Pathway to Carbon Neutrality in the Cement Industry: CO2 Uptake by Recycled Aggregates from Construction and Demolition Waste
Abstract
:1. Introduction
2. Research Significance
2.1. Environmental Parameters Affecting Carbonation Reaction
2.2. Effect of Carbonation on RCA Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
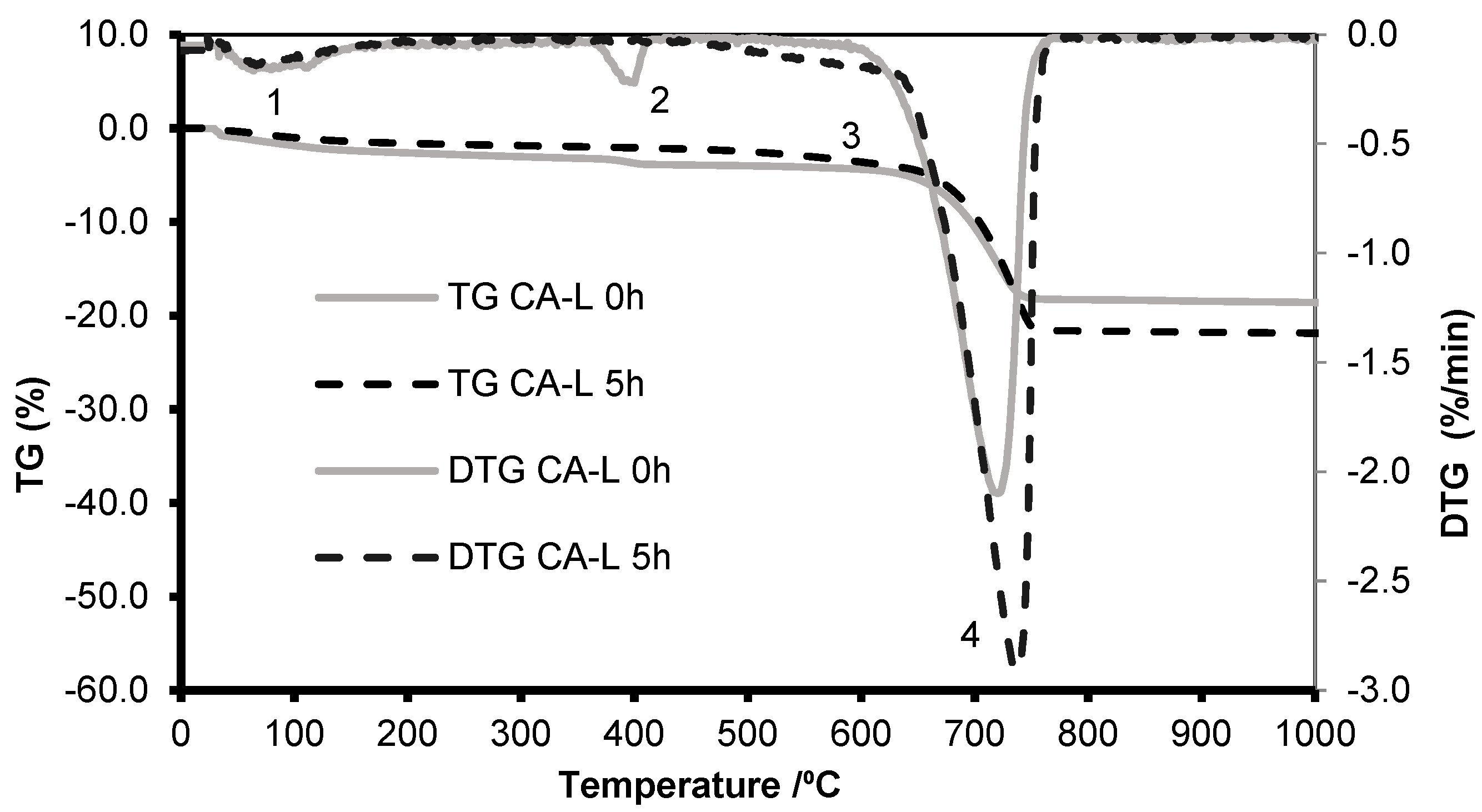
3.2.1. Thermogravimetric Analysis
- Z1, [20–400 °C]: temperature range that corresponds to release of moisture and dehydration of hydrated calcium silicate (C-S-H) and calcium aluminate hydrates (AFt and AFm phases);
- Z2, [400–500 °C]: temperature range that corresponds essentially to the dihydroxylation of portlandite (CH); allows to estimate the content of free portlandite, that can be used to estimate the carbonation potential of a cementitious material;
- Z3, [500–900 °C]: temperature range that corresponds to carbonates decarbonation, allowing to estimate CaCO3 content.
3.2.2. X-ray Diffraction Analysis
3.2.3. pH Evaluation
4. CO2 Capture of RAs
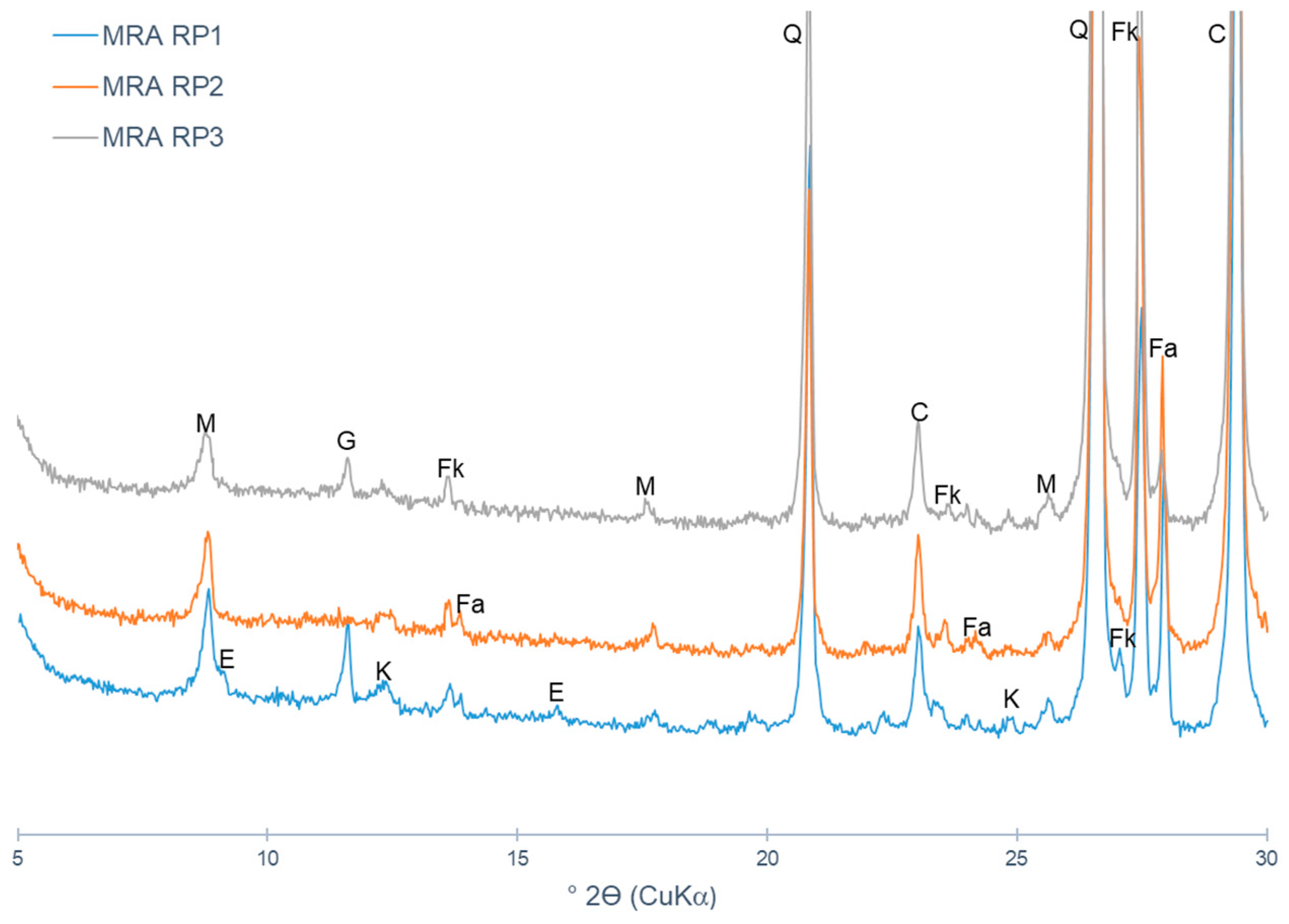
4.1. Mixed Recycled Aggregates
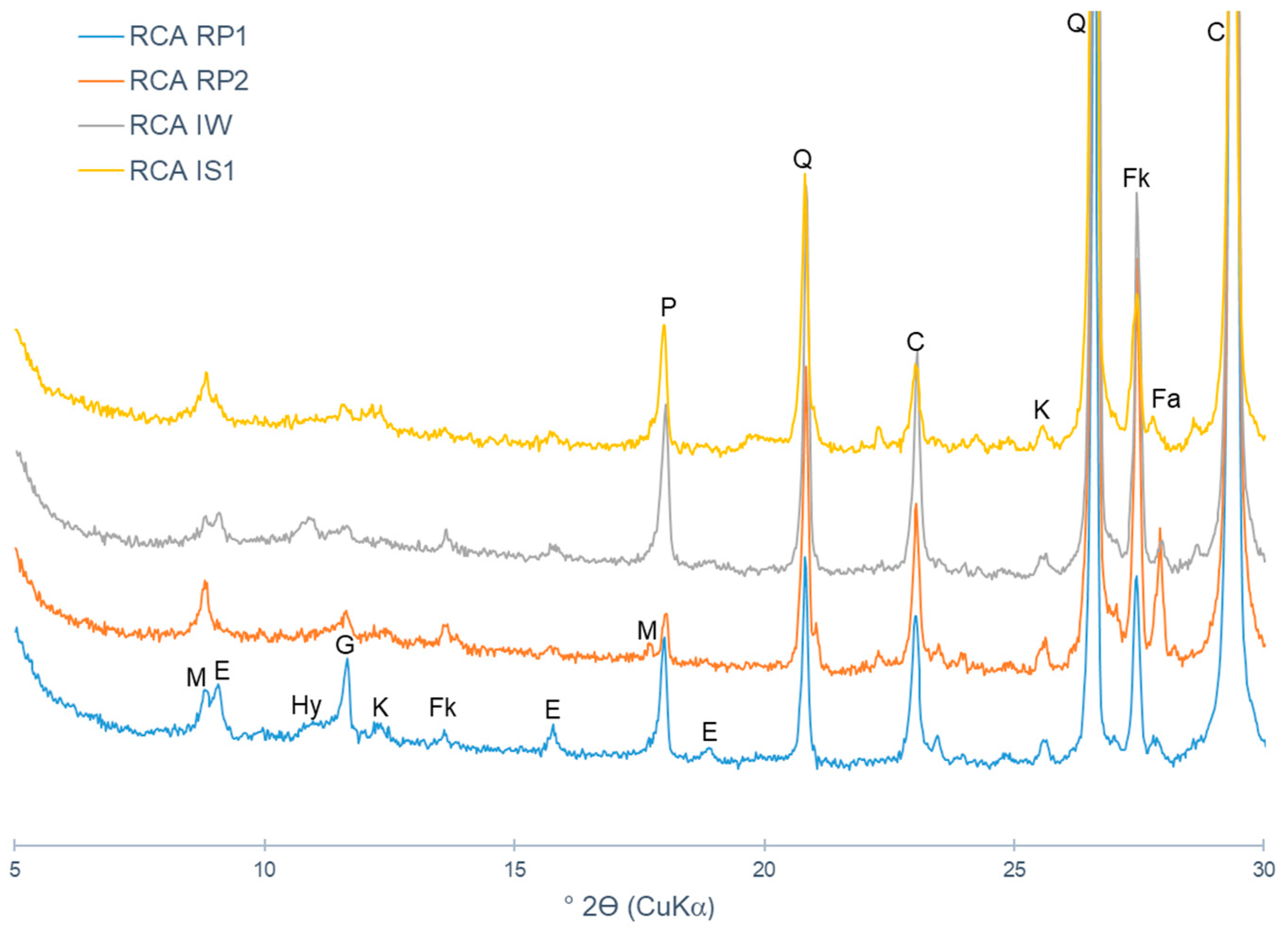
4.2. Recycled Concrete Aggregates from Recycling Plants
4.3. Concrete Aggregate from Lab Specimens
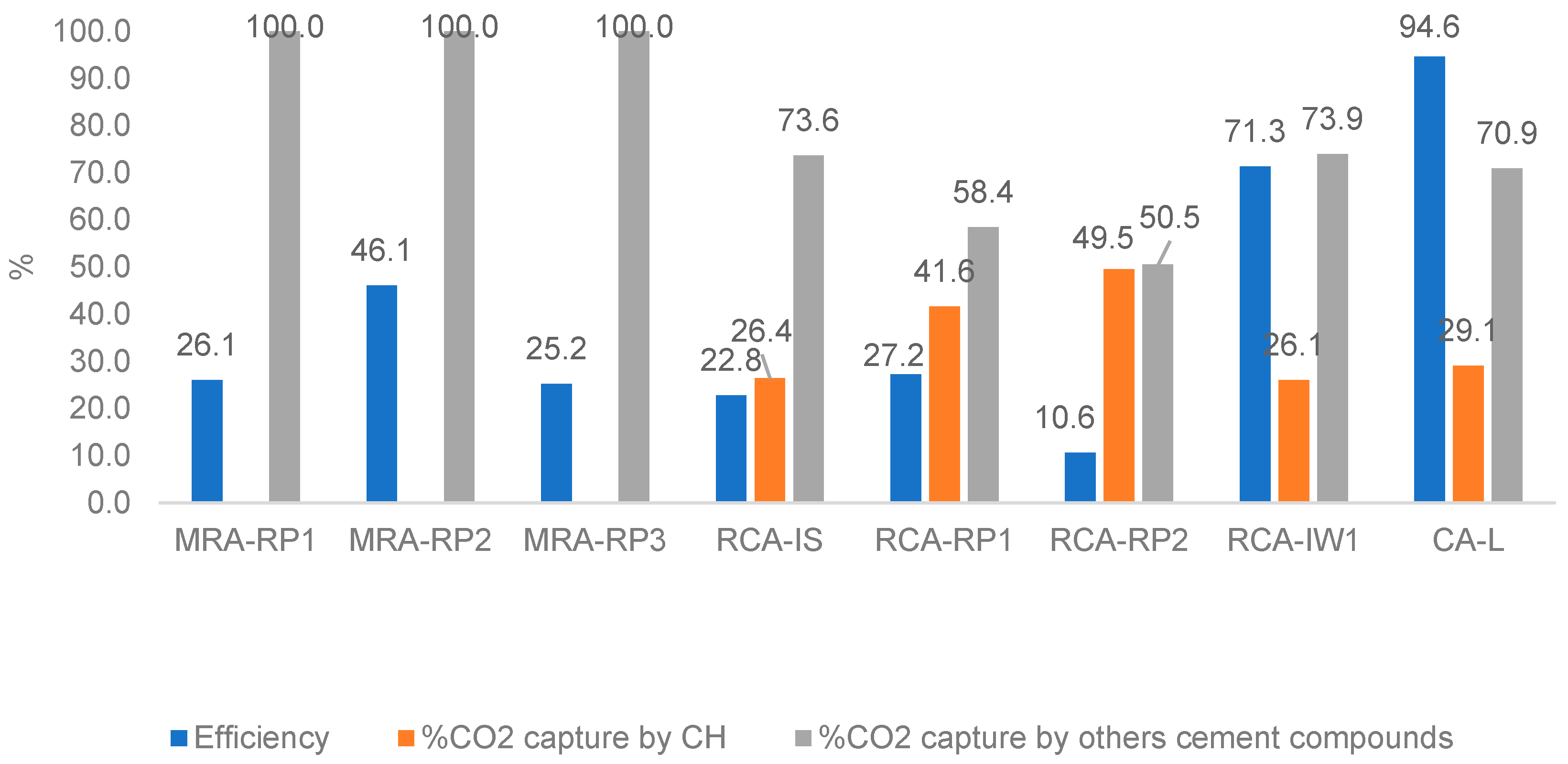
4.4. CO2 Uptake Efficiency of RAs and Calculation of Contribution of Cementitious Compounds for CO2 Capture
5. Acid Attack of RAs during Forced Carbonation
6. Conclusions
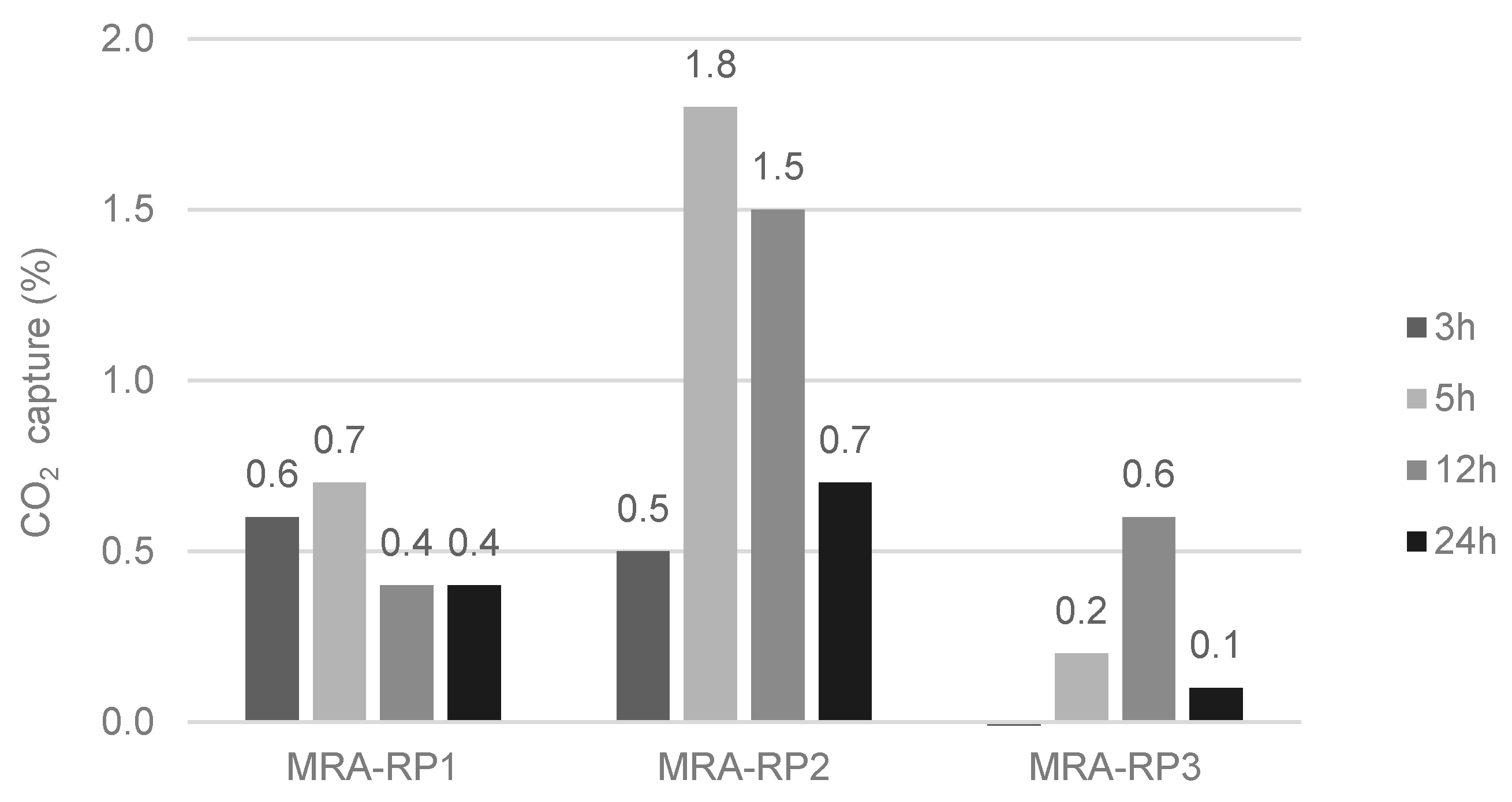
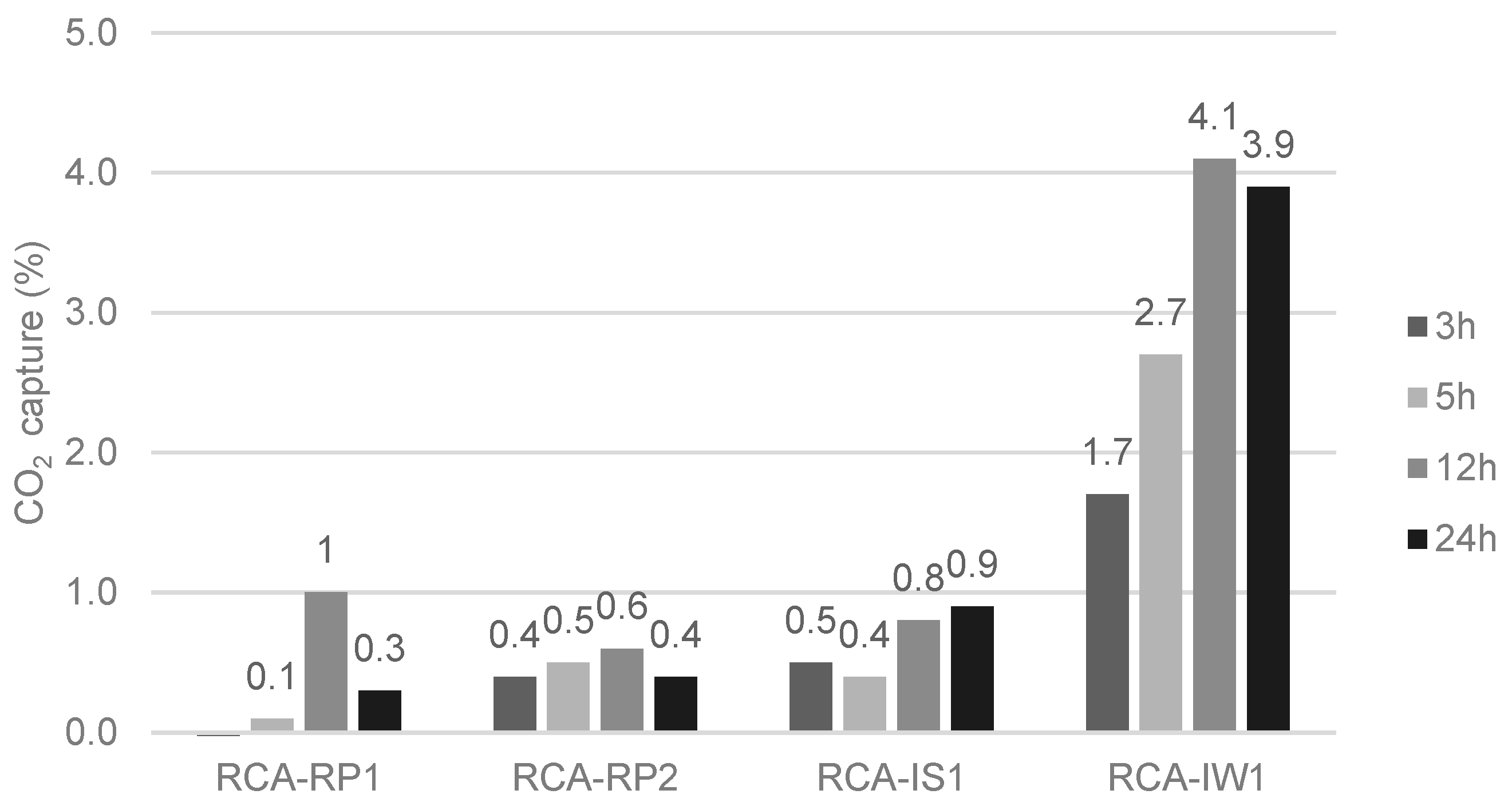
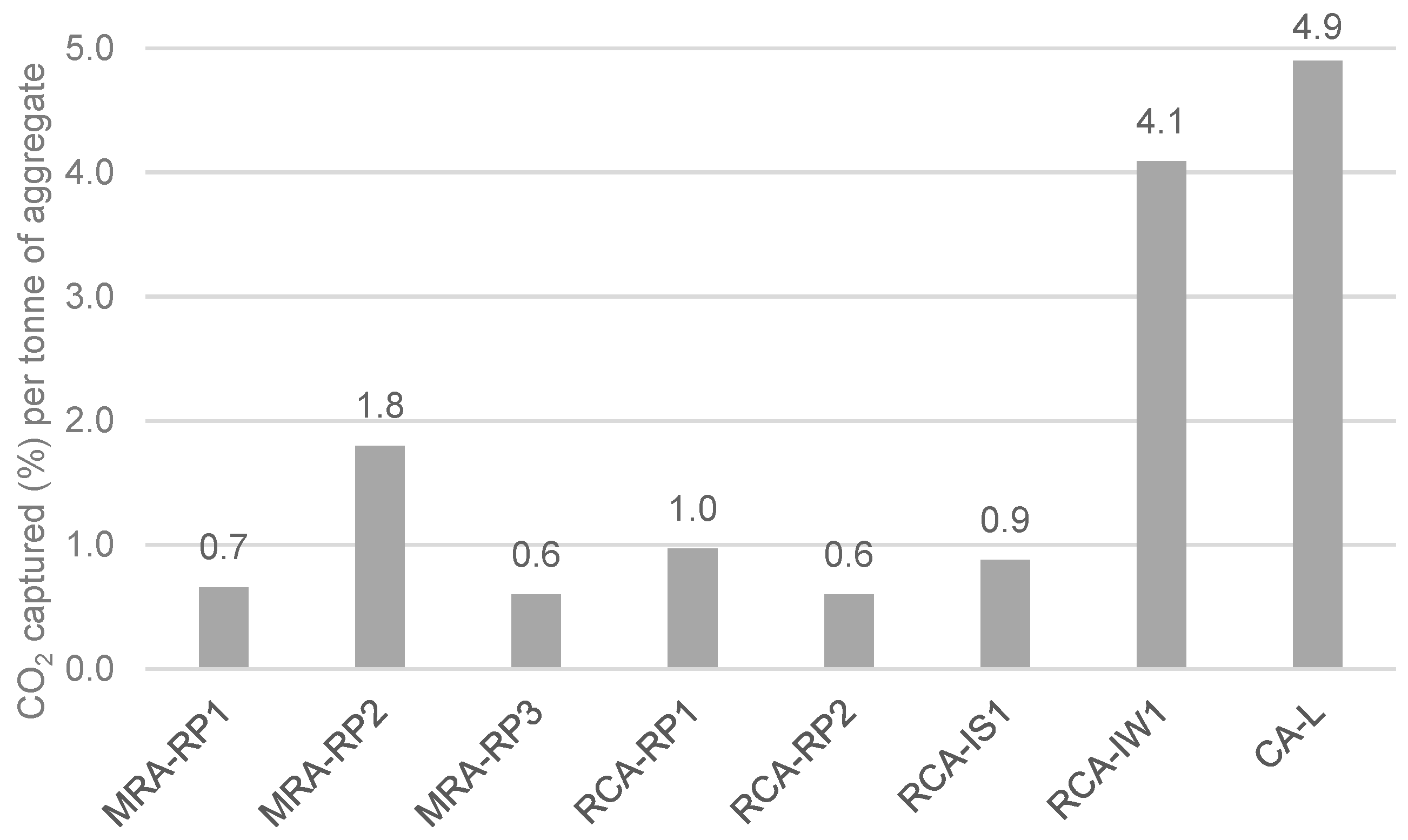
- CO2 capture depends on the type of CDW (mixed or mainly concrete). Indeed, the maximum CO2 uptake is related to the cementitious materials content and to the degree of natural carbonation. In short, CDW captured from 5 wt.% to 35 wt.% of CO2 per tonne of cement paste, which corresponds to 0.6% to 4.1% per tonne of aggregate.
- The lowest-captured CO2 value was 0.6% for MRA-RP3 and RCA-RP2 (corresponding to 6.0 kg per tonne of aggregate) and the highest was obtained in CA-L concrete produced in the laboratory with 4.9% (49 kg per tonne of aggregate). Considering that the MRAs did not present portlandite content, it was verified that the carbonation occurred by the reaction of CO2 and C-S-H.
- Taking into account that only 13% to 16% of Rc fraction is composed of cement paste, forced carbonation allowed the aggregates to absorb between 20% and 27% of cement process emissions (the ones that refer to decarbonisation of calcium carbonate).
- Forced carbonation does not continuously increase or stabilize over time. It reaches a maximum value of captured CO2 and then it goes down. After a given time, an acid attack occurs that reduces the calcium carbonate in the sample. The tested MRAs reached the maximum capture value after 5 h and the RCAs after 12 h.
- It was also verified that long periods of exposure to CO2 do not contribute to the increase in CO2 capture by RAs. An acid attack occurs at longer carbonation periods. This attack promotes the dissolution of CaCO3, decreasing the potential for CO2 capture for long carbonation periods.
- It was concluded that RAs, when submitted to forced carbonation, capture from 52 to 491 kg of CO2 per tonne of cement paste. Therefore, the forced carbonation of RAs, independently of the building life cycle (time and conditions of environmental exposure) and demolition carbonation phases, still has the potential to capture CO2.
- The forced carbonation of recycled aggregates has a great influence on their characteristics. The characterization of the aggregates before and after their carbonation made it possible to analyse the modification in their physical properties, namely water absorption and particle density. During the carbonation, the precipitation of calcium carbonate inside the pores causes an increment in the density of the aggregate and decreases the water absorption. The physical properties are related to the carbonation potential of the aggregate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holban, E.; Deák, G.Y.; Daescu, V.; Diacu, E.; Daescu, A.I.; Tanase, G.S.; Marinescu, P.; Sirbu, C.; Paceagiu, J. Ways to reduce CO2 emissions and energetic consumption at clinker producing under Romanian specific conditions. J. Environ. Prot. Ecol. 2015, 16, 479–488. [Google Scholar]
- Nganga, G.; Alexander, M.; Beushausen, H. The design of concrete mixes with reduced clinker content. In Proceedings of the 34th Cement and Concrete Science Conference, Sheffield, UK, 14–16 September 2014. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. Review-calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Gomes, R.I.; Bastos, D.; Farinha, C.B.; Pederneiras, C.M.; Veiga, R.; de Brito, J.; Faria, P.; Silva, A.S. Mortars with cdw recycled aggregates submitted to high levels of CO2. Infrastructures 2021, 6, 159. [Google Scholar] [CrossRef]
- Zhang, N.; Duan, H.; Miller, T.R.; Tam, V.W.Y.; Liu, G.; Zuo, J. Mitigation of carbon dioxide by accelerated sequestration in concrete debris. Renew. Sustain. Energy Rev. 2020, 117, 109495. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Shao, Y.; Ghoshal, S. Mathematical modeling of CO2 uptake by concrete during accelerated carbonation curing. Cem. Concr. Res. 2015, 67, 1–10. [Google Scholar] [CrossRef]
- Peter, M.A.; Muntean, A.; Meier, S.A.; Böhm, M. Competition of several carbonation reactions in concrete: A parametric study. Cem. Concr. Res. 2008, 38, 1385–1393. [Google Scholar] [CrossRef]
- Thiery, M.; Villain, G.; Dangla, P.; Platret, G. Investigation of the carbonation front shape on cementitious materials: Effects of the chemical kinetics. Cem. Concr. Res. 2007, 37, 1047–1058. [Google Scholar] [CrossRef]
- Vogler, N.; Drabetzki, P.; Lindemann, M.; Kühne, H.C. Description of the concrete carbonation process with adjusted depth-resolved thermogravimetric analysis. J. Therm. Anal. Calorim. 2022, 147, 6167–6180. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Rius, J.; Miravitlles, C. Supercritical carbonation of calcium aluminate cement. Cem. Concr. Res. 2008, 38, 1033–1037. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jensen, O.E.; Cliffe, K.A.; Maroto-Valer, M.M. A model of carbon dioxide dissolution and mineral carbonation kinetics. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 1265–1290. [Google Scholar] [CrossRef]
- Fernández Bertos, M.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar] [CrossRef]
- Alapati, P.; Kurtis, K.E. Carbonation in Alternative Cementitious Materials: Implications on Durability and Mechanical Properties. In Proceedings of the International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018; Volume 1. [Google Scholar]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Luan, C.; Zhang, G.; Fu, L.; El-Fatah Abomohra, A. Accelerated carbonation technology for enhanced treatment of recycled concrete aggregates: A state-of-the-art review. Constr. Build. Mater. 2021, 282, 122671. [Google Scholar] [CrossRef]
- Gholizadeh-Vayghan, A.; Bellinkx, A.; Snellings, R.; Vandoren, B.; Quaghebeur, M. The effects of carbonation conditions on the physical and microstructural properties of recycled concrete coarse aggregates. Constr. Build. Mater. 2020, 257, 119486. [Google Scholar] [CrossRef]
- Elsalamawy, M.; Mohamed, A.R.; Kamal, E.M. The role of relative humidity and cement type on carbonation resistance of concrete. Alexandria Eng. J. 2019, 58, 1257–1264. [Google Scholar] [CrossRef]
- Silva, R.V.; Neves, R.; De Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z. Effects of temperature, relative humidity and CO2 concentration on concrete carbonation. Mag. Concr. Res. 2020, 72, 936–947. [Google Scholar] [CrossRef]
- Shih, S.M.; Ho, C.S.; Song, Y.S.; Lin, J.P. Kinetics of the reaction of Ca(OH)2 with CO2 at low temperature. Ind. Eng. Chem. Res. 1999, 38, 1316–1322. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. Constr. 2017, 50, 30. [Google Scholar] [CrossRef]
- Wang, D.; Noguchi, T.; Nozaki, T. Increasing efficiency of carbon dioxide sequestration through high temperature carbonation of cement-based materials. J. Clean. Prod. 2019, 238, 117980. [Google Scholar] [CrossRef]
- Fang, X.; Xuan, D.; Poon, C.S. Empirical modelling of CO2 uptake by recycled concrete aggregates under accelerated carbonation conditions. Mater. Struct. Constr. 2017, 50, 200. [Google Scholar] [CrossRef]
- Humbert, P.S.; Castro-Gomes, J.P.; Savastano, H. Clinker-free CO2 cured steel slag based binder: Optimal conditions and potential applications. Constr. Build. Mater. 2019, 210, 413–421. [Google Scholar] [CrossRef]
- Hyvert, N.; Sellier, A.; Duprat, F.; Rougeau, P.; Francisco, P. Dependency of C-S-H carbonation rate on CO2 pressure to explain transition from accelerated tests to natural carbonation. Cem. Concr. Res. 2010, 40, 1582–1589. [Google Scholar] [CrossRef]
- Ye, X.; Chen, T.; Chen, J. Carbonation of cement paste under different pressures. Constr. Build. Mater. 2023, 370, 130511. [Google Scholar] [CrossRef]
- Lyubomirskiy, N.; Bahtin, A.; Lyubomirskiy, N.; Bahtin, A. Temperature Influence on Forced Carbonation Speed of Half-Dry Pressure Lime Stone. Motrol. Motoryz. Energetyka Rol. 2015, 17, 73–80. [Google Scholar]
- Liska, M.; Al-Tabbaa, A. Performance of magnesia cements in pressed masonry units with natural aggregates: Production parameters optimisation. Constr. Build. Mater. 2008, 22, 1789–1797. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Al-Tabbaa, A. Accelerated carbonation of reactive MgO cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Cui, H.; Tang, W.; Liu, W.; Dong, Z.; Xing, F. Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms. Constr. Build. Mater. 2015, 93, 522–527. [Google Scholar] [CrossRef]
- Castellote, M.; Fernandez, L.; Andrade, C.; Alonso, C. Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations. Mater. Struct. Constr. 2009, 42, 515–525. [Google Scholar] [CrossRef]
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Yu, J.; Yamaguchi, A. Effect of porosity on carbonation and hydration resistance of CaO materials. J. Eur. Ceram. Soc. 2007, 27, 1953–1959. [Google Scholar] [CrossRef]
- Zhao, Z.; Remond, S.; Damidot, D.; Courard, L.; Michel, F. Improving the properties of recycled concrete aggregates by accelerated carbonation. Proc. Inst. Civ. Eng. Constr. Mater. 2018, 171, 126–132. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27, 04015029. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.S.; Xie, Z. Influence of carbonated recycled concrete aggregate on properties of cement mortar. Constr. Build. Mater. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Nakarai, K.; Yoshida, T. Effect of carbonation on strength development of cement-treated Toyoura silica sand. Soils Found. 2015, 55, 857–865. [Google Scholar] [CrossRef]
- Tracz, T.; Zdeb, T. Effect of Hydration and Carbonation Progress on the Porosity and Permeability of Cement Pastes. Materials 2019, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Ikumapayi, C.M. Effects of Carbonation on the Properties of Concrete. Sci. Rev. 2019, 5, 205–214. [Google Scholar]
- Xuan, D.; Zhan, B.; Poon, C.S. Assessment of mechanical properties of concrete incorporating carbonated recycled concrete aggregates. Cem. Concr. Compos. 2016, 65, 67–74. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y.; Zhang, J.; Li, W.; Chong, L.; Xie, Z. Performance enhancement of recycled concrete aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- De Brito, J.; Agrela, F.; Silva, R.V. Construction and Demolition Waste; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081024805. [Google Scholar]
- Rao, A.; Jha, K.N.; Misra, S. Use of aggregates from recycled construction and demolition waste in concrete. Resour. Conserv. Recycl. 2007, 50, 71–81. [Google Scholar] [CrossRef]
- Walton, J.C.; Bin-Shafique, S.; Smith, R.W.; Gutierrez, N.; Tarquin, A. Role of carbonation in transient leaching of cementitious wasteforms. Environ. Sci. Technol. 1997, 31, 2345–2349. [Google Scholar] [CrossRef]
- Lange, L.C.; Hills, C.D.; Poole, A.B. The effect of accelerated carbonation on the properties of cement- solidified waste forms. Waste Manag. 1996, 16, 757–763. [Google Scholar] [CrossRef]
- Tam, V.W.; Butera, A.; Le, K.N.; Li, W. CO2 concrete and its practical value utilising living lab methodologies. Clean. Eng. Technol. 2021, 3, 100131. [Google Scholar] [CrossRef]
- Le, H.B.; Bui, Q.B. Recycled aggregate concretes—A state-of-the-art from the microstructure to the structural performance. Constr. Build. Mater. 2020, 257, 119522. [Google Scholar] [CrossRef]
- Li, Y.; Fu, T.; Wang, R.; Li, Y. An assessment of microcracks in the interfacial transition zone of recycled concrete aggregates cured by CO2. Constr. Build. Mater. 2020, 236, 117543. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wang, R.; Zhao, Y.; Men, C. Effects of carbonation treatment on the crushing characteristics of recycled coarse aggregates. Constr. Build. Mater. 2019, 201, 408–420. [Google Scholar] [CrossRef]
- Russo, N.; Lollini, F. Effect of carbonated recycled coarse aggregates on the mechanical and durability properties of concrete. J. Build. Eng. 2022, 51, 104290. [Google Scholar] [CrossRef]
- Gomes, H.C.; Reis, E.D.; Azevedo, R.C.d.; Rodrigues, C.d.S.; Poggiali, F.S.J. Carbonation of Aggregates from Construction and Demolition Waste Applied to Concrete: A Review. Buildings 2023, 13, 1097. [Google Scholar] [CrossRef]
- EN 197-1; Cement Composition, Specifications and Conformity Criteria for Common Cements. British Standards Institution: London, UK, 2011.
- EN 933-11; Tests for Geometrical Properties of Aggregates. Part 11: Classification Test for the Constituents of Coarse Recycled Aggregate. British Standards Institution: London, UK, 2009.
- EN 13242; Aggregates for Unbound and Hydraulically Bound Materials for Use in Civil Engineering Work and Road Construction. British Standards Institution: London, UK, 2007.
- EN 1015-1; Methods of Test for Mortar for Masonry—Part 1: Determination of Particle Size Distribution (by Sieve Analysis). British Standards Institution: London, UK, 1998.
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- LNEC E 403; Análise Mineralógica por Difratometria de Raios X. LNEC: Lisbon, Portugal, 1993.
- Vaitkus, A.; Merkys, A.; Grazulis, S. Validation of the Crystallography Open Database using the Crystallographic Information Framework. J. Appl. Crystallogr. 2021, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Gražulis, S.; Girdzijauskaitė, S.; Merkys, A.; Vaitkus, A. Using SMILES strings for the description of chemical connectivity in the Crystallography Open Database. J. Cheminform. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Merkys, A.; Vaitkus, A.; Butkus, J.; Okulič-Kazarinas, M.; Kairys, V.; Gražulis, S. COD::CIF::Parser: An error-correcting CIF parser for the Perl language. J. Appl. Crystallogr. 2016, 49, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Merkys, A.; Vaitkus, A.; Okulič-Kazarinas, M. Computing stoichiometric molecular composition from crystal structures. J. Appl. Crystallogr. 2015, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, 420–427. [Google Scholar] [CrossRef]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.; Shi, W. Random crowd-induced vibration in footbridge and adaptive control using semi-active TMD including crowd-structure interaction. Eng. Struct. 2024, 306, 117839. [Google Scholar] [CrossRef]
- Fang, X.; Zhan, B.; Poon, C.S. Enhancement of recycled aggregates and concrete by combined treatment of spraying Ca2+ rich wastewater and flow-through carbonation. Constr. Build. Mater. 2021, 277, 122202. [Google Scholar] [CrossRef]
- Saiz-Martínez, P.; Ferrández-Vega, D.; Morón-Fernández, C.; Payán De Tejada-Alonso, A. Comparative study of the influence of three types of fibre in the shrinkage of recycled mortar. Mater. Constr. 2018, 68, e168. [Google Scholar] [CrossRef]
- Garach, L.; López, M.; Agrela, F.; Ordóñez, J.; Alegre, J.; Moya, J.A. Improvement of bearing capacity in recycled aggregates suitable for use as unbound road sub-base. Materials 2015, 8, 8804–8816. [Google Scholar] [CrossRef] [PubMed]
- Bisciotti, A.; Jiang, D.; Song, Y.; Cruciani, G. Estimating attached mortar paste on the surface of recycled aggregates based on deep learning and mineralogical models. Clean. Mater. 2024, 11, 100215. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kuroda, Y. Carbon dioxide uptake in demolished and crushed concrete. J. Adv. Concr. Technol. 2011, 9, 115–124. [Google Scholar] [CrossRef]
- Sereng, M.; Djerbi, A.; Metalssi, O.O.; Dangla, P.; Torrenti, J.M. Improvement of recycled aggregates properties by means of CO2 uptake. Appl. Sci. 2021, 11, 6571. [Google Scholar] [CrossRef]
- Mahdi, Z.H.; Abbas, W.A.; Abdulhussein, F.K.; Abdulghani, M.R.; Hasan, M.Q. Compressive strength and shrinkage of concrete containing calcium oxide (CaO) powder. IOP Conf. Ser. Mater. Sci. Eng. 2020, 881, 012165. [Google Scholar] [CrossRef]
- Shahzadi, P.; Shahnaz, A.; Khan, M.S.; Rana, B.B.; Chaudry, M.Y.; Yasmeen, S. A Short Study to Test the Compliance of Various Pakistani Ordinary Portland Cements with ASTM Composition Standards. Pakistan J. Chem. 2013, 3, 163–169. [Google Scholar] [CrossRef]
- Brazão Farinha, C.; Pederneiras, C.M.; Bastos, D.; Infante Gomes, R.; Veiga, R.; Santos Silva, A.; Martins, I. CDW as sources of CO2 absorption. In Proceedings of the V International Conference Progress of Recycling in the Built Environment, Weimar, Germany, 10–12 October 2023; pp. 227–234. [Google Scholar]
- Hadigheh, S.A.; Gravina, R.J.; Smith, S.T. Effect of acid attack on FRP-to-concrete bonded interfaces. Constr. Build. Mater. 2017, 152, 285–303. [Google Scholar] [CrossRef]
- Lokesh, K.; Reddy, M.; Kotaiah, B. Degradation of Concrete Structures and Protective Measures. Int. J. Eng. Res. Technol. (IJERT) 2014, 3, 1415–1420. [Google Scholar]

| Aggregate Type | Designation | Origin (Collected at) | Location |
|---|---|---|---|
| Mixed recycled aggregates (MRAs) | MRA-RP1 | Recycling plant 1 | Pero Pinheiro, Portugal |
| MRA-RP2 | Recycling plant 2 | Figueira da Foz, Portugal | |
| MRA-RP3 | Recycling plant 3 | Seixal, Portugal | |
| Recycled concrete aggregates (RCAs) | RCA-IW1 | Concrete producer | Alhanda, Portugal |
| RCA-IS1 | Demolished in situ | Setúbal, Portugal | |
| RCA-RP1 | Recycling plant 1 | Pero Pinheiro, Portugal | |
| RCA-RP2 | Recycling plant 4 | Bucelas, Portugal | |
| Control concrete aggregates (CA) | CA-L | Concrete produced in laboratory | Lisbon, Portugal |
| Waste | Rc (%) | Ru (%) | Rb (%) | Ra (%) | Rg (%) | X (%) | Fl (%) | Classification According to EN 933-11 [55] * (as Per the Results Obtained in Laboratory) |
|---|---|---|---|---|---|---|---|---|
| MRA-RP1 | 42.5 | 27.5 | 21.7 | 9.6 | 0.2 | 0.8 | Fl5- | Rc declared Rcug70 Rb30- Ra10- Rg2- X1- Fl5- |
| MRA-RP2 | 61.5 | 26.7 | 9.2 | 0.2 | 0.0 | 2.4 | Fl5- | Rc50 Rg2- Rcug70 Rb10- Ra1- X1- Fl5- |
| MRA-RP3 | 37.4 | 16.3 | 26.6 | 6.2 | 6.9 | 6.7 | Fl5- | Rc declared Rg2- Rcug70 Rb 30- Ra1- X1- Fl5- |
| RCA-IS | 62.1 | 18.0 | 18.4 | 0.0 | 0.1 | 0.5 | Fl5- | Rc50 Rg2- Rcug70 Rb30- Ra1- X1- Fl5- |
| RCA-RP1 | 57.8 | 28.4 | 12.4 | 0.0 | 0.2 | 0.4 | Fl5- | Rc50 Rg2- Rcug70 Rb30- Ra1- X1- Fl5- |
| RCA-RP2 | 89.0 | 10.6 | 0.4 | 0.0 | 0.0 | 0.0 | Fl5- | Rc70 Rcug90 Rb10- Ra1- Rg2- X1- Fl5- |
| RCA-IW | 90.4 | 9.6 | 0.0 | 0.0 | 0.0 | 0.0 | Fl5- | Rc90 Rcug90 Rb10- Ra1- Rg2- X1- Fl5- |
| CA-L | 90.7 | 9.3 | 0.0 | 0.0 | 0.0 | 0.0 | Fl5- | Rc90 Rcug90 Rb10- Ra1- Rg2- X1- Fl5- |
| Aggregate | Water Absorption (%) | Particle Density (kg/m³) | ||
|---|---|---|---|---|
| Non-Carbonated | Carbonated | Non-Carbonated | Carbonated | |
| Natural sand | 0.25 ± 0.06 | - | 2627 ± 5 | - |
| MRA-RP1 | 7.05 ± 0.44 | 3.63 ± 0.23 | 2424 ± 66 | 2556 ± 48 |
| MRA-RP2 | - | - | - | - |
| MRA-RP3 | 6.89 ± 0.91 | 3.90 ± 0.88 | 2485 ± 52 | 2570 ± 40 |
| RCA-IS | 7.18 ± 0.20 | 4.87 ± 0.20 | 2426 ± 28 | 2640 ± 264 |
| RCA-RP2 | 3.81 ± 0.54 | 2.45 ± 0.13 | 2636 ± 57 | 2694 ± 44 |
| RCA-RP1 | 5.58 ± 1.18 | 2.48 ± 0.36 | 2510 ± 130 | 2514 ± 12 |
| RCA-IW | 5.01 ± 0.28 | 3.25 ± 0.34 | 2408 ± 18 | 2553 ± 5 |
| CA-L | 4.74 ± 0.16 | 0.46 ± 0.04 | 2622 ± 7 | 2670 ± 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, D.; Brazão Farinha, C.; Maia Pederneiras, C.; Veiga, R.; Bogas, J.A.; Infante Gomes, R.; Santos Silva, A. Pathway to Carbon Neutrality in the Cement Industry: CO2 Uptake by Recycled Aggregates from Construction and Demolition Waste. Appl. Sci. 2024, 14, 5224. https://doi.org/10.3390/app14125224
Bastos D, Brazão Farinha C, Maia Pederneiras C, Veiga R, Bogas JA, Infante Gomes R, Santos Silva A. Pathway to Carbon Neutrality in the Cement Industry: CO2 Uptake by Recycled Aggregates from Construction and Demolition Waste. Applied Sciences. 2024; 14(12):5224. https://doi.org/10.3390/app14125224
Chicago/Turabian StyleBastos, David, Catarina Brazão Farinha, Cinthia Maia Pederneiras, Rosário Veiga, José Alexandre Bogas, Ricardo Infante Gomes, and António Santos Silva. 2024. "Pathway to Carbon Neutrality in the Cement Industry: CO2 Uptake by Recycled Aggregates from Construction and Demolition Waste" Applied Sciences 14, no. 12: 5224. https://doi.org/10.3390/app14125224





