Effect of Nitric Acid on the Synthesis and Biological Activity of Silica–Quercetin Hybrid Materials via the Sol-Gel Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis via the Sol-Gel Route
2.2. Characterization of Hybrid Materials
2.2.1. FTIR Characterization
2.2.2. Bioactivity Study of Silica/Quercetin System
2.2.3. Encapsulation Efficiency and Drug Release Study
2.2.4. Antimicrobial Study
3. Results and Discussion
3.1. Structural Characterization of Si, Si-HNO3, SiQ5, and SiQ5-HNO3 Hybrid Materials
3.2. Bioactivity Study
3.3. Encapsulation Efficiency and In Vitro Release Study
3.4. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, R.; Yin, J.; Li, L.; Hong, R.; Chen, Y.; Zhao, Q.; Zhou, Y.; Huang, T.; Lin, J. 3D-Printed Scaffolds of Porous Amorphous Calcium Phosphate Nanospheres Loaded with Quercetin for Promoting Bone Repair via Synergistic Osteogenesis and Immunoregulation. ACS Appl. Nano Mater. 2024, 7, 10573–10590. [Google Scholar] [CrossRef]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Komaki, H.; Chazono, M.; Kitasato, S.; Kakuta, A.; Akiyama, S.; Marumo, K. Basic research and clinical application of beta-tricalcium phosphate (β-TCP). Morphologie 2017, 101, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Echazú, M.I.; Renou, S.J.; Alvarez, G.S.; Desimone, M.F.; Olmedo, D.G. Synthesis and Evaluation of a Chitosan–Silica-Based Bone Substitute for Tissue Engineering. Int. J. Molec. Sci. 2022, 23, 13379. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Luo, J.; Kong, Y.; Pan, B.; Zhao, J.; Wang, Y.; Yu, C. Cationic and Anionic Antimicrobial Agents Co-Templated Mesostructured Silica Nanocomposites with a Spiky Nanotopology and Enhanced Biofilm Inhibition Performance. Nano-Micro Lett. 2022, 14, 83. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Duan, Y.; Song, X.; Chang, M.; Feng, W.; Chen, Y. Silicon-Containing Nanomedicine and Biomaterials: Materials Chemistry, Multi-Dimensional Design, and Biomedical Application. Chem. Soc. Rev. 2024, 53, 1167–1315. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 1990; ISBN 978-0-12-134970-7. [Google Scholar]
- Innocenzi, P. Overview of the Sol–Gel Process. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M., Steiner Iii, S.A., Eds.; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2023; pp. 53–69. ISBN 978-3-030-27321-7. [Google Scholar]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Béland, F.; Ilharco, L.M.; Pagliaro, M. The Sol–Gel Route to Advanced Silica-Based Materials and Recent Applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; D’Errico, Y.; D’Angelo, A.; Clarke, R.J.; Blanco, I. Antibacterial Activity and Iron Release of Organic-Inorganic Hybrid Biomaterials Synthesized via the Sol-Gel Route. Appl. Sci. 2021, 11, 9311. [Google Scholar] [CrossRef]
- Vertuccio, L.; Guadagno, L.; D’Angelo, A.; Viola, V.; Raimondo, M.; Catauro, M. Sol-Gel Synthesis of Caffeic Acid Entrapped in Silica/Polyethylene Glycol Based Organic-Inorganic Hybrids: Drug Delivery and Biological Properties. Appl. Sci. 2023, 13, 2164. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Variation of the Properties of Sol–Gel Synthesized Bioactive Glass 45S5 in Organic and Inorganic Acid Catalysts. Mater. Adv. 2021, 2, 413–425. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Pope, E.J.A.; Mackenzie, J.D. Sol-Gel Processing of Silica. J. Non Cryst. Solids 1986, 87, 185–198. [Google Scholar] [CrossRef]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H. Quercetin Oxidation Paradoxically Enhances Its Antioxidant and Cytoprotective Properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Pérez, H.; Miranda, R.; Saavedra-Leos, Z.; Zarraga, R.; Alonso, P.; Moctezuma, E.; Martínez, J. Green and Facile Sol–Gel Synthesis of the Mesoporous SiO 2 –TiO 2 Catalyst by Four Different Activation Modes. RSC Adv. 2020, 10, 39580–39588. [Google Scholar] [CrossRef]
- Das, S.S.; Hussain, A.; Verma, P.R.P.; Imam, S.S.; Altamimi, M.A.; Alshehri, S.; Singh, S.K. Recent Advances in Liposomal Drug Delivery System of Quercetin for Cancer Targeting: A Mechanistic Approach. CDD 2020, 17, 845–860. [Google Scholar] [CrossRef]
- Michala, A.-S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, V.; Dell’Anna, M.M.; Mastrorilli, P.; Viola, V.; Catauro, M.; D’Angelo, A. Synthesis by Sol–Gel Route of Organic–Inorganic Hybrid Material: Chemical Characterization and In vitro Release Study. Appl. Sci. 2023, 13, 8410. [Google Scholar] [CrossRef]
- Raffaini, G.; Pirozzi, P.; Catauro, M.; D’Angelo, A. Hybrid Organic–Inorganic Biomaterials as Drug Delivery Systems: A Molecular Dynamics Study of Quercetin Adsorption on Amorphous Silica Surfaces. Coatings 2024, 14, 234. [Google Scholar] [CrossRef]
- Trzeciak, K.; Chotera-Ouda, A.; Bak-Sypien, I.I.; Potrzebowski, M.J. Mesoporous Silica Particles as Drug Delivery Systems—The State of the Art in Loading Methods and the Recent Progress in Analytical Techniques for Monitoring These Processes. Pharmaceutics 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; De Souza, A.L.R.; Silva, A.M.; Souto, E.B. Sol–Gel Carrier System: A Novel Controlled Drug Delivery. In Patenting Nanomedicines; Souto, E.B., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 151–166. ISBN 978-3-642-29264-4. [Google Scholar]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Catauro, M.; D’Angelo, A.; Fiorentino, M.; Pacifico, S.; Latini, A.; Brutti, S.; Vecchio Ciprioti, S. Thermal, Spectroscopic Characterization and Evaluation of Antibacterial and Cytotoxicity Properties of Quercetin-PEG-Silica Hybrid Materials. Ceram. Int. 2023, 49, 14855–14863. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, J.; Zhao, S.; Huang, T.; Ying, H.; Trujillo, C.; Molinaro, G.; Zhou, Z.; Jiang, T.; Liu, W.; et al. High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat. Commun. 2022, 13, 1262. [Google Scholar] [CrossRef]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Jackson, N.; Ortiz, A.C.; Jerez, A.; Morales, J.; Arriagada, F. Kinetics and Mechanism of Camptothecin Release from Transferrin-Gated Mesoporous Silica Nanoparticles through a pH-Responsive Surface Linker. Pharmaceutics 2023, 15, 1590. [Google Scholar] [CrossRef]
- Hudziky, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Caruso, A.A.; Viola, V.; Del Prete, S.; Leo, S.; Marasco, D.; Fulgione, A.; Naviglio, D.; Gallo, M. Identification and Characterization of Nasal Polyposis and Mycoplasma Superinfection by Scanning Electron Microscopy and Nasal Cytology with Optical Microscopy: A Case Report. Diagnostics 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Righi, C.; Barbieri, F.; Sgarbi, E.; Maistrello, L.; Bertacchini, A.; Andreola, F.N.; D’Angelo, A.; Catauro, M.; Barbieri, L. Suitability of Porous Inorganic Materials from Industrial Residues and Bioproducts for Use in Horticulture: A Multidisciplinary Approach. Appl. Sci. 2022, 12, 5437. [Google Scholar] [CrossRef]
- Saputra, O.A.; Lestari, W.A.; Kurniansyah, V.; Lestari, W.W.; Sugiura, T.; Mukti, R.R.; Martien, R.; Wibowo, F.R. Organically Surface Engineered Mesoporous Silica Nanoparticles Control the Release of Quercetin by pH Stimuli. Sci. Rep. 2022, 12, 20661. [Google Scholar] [CrossRef] [PubMed]
- AbouAitah, K.E.A.; Farghali, A.A.; Swiderska-Sroda, A.; Lojkowski, W.; Razin, A.M.; Khedr, M.K. Mesoporous Silica Materials in Drug Delivery System: pH/Glutathione- Responsive Release of Poorly Water-Soluble Pro-Drug Quercetin from Two and Three-Dimensional Pore-Structure Nanoparticles. J. Nanomed. Nanotechnol. 2016, 7, 1000360. [Google Scholar] [CrossRef]
- Mondragón, M.A.; Castaño, V.M.; Garcia, M.J.; Téllez, S.C.A. Vibrational Analysis of Si(OC2H5)4 and Spectroscopic Studies on the Formation of Glasses via Silica Gels. Vib. Spectrosc. 1995, 9, 293–304. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-0-470-61637-6. [Google Scholar]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Bondžić, B.P.; Čolović, M.B.; Jadranin, M.B.; Vasić, V.M. Investigation of Reaction between Quercetin and Au(Iii) in Acidic Media: Mechanism and Identification of Reaction Products. New J. Chem. 2013, 37, 901. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Miolo, G.; Innocenti, G.; Caffieri, S. The Photodegradation of Quercetin: Relation to Oxidation. Molecules 2012, 17, 8898–8907. [Google Scholar] [CrossRef]
- Zhai, G.; Zhu, W.; Duan, Y.; Qu, W.; Yan, Z. Synthesis, characterization and antitumor activity of the germanium-quercetin complex. Main Group Met. Chem. 2012, 35, 103–109. [Google Scholar] [CrossRef]
- Skoko, S.; Ambrosetti, M.; Giovannini, T.; Cappelli, C. Simulating Absorption Spectra of Flavonoids in Aqueous Solution: A Polarizable QM/MM Study. Molecules 2020, 25, 5853. [Google Scholar] [CrossRef] [PubMed]
- Sokolová, R.; Degano, I.; Ramešová, Š.; Bulíčková, J.; Hromadová, M.; Gál, M.; Fiedler, J.; Valášek, M. The oxidation mechanism of the antioxidant quercetin in nonaqueous media. Electrochim. Acta 2011, 56, 7421–7427. [Google Scholar] [CrossRef]
- Jørgensen, L.V.; Cornett, C.; Justesen, U.; Skibsted, L.H.; Dragsted, L.O. Two-Electron Electrochemical Oxidation of Quercetin and Kaempferol Changes Only the Flavonoid C-Ring. Free. Radic. Res. 1998, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the Stability of the Bioactive Flavonoids Quercetin and Luteolin under Oxygen-Free Conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Sadik, O.A. Comparative Analysis of Quercetin Oxidation by Electrochemical, Enzymatic, Autoxidation, and Free Radical Generation Techniques: A Mechanistic Study. J. Agric. Food Chem. 2008, 56, 12081–12091. [Google Scholar] [CrossRef] [PubMed]
- Metodiewa, D.; Jaiswal, A.K.; Cenas, N.; Dickancaité, E.; Segura-Aguilar, J. Quercetin May Act as a Cytotoxic Prooxidant after Its Metabolic Activation to Semiquinone and Quinoidal Product. Free. Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.; Sarfraz, S.; Amin, S. On the Formation of Hydroxyapatite Nano Crystals Prepared Using Cationic Surfactant. Mat. Res. 2015, 18, 468–472. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, S.-G.; Balázsi, C.; Chae, W.-S.; Lee, H.-O. Comparative Study of Hydroxyapatite from Eggshells and Synthetic Hydroxyapatite for Bone Regeneration. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Roviello, G.; Ferone, C.; Bollino, F.; Trifuoggi, M.; Aurilio, C. Synthesis of SiO2 and CaO rich calcium silicate systems via sol-gel process: Bioactivity, biocompatibility, and drug delivery tests. J. Biomed. Mater. Res. Part A 2014, 102A, 3087–3092. [Google Scholar] [CrossRef]
- Sahadat Hossain, M.; Ahmed, S. FTIR Spectrum Analysis to Predict the Crystalline and Amorphous Phases of Hydroxyapatite: A Comparison of Vibrational Motion to Reflection. RSC Adv. 2023, 13, 14625–14630. [Google Scholar] [CrossRef]
- Vechietti, F.A.; Marques, D.; Muniz, N.O.; Santos, L.A. Fibers Obtaining and Characterization Using Poly (Lactic-Co-Glycolic Acid) and Poly (Isoprene) Containing Hydroxyapatite and α TCP Calcium Phosphate by Electrospinning Method. KEM 2014, 631, 173–178. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Zhou, Z.; Wang, Z.; Wang, G. The Influence of Process Parameters on the Structure, Phase Composition, and Texture of Micro-Plasma Sprayed Hydroxyapatite Coatings. Coatings 2018, 8, 106. [Google Scholar] [CrossRef]
- Singh, R.; Tan, C.; Abd Shukor, M.; Sopyan, I.; Teng, W. The Influence of Ca/P Ratio on the Properties of Hydroxyapatite Bioceramics. In Proceedings of the International Conference on Smart Materials and Nanotechnology in Engineering A, Harbin, China, 1–4 July 2007; Proceedings SPIE: Bellingham, WA, USA, 2007; Volume 6423. [Google Scholar] [CrossRef]
- Sadžak, A.; Eraković, M.; Šegota, S. Kinetics of Flavonoid Degradation and Controlled Release from Functionalized Magnetic Nanoparticles. Mol. Pharm. 2023, 20, 5148–5159. [Google Scholar] [CrossRef] [PubMed]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An Overview of the Fundamentals of the Chemistry of Silica with Relevance to Biosilicification and Technological Advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Papan, P.; Kantapan, J.; Sangthong, P.; Meepowpan, P.; Dechsupa, N. Iron (III)-Quercetin Complex: Synthesis, Physicochemical Characterization, and MRI Cell Tracking toward Potential Applications in Regenerative Medicine. Contrast Media Mol. Imaging 2020, 2020, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Huck-Iriart, C.; Morales, N.J.; Herrera, M.L.; Candal, R.J. Micro to Mesoporous SiO2xerogels: The Effect of Acid Catalyst Type in Sol–Gel Process. J. Sol Gel Sci. Technol. 2022, 102, 197–207. [Google Scholar] [CrossRef]
- Takeda, Y.; Hashimoto, T.; Nasu, H.; Kamiya, K. Crystallization Behavior of Alumina Gels Prepared by Sol-Gel Method Using Nitric Acid as a Catalyst. Complete .ALPHA.-Transformation at 800.DEG.C.: Complete α-Transformation at 800 °C. J. Ceram. Soc. Jpn. 2002, 110, 1025–1028. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Wang, W.; Gao, H. Nitric Oxide, Nitric Oxide Formers and Their Physiological Impacts in Bacteria. Int. J. Mol. Sci. 2022, 23, 10778. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Coleman, C.M.; Gordon, A.L.; Lim, W.S.; Webb, A.J. Nitric Oxide for the Prevention and Treatment of Viral, Bacterial, Protozoal and Fungal Infections. F1000Research 2021, 10, 536. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chethana, K.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
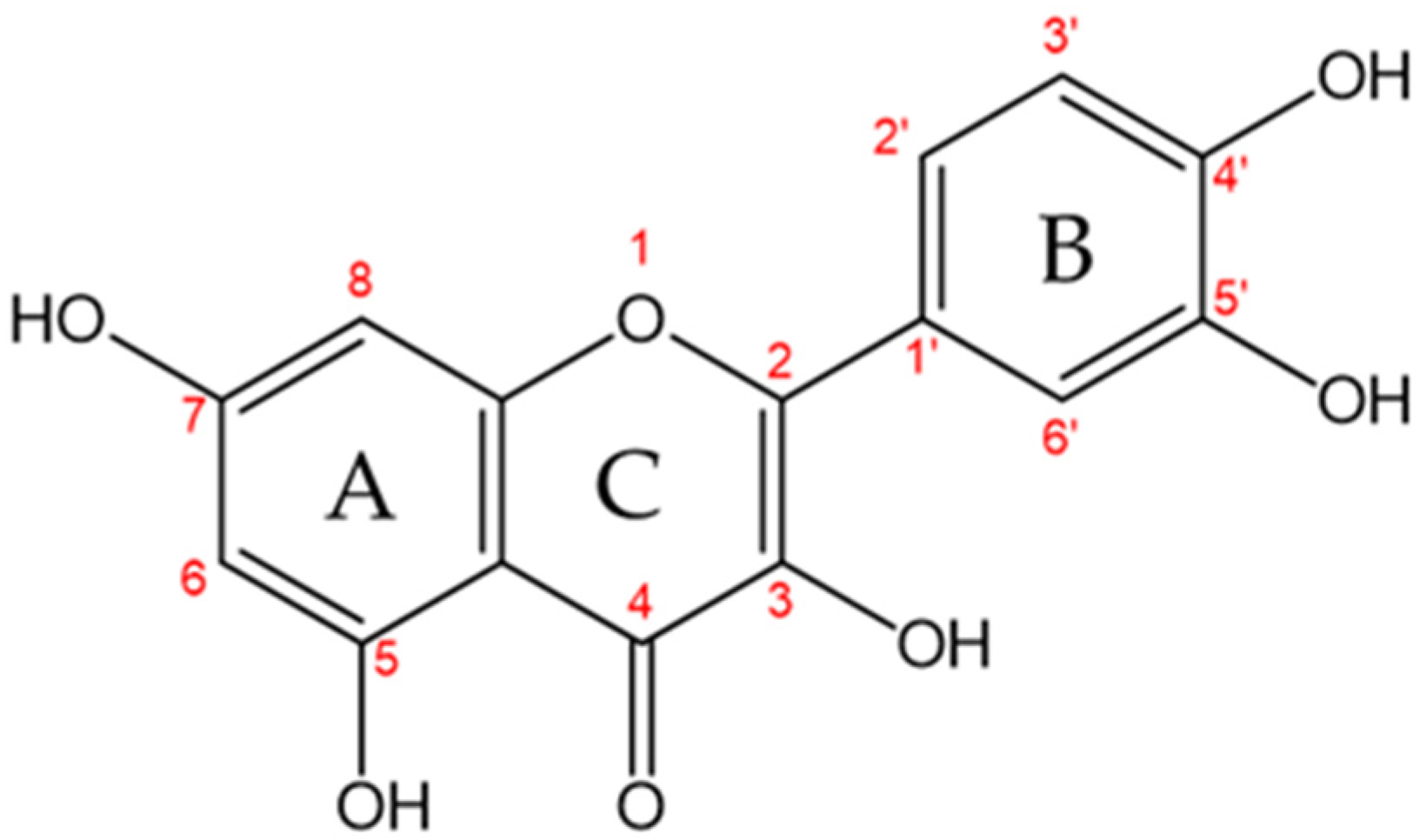


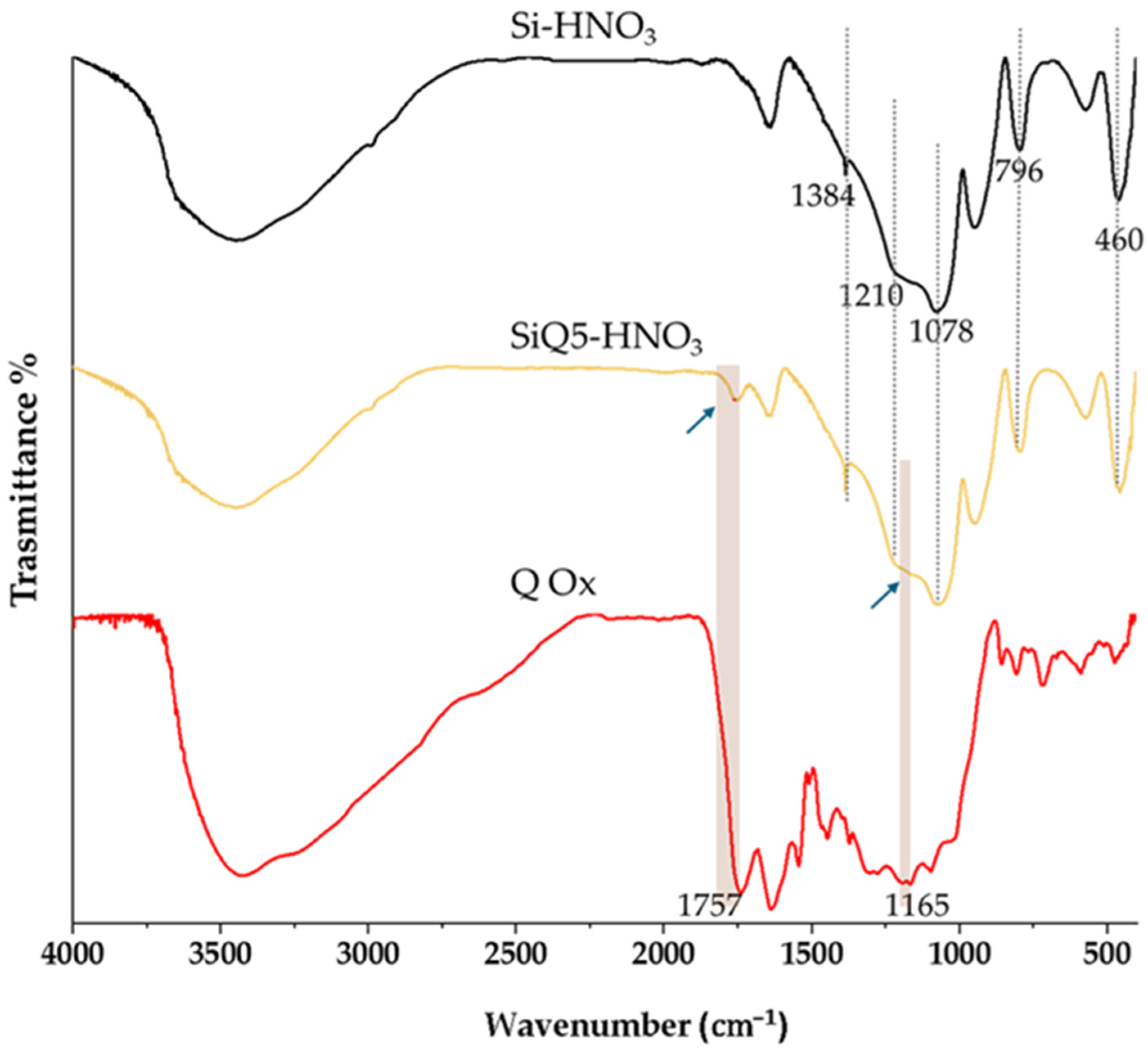

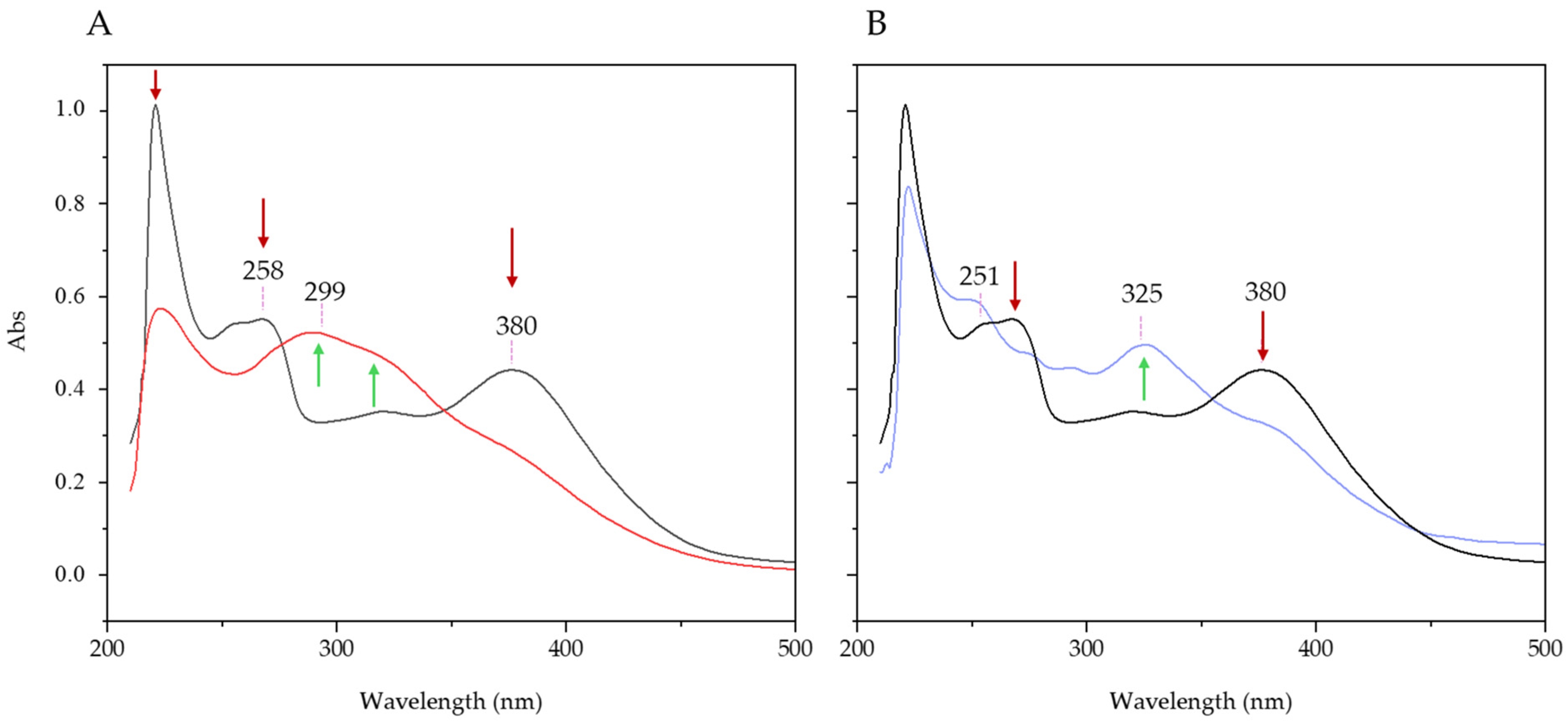
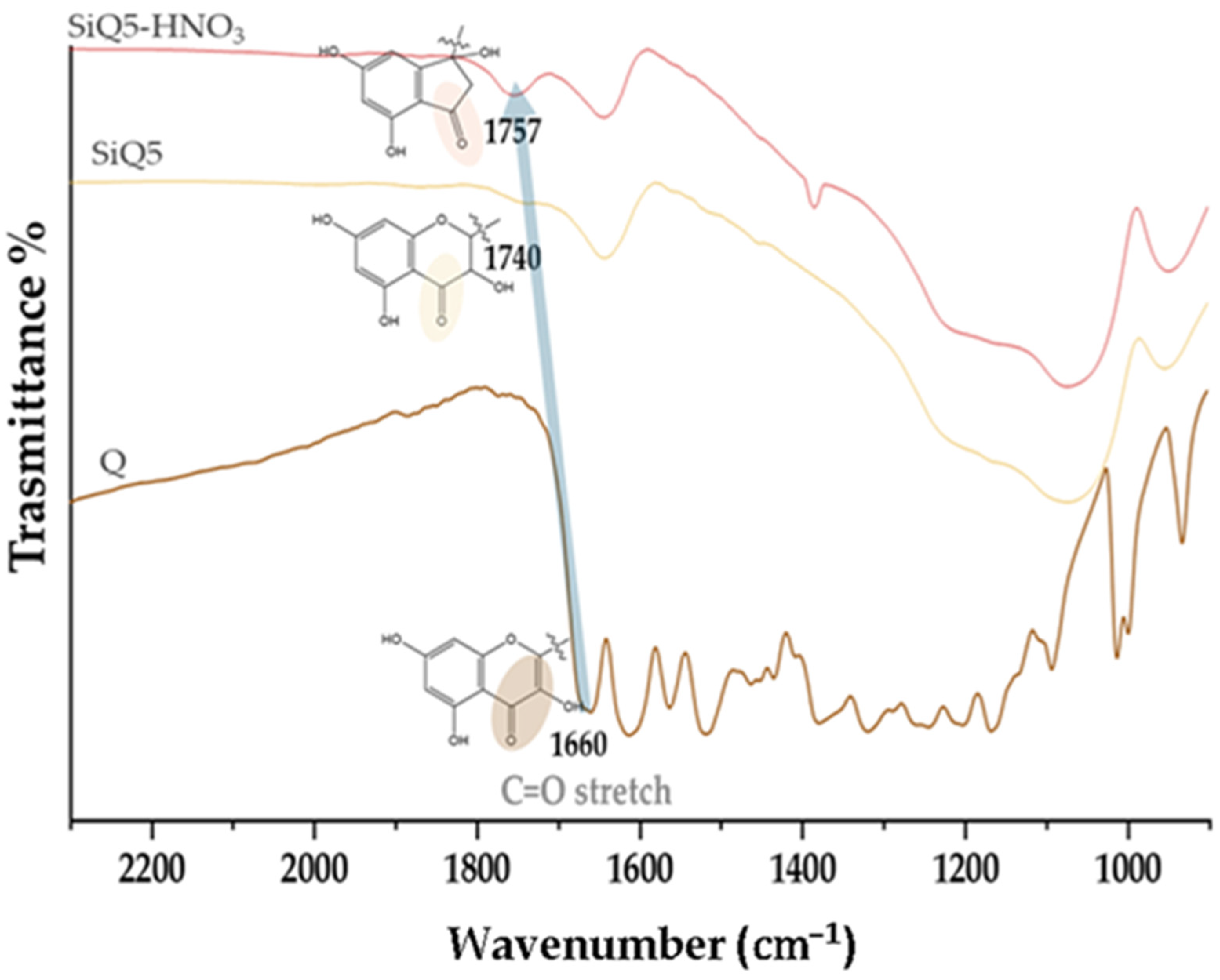

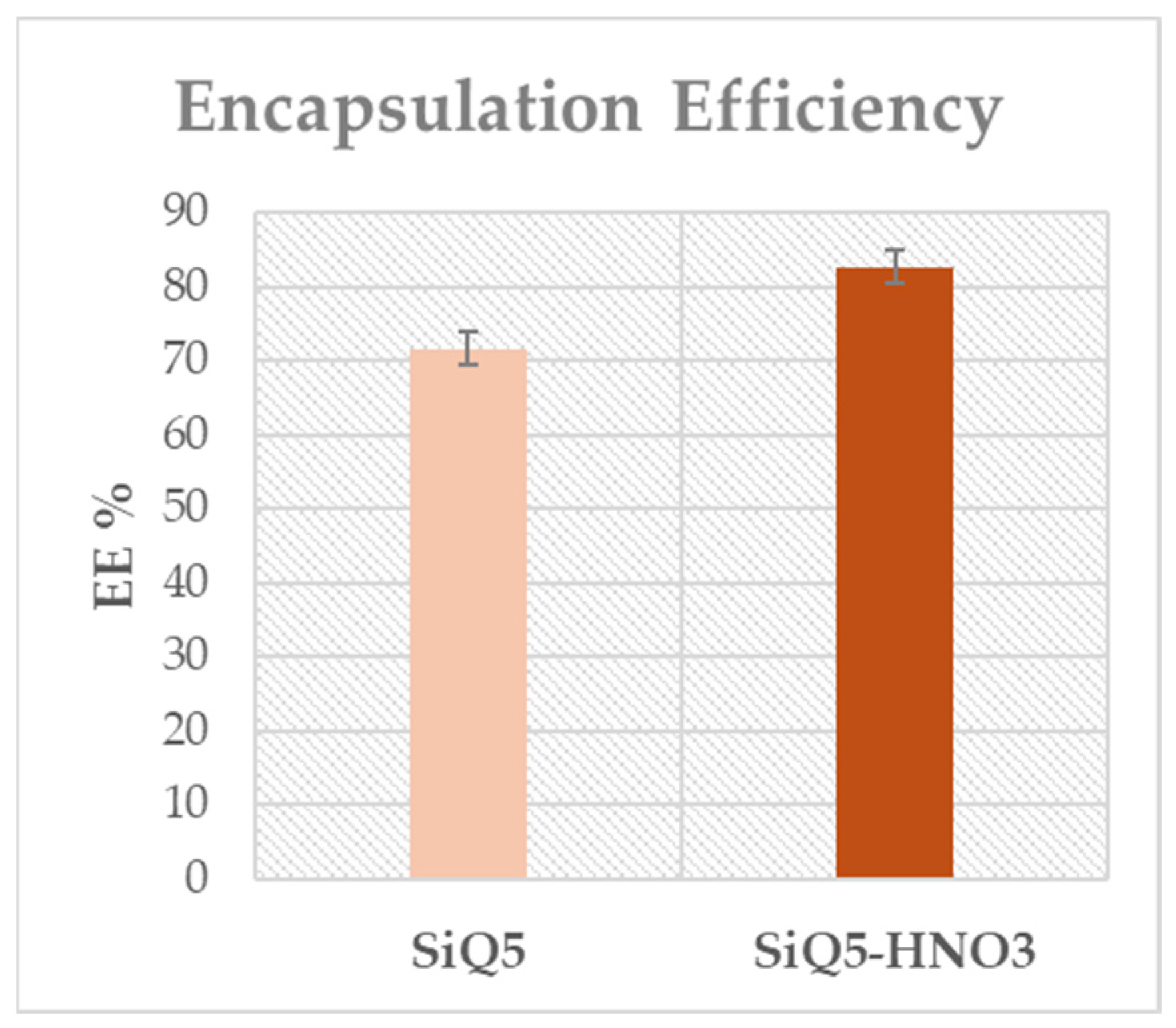
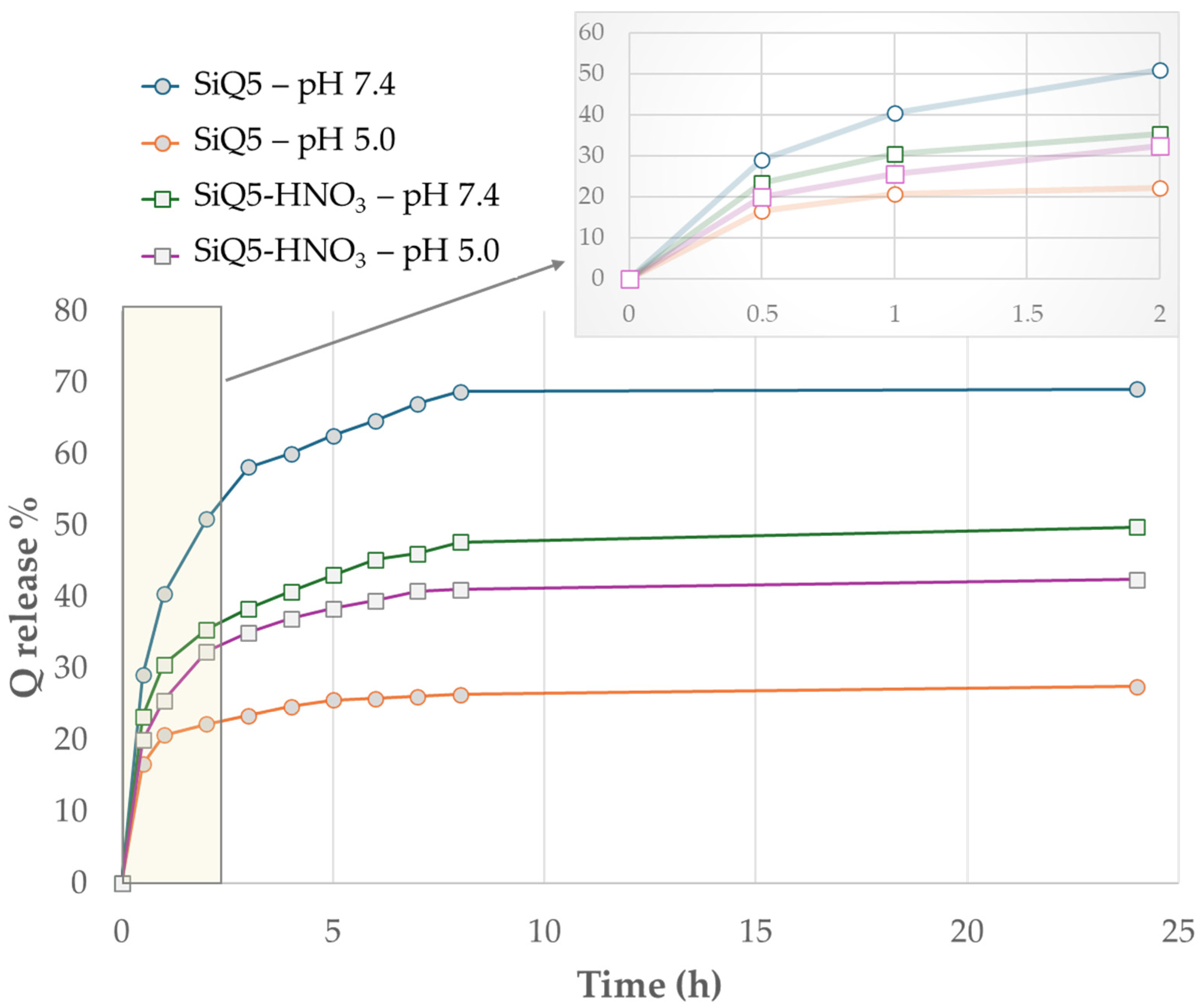

| Samples | EDS Measured Ca/P Ratio |
|---|---|
| SiQ5 | 1.73 |
| SiQ5-HNO3 | 1.67 |
| Sample | pH Value after 24 h Release |
|---|---|
| SiQ5 in SBF pH 7.4 | 7.47 ± 0.06 |
| SiQ5 in SBF pH 5.0 | 5.20 ± 0.12 |
| SiQ5-HNO3 in SBF pH 7.4 | 7.43 ± 0.11 |
| SiQ5-HNO3 in SBF pH 5.0 | 4.80 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, A.; Fiorentino, M.; Viola, V.; Vertuccio, L.; Catauro, M. Effect of Nitric Acid on the Synthesis and Biological Activity of Silica–Quercetin Hybrid Materials via the Sol-Gel Route. Appl. Sci. 2024, 14, 5268. https://doi.org/10.3390/app14125268
D’Angelo A, Fiorentino M, Viola V, Vertuccio L, Catauro M. Effect of Nitric Acid on the Synthesis and Biological Activity of Silica–Quercetin Hybrid Materials via the Sol-Gel Route. Applied Sciences. 2024; 14(12):5268. https://doi.org/10.3390/app14125268
Chicago/Turabian StyleD’Angelo, Antonio, Marika Fiorentino, Veronica Viola, Luigi Vertuccio, and Michelina Catauro. 2024. "Effect of Nitric Acid on the Synthesis and Biological Activity of Silica–Quercetin Hybrid Materials via the Sol-Gel Route" Applied Sciences 14, no. 12: 5268. https://doi.org/10.3390/app14125268
APA StyleD’Angelo, A., Fiorentino, M., Viola, V., Vertuccio, L., & Catauro, M. (2024). Effect of Nitric Acid on the Synthesis and Biological Activity of Silica–Quercetin Hybrid Materials via the Sol-Gel Route. Applied Sciences, 14(12), 5268. https://doi.org/10.3390/app14125268








