Protective Effects of a Combined Herbal Medicine against Amyotrophic Lateral Sclerosis-Associated Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of CHM Extracts
2.3. Footprint Analysis
2.4. Western Blotting
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
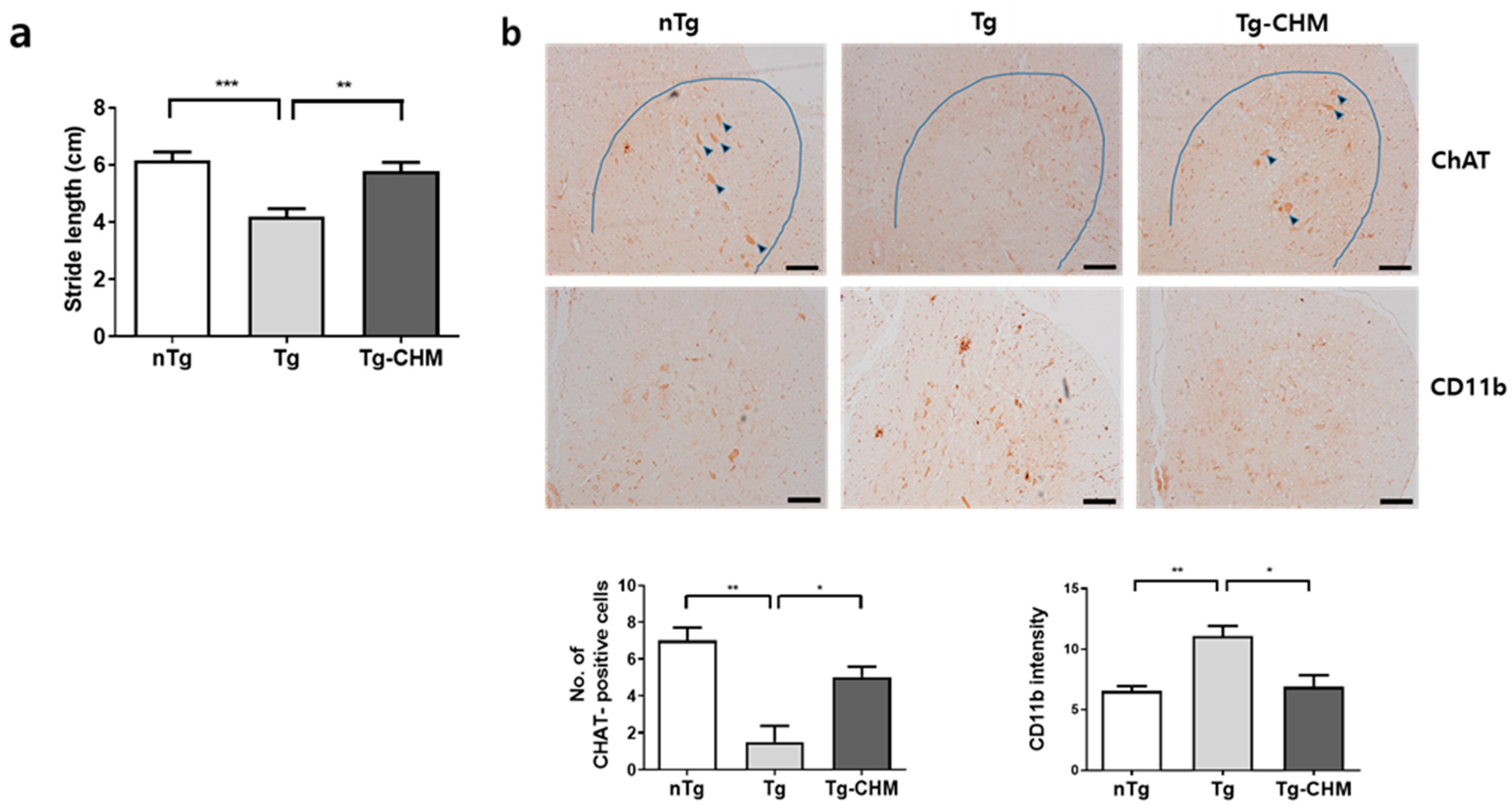
3.1. CHM Treatment Improves Movement in hSOD1G93A Mice
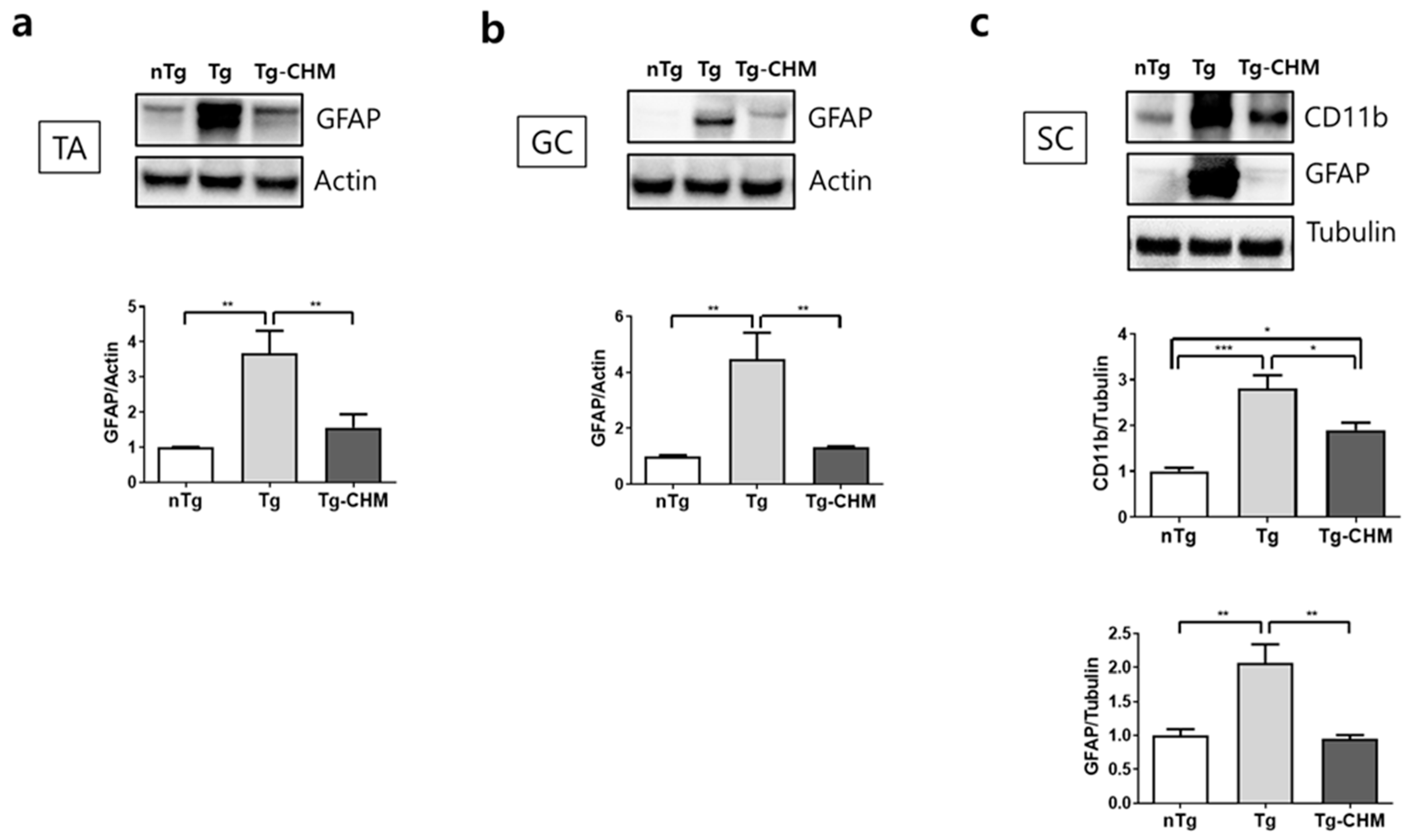
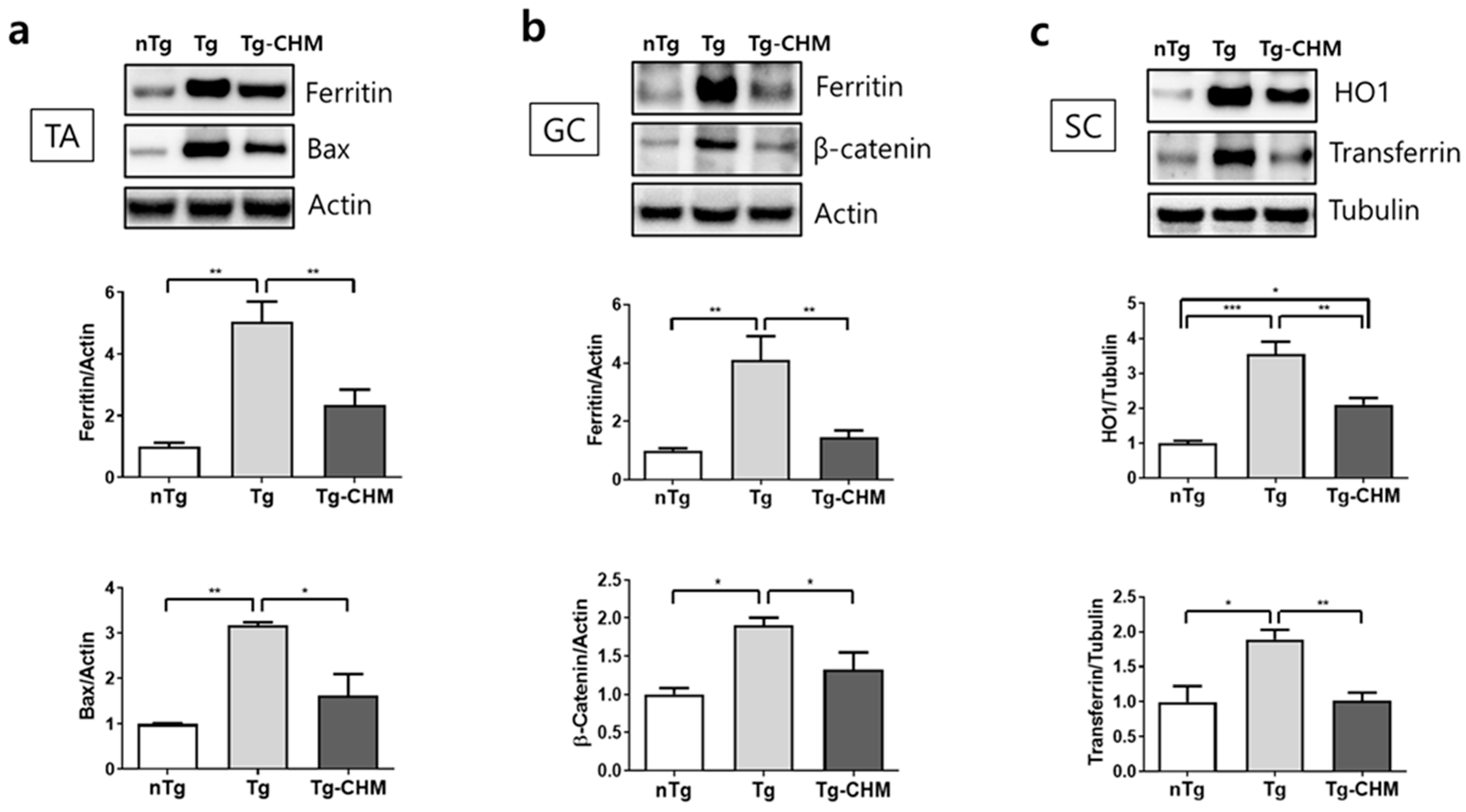
3.2. CHM Treatment Suppresses the Occurrence of Inflammation-Related Events in the Muscles and SC of hSOD1G93A Mice
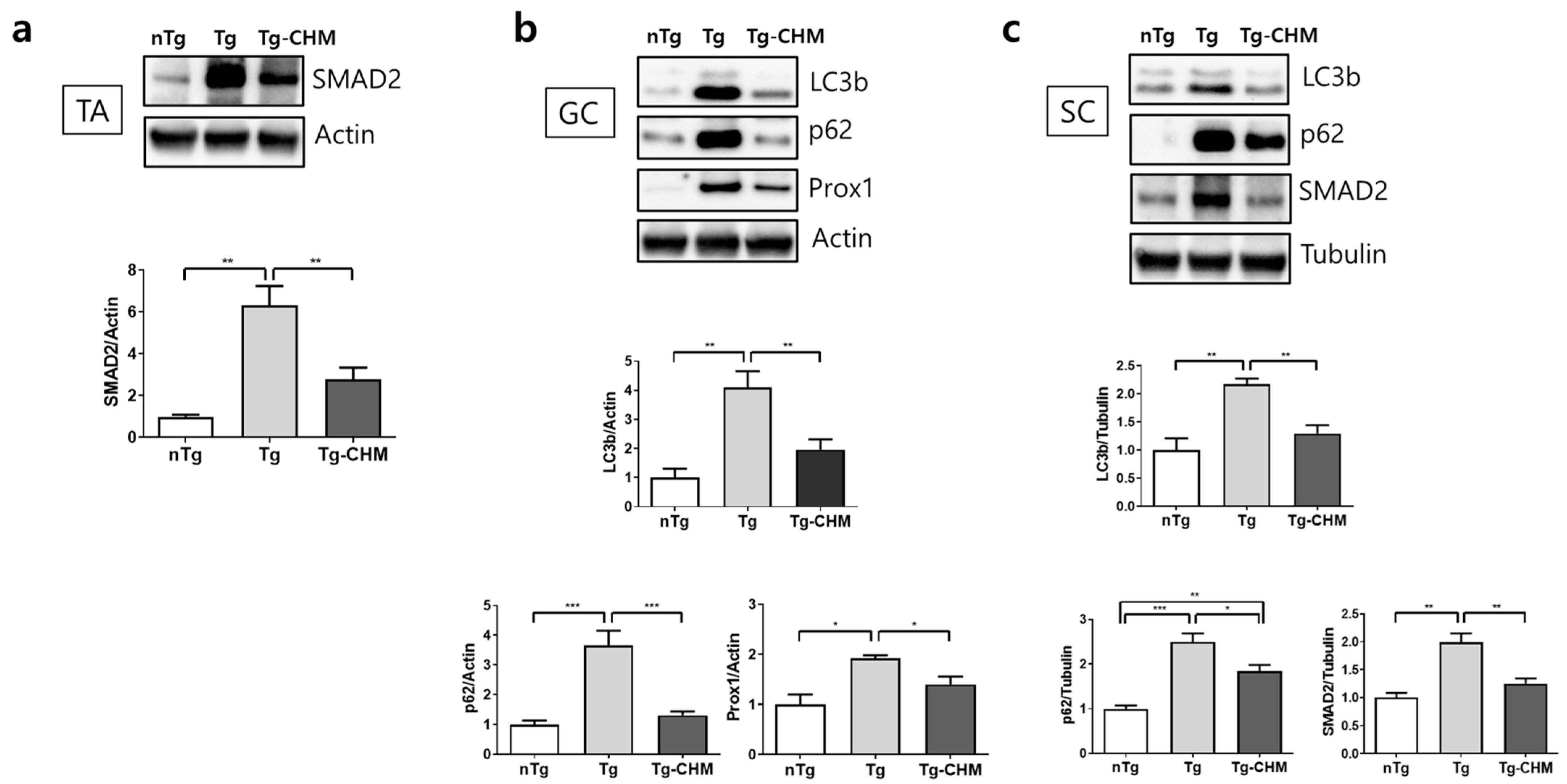
3.3. CHM Treatment Ameliorates Autophagy Dysfunction in the Muscle and SC of hSOD1G93A Mice
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef]
- Oakes, J.A.; Davies, M.C.; Collins, M.O. TBK1: A new player in ALS linking autophagy and neuroinflammation. Mol. Brain 2017, 10, 5. [Google Scholar] [CrossRef]
- Cho, H.; Shukla, S. Role of edaravone as a treatment option for patients with amyotrophic lateral sclerosis. Pharmaceuticals 2020, 14, 29. [Google Scholar] [CrossRef]
- Gupta, D.; Vagha, S.; Dhingra, H.; Shirsath, H. Advances in understanding and treating amyotrophic lateral sclerosis (ALS): A comprehensive review. Cureus 2023, 15, e48691. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Chiu, I.M.; Chen, A.; Zheng, Y.; Kosaras, B.; Tsiftsoglou, S.A.; Vartanian, T.K.; Brown, R.H.; Carroll, M.C. T lymphocytes po tentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl. Acad. Sci. USA 2008, 105, 17913–17918. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, W.; Beers, D.R.; Yen, A.A.; Xie, W.; Henkel, J.S.; Appel, S.H. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J. Neurochem. 2007, 102, 2008–2019. [Google Scholar] [CrossRef]
- Gowing, G.; Philips, T.; Van Wijmeersch, B.; Audet, J.N.; Dewil, M.; Van Den Bosch, L.; Billiau, A.D.; Robberecht, W.; Julien, J.P. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J. Neurosci. 2008, 28, 10234–10244. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Damian Holsinger, R.M. Oxidative Stress and Antioxidants in neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Ryu, J.K.; Bardehle, S.; Meyer-Franke, A.; Ang, K.K.; Wilson, C.; Baeten, K.M.; Hanspers, K.; Merlini, M.; Thomas, S.; et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 2020, 21, 513–524. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, P.S.; Shaw, I.C.; Kleiner, H.E.; Miller, R.T.; Monks, T.J.; Lau, S.S.; Mitchell, J.D.; Lynch, P.G. Evidence for DNA damage in amyotrophic lateral sclerosis. Muscle Nerve 1996, 19, 797–798. [Google Scholar] [PubMed]
- Abe, K.; Pan, L.H.; Watanabe, M.; Kato, T.; Itoyama, Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci. Lett. 1995, 199, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Shaw, P.J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010, 48, 62–641. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.D.; Yin, H.Z.; Weiss, J.H. Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons. J. Neurosci. 2003, 23, 2627–2633. [Google Scholar] [CrossRef]
- Orrell, R.W.; Lane, R.J.; Ross, M. Antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst. Rev. 2007, 2007, CD002829. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Machado, M.; Dias-Teixeira, M.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. The neuroprotective effect of traditional Chinese medicinal plants—A critical review. Acta Pharm. Sin. B 2023, 13, 3208–3237. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jia, Q.; Guan, X.; Kazuo, S.; Liu, J.; Duan, W.; Feng, L.; Zhang, C.; Gao, Y. Herbal medicine for amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 946548. [Google Scholar] [CrossRef]
- Wang, S.; Long, S.; Deng, Z.; Wu, W. Positive role of Chinese herbal medicine in cancer immune regulation. Am. J. Chin. Med. 2020, 48, 1577–1592. [Google Scholar] [CrossRef]
- Cai, M.; Yang, E.J. Complementary and alternative medicine for treating amyotrophic lateral sclerosis: A narrative review. Integr. Med. Res. 2019, 8, 234–239. [Google Scholar] [CrossRef]
- Wu, Y.N.; Wen, S.H.; Zhang, W.; Yu, S.S.; Yang, K.; Liu, D.; Zhao, C.B.; Sun, J. Gastrodia elata BI.: A comprehensive review of its traditional use, botany, phytochemistry, pharmacology, and pharmacokinetics. Evid. Based Complement. Alternat. Med. 2023, 2023, 5606021. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mori, A. Antioxidant and free radical scavenging activities of Gastrodia elata Bl. and Uncaria rhynchophylla (Miq.) Jacks. Neuropharmacology 1992, 31, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kwon, J.; Lee, K.; Lee, J.W.; Jang, D.S.; Kwon, H.C. Constituents of Gastrodia elata and their neuroprotective effects in HT22 hippocampal neuronal, R28 retinal cells, and BV2 microglial cells. Plants 2020, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluid 2010, 52, 62–70. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Wu, W.; Feng, X.; Du, K. Gastrodia elata blume polysaccharides attenuate vincristine-evoked neuropathic pain through the inhibition of neuroinflammation. Mediators Inflamm. 2021, 2021, 9965081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y. Neuroprotective effect of polysaccharides from Gastrodia elata Blume against corticosterone-induced apoptosis in PC12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Mikamo, H.; Kiwazoe, H.; Sato, Y.; Hayaski, Y.; Izumi, K.; Tamaya, T. Effects of crude herbal ingredients on serum levels of inflammatory cytokines in a rat uterine endometritis model. Curr. Ther. Res. 2000, 60, 105–110. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jung, D.H.; Lee, H.W. Therapeutic effect of Cnidium officinale Makino extract on ovariectomized hind-limb ischemic mice. Integr. Med. Res. 2019, 8, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shin, S. In vitro effects of essential oils from Ostericum koreanum against antibiotic-resistant Salmonella spp. Arch. Pharm. Res. 2005, 28, 765–769. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, H.J.; Lee, S.J.; Choi, H.Y.; Jin, C.; Lee, Y.S. A new chromone, 11-hydroxy-sec-O-glucosylhamaudol from Ostericum koreanum. Chem. Pharm. Bull. 2007, 55, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Bae, G.S.; Kim, D.Y.; Seo, S.W.; Park, K.B.; Kim, B.J. Inhibitory effect of extract from Ostericum koreanum on LPS-induced proinflammatory cytokines production in RAW 264.7 cells. Korea J. Herbol. 2008, 23, 127–134. [Google Scholar]
- Jung, H.W.; Mahesh, R.; Park, J.H.; Boo, Y.C.; Park, K.M.; Park, Y.K. Bisabolangelone isolated from Ostericum koreanum inhibits the production of inflammatory mediators by down-regulation of NF-κB and ERK MAP kinase activity in LPS-stimulated RAW264.7 cells. Int. Immunopharmacol. 2010, 10, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, S.J.; Baek, J.M.; Yoon, K.H.; Lee, M.S.; Oh, J. Ostericum koreanum reduces LPS-induced bone loss through inhibi tion of osteoclastogenesis. Am. J. Chin. Med. 2015, 43, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Gurney, M.E.; Pu, H.; Chiu, A.Y.; Dal Canto, M.C.; Polchow, C.Y.; Alexander, D.D.; Caliendo, J.; Hentati, A.; Kwon, Y.W.; Deng, H.X. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 1994, 264, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cai, M.; Yang, E.J. Anti-inflammatory effects of a novel herbal extract in the muscle and spinal cord of an amyotrophic lateral sclerosis animal model. Front. Neurosci. 2021, 15, 743705. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Brännström, T.; Andersen, P.M.; Pedrosa-Domellöf, F. Distinct changes in synaptic protein composition at neuromas cular junctions of extraocular muscles versus limb muscles of ALS donors. PLoS ONE 2013, 8, e57473. [Google Scholar]
- Lee, J.K.; Shin, J.H.; Lee, J.E.; Choi, E.J. Role of autophagy in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta 2015, 1852, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69–75. [Google Scholar]
- Leńska-Mieciek, M.; Madetko-Alster, N.; Alster, P.; Królicki, L.; Fiszer, U.; Koziorowski, D. Inflammation in multiple system atrophy. Front. Immunol. 2023, 14, 1214677. [Google Scholar] [CrossRef]
- Beers, D.R.; Zhao, W.; Wang, J.; Zhang, X.; Wen, S.; Neal, D.; Thonhoff, J.R.; Alsuliman, A.S.; Shpall, E.J.; Rezvani, K.; et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight 2017, 2, e89530. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Pollari, E.; Goldsteins, G.; Bart, G.; Koistinaho, J.; Giniatullin, R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, A.; Masoumeh, T.F.; Woan, S.T.; Si-vapragasam, G.; Sharida, F.; Mohd, M.N.; Suresh, K. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Münch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Shefner, J.M.; Andrews, J.A.; Genge, A.; Jackson, C.; Lechtzin, N.; Miller, T.M.; Cockroft, B.M.; Meng, L.; Wei, J.; Wolff, A.A.; et al. A phase 2, double-blind, randomized, dose-ranging trial of reldesemtiv in patients with ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 2021, 22, 287–299. [Google Scholar] [CrossRef]
- Chaves-Filho, A.B.; Pinto, I.F.D.; Dantas, L.S.; Xavier, A.M.; Inague, A.; Faria, R.L.; Medeiros, M.H.G.; Glezer, I.; Yoshinaga, M.Y.; Miyamoto, S. Alterations in lipid metabolism of spinal cord linked to amyotrophic lateral sclerosis. Sci. Rep. 2019, 9, 11642. [Google Scholar] [CrossRef] [PubMed]
- Scaricamazza, S.; Salvatori, I.; Giacovazzo, G.; Loeffler, J.P.; Renè, F.; Rosina, M.; Quessada, C.; Proietti, D.; Heil, C.; Rossi, S.; et al. Skeletal-muscle metabolic reprogramming in ALS-SOD1G93A mice predates disease onset and is a promising therapeutic target. iScience 2020, 23, 101087. [Google Scholar] [CrossRef]
- De Vocht, J.; Van Weehaeghe, D.; Ombelet, F.; Masrori, P.; Lamaire, N.; Devrome, M.; Van Esch, H.; Moisse, M.; Koole, M.; Dupont, P.; et al. Differences in cerebral glucose metabolism in ALS patients with and without C9orf72 and SOD1 mutations. Cells 2023, 12, 933. [Google Scholar] [CrossRef]
- Lanznaster, D.; Bruno, C.; Bourgeais, J.; Emond, P.; Zemmoura, I.; Lefèvre, A.; Reynier, P.; Eymieux, S.; Blanchard, E.; Vourc’h, P.; et al. Metabolic profile and pathological alterations in the muscle of patients with early-stage amyotrophic lateral sclerosis. Biomedicines 2022, 10, 1307. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Boss, J.; Guo, K.; Alakwaa, F.M.; Patterson, A.; Kim, S.; Savelieff, M.G.; Hur, J.; Feldman, E.L. Untargeted metabolomics yields insight into ALS disease mechanisms. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, S.; Parone, P.A.; Lopes, V.S.; Lillo, C.; McAlonis-Downes, M.; Lee, S.K.; Vetto, A.P.; Petrosyan, S.; Marsala, M.; Murphy, A.N.; et al. Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab. 2012, 1, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, L.; Lenzi, P.; Biagioni, F.; Siciliano, G.; Fornai, F. Cell to cell spreading of misfolded proteins as a therapeutic target in motor neuron disease. Curr. Med. Chem. 2014, 21, 3508–3534. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Nagai, M.; Ohta, Y.; Miyazaki, K.; Kurata, T.; Morimoto, M.; Murakami, T.; Takehisa, Y.; Ikeda, Y.; Kamiya, T.; et al. Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 2007, 1167, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gal, J.; Ström, A.L.; Kilty, R.; Zhang, F.; Zhu, H. p62 accumulates and enhances aggregate formation in model systems of familial amyotrophic lateral sclerosis. J. Biol. Chem. 2007, 282, 11068–11077. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Chen, S.; Yang, D.; Wang, Y.; Zhang, X.; Wang, Z.; Le, W. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy 2011, 7, 412–425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.J. Protective Effects of a Combined Herbal Medicine against Amyotrophic Lateral Sclerosis-Associated Inflammation and Oxidative Stress. Appl. Sci. 2024, 14, 5386. https://doi.org/10.3390/app14135386
Yang EJ. Protective Effects of a Combined Herbal Medicine against Amyotrophic Lateral Sclerosis-Associated Inflammation and Oxidative Stress. Applied Sciences. 2024; 14(13):5386. https://doi.org/10.3390/app14135386
Chicago/Turabian StyleYang, Eun Jin. 2024. "Protective Effects of a Combined Herbal Medicine against Amyotrophic Lateral Sclerosis-Associated Inflammation and Oxidative Stress" Applied Sciences 14, no. 13: 5386. https://doi.org/10.3390/app14135386



