Electroencephalography Response during an Incremental Test According to the V̇O2max Plateau Incidence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants Recruitment and Ethical Approval
2.2. Participants Recruitment and Ethical Approval
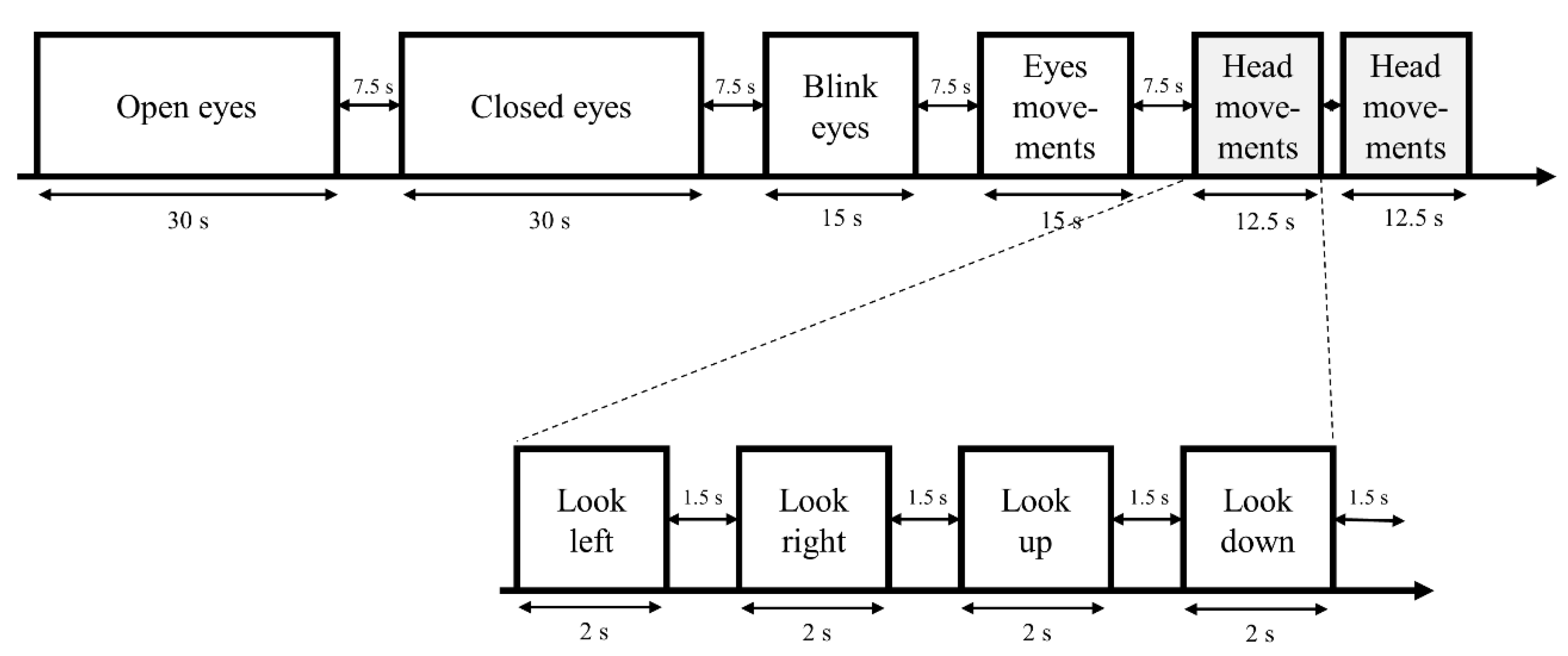
2.3. Experimental Design
Rating of Perceived Exertion (RPE) Scale
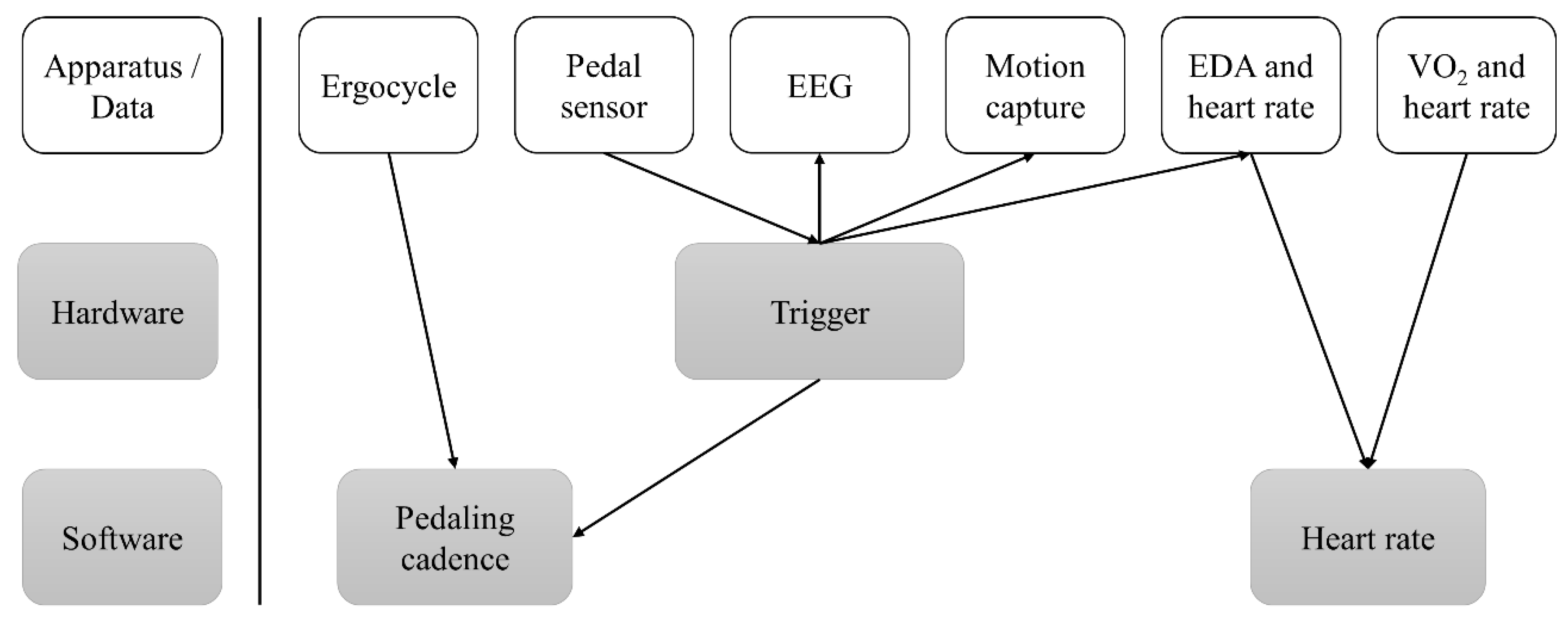
2.4. Measurements
2.4.1. Ergocycle Data
2.4.2. Electroencephalography Measurements
2.4.3. Heart Rate (HR) Measurements
2.4.4. Gas Measurements
2.4.5. Determination of the O2max Plateau
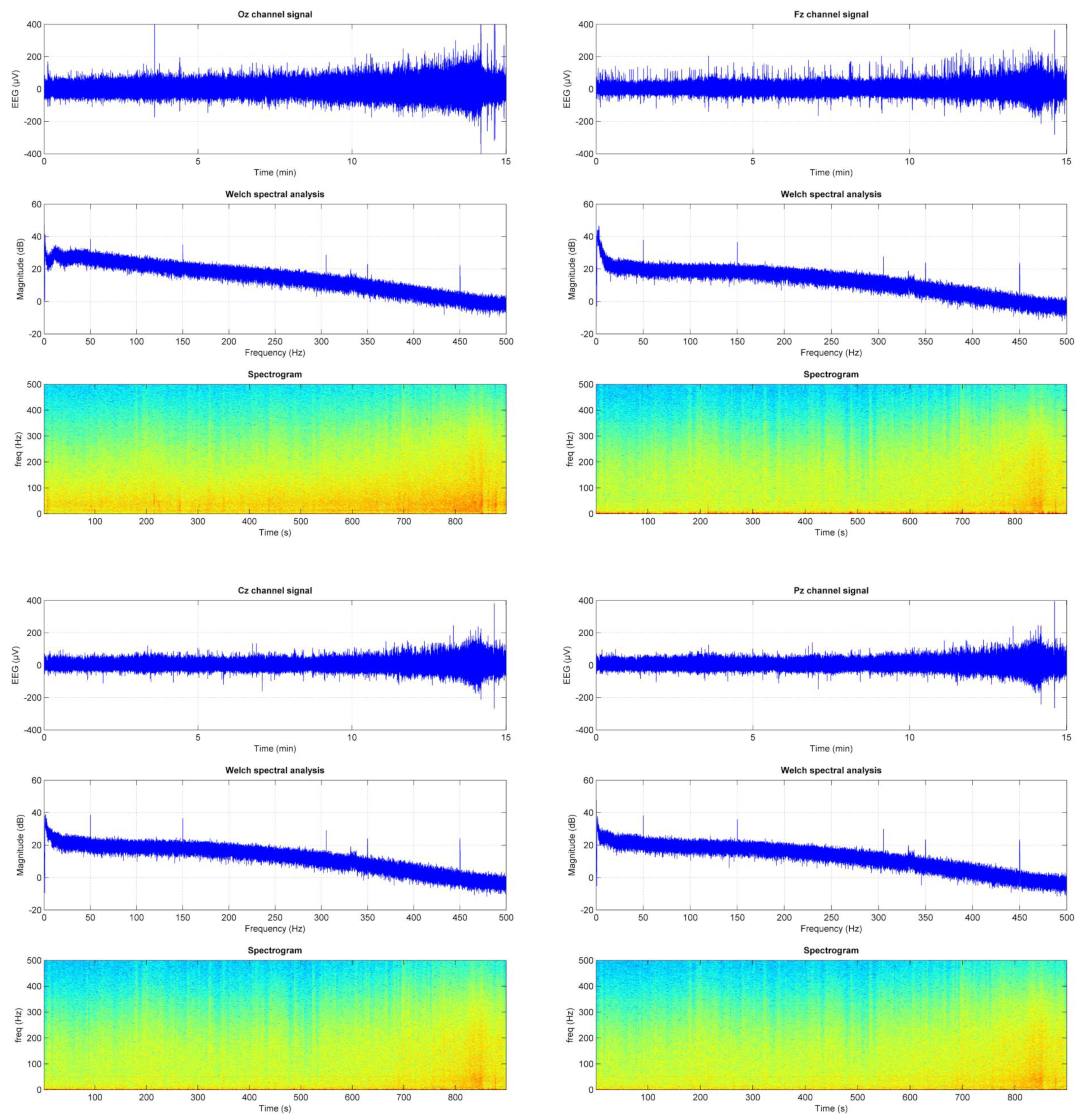
2.5. Signal Processing
EEG: Data Reduction Procedures for Artifact Correction and Removal
- Increases systematically towards the end of the recording and the highest effort.
- Is higher on the occipital derivation, probably due to muscle contraction.
- Is kept to an acceptable level (approximately 20 dB at 50 Hz).
2.6. Statistics
3. Results
3.1. Occurrence of O2pl in the Whole Group (n = 42)
3.2. Difference in Physiological and Training Parameters between the NONPLAT and PLAT Groups or NONEND and END Ones
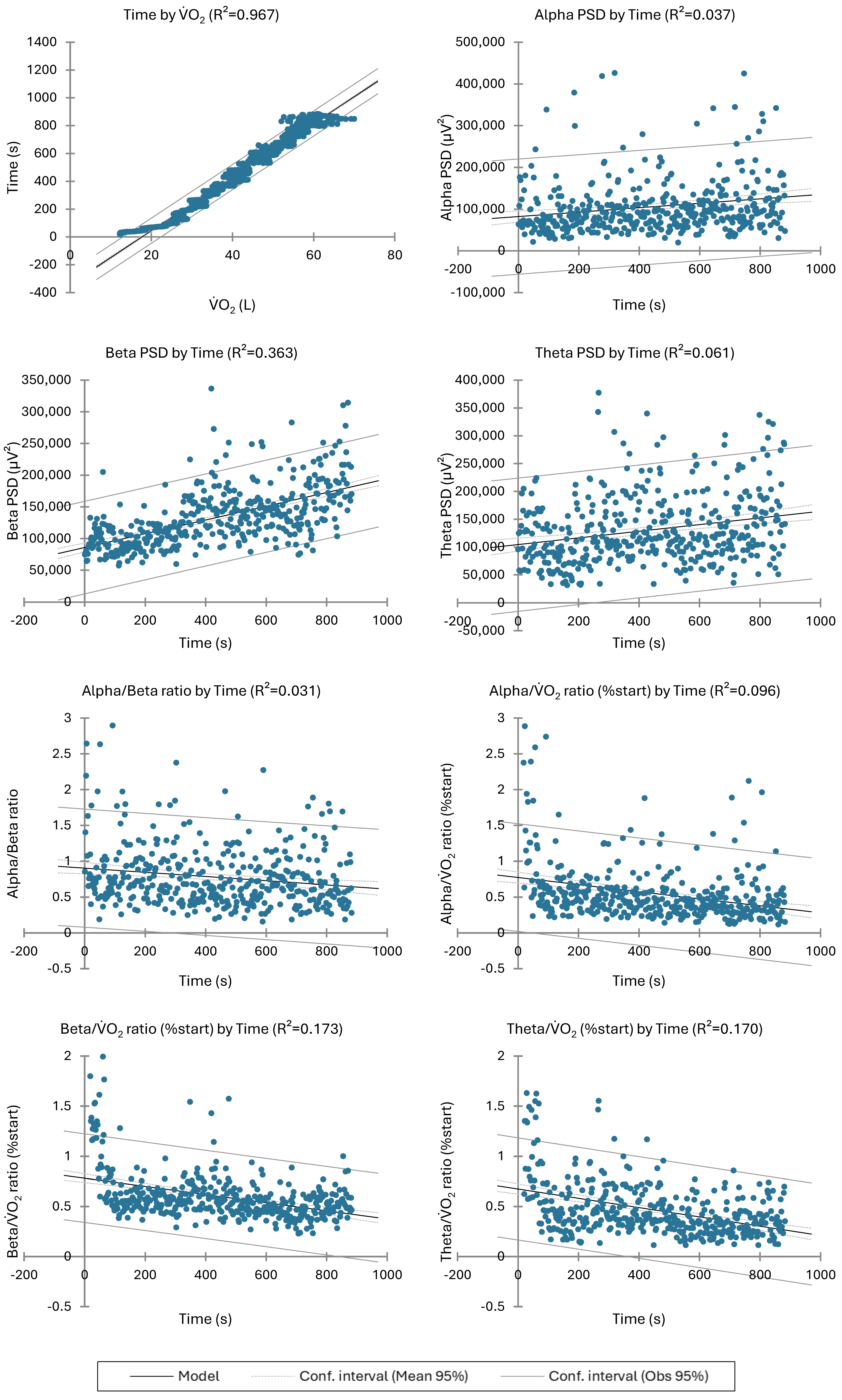
3.3. EEG Responses vs. O2 Increase, between the NONPLAT and PLAT Groups or NONEND and END Ones during the IET
4. Discussion
- Difference in maximal value of O2, heart rate, RPE, and training parameters between the NONPLAT and PLAT groups or NONEND and END ones.
- The occurrence of O2pl
- RPE response between the groups
- EEG responses between the NONPLAT and PLAT groups or NONEND and END ones during the IET
- Impact of the cadence on the EEG responses between the NONPLAT and PLAT groups or NONEND and END ones during the IET
- Application of the EEG/O2 ratio approach in exercise physiology and medicine.
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, A.V.; Lupton, H. Muscular Exercise, Lactic Acid, and the Supply and Utilization of Oxygen. QJM Int. J. Med. 1923, 16, 135–171. [Google Scholar] [CrossRef]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Despres, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e653–e699. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Jones, A.M. Measurement of the maximum oxygen uptake VO2max: Vo2peak is no longer acceptable. J. Appl. Physiol. 2017, 122, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, M.; Knaier, R.; Beneke, R. The Oxygen Uptake Plateau—A Critical Review of the Frequently Misunderstood Phenomenon. Sports Med. 2021, 51, 1815–1834. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vella, C.A.; Marks, D.; Robergs, R.A. Oxygen cost of ventilation during incremental exercise to VO2 max. Respirology 2006, 11, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R., Jr. Scientific contributions of A. V. Hill: Exercise physiology pioneer. J. Appl. Physiol. 2002, 93, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Willey, J.; Kinnahan, J.; Larsson, S.M.; Welch, H.; Dalleck, L.C.; Shephard, R.J. Elucidating determinants of the plateau in oxygen consumption at VO2max. Br. J. Sports Med. 2005, 39, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Rossiter, H.B.; Coats, E.M.; Skasick, A.; Whipp, B.J. The maximally attainable Vo2during exercise in humans: The peak vs. maximum issue. J. Appl. Physiol. 2003, 95, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.E.; Howley, E.T.; Johnson, B.N. Applicability of VO2max criteria: Discontinuous versus continuous protocols. Med. Sci. Sports Exerc. 1997, 29, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, H.B.; Kowalchuk, J.M.; Whipp, B.J. A test to establish maximum O2uptake despite no plateau in the O2uptake response to ramp incremental exercise. J. Appl. Physiol. 2006, 100, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A. Alterations in VO2max and the VO2 plateau with manipulation of sampling interval. Clin. Physiol. Funct. Imaging 2009, 29, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; White, A.C.; Dalleck, L.C. Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int. J. Sports Med. 2009, 30, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Wilkerson, D.P.; Jones, A.M. Validity of criteria for establishing maximal O2 uptake during ramp exercise tests. Eur. J. Appl. Physiol. 2008, 102, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Nobbs, L.; Noakes, T.D. Low frequency of the “plateau phenomenon” during maximal exercise in elite British athletes. Eur. J. Appl. Physiol. 2003, 89, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Askew, C.D. VO2peak is an acceptable estimate of cardiorespiratory fitness but not VO2max. J. Appl. Physiol. 2018, 125, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.; Bhammar, D.M.; Babb, T.G.; Bowen, T.S.; Witte, K.K.; Rossiter, H.B.; Brugniaux, J.V.; Perry, B.D.; Dantas de Lucas, R.; Turnes, T.; et al. Commentaries on Viewpoint: VO2peak is an acceptable estimate of cardiorespiratory fitness but not VO2max. J. Appl. Physiol. 2018, 125, 970. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, M.; Leithaeuser, R.; Beneke, R. Oxygen uptake plateau occurrence depends on oxygen kinetics and oxygen deficit accumulation. Scand. J. Med. Sci. Sports 2019, 29, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, M.; Bergmann, T.G.J.; Beneke, R. Oxygen uptake plateau: Calculation artifact or physiological reality? Eur. J. Appl. Physiol. 2020, 120, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Hopkins, S.; King, C.; Keiller, D.; Barnes, R.J. Incidence of the plateau at VO2max is dependent on the anaerobic capacity. Int. J. Sports Med. 2011, 32, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Demarle, A.P.; Slawinski, J.J.; Laffite, L.P.; Bocquet, V.G.; Koralsztein, J.P.; Billat, V.L.; Cruz, R.S.d.O.; de Aguiar, R.A.; Turnes, T.; Pereira, K.L.; et al. Decrease of O2 deficit is a potential factor in increased time to exhaustion after specific endurance training. J. Appl. Physiol. 2001, 90, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Lanao, J.; Lucia, A.; Dekoning, J.J.; Foster, C. How Do Humans Control Physiological Strain during Strenuous Endurance Exercise? PLoS ONE 2008, 3, e2943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcora, S.M. Do we really need a central governor to explain brain regulation of exercise performance? Eur. J. Appl. Physiol. 2008, 104, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.; Noakes, T.D. The physiological regulation of pacing strategy during exercise: A critical review. Br. J. Sports Med. 2009, 43, e1. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P.; Beck, T.W.; Cramer, J.T.; Housh, T.J. Is fatigue all in your head? A critical review of the central governor model. Br. J. Sports Med. 2006, 40, 573–586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noakes, T.D. Fatigue is a Brain-Derived Emotion that Regulates the Exercise Behavior to Ensure the Protection of Whole Body Homeostasis. Front. Physiol. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ganesan, G.; Leu, S.-Y.; Cerussi, A.; Tromberg, B.; Cooper, D.M.; Galassetti, P. Cerebral and Muscle Tissue Oxygenation During Incremental Cycling in Male Adolescents Measured by Time-Resolved Near-Infrared Spectroscopy. Pediatr. Exerc. Sci. 2016, 28, 275–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, R.; Moser, M.; Baucsek, S.; Dern, S.; Schneider, S. Activation patterns of different brain areas during incremental exercise measured by near-infrared spectroscopy. Exp. Brain Res. 2015, 233, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Buchheit, M.; Girard, O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front. Physiol. 2014, 5, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rooks, C.R.; Thom, N.J.; McCully, K.K.; Dishman, R.K. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: A systematic review. Prog. Neurobiol. 2010, 92, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.P.; E Hall, E.; E Folger, S.; Miller, P.C. Changes in EEG during graded exercise on a recumbent cycle ergometer. J. Sports Sci. Med. 2008, 7, 505–511. [Google Scholar] [PubMed]
- Brümmer, V.; Schneider, S.; Strüder, H.; Askew, C. Primary motor cortex activity is elevated with incremental exercise intensity. Neuroscience 2011, 181, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.V.; Marino, F.E. Prefrontal and motor cortex EEG responses and their relationship to ventilatory thresholds during exhaustive incremental exercise. Eur. J. Appl. Physiol. 2015, 115, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Enders, H.; Nigg, B.M. Measuring human locomotor control using EMG and EEG: Current knowledge, limitations and future considerations. Eur. J. Sport Sci. 2016, 16, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Steffert, T.; Ros, T.; Leach, J.; Gruzelier, J. EEG applications for sport and performance. Methods 2008, 45, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Park, J.L.; Fairweather, M.M.; Donaldson, D.I. Making the case for mobile cognition: EEG and sports performance. Neurosci. Biobehav. Rev. 2015, 52, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Schimke, H.; Pachinger, T. Alpha frequency, reaction time, and the speed of processing information. J. Clin. Neurophysiol. 1996, 13, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Samaha, J.; Bauer, P.; Cimaroli, S.; Postle, B.R. Top-down control of the phase of alpha-band oscillations as a mechanism for temporal prediction. Proc. Natl. Acad. Sci. USA 2015, 112, 8439–8444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De, A.; Mondal, S. Yoga and Brain Wave Coherence: A Systematic Review for Brain Function Improvement. Heart. Mind 2020, 4, 33–39. [Google Scholar] [CrossRef]
- Kropotov, J.D. Beta and gamma rhythms. In Functional Neuromarkers for Psychiatry: Applications for Diagnosis and Treatment; Academic Press: Cambridge, MA, USA, 2016; pp. 107–119. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Introduction to EEG- and Speech-Based Emotion Recognition; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hilty, L.; Langer, N.; Pascual-Marqui, R.; Boutellier, U.; Lutz, K. Fatigue-induced increase in intracortical communication between mid/anterior insular and motor cortex during cycling exercise. Eur. J. Neurosci. 2011, 34, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gronwald, T.; Hottenrott, K. Effects of high vs. low cadence training on cyclists’ brain cortical activity during exercise. J. Sci. Med. Sport 2016, 19, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Hillman, C.H. Neuroelectric and behavioral indices of interference control during acute cycling. Clin. Neurophysiol. 2007, 118, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Rouffet, D.M.; Billaut, F.; Strüder, H.K. Cortical current density oscillations in the motor cortex are correlated with muscular activity during pedaling exercise. Neuroscience 2013, 228, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Brümmer, V.; Schneider, S.; Abel, T.; Vogt, T.; Strüder, H.K. Brain cortical activity is influenced by exercise mode and intensity. Med. Sci. Sports Exerc. 2011, 43, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Mechau, D.; Liesen, H.; Mücke, S.; Weiß, M. Effect of increasing running velocity on electroencephalogram in a field test. Eur. J. Appl. Physiol. 1998, 78, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, R.M.; Hanson, N.J.; Miller, M.G. Brain activity during self-paced vs. fixed protocols in graded exercise testing. Exp. Brain Res. 2019, 237, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.V.; Marino, F.E. A role for the prefrontal cortex in exercise tolerance and termination. J. Appl. Physiol. 2016, 120, 464–466. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.V.; Marino, F.E. Last Word on Viewpoint: A role for the prefrontal cortex in exercise tolerance and termination. J. Appl. Physiol. 2016, 120, 470. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Maby, E. Practical Guide to Performing an EEG Experiment. In Brain–Computer Interfaces 2: Technology and Applications; Clerc, M., Bougrain, L., Lotte, F., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 163–177. [Google Scholar]
- Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Fowles, D.C.; Christie, M.J.; Edelberg, R.; Grings, W.W.; Lykken, D.T.; Venables, P.H. Committee report. Publication recommendations for electrodermal measurements. Psychophysiology 1981, 18, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.; Hassmén, P.; Lagerström, M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1987, 56, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Midgley, A.W.; McNaughton, L.R.; Carroll, S. Time at VO2max during intermittent treadmill running: Test protocol dependent or methodological artefact? Int. J. Sports Med. 2007, 28, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Hillman, C.H. Neuroelectric measurement of cognition during aerobic exercise. Methods 2008, 45, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Damon, C.; Liutkus, A.; Gramfort, A.; Essid, S. Non-negative Tensor Factorization for single-channel EEG artifact rejection. In Proceedings of the 2013 IEEE International Workshop on Machine Learning for Signal Processing (MLSP), Southampton, UK, 22–25 September 2013; pp. 1–6. [Google Scholar]
- Noakes, T.D. Time to move beyond a brainless exercise physiology: The evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 2011, 36, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Tailor, A.; Bhatt, T. Effects of yoga on brain waves and structural activation: A review. Complement. Ther. Clin. Pract. 2015, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Schimke, H.; Pfurtscheller, G. Alpha frequency, cognitive load and memory performance. Brain Topogr. 1993, 5, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008, 32, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Gola, M.; Magnuski, M.; Szumska, I.; Wróbel, A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly subjects. Int. J. Psychophysiol. 2013, 89, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kim, S. Influence of Fragrances on Human Psychophysiological Activity: With Special Reference to Human Electroencephalographic Response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baumeister, J.; Reinecke, K.; Schubert, M.; Schade, J.; Weiss, M. Effects of induced fatigue on brain activity during sensorimotor control. Eur. J. Appl. Physiol. 2012, 112, 2475–2482. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Blomqvist, G. Maximal Oxygen Uptake. N. Engl. J. Med. 1971, 284, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.L.; Buskirk, E.; Henschel, A.; Poole, D.C.; Jones, A.M.; Périard, J.D.; Racinais, S.; Joyner, M.J.; Casey, D.P.; Tipton, C.M.; et al. Maximal Oxygen Intake as an Objective Measure of Cardio-Respiratory Performance. J. Appl. Physiol. 1955, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, J.C.; Malina, R.M. Secular trend in peak oxygen consumption among United States youth in the 20th century. Am. J. Hum. Biol. 2002, 14, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; St Clair Gibson, A. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br. J. Sports Med. 2004, 38, 648–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lacour, J.-R.; Messonnier, L.; Bourdin, M. The leveling-off of oxygen uptake is related to blood lactate accumulation. Retrospective study of 94 elite rowers. Eur. J. Appl. Physiol. 2007, 101, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Maximal oxygen uptake: “classical” versus “contemporary” viewpoints: A rebuttal. Med. Sci. Sports Exerc. 1998, 30, 1381–1398. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.R., Jr.; Howley, E.T. Maximal oxygen uptake: “classical” versus “contemporary” viewpoints. Med. Sci. Sports Exerc. 1997, 29, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S. Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 2009, 106, 2060–2062. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Marino, F.E. Does a central governor regulate maximal exercise during combined arm and leg exercise? A rebuttal. Eur. J. Appl. Physiol. 2008, 104, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998; p. 104. [Google Scholar]
- Chen, M.J.; Fan, X.; Moe, S.T. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: A meta-analysis. J. Sports Sci. 2002, 20, 873–899. [Google Scholar] [CrossRef] [PubMed]
- Coquart, J.B.; Legrand, R.; Robin, S.; Duhamel, A.; Matran, R.; Garcin, M. Influence of successive bouts of fatiguing exercise on perceptual and physiological markers during an incremental exercise test. Psychophysiology 2009, 46, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Davies, B.L.; Williams, J.G. Use of perceived effort ratings to control exercise intensity in young healthy adults. Eur. J. Appl. Physiol. 1987, 56, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Faulkner, J.A.; Mason, E.A.; Parfitt, G. The validity of predicting maximal oxygen uptake from perceptually regulated graded exercise tests of different durations. Eur. J. Appl. Physiol. 2006, 97, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Hosang, L.; Mouchlianitis, E.; Guérin, S.M.R.; Karageorghis, C.I. Effects of exercise on electroencephalography-recorded neural oscillations: A systematic review. Int. Rev. Sport Exerc. Psychol. 2022, 1–54. [Google Scholar] [CrossRef]
- Nielsen, B.; Nybo, L. Cerebral changes during exercise in the heat. Sports Med. 2003, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nybo, L.; Nielsen, B.; Sato, K.; Oue, A.; Yoneya, M.; Sadamoto, T.; Ogoh, S.; De Pauw, K.; Roelands, B.; Marušič, U.; et al. Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J. Appl. Physiol. 2001, 91, 2017–2023. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Oshiro, M.; Namba, M.; Shibasaki, M. Effects of aerobic exercise under different thermal conditions on human somatosensory processing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R629–R636. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Namba, M.; Oshiro, M.; Kakigi, R.; Nakata, H. Suppression of cognitive function in hyperthermia; From the viewpoint of executive and inhibitive cognitive processing. Sci. Rep. 2017, 7, srep43528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, J.W.; Ofori, E.; Misra, G.; Hess, C.W.; Vaillancourt, D.E. Beta-band activity and connectivity in sensorimotor and parietal cortex are important for accurate motor performance. NeuroImage 2017, 144, 164–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jenkinson, N.; Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Demiralp, T.; Ademoglu, A.; Istefanopulos, Y.; Başar-Eroglu, C.; Başar, E. Wavelet analysis of oddball P300. Int. J. Psychophysiol. 2001, 39, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Hämäläinen, H.; Krause, C.M. Brain oscillatory 4–30 Hz responses during a visual n-back memory task with varying memory load. Brain Res. 2007, 1138, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hottenrott, K.; Taubert, M.; Gronwald, T. Cortical Brain Activity is Influenced by Cadence in Cyclists. Open Sports Sci. J. 2013, 6, 9–14. [Google Scholar] [CrossRef]
- Ludyga, S.; Hottenrott, K.; Gronwald, T. Four weeks of high cadence training alter brain cortical activity in cyclists. J. Sports Sci. 2017, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Okazaki, K. Body Temperature Regulation During Exercise and Hyperthermia in Diabetics. In Diabetes and Its Complications; IntechOpen: London, UK, 2018; pp. 88–109. [Google Scholar] [CrossRef]
- Cantero, J.L.; Atienza, M.; Salas, R.M. Human alpha oscillations in wakefulness, drowsiness period, and REM sleep: Different electroencephalographic phenomena within the alpha band. Neurophysiol. Clin. 2002, 32, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Plattner, K.; Lambert, M.I.; Tam, N.; Lamberts, R.P.; Baumeister, J. Changes in cortical beta activity related to a biceps brachii movement task while experiencing exercise induced muscle damage. Physiol. Behav. 2014, 123, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Büchel, D.; Sandbakk, Ø.; Baumeister, J. Exploring intensity-dependent modulations in EEG resting-state network efficiency induced by exercise. Eur. J. Appl. Physiol. 2021, 121, 2423–2435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaire, A.; Becke, A.; Düzel, E. Effects of Physical Exercise on Working Memory and Attention-Related Neural Oscillations. Front. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kao, S.-C.; Wang, C.-H.; Hillman, C.H. Acute effects of aerobic exercise on response variability and neuroelectric indices during a serial n-back task. Brain Cogn. 2020, 138, 105508. [Google Scholar] [CrossRef] [PubMed]
- Kubitz, K.A.; Mott, A.A. EEG power spectral densities during and after cycle ergometer exercise. Res. Q. Exerc. Sport 1996, 67, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mierau, A.; Schneider, S.; Abel, T.; Askew, C.; Werner, S.; Strüder, H.K. Improved sensorimotor adaptation after exhaustive exercise is accompanied by altered brain activity. Physiol. Behav. 2009, 96, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Zimmer, P.; Hülsdünker, T.; Lefebvre, J.; Binnebößel, S.; Oberste, M.; Bloch, W.; Strüder, H.; Mierau, A. The effects of exercise intensity and post-exercise recovery time on cortical activation as revealed by EEG alpha peak frequency. Neurosci. Lett. 2018, 668, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Amann, M. Central and peripheral fatigue: Interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 2011, 43, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Keogh, A.; Davenport, J.; Lawlor, A.; Smyth, B.; Caulfield, B. An evaluation of the training determinants of marathon performance: A meta-analysis with meta-regression. J. Sci. Med. Sport 2020, 23, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W. The limit to exercise tolerance in humans: Mind over muscle? Eur. J. Appl. Physiol. 2010, 109, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Meyniel, F.; Sergent, C.; Rigoux, L.; Daunizeau, J.; Pessiglione, M. Neurocomputational account of how the human brain decides when to have a break. Proc. Natl. Acad. Sci. USA 2013, 110, 2641–2646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Subjects | Mean | Standard Deviation (SD) |
|---|---|---|
| Age (years) | 25.81 | 4.92 |
| Height (cm) | 180.55 | 6.78 |
| Weight (kg) | 73.90 | 10.71 |
| Body mass index (kg/m2) | 22.59 | 2.27 |
| O2max (mL·min−1·kg−1) | 55.74 | 7.51 |
| Variable | Mean | SD | Range |
|---|---|---|---|
| O2max (mL·min−1·kg−1) | 55.74 | 7.51 | 40.0 |
| Maximal Aerobic Power (W) | 273.78 | 42.49 | 180.0 |
| Relative Maximal Aerobic Power (W/kg) | 3.74 | 0.67 | 3.4 |
| HRmax (bpm) | 188.52 | 9.83 | 45.0 |
| Time (s) | 967.44 | 162.52 | 713.0 |
| RPE | 16.79 | 1.50 | 5.0 |
| n | O2max (mL·min−1·kg−1) | Age (Years) | Height (cm) | Training/Week (Hour) | Final RPE | RCP O2max) | |
|---|---|---|---|---|---|---|---|
| NONEND | n = 22 | 51.77 ± 6.41 | 24.23 ± 4.34 | 181.95 ± 8.00 | 6.95 ± 4.74 | 16.55 ± 1.72 | 73.97 ± 11.89 |
| END | n = 20 | 60.10 ± 6.16 | 27.55 ± 5.04 | 179.00 ± 4.87 | 8.05 ± 4.75 | 17.08 ± 3.99 | 77.02 ± 7.52 |
| F value | −4.10 | −2.25 | 1.08 | −0.91 | −1.04 | −0.557 | |
| p value | <0.001 | 0.03 | 0.285 | 0.368 | 0.305 | 0.581 | |
| NONPLAT | n = 20 | 55.00 ± 5.64 | 25.85 ± 5.17 | 181.75 ± 6.84 | 8.15 ± 5.82 | 16.66 ± 4.02 | 73.88 ± 11.08 |
| PLAT | n = 22 | 56.41 ± 8.96 | 25.77 ± 4.81 | 179.45 ± 6.70 | 6.86 ± 3.45 | 16.91 ± 1.47 | 76.82 ± 9.05 |
| F value | 0.353 | 0.684 | 0.665 | 1.149 | −0.271 | −1.11 | |
| p value | 0.72 | 0.498 | 0.510 | 0.258 | 0.788 | 0.273 |
| Alpha/Beta Ratio | Theta Increase (%start) | Beta Increase (%start) | ||
|---|---|---|---|---|
| NONEND | n = 22 | −20.30 ± 12.93 | 20.39 ± 16.43 | 40.06 ± 19.97 |
| END | n = 20 | −15.74 ± 11.93 | 20.24 ± 16.43 | 47.00 ± 16.14 |
| F value | 1.36 | 0.01 | 1.33 | |
| p value | 0.250 | 0.907 | 0.256 | |
| NONPLAT | n = 20 | −18.58 ± 12.71 | 18.29 ± 13.31 | 44.26 ± 18.59 |
| PLAT | n = 22 | −17.93 ± 12.67 | 22.25 ± 18.65 | 42.34 ± 18.93 |
| F value | <0.001 | 0.74 | 0.08 | |
| p value | 0.988 | 0.395 | 0.778 |
| O2 | Alpha PSD | Beta PSD | Theta PSD | Alpha/Beta Ratio | O2 | O2 | O2 | |
|---|---|---|---|---|---|---|---|---|
| R2 | 0.967 | 0.037 | 0.363 | 0.061 | 0.031 | 0.096 | 0.173 | 0.170 |
| F | 25,011.7 | 16.0 | 238.1 | 27.0 | 13.1 | 42.7 | 85.6 | 84.0 |
| Pr > F | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Positive Alpha Tendency | Negative Alpha Tendency | p Value | |
|---|---|---|---|
| NONEND | 12 | 10 | 0.07 |
| END | 15 | 5 | |
| NOPLAT | 14 | 6 | 0.75 |
| PLAT | 12 | 10 |
| Chi-Square (Observed Value) | Chi-Square (Critical Value) | DF | p Value | Alpha |
|---|---|---|---|---|
| 3.663 | 7.815 | 3 | 0.300 | 0.05 |
| Cadence | O2 | O2 | O2 | α/β Ratio | All | |
|---|---|---|---|---|---|---|
| Chi-square (Observed value) | 0.005 | 0.225 | 0.431 | 0.558 | 0.288 | 7.61 |
| Chi-square (Critical value) | 3.84 | 3.85 | 3.86 | 3.84 | 3.87 | 16.9 |
| p value | 0.945 | 0.636 | 0.512 | 0.455 | 0.591 | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billat, V.; Berthomier, C.; Clémençon, M.; Brandewinder, M.; Essid, S.; Damon, C.; Rigaud, F.; Bénichoux, A.; Maby, E.; Fornoni, L.; et al. Electroencephalography Response during an Incremental Test According to the V̇O2max Plateau Incidence. Appl. Sci. 2024, 14, 5411. https://doi.org/10.3390/app14135411
Billat V, Berthomier C, Clémençon M, Brandewinder M, Essid S, Damon C, Rigaud F, Bénichoux A, Maby E, Fornoni L, et al. Electroencephalography Response during an Incremental Test According to the V̇O2max Plateau Incidence. Applied Sciences. 2024; 14(13):5411. https://doi.org/10.3390/app14135411
Chicago/Turabian StyleBillat, Véronique, Christian Berthomier, Michel Clémençon, Marie Brandewinder, Slim Essid, Cécilia Damon, François Rigaud, Alexis Bénichoux, Emmanuel Maby, Lesly Fornoni, and et al. 2024. "Electroencephalography Response during an Incremental Test According to the V̇O2max Plateau Incidence" Applied Sciences 14, no. 13: 5411. https://doi.org/10.3390/app14135411








