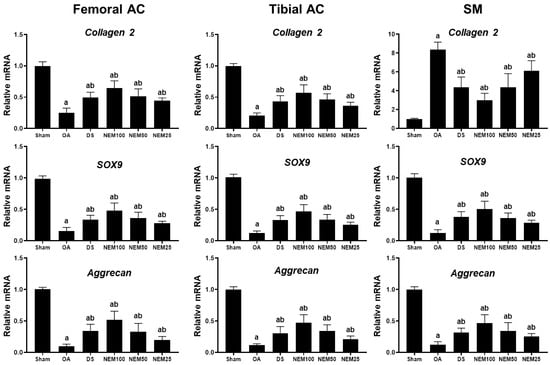
Figure 6.
Gene expression levels of Col-2, aggrecan, and Sox9 in the femoral and tibial articular cartilage (AC) and the synovial membrane (SM). We assessed the gene expression related to cartilage composition from the femoral and tibial articular cartilage (AC) and synovial membrane (SM). Total RNA was isolated and the mRNA levels were measured using real-time PCR. Gene expression levels were quantified relative to a vehicle control and normalized to β-actin mRNA. Values are expressed as means ± SD in 10 rats. a p < 0.01 as compared with sham by DT3; b p < 0.01 as compared with OA by DT3.
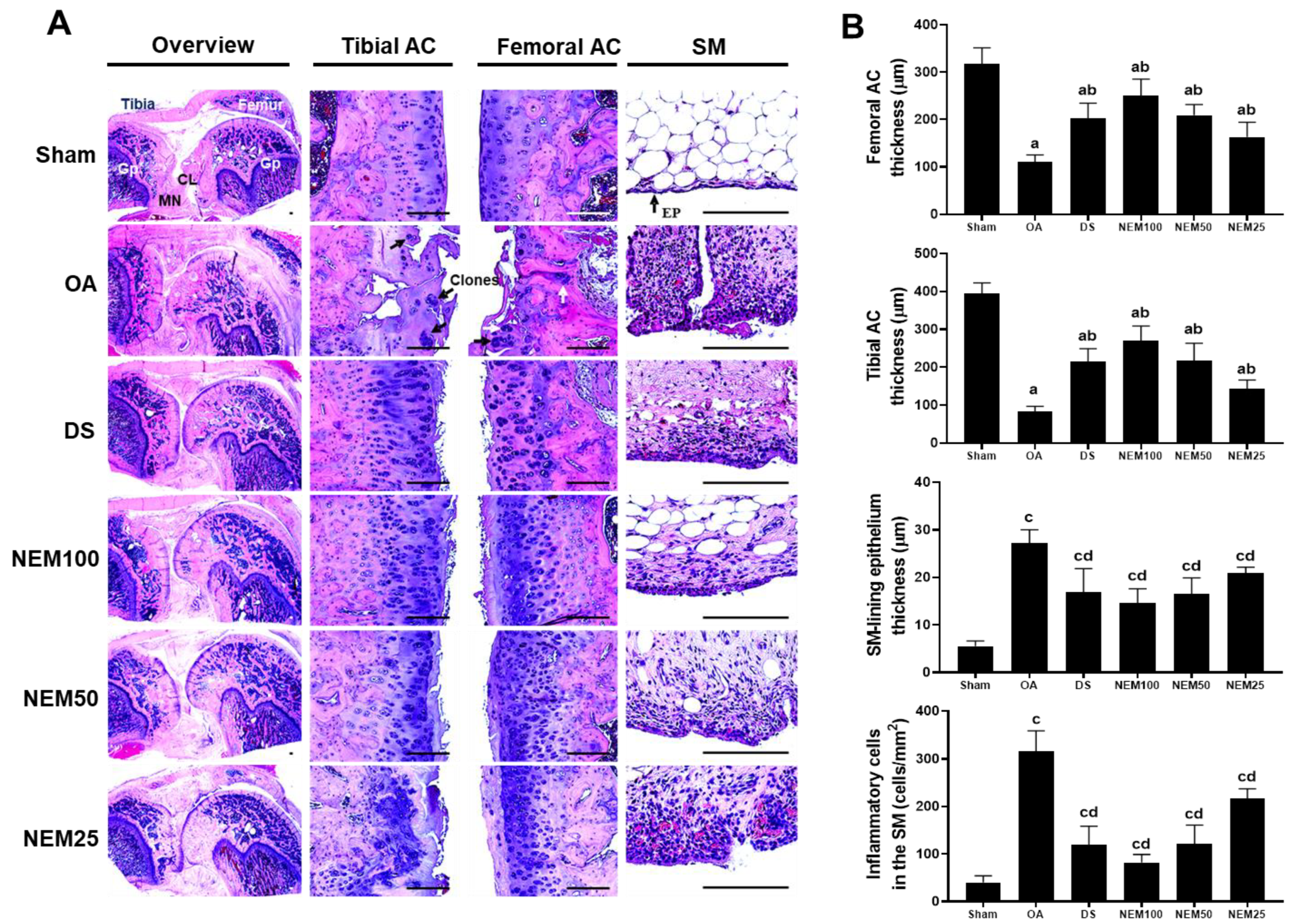
3.7. Effect of NEM on Histopathological Changes and Infiltrated Inflammatory Cells in Femoral and Tibial Articular Cartilage and Sinovial Membrane In Vivo
The histopathological changes were examined in the AC and SM stained with H&E and SO. After H&E staining, the OA group clearly showed a thin cartilage layer, clonal formation, and severe cartilage damage on the femur and tibia articular surfaces, as well as an increase in the amount of SM-lining epithelium and number of infiltrated inflammatory cells (Figure 7A,B). However, these histopathological changes were improved in the DS and NEM treatment groups. In the surgically induced OA group, the decreased thickness of the femoral and tibial AC increased after DS, NEM100, or NEM50 administration (Figure 7A,B). In particular, the NEM100 group showed a greater increase in the thickness of the AC and a more prominently reduced thickness of the SM-lining epithelium than the DS group.
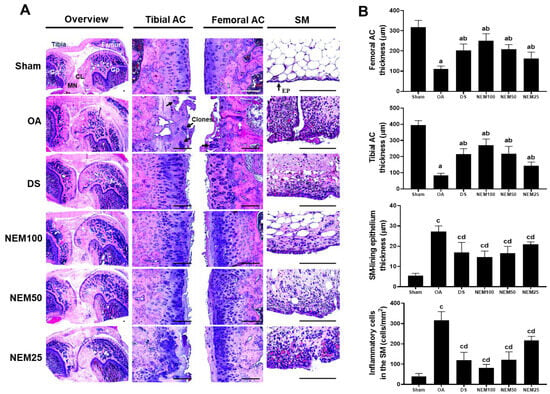
Figure 7.
Histopathological analysis in femoral and tibial articular cartilage (AC) and synovial membrane (SM). (A) Representative histological images of the femoral and tibial AC with SM stained with hematoxylin and eosin (H&E). Black and white arrows indicate the clone formation in the articular surfaces and SM-lining epithelium, respectively. GP = Growth plate; MN = Meniscus; CL = Crucial ligament. Scale bars = 160 µm. (B) Thickness of both AC and the SM-lining epithelium and the number of infiltrated inflammatory (IF) cells in the SM. Values are expressed as means ± SD in 10 rats. a p < 0.01 as compared with sham by THSD; b p < 0.01 as compared with OA by THSD; c p < 0.01 as compared with sham by DT3; d p < 0.01 as compared with OA by DT3.
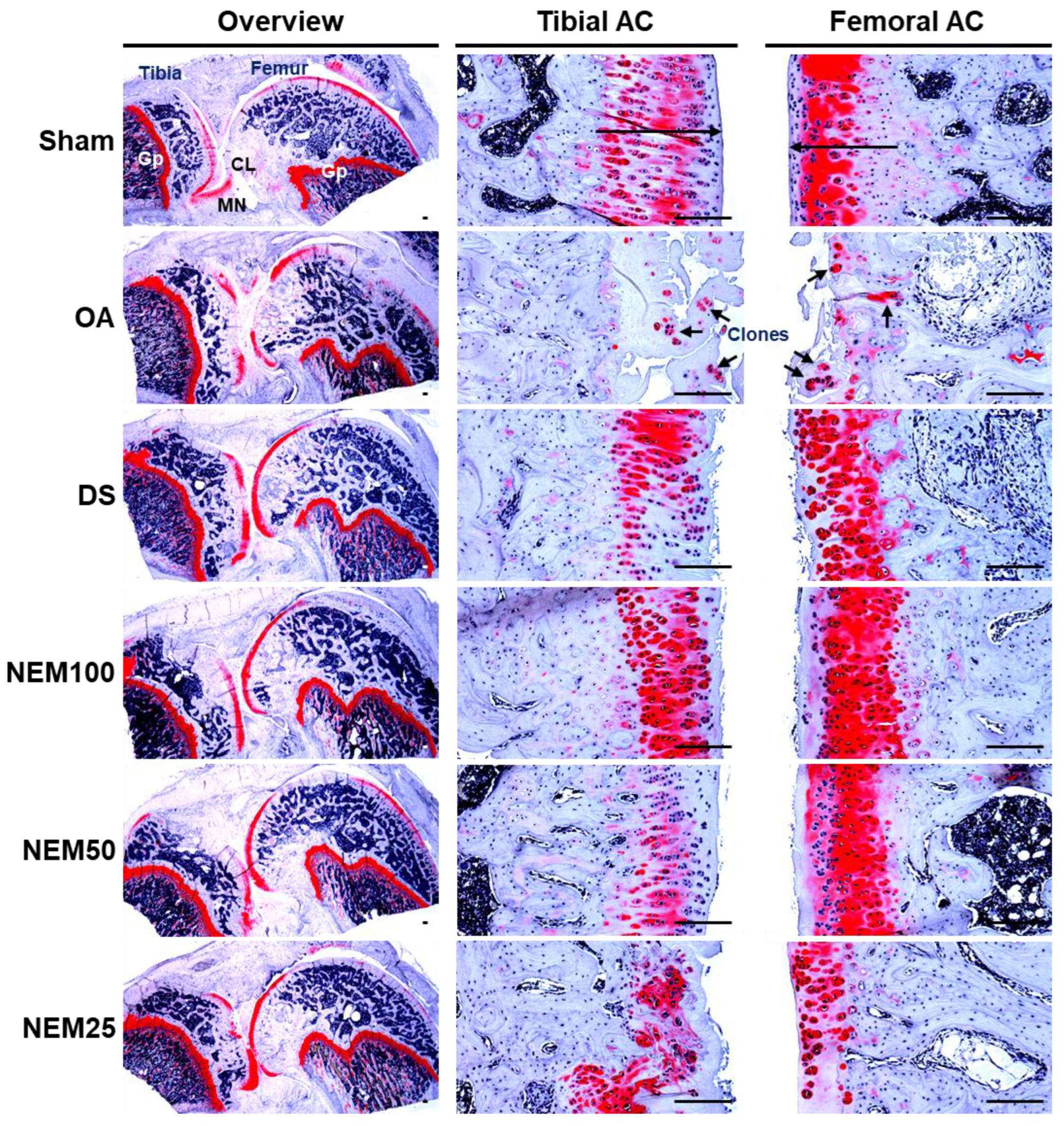
After SO staining, OA rats showed a significant increase in superficial cartilage damage, a decrease in chondrocyte and clonal formation, and a decrease in SO staining intensity in both the femoral and tibial AC, resulting in a significant increase in femoral and tibial AC Mankin scores (Figure 8 and Table 2 and Table 3). However, the scores were significantly reduced in the DS and NEM compared with in the OA group and were further reduced in the NEM200 group compared with in the DS group.
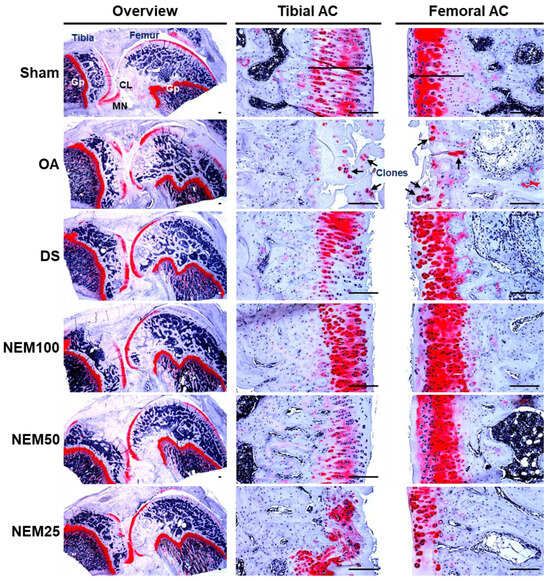
Figure 8.
Histopathological analysis in femoral and tibial articular cartilage (AC). Representative safranin O (SO)-stained histological images of the femoral and tibial AC with SM. Long arrows indicated the thicknesses of tibia or femur AC. And short arrows indicate the clone formation in the articular surfaces. GP = Growth plate; MN = Meniscus; CL = Crucial ligament. Scale bars = 160 µm.

Table 2.
Femoral AC Mankin scores in sham-operated or OA rats.

Table 3.
Tibial AC Mankin scores in sham-operated or OA rats.
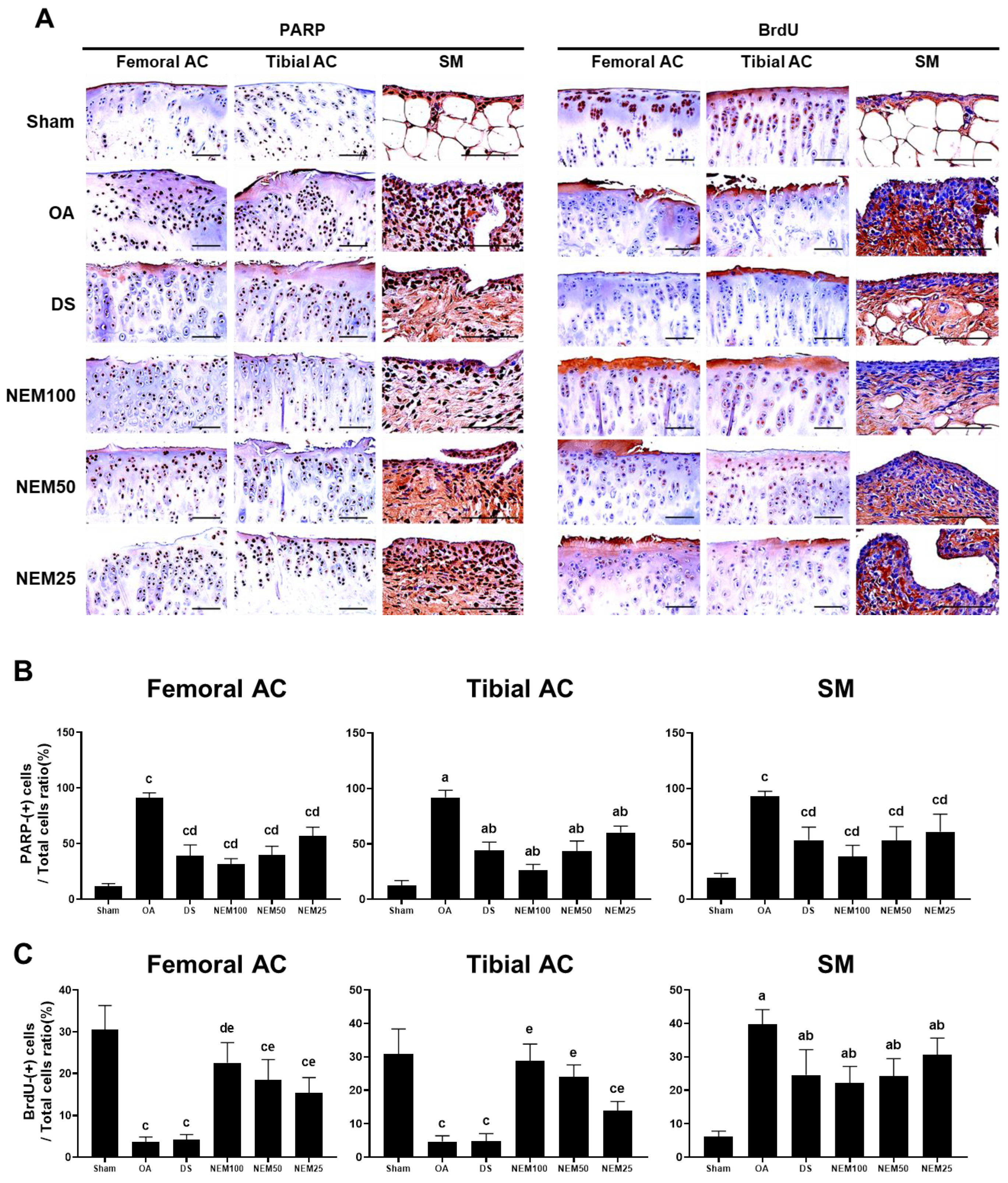
3.8. Effects of NEM on Inflammation, Apoptosis, and Cell Proliferation In Vivo
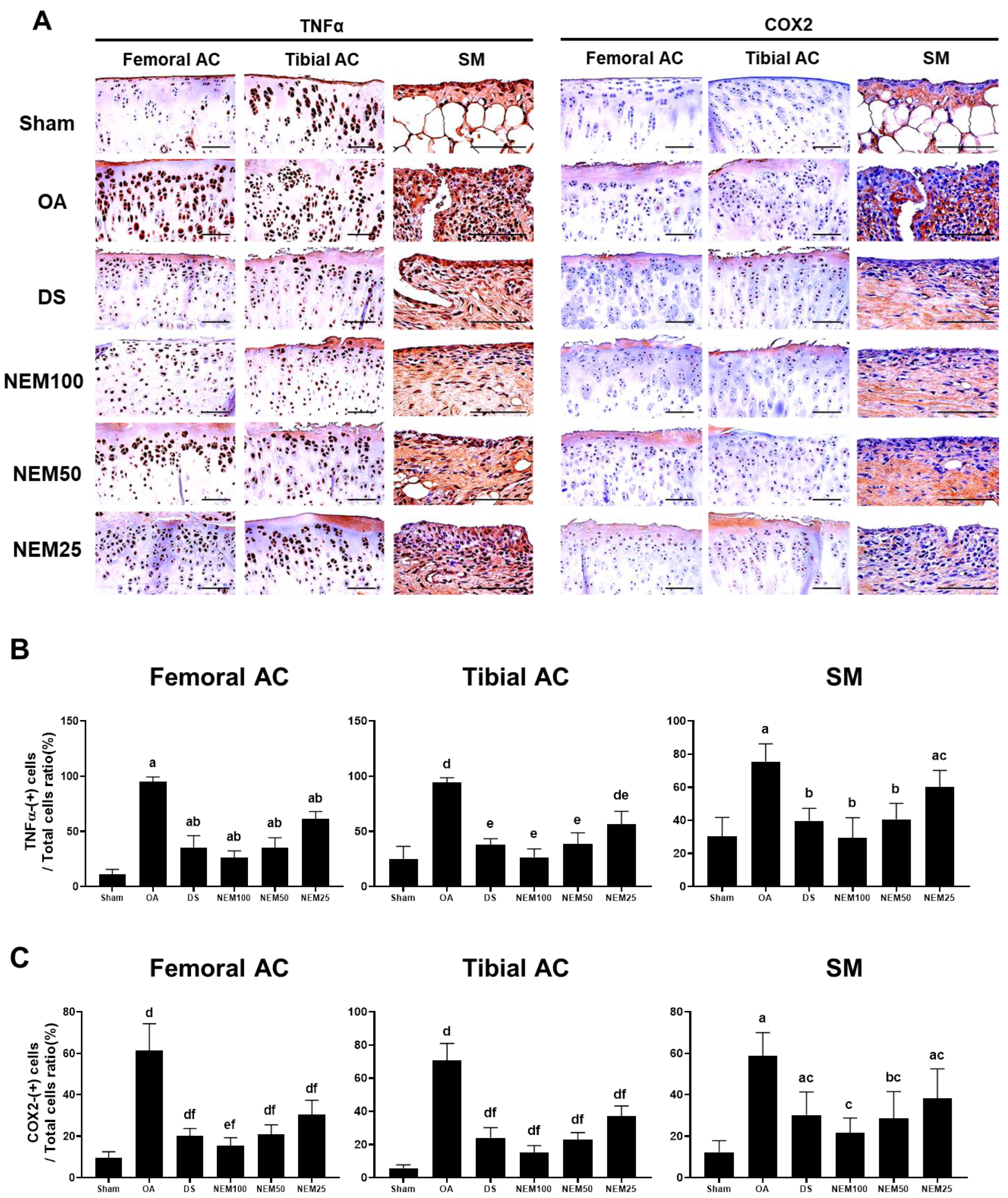
Figure 9 and Figure 10 show the immunostained images of the inflammatory markers TNFα and COX-2, the apoptosis marker PARP, and the cell proliferation marker BrdU in the femoral and tibial AC and SM. In the OA group, the number of immunopositive cells for TNFα, COX-2, and PARP was significantly increased in the femoral and tibial AC and SM. However, this increment was attenuated in the DS, NEM100, and NEM50 groups.
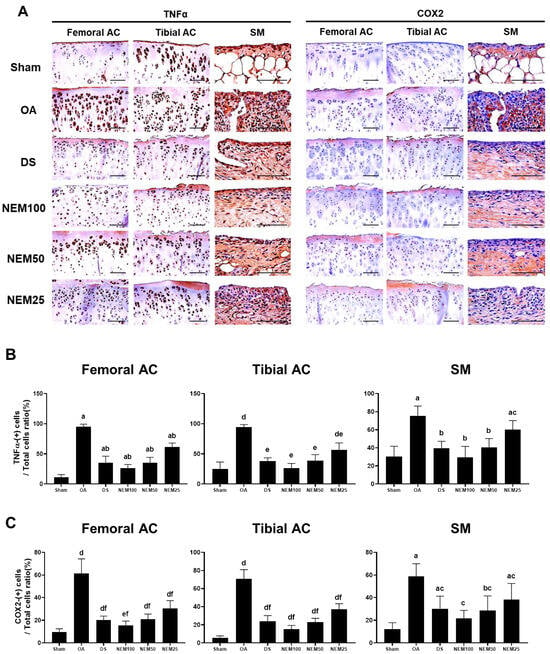
Figure 9.
Immunohistochemistry for inflammation in vivo. (A) Representative immunohistochemistrical images of TNFα and COX-2 cells on the femoral and tibial articular cartilage (AC) with synovial membrane (SM). Scale bars = 80 µm. (B) The proportion of TNFα(+) cells was calculated as a ratio of positive cells to total cells. Values are expressed as means ± SD in 10 rats. a p < 0.01 as compared with sham by THSD; b p < 0.01 and c p < 0.05 as compared with OA by THSD; d p < 0.01 as compared with sham by DT3; e p < 0.01 as compared with the OA by DT3. (C) The proportion of COX-2(+) cells was calculated as a ratio of positive cells to total cells. Values are expressed as means ± SD in 10 rats. a p < 0.01 and b p < 0.05 as compared with sham by THSD; c p < 0.01 as compared with OA by THSD; d p < 0.01 and e p < 0.05 as compared with sham by DT3; f p < 0.01 as compared with the OA by DT3.
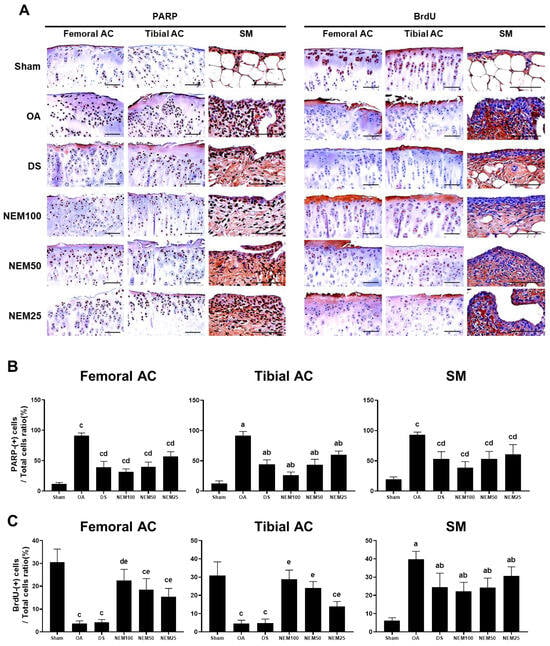
Figure 10.
Immunohistochemistry for apoptosis and cell proliferation in vivo. (A) Representative immunohistochemistrical images of the cleaved poly(ADP-ribose) polymerase (PARP)- and bromodeoxyuridine (BrdU)-immunoreactive cells on the femoral and tibial articular cartilage (AC) with SM. Scale bars = 100 µm. (B) The proportion of PARP cells was calculated as a ratio of positive cells to total cells. Values are expressed as means ± SD in 10 rats. a p < 0.01 as compared with sham by THSD; b p < 0.01 as compared with OA by THSD; c p < 0.01 as compared with sham by DT3; d p < 0.01 as compared with the OA by DT3. (C) The proportion of BrdU cells was calculated as a ratio of positive cells to total cells. Values are expressed as means ± SD in 10 rats. a p < 0.01 as compared with sham by THSD; b p < 0.01 as compared with OA by THSD; c p < 0.01 and d p < 0.05 as compared with sham by DT3; e p < 0.01 as compared with the OA by DT3.
The immune response to BrdU showed differences in the cartilage and synovium in the OA compared with in the sham group. BrdU-immunopositive cells were decreased in the two types of AC of the OA group but were significantly increased in a dose-dependent manner in the NEM group, excluding DS administration. Conversely, the immunoreactive cells in the SM were increased in the OA group compared with in the sham group, but the DS, NEM100, and NEM50 treatments inhibited this increase. Specifically, in the OA model, NEM100 exhibited more significant beneficial effects on anti-inflammation, anti-apoptosis, and chondrogenesis than DS.
4. Discussion
This study demonstrated that NEM exhibits dose-dependent anti-inflammatory and cytoprotective effects on extracellular matrix preservation in primary cultured rat articular chondrocytes, without cytotoxicity up to a concentration of 10 mg/mL in vitro. These findings provide direct evidence of NEM’s beneficial effects in mitigating chondrocyte damage induced by inflammatory stress under experimental conditions. Historically, surgical techniques have been utilized in animal models to induce OA, facilitating the preclinical evaluation of potential disease-modifying drugs. Such models, developed in rodents since the late 1970s, enable researchers to assess drug efficacy within specific joint tissues and determine associated side effects [32]. Surgical methods, including anterior cruciate ligament transection and partial medial meniscectomy in rats, effectively mimic post-traumatic OA, offering critical insights into disease progression and the potential of therapeutic interventions. These surgical models are instrumental in reproducing the conditions of post-traumatic OA, thereby enhancing the translational relevance of preclinical findings for therapeutic development [33]. Incorporating surgical techniques in OA models aids in the practical reproduction of post-traumatic OA conditions, enhancing the relevance of preclinical trials for potential therapeutic interventions [29,30]. In our study, NEM demonstrated a dose-dependent mitigation of surgically induced OA in Sprague–Dawley rats, suggesting its potential as an alternative therapy or functional food component for OA management. Comparing NEM’s effects to those of diclofenac sodium (DS), a well-established COX inhibitor and NSAID, further underscores NEM’s therapeutic promise. Ten animals per group, exhibiting consistent weight changes 13 days post-OA surgery, were selected for analysis. At the study’s conclusion, no significant differences were observed in body weight and weight gain among the test substances, including the highest NEM concentration tested (NEM100), indicating the absence of adverse effects at therapeutic doses.
The metabolism of arachidonic acid is pivotal in maintaining bodily homeostasis and orchestrating inflammatory responses. This metabolic pathway is responsible for the synthesis of prostaglandins, which serve as chemical mediators of inflammation, and leukotrienes, which modulate immune responses, including those linked to inflammation, asthma, and allergies [34]. The enzyme 5-LPO aids in leukotriene biosynthesis [35], while PGE2 is produced by the enzyme COX-2, in coordination with IL-1 and TNF-α. PGE2 synthesis is implicated in the etiopathogenesis of OA, contributing to AC degradation [36,37]. Additionally, PGE2 is implicated in abnormal bone growth and osteophyte formation, hallmark features of OA, leading to joint pain and discomfort [28,38]. These inflammatory processes induce edema in surrounding tissues, significantly increasing joint thickness—a symptom commonly observed in OA patients. Such insights underscore the complex interplay between arachidonic acid metabolism and OA development, highlighting potential therapeutic targets for mitigating disease progression and associated symptoms [28,31,38,39].
In some instances, NSAIDs may adversely affect cartilage health by hindering the synthesis of PGs, which are essential for maintaining cartilage integrity and function [19]. Considering this, it is important to discuss the implications of NEM treatment on either increasing or inhibiting PG synthesis. To better understand the impact of NEM on PG synthesis and cartilage health, future studies should investigate the long-term effects of NEM treatment on cartilage function and structure. Additionally, exploring the specific pathways through which NEM modulates PG synthesis will provide deeper insights into its mechanisms of action. MMPs are pivotal proteolytic enzymes in the degradation of extracellular matrix components, including proteoglycans, within chondrocytes and bone tissues [40]. Since MMPs act as enzymes that degrade the extracellular matrix, particularly proteoglycans (PGs) [41], they represent a significant target for OA treatment [9,31,42]. In this study, control rats with OA demonstrated significant increases in knee thickness, reductions in the thickness of femoral and tibial articular cartilage, and a marked rise in COX-2- and TNF-α-immunolabeled cells upon histopathological examination. Furthermore, these OA rats exhibited significant increases in COX-2- and TNF-α-immunopositive cells within the synovial membrane, alongside notable elevations in PGE2, 5-LPO, MMP-2, and MMP-9 levels in both femoral and tibial articular cartilage and the synovial membrane. Our findings reveal that the administration of DS and NEM effectively inhibited the activity of proinflammatory enzymes (PGE2, 5-LPO, TNF-α, and COX-2) and enzymes responsible for cartilage extracellular matrix degradation (MMP-2 and MMP-9), which were upregulated due to OA in the femoral and tibial cartilage and synovial membrane. Overall, NEM can prevent OA-induced cartilage damage by reducing MMPs that degrade proteoglycans, which are essential components of articular cartilage, and by reducing inflammation. Notably, the results indicate that NEM100 demonstrates more potent anti-inflammatory effects compared to DS, offering substantial evidence of its therapeutic potential in mitigating OA-induced inflammatory and degradative processes.
In OA, fibrosis arising from chronic inflammatory processes imposes limitations on joint motion, with joint stiffness being a prominent symptom. In assessing joint stiffness, we evaluated the maximum extension angle of the joint, considering 0° as the maximum extension. A lower value indicates better knee function [29,30,31,43]. In our study, the maximum extension angles significantly increased in OA-induced knees, strongly indicating the successful induction of OA through appropriate surgical procedures. Moreover, animals with OA demonstrated substantial hypertrophy and hyperplasia in the SM; both processes are associated with SM fibrosis and joint stiffness [31,44,45]. These findings were also evident in our OA rats. Overall, such findings provide strong evidence that, similar to the effects of DS, NEM has favorable antifibrotic and spasticity-relieving activities in OA.
As a part of the OA process, physical damage results in significant loss in the joint region, cartilage erosion, and the formation of osteophytes, which are easily detected through gross examination and X-ray imaging [46]. Hence, the reduction or inhibition of cartilage damage observed through gross and radiographic assessments provides a straightforward and valuable metric for evaluating the effectiveness of anti-OA medications [47]. BMD provides good predictable information regarding anti-OA drug efficacy in bone or bone-related diseases [48]. In addition, the focal BMDs of joint surfaces are useful in diagnosing OA progression [49,50]. Bone strength also provides good predictable information on the efficacy of drugs targeting bone or bone-related diseases [51,52]. Similarly, we considered that the compressive strength of the surface cartilage provides information on ECM composition and chondrocyte status on the joints. In our study, subcutaneous administration of DS or oral administration of NEM significantly mitigated surgically induced OA-related edematous changes in the knee joint, femur, and tibia, as well as erosive signs in the AC. Additionally, we noted a considerable suppression of related decreases in BMD and joint surface compression strength with these treatments.
The ECM of AC is primarily composed of collagen and glycosaminoglycans, including proteoglycans (PGs), aggrecan, and glycosaminoglycan (GAG) [8,53]. OA is characterized by the loss of AC components, notably PGs, leading to tissue degradation, hypocellularity, and eventual loss of joint function [12]. The inflammation-induced degradation of ECM components such as collagen, aggrecan, and the transcription factor SOX9—essential for chondrogenic differentiation and cartilage integrity—contributes to homeostasis disruption and functional limitations in OA [54]. SOX9, a transcription factor that plays a crucial role in chondrogenic differentiation, along with aggrecan and collagen, which are essential components of a healthy cartilage ECM [53], are crucial for maintaining cartilage integrity. Preventing ECM degradation represents a critical target for anti-OA treatments aimed at preserving the structural and functional integrity of AC [55,56]. In this context, our study demonstrates that NEM treatment notably improved the expression of Col-2, SOX9, and aggrecan, which were diminished in the OA model. Interestingly, while Col-2 expression was upregulated in the synovial membrane of control OA models, it was downregulated in the DS, NEM100, and NEM50 groups, potentially contributing to the mitigation of joint fibrosis. These results provide clear and direct evidence that NEM has beneficial effects on ECM formation, facilitated through its anti-inflammatory and chondrocyte-protective activities in OA. Furthermore, the observed increase in mRNA expression of Col-2, SOX9, and aggrecan in the femoral and tibial AC in the DS group suggests secondary changes attributable to potent anti-inflammatory and cell-protective activities, rather than direct proliferative effects on cells. The absence of significant increases in BrdU-immunolabeled cells in both femoral and tibial AC aligns with our in vivo observations, providing compelling evidence of NEM’s beneficial effects on OA through mechanisms beyond mere cell proliferation.
The Mankin scoring system, a widely recognized histopathological tool, evaluates AC injuries based on criteria including surface damage, chondrocyte density, clone formation, and safranin O staining intensity. Higher scores signify a more severe progression of OA [57]. Our findings indicate that OA notably increased Mankin scores in femoral and tibial AC, alongside a reduction in cartilage thickness. Remarkably, treatment with DS or NEM, particularly at the NEM100 dosage, effectively reversed these pathological changes. This reversal suggests the potential of DS and NEM, especially NEM100, as therapeutic agents in mitigating the histopathological manifestations of OA, underscoring their role in preserving cartilage integrity and offering a promising avenue for OA treatment strategies.
PARP is a marker of apoptosis, and excessively increased apoptosis induces damage to various organs, including the bone (OA) [58]. Meanwhile, among the methods used to evaluate cell proliferation in histological sections, BrdU staining is simple and enables specific observation of cell proliferation [59,60,61] This study demonstrated that NEM100 improves OA by protecting the cartilage and SM cells through the inhibition of apoptosis and promotion of cartilage proliferation.
In future studies, given its proven anti-inflammatory, cytoprotective, and chondrogenic properties, NEM could be evaluated for its efficacy in addressing other musculoskeletal disorders characterized by inflammation and tissue degeneration. Additionally, if NEM is utilized for biocompatible materials, it can provide new research materials for regenerative medicine and orthopedic applications. To exploit the potential of NEM as a versatile therapeutic agent, further studies focusing on elucidating the underlying mechanism of action and assessing long-term safety are essential. To assess the extent of the in vivo absorption of the active components of NEM, we conducted observations using various dosages, and the effectiveness was evident at specific dosages, helping to address doubts regarding absorption. Despite these findings, the study falls short of providing a comprehensive explanation of the absorption mechanism, marking a limitation. Specifically, a detailed understanding of the digestion and breakdown processes that NEM undergoes during absorption after oral administration remains elusive. For this reason, further research is needed to investigate the mechanism of digestion and absorption of NEM.
5. Conclusions
This study highlights the concentration-dependent anti-inflammatory and cytoprotective effects of NEM on primary cultured rat articular chondrocytes, demonstrating ECM-protective effects without inducing cytotoxicity.
In a surgically induced OA model, typical inflammatory-related OA signs were significantly inhibited by DS (2 mg/kg) and NEM (100, 50, and 25 mg/kg) over 56 days. Notably, the 100 mg/kg dose showed the most significant effect, while the 50 mg/kg dose demonstrated a comparable improvement to DS 2 mg/kg, highlighting it as the minimum effective dose.
Therefore, NEM may be a potent alternative agent or functional food ingredient for mitigating various OA signs, emphasizing the need for further exploration through preclinical and clinical trials.
Author Contributions
S.-K.K. and Y.-S.K. designed research; J.-I.K., J.-H.C., M.-S.S., J.-K.K. and Y.-S.C. performed research (in vitro and in vivo); J.-I.K., J.-H.C., S.-K.K. and Y.-S.K. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All laboratory animals were preapproved by the Institutional Animal Care and Use Committee at Daegu Haany University, Gyeongsan, Korea, prior to the initiation of the experiments, with the approval numbers DHU2021-068 and DHU2021-072 for the in vivo and in vitro studies, respectively.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
Authors Jong-Kyu Kim and Yoon-Seok Chun were employed by the company AriBnC, Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Dougados, M.; Nguyen, M.; Berdah, L.; Maziéres, B.; Vignon, E.; Lequesne, M.; ECHODIAH Investigators Study Group. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Arthritis Rheum. 2001, 44, 2539–2547. [Google Scholar] [CrossRef] [PubMed]
- Pavelká, K.; Gatterová, J.; Olejarová, M.; Machacek, S.; Giacovelli, G.; Rovati, L.C. Glucosamine sulfate use and delay of progression of knee osteoarthritis: A 3-year, randomized, placebo-controlled, double-blind study. Arch. Intern. Med. 2002, 162, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Fiorito, S.; Magrini, L.; Adrey, J.; Mailhe, D.; Brouty-Boye, D. Inflammatory status and cartilage regenerative potential of synovial fibroblasts from patients with osteoarthritis and chondropathy. Rheumatology 2005, 44, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Badley, E. The effect of osteoarthritis on disability and health care use in Canada. J. Rheumatol. Suppl. 1995, 43, 19–22. [Google Scholar] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Jotanovic, Z.; Mihelic, R.; Sestan, B.; Dembic, Z. Role of interleukin-1 inhibitors in osteoarthritis: An evidence-based review. Drugs Aging 2012, 29, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, Y.-S.; Liu, J.; Li, J.; Tan, Y.; Li, X.-J.; Magdalou, J.; Mei, Q.-B.; Wang, H.; Chen, L.-B. Effect of Angelica sinensis polysaccharides on osteoarthritis in vivo and in vitro: A possible mechanism to promote proteoglycans synthesis. Evid. Based Complement. Alternat. Med. 2013, 2013, 794761. [Google Scholar] [CrossRef] [PubMed]
- Na, J.Y.; Song, K.B.; Kim, S.H.; Kwon, Y.B.; Kim, D.G.; Lee, J.K.; Jo, H.K.; Kwon, J.K. Effects of HPL-04 on degenerative osteoarthritis. J. Korean Soc. Food Sci. Nutr. 2014, 43, 30–39. [Google Scholar] [CrossRef]
- Choi, B.R.; Ku, S.K.; Kang, S.J.; Park, H.R.; Sung, M.S.; Lee, Y.J.; Park, K.M. Anti-osteoarthritis effects of Pomegranate, Eucommiae cortex and Achyranthis radix extracts on the primary cultured rat articular chondrocytes. Soc. Prev. Korean Med. 2017, 21, 87–98. [Google Scholar] [CrossRef]
- Choi, B.R.; Ku, S.K.; Kang, S.J.; Park, H.R.; Sung, M.S.; Lee, Y.J.; Park, K.M. Concentration-dependent in vitro anti-osteoarthritis effects of mixed formula-pomegranate concentrate powder: Eucommiae Cortex: Achyranthis Radix 5: 4: 1 (g/g) on the primary cultured rat articular chondrocytes. J. Physiol. Pathol. Korean Med. 2019, 33, 131–140. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998, 41, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Loo, F.A.V.D.; Joosten, L.A.; Van Lent, P.L.; Arntz, O.J.; Van Den Berg, W.B. Role of interleukin-1, tumor necrosis factor α, and interleukin-6 in cartilage proteoglycan metabolism and destruction effect of in situ blocking in murine antigen-and zymosan-induced arthritis. Arthritis Rheum. 1995, 38, 164–172. [Google Scholar] [CrossRef]
- Berenbaum, F.; Jacques, C.; Thomas, G.; Corvol, M.T.; Béréziat, G.; Masliah, J. Synergistic effect of interleukin-1β and tumor necrosis factor α on PGE2 Production by articular chondrocytes does not involve PLA2 Stimulation. Exp. Cell Res. 1996, 222, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, P.; Macpherson, H.; Ralston, S. Nitric oxide production in cells derived from the human joint. Rheumatology 1996, 35, 207–212. [Google Scholar] [CrossRef]
- Stefanovic-Racic, M.; Morales, T.; Taskiran, D.; McIntyre, L.; Evans, C. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. J. Immunol. 1996, 156, 1213–1220. [Google Scholar] [CrossRef]
- Koolpe, M.; Pearson, D.; Benton, H.P. Expression of both P1 and P2 purine receptor genes by human articular chondrocytes and profile of ligand-mediated prostaglandin E2 release. Arthritis Rheum. 1999, 42, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.T.G.; McErlain, D.D.; Pitelka, V.; Schwartz, N.; Bernier, S.M.; Henry, J.L.; Holdsworth, D.W.; Beier, F. Forced mobilization accelerates pathogenesis: Characterization of a preclinical surgical model of osteoarthritis. Arthritis Res. Ther. 2007, 9, R13. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Ohmori, K. Rhein, an active metabolite of diacerein, suppresses the interleukin-1α-induced proteoglycan degradation in cultured rabbit articular chondrocytes. Jpn. J. Pharmacol. 2001, 85, 101–104. [Google Scholar] [CrossRef]
- Furukawa, K.; Kono, M.; Kataoka, T.; Hasebe, Y.; Jia, H.; Kato, H. Effects of eggshell membrane on keratinocyte differentiation and skin aging in vitro and in vivo. Nutrients 2021, 13, 2144. [Google Scholar] [CrossRef]
- Ruff, K.J.; DeVore, D.P.; Leu, M.D.; Robinson, M.A. Eggshell membrane: A possible new natural therapeutic for joint and connective tissue disorders. Results from two open-label human clinical studies. Clin. Interv. Aging 2009, 4, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hanate, M.; Aw, W.; Itoh, H.; Saito, K.; Kobayashi, S.; Hachimura, S.; Fukuda, S.; Tomita, M.; Hasebe, Y. Eggshell membrane powder ameliorates intestinal inflammation by facilitating the restitution of epithelial injury and alleviating microbial dysbiosis. Sci. Rep. 2017, 7, 43993. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.S.; Jia, H.; Sekine, A.; Lyu, W.; Furukawa, K.; Saito, K.; Hasebe, Y.; Kato, H. Eggshell membrane powder lowers plasma triglyceride and liver total cholesterol by modulating gut microbiota and accelerating lipid metabolism in high-fat diet-fed mice. Food Sci. Nutr. 2020, 8, 2512–2523. [Google Scholar] [CrossRef] [PubMed]
- Kiers, J.L.; Bult, J.H.F. Mildly processed natural eggshell membrane alleviates joint pain associated with osteoarthritis of the knee: A randomized double-blind placebo-controlled study. J. Med. Food 2021, 24, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Enomoto, M.; Buono, A.; Steiner, J.; Lascelles, B. Placebo-controlled pilot study of the effects of an eggshell membrane-based supplement on mobility and serum biomarkers in dogs with osteoarthritis. Vet. J. 2019, 253, 105379. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pachaiappan, R.; Tamboli, E.; Acharya, A.; Su, C.H.; Gopinath, S.C.B.; Chen, Y.; Velusamy, P. Separation and identification of bioactive peptides from stem of Tinospora cordifolia (Willd.) Miers. PLoS ONE 2018, 13, e0193717. [Google Scholar] [CrossRef]
- Nam, D.E.; Kim, O.K.; Shim, T.J.; Kim, J.H.; Lee, J. Effect of Boswellia Serrata Extracts on Degenerative Osteoarthritis in vitro and in vivo model. J. Korean Soc. Food Sci. Nutr. 2014, 43, 347. [Google Scholar] [CrossRef]
- Moon, C.H.; Kwon, O.; Woo, C.H.; Ahn, H.D.; Kwon, Y.S.; Park, S.J.; Song, C.H.; Ku, S.K. Therapeutic effect of irradiation of magnetic infrared laser on osteoarthritis rat model. Photochem. Photobiol. 2014, 90, 1150–1159. [Google Scholar] [CrossRef]
- Choi, J.S.; Shin, H.S.; Kim, K.Y.; Ku, S.K.; Choi, I.S.; Kim, J.W. Effect of Polycalcium, a mixture of Polycan and calcium lactate-gluconate in a 1: 9 weight ratio, on rats with surgery-induced osteoarthritis. Exp. Ther. Med. 2015, 9, 1780–1790. [Google Scholar] [CrossRef]
- Kim, C.G.; Lee, D.G.; Oh, J.; Lee, Y.H.; Lee, Y.J.; Song, P.H.; Song, C.H.; Ku, S.K. Effects of balneotherapy in Jeju magma-seawater on knee Osteoarthritis model. Sci. Rep. 2020, 10, 6620. [Google Scholar] [CrossRef]
- Goldberg, V.; Buckwalter, J. Hyaluronans in the treatment of osteoarthritis of the knee: Evidence for disease-modifying activity. Osteoarthr. Cartil. 2005, 13, 216–224. [Google Scholar] [CrossRef]
- Wei, L.; Hjerpe, A.; Brismar, B.; Svensson, O. Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthr. Cartil. 2001, 9, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Myers, C.E. Arachidonic acid stimulates prostate cancer cell growth: Critical role of 5-lipoxygenase. Biochem. Biophys. Res. Commun. 1997, 235, 418–423. [Google Scholar] [CrossRef]
- Sailer, E.R.; Schweizer, S.; Boden, S.E.; Ammon, H.P.; Safayhi, H. Characterization of an acetyl-11-keto-β-boswellic acid and arachidonate-binding regulatory site of 5-lipoxygenase using photoaffinity labeling. Eur. J. Biochem. 1998, 256, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.; Bakhle, Y.; Botting, R. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Bensen, W.G.; Fiechtner, J.J.; McMillen, J.I.; Zhao, W.W.; Yu, S.S.; Woods, E.M.; Hubbard, R.C.; Isakson, P.C.; Verburg, K.M.; Geis, G.S. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: A randomized controlled trial. Mayo Clin. Proc. 1999, 74, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.M.; Seibert, K.; Manning, P.T.; Currie, M.G.; Woerner, B.M.; Edwards, D.; Koki, A.; Tripp, C.S. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002, 46, 1789–1803. [Google Scholar] [CrossRef]
- Guo, J.S.; Ou, L.; Zhou, J.; Wang, X.J.; Guo, X. Impact on the model of rat osteoarthritis of jingu tablet. China J. Chin. Mater. Medica 2006, 31, 232–235. [Google Scholar]
- Bresnihan, B. Pathogenesis of joint damage in rheumatoid arthritis. J. Rheumatol. 1999, 26, 717–719. [Google Scholar]
- Nagase, H.; Woessner, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oral inflammation and reactive species: A missed opportunity? Oral Dis. 2000, 6, 136–137. [Google Scholar] [CrossRef] [PubMed]
- Rezende, M.U.D.; Gurgel, H.M.D.C.; Vilaça Junior, P.R.; Kuroba, R.K.; Lopes, A.S.S.; Phillipi, R.Z.; Hernandez, A.J. Diacerhein versus glucosamine in a rat model of osteoarthritis. Clinics 2006, 61, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; Guede, D.; López-Peña, M.; Muñoz, F.; Caeiro, J.-R.; González-Cantalapiedra, A. Effects of diacerein on cartilage and subchondral bone in early stages of osteoarthritis in a rabbit model. BMC Vet. Res. 2015, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; Guede, D.; López-Peña, M.; Muñoz, F.; Caeiro, J.-R.; González-Cantalapiedra, A. Comparison of various SYSADOA for the osteoarthritis treatment: An experimental study in rabbits. BMC Musculoskelet. Disord. 2015, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Sakano, Y.; Terada, N.; Ueda, H.; Fujii, Y.; Hamada, Y.; Akamatsu, N.; Ohno, S. Histological study of articular cartilage in experimental rat knee arthritis induced by intracapsular injection of cationic polyethyleneimine. Med. Electron Microsc. 2000, 33, 246–257. [Google Scholar] [CrossRef]
- Jiang, D.; Zou, J.; Huang, L.; Shi, Q.; Zhu, X.; Wang, G.; Yang, H. Efficacy of intra-articular injection of celecoxib in a rabbit model of osteoarthritis. Int. J. Mol. Sci. 2010, 11, 4106–4113. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.; Khan, A. Bone densitometry: Applications and limitations. J. Obstet. Gynaecol. Can. 2002, 24, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Karystinou, A.; Roelofs, A.J.; Neve, A.; Cantatore, F.P.; Wackerhage, H.; De Bari, C. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res. Ther. 2015, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Tsouknidas, A.; Anagnostidis, K.; Panagiotidou, S.; Michailidis, N. The effect of osteoarthritis on the regional anatomical variation of subchondral trabecular bone in the femoral head. Clin. Biomech. 2015, 30, 418–423. [Google Scholar] [CrossRef]
- Horcajada-Molteni, M.N.; Crespy, V.; Coxam, V.; Davicco, M.J.; Rémésy, C.; Barlet, J.P. Rutin inhibits ovariectomy-induced osteopenia in rats. J. Bone Miner. Res. 2000, 15, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Bilston, L.; Little, D.; Smith, N.; Williams, P.; Briody, J. Zoledronic acid improves the mechanical properties of normal and healing bone. Clin. Biomech. 2002, 17, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.; Gordon, C.; Vaughan-Thomas, A.; Rhodes, N.; Clegg, P. Evaluation of cartilage, synovium and adipose tissue as cellular sources for osteochondral repair. Vet. J. 2013, 197, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Graeve, T.; Twardziok, S.; Schmidt, M.F. Evidence for regulated interleukin-4 expression in chondrocyte-scaffolds under in vitro inflammatory conditions. PLoS ONE 2011, 6, e25749. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.J.; Spina, J.; Bobe, G.; Duesterdieck-Zellmer, K.F.; Ott, J.; Baltzer, W.I.; Bay, B.K. Culture of canine synoviocytes on porcine intestinal submucosa scaffolds as a strategy for meniscal tissue engineering for treatment of meniscal injury in dogs. Vet. J. 2014, 199, 49–56. [Google Scholar] [CrossRef]
- Armstrong, S.; Read, R.; Ghosh, P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis. J. Rheumatol. 1994, 21, 680–688. [Google Scholar] [PubMed]
- Cole, K.K.; Perez-Polo, J.R. Poly (ADP-ribose) polymerase inhibition prevents both apoptotic-like delayed neuronal death and necrosis after H2O2 injury. J. Neurochem. 2002, 82, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Ganey, T.; Libera, J.; Moos, V.; Alasevic, O.; Fritsch, K.G.; Meisel, H.J.; Hutton, W.C. Disc chondrocyte transplantation in a canine model: A treatment for degenerated or damaged intervertebral disc. Spine 2003, 28, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Bendele, A.; Thompson, D.; Littau, A.; Waggie, K.; Reardon, B.; Ellsworth, J. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthr. Cartil. 2005, 13, 623–631. [Google Scholar] [CrossRef]
- Hwang, Y.I.; Yoo, Y.B.; Baik, S.H. Comparative study of rat thyroid regeneration using PCNA and BrdU immunohistochemistry. Korean J. Anat. 2000, 33, 247–254. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).