Featured Application
Featured Application: The aim of our research is to identify biomarkers with a high specificity and sensitivity for IBD. The identification of new diagnostic methods would support the early diagnosis of IBD in outpatient settings, therefore limiting the need for hospitalization. Introducing novel biomarkers would ensure the non-invasive and precise monitoring of the disease activity. Moreover, the results of our research provide new information about the possible interactions between proinflammatory mediators in IBD, and reveal a critical difference in inflammatory patterns during CD and UC. The precise understanding of pathological mechanisms during development of the disease is crucial for inventing modern therapies and improving health care.
Abstract
Inflammatory Bowel Disease (IBD) is a group of chronic intestinal diseases, among which Crohn’s disease (CD) and ulcerative colitis (UC) represent the two main types. The differential diagnosis of these two disorders is often a significant challenge, as there is a lack of specific and non-invasive biomarkers. In this study, we assessed the serum profile of proinflammatory mediators (E- and P-selectin, CCL2, IL-1α, IL-12p70, TNF-α) in patients with IBD to identify biomarkers helpful in the differential diagnosis of CD and UC. The conducted statistical analyses revealed a significant increase in E-selectin, P-selectin, IL-1α, and IL-12p70 levels in the serum of CD patients compared to UC. The performed ROC curve analysis identified moderate values of E-selectin (AUC 0.752), P-selectin (AUC 0.733), and IL-1α (AUC 0.731) in differentiating CD from UC, while IL-12p70 presented a satisfactory value (AUC 0.695). Simultaneous measurements of each biomarker with serum calprotectin improved the ability of E-selectin (AUC 0.752 vs. 0.829), P-selectin (AUC 0.733 vs. 0.75), IL-1α (AUC 0.731 vs. 0.778), and IL-12p70 (AUC 0.695 vs. 0.714) to differentiate CD from UC. Moreover, we identified a significant relationship between the concentration of CCL2 (r = 0.566, p < 0.005) and TNF-α (r = 0.431, p < 0.05) and the disease activity expressed as the Mayo score in the UC group. We also identified a significant relationship between the concentration of E-selectin (r = 0.372, p < 0.05), CCL-2 (r = 0.55, p < 0.05), IL-1α (r = 0.637, p < 0.005), and TNF-α in the group of patients with UC. Another significant correlation in the UC group was noted in the case of E-selectin and IL-12p70 (r = 0.542, p < 0.05), as well as between IL1-α and P-selectin (r = 0.514, p < 0.05). The results obtained in this study indicate the potential use of E-selectin, P-selectin, IL-1α, and IL-12p70 serum profiles in differentiating CD from UC. Regarding the significant relationship of CCL2 and TNF-α with the Mayo score, these two biomarkers might be useful in assessing and monitoring the disease activity during UC.
Keywords:
Crohn’s disease; ulcerative colitis; differential diagnosis; E-selectin; P-selectin; CCL2; IL-1α; IL-12p70; TNF-α 1. Introduction
Inflammatory Bowel Disease (IBD) comprises a spectrum of chronic inflammatory disorders affecting the intestines, among which Crohn’s disease (CD) and ulcerative colitis (UC) represent the two major diseases. The pathomechanism of CD and UC is multifaced and involves immunological, genetical, microbial, and environmental factors. Despite some similarities in disease etiology, these conditions manifest distinct presentations. In Crohn’s disease, lesions typically exhibit transmural involvement and can be located in any part of the gastrointestinal tract. In contrast, ulcerative colitis is usually limited to the intestinal mucosa typically progressing from the rectum to proximal segments of the intestines [1]. Key differences in the course of CD and UC are also related to the risk of stricture and fistula formation, being more frequent in Crohn’s disease. Consequently, the subclassification of IBD into CD and UC represents critical procedural steps in clinical settings, as the former determines the implemented treatment and the prognosis of the course of the disease. Misdiagnoses may not only be related to the lower effectiveness of the implemented medicines, but may also affect the choice of preferable surgical treatment and enhance the risk of future complications. The initial diagnosis of IBD and further differential diagnosis between CD or UC is typically established based on clinical symptoms, endoscopic examination, and laboratory tests. Endoscopic examination is a valuable diagnostic tool; however, certain disease manifestations occur such as erythema, loss of vascularity, erosions, and ulcers. Consequently, endoscopic examination particularly in the early stage of the disease may not provide adequate diagnostic precision. Fecal calprotectin represents the most commonly used diagnostic test in IBD, being a highly sensitive disease marker. Nonetheless, increased fecal calprotectin levels lack specificity for IBD and are insufficient for differentiating CD from UC. Moreover, the difficulties in the differential diagnosis may be further underlined by the observation that 10–15% of patients initially diagnosed with Crohn’s disease are subsequently reclassified as exhibiting ulcerative colitis within the first year of diagnosis [1,2,3]. Therefore, there is a serious need to develop new diagnostic methods, which could support the differential diagnosis of IBD to Crohn’s disease or ulcerative colitis and could be used to monitor the disease activity.
Regarding the crucial role of the inflammatory response in the initiation and development of Crohn’s disease, as well as ulcerative colitis, in this research, we verified the potential of proinflammatory mediators as biomarkers used in differential diagnosis of these two diseases. The inflammatory response in CD and UC develops as a result of an excessive immune response; however, it occurs through different molecular and cellular pathways. At the cellular level, one of the differences between CD and UC is that Crohn’s disease is related to the excessive response of Th1 and Th17 cells, while ulcerative colitis is linked to upregulation of the Th2 cells. During Crohn’s disease, the differentiation of Th1 cells from naïve T cells is triggered by the release of, among others, IL-12 from the antigen-presenting cells. Activated Th1 cells secrete proinflammatory cytokines like IL-6, IFN-γ, and TNF-α, which further enhance the inflammatory process. Naïve T cells, upon exposure to IL-6 and TGF-β, may polarize to Th17 cells, being engaged in the pathogenesis of CD. In the presence of IL-6, IL-23, and IL-1β, Th17 cells may induce the release of proinflammatory molecules such as granulocyte–macrophage colony-stimulating factor, chemokines, IL-6, and TNF-α, resulting in the uncontrolled inflammation. Additionally, Th17 cells present high plasticity; therefore, they can easily polarize into Th1 cells, which has been reported in some experimental animal models of IBD [4,5]. In the course of UC, the polarization of naïve T cells to Th2 cells stimulates the synthesis of proinflammatory cytokines such as IL-4, IL-13, and TNF-α. The observed differences in cytokine profiles between the diseases indicate a potential role of IL-12, as a biomarker used in differentiating CD from UC. Despite the observed upregulation of TNF-α in both Crohn’s disease and ulcerative colitis, the cellular sources of TNF-α and the local profile of other cytokines are divergent. Therefore, the level of TNF-α, as well as its influence on immune cells and the development of the inflammatory response, differs between CD and UC, suggesting the potential utility of this parameter in the diagnosis of the diseases. The precise differences in TNF-α action during the development of CD and UC are not clear; however, TNF-α is able to induce the proinflammatory pathways such as nuclear factor κ B (NF-κB) or mitogen-associated protein kinase (MAPK), resulting in the synthesis of other cytokines and chemokines like CCL2 (also known as monocyte chemoattractant protein-1, MCP-1). CCL-2 plays an important role in CD and UC as it recruits the monocytes, which are cells with a significant contribution to the pathogenesis of the diseases, to the inflammatory site and participates in the polarization of naïve T cells to Th2 cells, being a phenotype characteristic for UC. Regarding the influence of CCL-2 on the immune cells, the serum profile of this biomarker might be useful in differentiating UC from CD [6,7,8,9]. The development of the inflammatory process is also related to the alterations of endothelial cells, which, upon inflammatory triggers, express the adhesion molecules such as E- and P-selectin. E-selectin is exclusively expressed on the surface of endothelial cells, where it binds to the neutrophils, as well as to monocytes, basophils, and T cells. The expression of E-selectin on the cell surface occurs in the early stage of inflammation in response to IL-1 and TNF-α. Another adhesive molecule, P-selectin, is stored in the Weibel–Palade bodies of endothelial cells and in platelet granules. Factors triggering the release of P-selectin and its relocation to the cell membrane include histamine, thrombin, and TNF-α. The ligands for P-selectin are located on the surface of leukocytes and platelets; hence, the circulating level of P-selectin may not only reflect the activity of the inflammatory process, but also the activity of platelets, identifying the risk of thrombotic events [10,11,12]. One of the mediators of leukocyte–endothelial adhesion is IL-1α, which enhances the expression of vascular cell adhesion molecule-1 on endothelial cells. IL-1α can act as a transcription factor but can also be expressed on the surface of numerous cells, including endothelial cells and epithelial cells of the gastrointestinal tract. This cytokine fulfills the role of “alarmin” since, upon release from necrotic cells, it can initiate and propagate local inflammatory processes [13,14]. Considering the contrast in the activation and recruitment of leukocytes, as well as potential differences in leukocyte–endothelial interactions between Crohn’s disease and ulcerative colitis, the assessment of adhesion molecules and their mediators in the serum of patients could be used in the differential diagnosis of the diseases.
The aim of this study is to identify diagnostic methods that could be used in the differential diagnosis of Crohn’s disease and ulcerative colitis. Therefore, in this research, we analyzed the serum profile of proinflammatory markers related to the pathogenesis of the disease (IL-12, TNF-α, CCL-2, E-selectin, P-selectin, IL-1α) in patients with CD and UC to assess their utility in the differential diagnosis of the diseases. Moreover, another aim of this study is to identify to usefulness of the introduced biomarkers in the evaluation of the disease activity.
2. Materials and Methods
2.1. Study Population
The study group of this research included 46 newly diagnosed patients with Inflammatory Bowel Disease, among which 30 were identified with ulcerative colitis (UC) and 16 with Crohn’s disease (CD). The diagnosis of the disease was made at the Department of Gastroenterology of St. Barbara’s Regional Specialist Hospital in Sosnowiec on the basis of laboratory tests, endoscopic examination, and clinical symptoms. The activity of the disease was assessed with the Mayo endoscopic scale in the case of patients with ulcerative colitis and with the Crohn’s Disease Activity Index (CDAI) in patients with Crohn’s disease. The inclusion criteria of this study were an age above 18 years old and newly diagnosed ulcerative colitis or Crohn’s disease, while the exclusion criteria included unstable coronary disease; chronic liver or kidney disease; toxic or fulminant colitis; unstable coronary disease; severe fungal, viral, or bacterial infections; and pregnancy and breastfeeding. The material investigated in this study was venous blood collected from patients with UC and CD before treatment, for which consent from the Bioethics Committee was obtained (KNW/0022/KB/309/15). Venous blood samples with a volume of 9 mL were collected into tubes with no added anticoagulant and centrifuged for 10 min at 1500× g at 4 °C to separate serum. The collected serum was portioned and preserved at −80 °C for biochemical analysis.
2.2. Methods
In this study, the profile of proinflammatory mediators including E-and P-selectin, IL-1α, IL-12p70 (active form of IL-12), TNF-α, and chemokine CCL2 was assessed in the serum of patients with UC and CD. Regarding the fact that the implication of new diagnostic methods in daily clinical practice is dependent on the sensitivity and specificity of the biomarkers to the disease, but also on the simplicity of the methodology, the measurement of analyzed markers was performed using the bead-based multiplex assay of the Luminex 200 Flow cytometry system (Luminex Corporation, Austin, TX, USA). This methodology can be easily integrated into routine use in medical laboratories in the future, as it allows for analysis of multiple parameters using a minimal sample volume. Additionally, the assessment process remains straightforward. The measurements of serum calprotectin were conducted using the ELISA test from the Immunodiagnostik AG company (Bensheim, Germany). The test used in this research measured both subunits of calprotectin—S100A8 and S100A9. The intra-assay variability of this test was 3.3%, while the sensitivity equalled 0.897 ng/mL.
2.3. Statistical Analyses
The obtained data were evaluated with the use of STATISTICA software (StatSoft 13.3, Kraków, Poland). The data distribution was assessed with the Shapiro–Wilk test. The characteristics of the parameters are presented as the mean values and standard deviation in the case of normally distributed data and the median and interquartile range in the case of non-normally distributed ones. The statistical significance of difference between analyzed groups was performed with the use of Student’s t test (normally distributed data) and the Mann–Whitney U test (non-normally distributed data). The diagnostic utility of analyzed biomarkers was estimated using ROC curve analysis, which evaluates the sensitivity and specificity of the parameters in differentiating patients with Crohn’s disease from those with ulcerative colitis. The cut-off values proposed in this article were defined using Youden’s index. The correlation between analyzed parameters was evaluated using the Pearson test. The significance level of p < 0.05 was used with all conducted statistical analyses.
3. Results
3.1. Patients Characteristics
Patients included in this study were divided into two groups according to the diagnosed disease. The clinical characteristics of each group are presented in Table 1. The data presented in Table 1 have already been published and, as part of this research group, were also included in our previously conducted studies [15]. The activity of the disease in the group of patients with Crohn’s disease was assessed with the use of the CDAI score, in which severe disease was classified as more than 450 points of that scale, moderate as 220–450 points, and mild disease as 150–220 points [16]. In this group of patients, severe disease was noted in the case of 1 (6%) individual and moderate disease in 15 (94%) individuals. The intensity of inflammatory processes was assessed by the measurement of the C-reactive protein (CRP) level in the serum. The reference range for CRP concentration was estimated as lower than 10 mg/L. The increased level of CRP was noted in the case of 8 out of 16 patients with CD, while the median level equaled 18.8 (±20.1) mg/L. In the group of patients with ulcerative colitis, severe disease was defined as 3 points on the Mayo endoscopic scale, moderate as 2 points, and mild disease as 0–1 point [17]. Severe disease was noted in 18 (60%) patients, while 12 (40%) patients were classified with moderate disease. The median CRP level in this group equaled 14.4 (±24.5) mg/L; however, an increased CRP level was noted in the case of 10 patients.

Table 1.
Clinical characteristics of patients with Crohn’s disease and ulcerative colitis.
3.2. Comparison of Serum Proinflammatory Mediators Level between Patients with Crohn’s Disease and Ulcerative Colitis
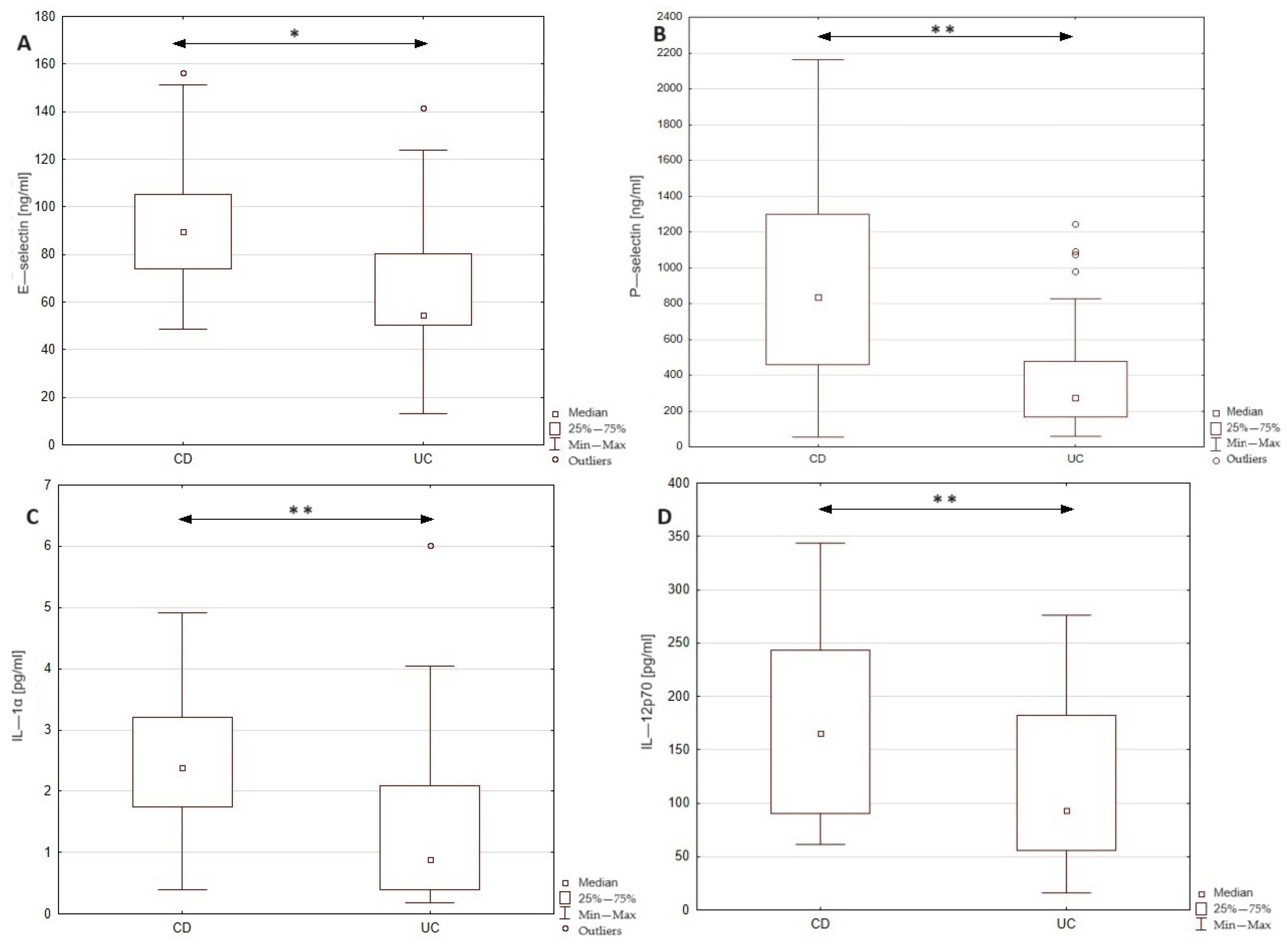
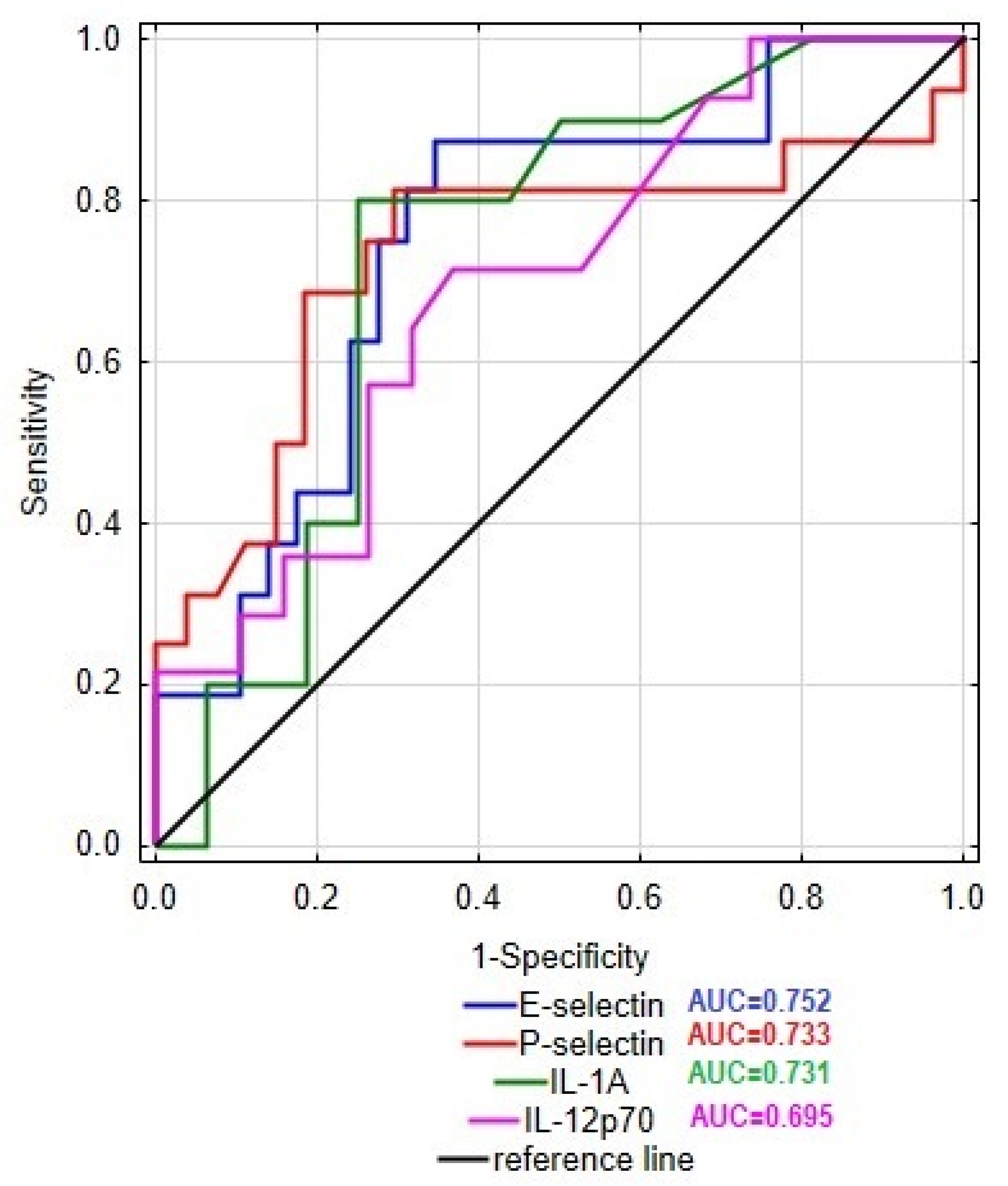
In this study, we examined the level E- and P-selectins, CCL-2, IL-1α, IL-12p70, and TNF-α in the serum of patients with Crohn’s disease and ulcerative colitis in order to identify the differences in the profile of inflammatory mediators between these two groups of patients. The obtained results together with the statistical significance of dissimilarities between analyzed groups are presented in Figure 1. The conducted statistical analysis revealed a significance difference in the serum profile of E- and P-selectin, IL-1α, and IL-12p70 between patients with CD and UC. In the group of CD patients, the level of E-selectin was increased by 64% compared to the UC group (89.6 vs. 54.5 ng/mL). In the case of P-selectin, the observed increase in the serum of CD patients was even higher, reaching 204% compared to the UC group (838.1 vs. 275.4 ng/mL). To identify the potential source (endothelial cells or platelets) of P-selectin, we additionally evaluated the correlation between P-selectin and platelet count in both groups of patients. Statistical analyses revealed a significant moderate correlation between the serum level of P-selectin and platelet count in the group of patients with CD (r = 0.519, p < 0.05); however, no such relationship was noted in the UC group. Thus, the presented results may indicate differences in the source of P-selectin among these two groups. Significant differences were noted also in the cases of IL-1α and IL-12p70, where subjects with CD presented a higher concentration of the mentioned cytokines compared to patients with UC (IL-1α: 2.4 vs. 0.9; IL-12p-70: 165.7 vs. 94.2) by 167 and 76%, respectively. No significant difference was, however, noted in the level of CCL-2 and TNF-α between the analyzed groups. In order to assess the specificity and sensitivity of the analyzed parameters in the differential diagnosis of CD and UC, we drew the receiver operating characteristic (ROC) curve, as presented as Figure 2. The ROC curve analysis revealed that the ability of E-selectin, P-selectin, IL-1α, and IL-12p70 in differentiating Crohn’s disease from ulcerative colitis was statistically significant. The serum profile of E-selectin presented a moderate value in differentiating CD from UC with AUC 0.752 (95% CI 0.604–0.900). The proposed cut-off value for E-selectin was estimated as 61.7 ng/mL with a Youden index of 0.53, which led to 10 false positive cases of CD and 2 false negative cases of CD. A similar sensitivity and specificity was noted in the case of P-selectin with AUC 0.733 (95% CI 0.556–0.909) and IL-1α with AUC 0.731 (95% CI 0.532–0.930). The cut-off value for P-selectin was defined as 441.2 ng/mL with a Youden index of 0.52, and it was related to 8 cases of false positive and 3 cases of false negative diagnoses of CD. The cut-off value of IL-1α was 1.7 pg/mL with a Youden index of 0.55, resulting in 4 false positive and 2 false negative diagnoses of CD. In the case of IL-12p70, the value of differentiating CD from UC was estimated as satisfactory with AUC 0.695 (95% CI 0.515–0.876). The cut-off value was defined as 144.1 with a Youden index of 0.35, and this was related to 7 false positive and 4 false negative diagnoses of CD. In this study, serum calprotectin was measured in both groups of patients (CD group: 3537.5 ± 1893.8; UC group: 3337.1 ± 1775), together with ROC curve analysis for this biomarker. The performed analysis revealed that serum calprotectin presented a satisfactory value (AUC 0.604; 95% CI 0.424–0.783) for differentiating CD from UC. To enhance the sensitivity and specificity of the analyzed biomarkers in the differential diagnosis of IBD, we conducted ROC curve analyses including each biomarker paired with serum calprotectin level. The cut-off value of serum calprotectin was estimated as 2890 ng/mL to achieve optimal sensitivity and specificity. The inclusion of serum calprotectin level in the ROC curve analysis improved the AUC values of the analyzed biomarkers. In patients with serum calprotectin levels below 2890 ng/mL, the AUC of E-selectin was 0.829 (95% CI 0.641–1), that of P-selectin was 0.75 (95% CI 0.47–1), that of IL-1α was 0.778 (95% CI 0.386–1), and that of IL-12p70 was 0.85 (95% CI 0.633–1). In patients with serum calprotectin levels above 2890 ng/mL, the AUC value of E-selectin was 0.769 (95% CI 0.534–1), that of P-selectin was 0.782 (95% CI 0.567–0.997), that of IL-1α was 0.575 (0.255–0.895), and that of IL-12p70 was 0.714 (0.423–1).
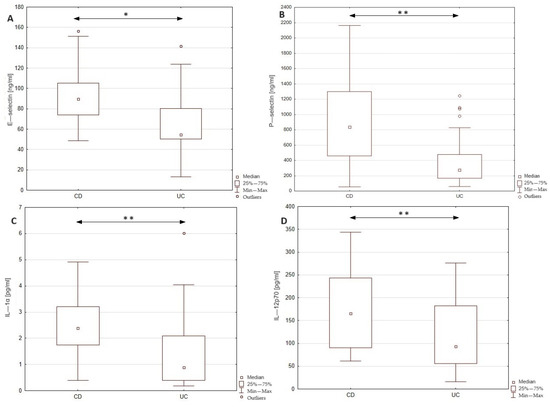
Figure 1.
Comparison of serum E—selectin, P—selectin, IL—1α, and IL—12p70 levels between patients with Crohn’s disease and ulcerative colitis. * p < 0.01; ** p < 0.05; CD—patients with Crohn’s disease; UC—patients with ulcerative colitis.
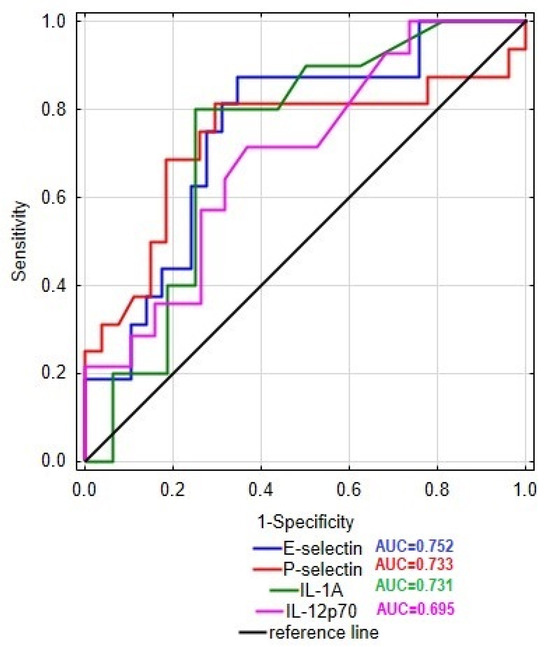
Figure 2.
ROC curve of E-selectin, P-selectin, IL-1α, and IL-12p70 in differentiating patients with Crohn’s disease from patients with ulcerative colitis.
3.3. Relationship between Profile of Proinflammatory Mediators and Disease Activity
One of the aims of our research was to assess the utility of proinflammatory mediators in reflecting disease activity in patients with CD and UC. The results obtained from the statistical analyses of these relationships are presented in Table 2. A significant correlation between analyzed markers and disease activity was noted only in the case of patients with ulcerative colitis. In that group, the serum profile of CCL-2 and TNF-α correlated with disease activity assessed by the Mayo endoscopic scale. Statistical analysis revealed that the relationship between disease activity and CCL-2 level was moderate (r = 0.566, p < 0.005), similar to the correlation between disease activity and TNF-α (r = 0.431, p < 0.05) concentration in the serum of UC patients. In this study, we did not identify a valid correlation between the level of analyzed biomarkers and CRP in the serum of both CD and UC patients. Additionally, the relationship between analyzed biomarkers with serum calprotectin level was analyzed. In the group of patients with Crohn’s disease, the correlation between serum calprotectin and proinflammatory mediators was estimated as not statistically significant; however, in the group of patients with ulcerative colitis, a significant correlation was noted between serum calprotectin and IL-1α (r = 0.753, p < 0.005), as well as TNF-α (r = 0.465, p < 0.05).

Table 2.
Relationship between analyzed proinflammatory mediators and disease activity in patients with Crohn’s disease and ulcerative colitis.
3.4. Interrelationship between Analyzed Proinflammatory Mediators in Patients with Crohn’s Disease and Ulcerative Colitis
In this study, we also analyzed the significance of the relationship between the analyzed parameters since their expression depends, among other factors, on the cytokine and chemokine environment. In the group of patients with Crohn’s disease, we noted no significant relationship between the assessed proinflammatory mediators; however, some valid correlations were noted in the UC group (presented in Table 3). The cytokine that relates to the most proinflammatory markers assessed in this study was TNF-α. The concentration of TNF-α correlated with the level of E-selectin, CCL-2, and IL-1α in the serum of patients with UC. The correlation between TNF-α and IL-1α was classified as strong (r = 0.637, p < 0.005), and in the case of E-selectin (r = 0.372, p < 0.05) and CCL-2 (r = 0.55, p < 0.05), the relationship with TNF-α was defined as moderate. Another significant relationship was noted in the case of IL-1α, whose level in the serum of patients with UC correlated moderately with the concentration of P-selectin (r = 0.514, p < 0.05). Similarly, a moderate relationship was noted in the case of IL-12p70 and E-selectin (r = 0.542, p < 0.05) serum levels in the UC group.

Table 3.
Relationship between analyzed biomarkers in patients with ulcerative colitis.
4. Discussion
4.1. Comparison of Serum Proinflammatory Mediators Level between Patients with Crohn’s Disease and Ulcerative Colitis
The aim of our study was to identify biomarkers, which could be used in the differential diagnosis of CD and UC. The conducted statistical analysis revealed a significant difference in the serum level of IL-1α, IL-12p70, and E- and P-selectin between the two groups of patients, indicating a potential use of the mentioned biomarkers in differentiating Crohn’s disease from ulcerative colitis. All of the mentioned parameters were upregulated in the group of patients with CD compared to the UC group. The increase in IL12p70 level in the serum of CD patients can be related to the excessive activation of both innate and adaptive immune cells. The activated innate cells like dendritic cells and macrophages secrete IL-12p70, which is engaged in the polarization of T-naïve cells to Th1 cells, with the pathogen cells being characteristic for CD but not UC. This differentiation of T cells enable them to synthesize and release IFN-γ, being one of the inductors of IL-12p70 synthesis, creating a positive feedback loop [18]. The observed increase in the active IL-12 form (IL-12p70) may be compared to the results of Monteleone et al.’s [19] study, in which the expression of that cytokine was assessed in the mononuclear cells of the intestinal lamina propria collected from CD and UC patients. The expression of active IL-12 in the samples from CD patients was elevated compared to the UC and control groups, which seems to be consistent with our results. Both ours and Monteleone et al.’s studies indicate crucial differences in the IL-12p70 profile in circulation and at the tissue level between Crohn’s disease and ulcerative colitis. Given the satisfactory value of IL-12p70 to differentiate CD from UC, this parameter may be useful in the diagnosis of these two types of IBD.
The conducted statistical analyses revealed a significant increase in E- and P-selectin levels among patients with CD in comparison to the UC group. Considering that P-selectin can be expressed by both platelets and endothelial cells, we have analyzed the correlation between serum P-selectin level and platelet count in CD and UC patients. The conducted analyses revealed a significant moderate correlation between the P-selectin and platelet count, but only in group of patients with Crohn’s disease. The obtained results may indicate that circulating P-selectin in CD patients is mostly cleaved from the surface of platelets, while in UC, the main source of P-selectin could be the endothelial cells. Regarding the lack of significant difference in PLT count between patients with CD and UC, this hypothesis should be further analyzed. The performed analysis of the ROC curve in our study indicated that the P-selectin profile allowed Crohn’s disease to be differentiated from ulcerative colitis with a moderate value, indicating a potential use of this parameter in differential diagnosis. Moreover, another advantage of this biomarker is that the serum P-selectin level can reflect the risk of thrombotic events among patients with both CD and UC, as its level is related to the activation of platelets, often occurring during the course of IBD. The serum P-selectin level in healthy individuals usually equals 100 ng/mL, while in the CD group, the median level was 838.1 ng/mL, and 275.4 ng/mL in the UC group, indicating the increased activation of platelets in both CD and UC [12].
Another adhesive molecule that increased in the group of CD patients compared to UC patients is E-selectin, being expressed on the surface of endothelial cells. The observed increase in E-selectin in the serum of CD patients, together with the good ability of this biomarker of differentiating the CD from UC, demonstrates a potential use of this parameter in diagnosis of the diseases. The observed changes in E-selectin level between CD and UC patients may be explained by the study conducted by Pooley et al.’s [10], in which, upon exposure of the intestinal tissue collected from CD and UC patients to a proinflammatory medium, the expression of E-selectin increased 5.5 times in CD patients and 2 times in UC patients. These results indicate valid differences in the excess of E-selectin upregulation between CD and UC patients in response to the inflammatory microenvironment. One of the factors enhancing the expression of E-selectin is a well-known proinflammatory cytokine—IL-1β—whose activation and release were enhanced in CD compared to UC in Lazaridis et al.’s study [20]. These results suggest that the activation and release of IL-1β are enhanced in CD compared to UC; therefore, the observed enhanced expression of E-selectin can be related to the upregulation of IL-1β. Regarding the valid increase in E-selectin level upon exposition to the proinflammatory environment on the tissue level, as well as in the serum of CD patients compared to UC, this parameter might be used for the differential diagnosis of these two diseases. This suggestion can be further confirmed by the ROC curve analysis, which showed that E-selectin presented a moderate value of sensitivity and specificity in differentiating Crohn’s disease from ulcerative colitis.
In the group of patients with CD, we also noted a significant increase in serum IL-1α compared to the UC group. IL-1α is expressed constantly in the epithelial cells; however, it can also be upregulated in response to different triggers such as oxidative stress, agonists of Toll-like receptors, and inflammatory cytokines including IL-1β [21]. Regarding the increased release of IL-1β from circulating mononuclear cells noted in the CD group compared to UC in Lazaridis er al.’s study [20], the elevation of IL-1α during Crohn’s disease may also be triggered by the upregulation of IL-1β. Another explanation for the increased IL-1α in the serum of CD patients may be the interactions of P-selectin with leukocytes. The binding of P-selectin to its ligand on the surface of leukocytes may induce the generation of oxidative free radicals in neutrophils, which could serve as a potential source of oxidative stress triggering the release of IL-1α. Considering that IL-1α is typically located inside cells, its extracellular location can indicate cell damage and trigger the inflammatory response. Therefore, it plays a role as an “alarmin”, further enhancing the inflammatory response [21,22]. In contrast to our results, the serum level of IL-1α did not differ between pediatric patients with CD and UC in the study conducted by Ott et al. [8]. At the same time, in Ott et al.’s study, the CCL2 serum profile was different between CD and UC pediatric groups, while no such difference was noted in our study. The observed inconsistency between our study and Ott et al.'s may be related to the age of the patients, as Ott et al. included only patients below 17 years old, while the mean age of patients included in our study was 32 in the CD group and 33 in the UC group. Moreover, in Ott et al.'s [8] study, the activity of the disease was assessed with pediatric scales, and patients included in the research had to be in remission for at least 3 months, while patients in our study presented active disease. Therefore, the differences in the patients' inclusion criteria may be responsible for the observed differences between our study and Ott et al.'s [8] study.
The differences identified in our study in IL-1α serum profile between CD and UC patients might be related to the greater role of that cytokine in the pathogenesis of CD among adults; however, this hypothesis should be further confirmed. The results of our study indicated, however, a valid role of IL-1α in the diagnosis of IBD, as this parameter presented a moderate specificity and sensitivity in differentiating Crohn’s disease from ulcerative colitis.
This study has some limitations, among which study size can be mentioned. Unfortunately, we were not able to confirm our results by comparing them with an independent cohort or “in silico”. Expanding the number of patients included in study groups will enable a more precise evaluation of the specificity and sensitivity of the analyzed biomarkers, as well as allowing for the establishment of cut-off values of these biomarkers for differentiating Crohn’s disease from ulcerative colitis with higher precision. Considering the role of analyzed biomarkers in the inflammatory process, their serum profile may also be elevated in other inflammatory diseases. Therefore, further studies, including patients with different inflammatory diseases, are needed to elucidate the specificity of these biomarkers towards IBD. The performed ROC curve analysis of serum calprotectin and proinflammatory mediators revealed that E-selectin, P-selectin, IL-1α, and IL-12p70 presented a better specificity and sensitivity in differentiating CD from UC than serum calprotectin. However, pairing each parameter with the serum calprotectin level significantly increased the sensitivity and specificity of the diagnosis for both CD and UC. Therefore, simultaneous measurements of serum calprotectin with E-selectin, P-selectin, IL-1α, or IL-12p70 may present greater usefulness in the differential diagnosis of IBD than the measurement of each parameter individually. Unfortunately, our study did not include patients with unclassified colitis, preventing us from elucidating the usefulness of the analyzed proinflammatory mediators in the intermediate type of IBD. Thus, further research should include patients with unclassified colitis as another study group. One of the main advantages of our study is the simplicity of the methodology, particularly the multiplex test using the Luminex 200 flow cytometry system. This approach can be easily integrated into routine clinical practice because it is not time-consuming and allows the assessment of multiple parameters in a small sample volume.
4.2. Relationship between Profile of Proinflammatory Mediators and Disease Activity
In this research, we analyzed the correlation between the assessed serum proinflammatory mediators (E- and P-selectin, CCL2, IL-1α, IL-12p70, TNF-α) and disease activity to identify the utility of these biomarkers in monitoring the course of Crohn’s disease and ulcerative colitis. Statistical analyses revealed a significant moderate correlation between the serum level of CCL2 and TNF-α with disease activity, but only in patients with ulcerative colitis, while no such relationship was noted in the group of patients with CD. The relationship between the Mayo score and CCL2 serum level in the UC group may be related to the dominance of the Th2 phenotype observed during ulcerative colitis. Not only is CCL2 engaged in the differentiation of naïve T cells into Th2 cells, but it also stimulates these cells to release Th2-related proinflammatory cytokines such as IL-4 [9]. The obtained results can be compared to the study conducted by Magnusson et al. [23], in which the researchers analyzed the changes in CCL2 expression in the serum and intestinal tissue collected from patients with UC during anti-inflammatory biological treatment (with infliximab). In the group of patients responding to the implemented treatment, the expression of CCL2 decreased significantly after 14 weeks of therapy in both the serum and intestinal tissue, whereas no such difference was noted in the group of non-responders. The results of our study and Magnusson et al.’s study may indicate the use of serum CCL2 profiling in monitoring the activity of ulcerative colitis, as well as in assessing the effectiveness of the implemented biological treatment.
The role of TNF-α in the pathogenesis of both CD and UC is well established. Due to its crucial role in the inflammatory process, numerous studies have shown an increased expression of TNF-α in biopsies collected from patients with CD and UC [24]. Therefore, one of the aims of our study was to identify the utility of TNF-α in assessing the disease activity. The obtained results presented a significant correlation between TNF-α serum profile and Mayo score in the group of UC patients; however, the level of that cytokine did not correlate with disease activity in the CD group. This observation may indicate that the TNF-α serum profile reflects the activity of the disease better in ulcerative colitis than in Crohn’s disease. Regarding the common use of anti-TNF-α biological treatment during IBD, the measurements of TNF-α might be beneficial in not only monitoring the disease activity but also the response to treatment at the same time. However, this hypothesis needs to be tested.
4.3. Interrelationship between Analyzed Proinflammatory Mediators in Patients with Crohn’s Disease and Ulcerative Colitis
During inflammation, cytokines and chemokines present a unique network of interactions, where the local microenvironment affects the actions of not only immune cells but also inflammatory mediators. Therefore, in order to identify new mechanisms engaged in the initiation and development of the inflammatory process during Crohn’s disease and ulcerative colitis, we evaluated the relationship between the analyzed proinflammatory mediators, being E- and P-selectin, CCL2, IL-1β, IL-12p70, and TNF-α. Significant correlations were noted, but only in the UC group in the case of E-selectin and IL-12p70; P-selectin and IL-1α; and TNF-α with E-selectin, CCL2, and IL-1α.
Considering the fact that P-selectin level did not correlate with platelet count in UC patients, the most likely explanation of the observed correlation between P-selectin and IL-1α is the interactions of P-selectin with leukocytes. The presented moderate relationship between P-selectin and IL-1α may be related to the hypothesis that the increase in IL-1α emerges from the neutrophil-associated generation of free radicals, which could be mediated by the interactions of P-selectin with immune cells. The rise in oxidative stress in local inflamed tissue can stimulate the release of IL-1α [21,22]. Considering that the correlation between P-selectin and IL-1α was noted only in the UC group, the P-selectin–neutrophil–IL-1α axis may be involved in the development of the inflammatory state during UC, but not CD. The greater role of neutrophils in the inflammatory process during UC may be confirmed by the study conducted by Dinallo et al. [25], in which the researchers analyzed the contribution of neutrophils to the pathogenesis of Crohn’s disease and ulcerative colitis. Neutrophils, upon activation, release reactive oxygen species, store enzymes in their granules, and form neutrophil extracellular traps (NETs) to neutralize potential pathogens. In Dinallo et al.’s study, the expression of NET-associated proteins was increased in intestinal biopsies of UC patients compared to patients with CD. Additionally, upon stimulation by lipopolysaccharide and TNF-α, the formation of NETs by neutrophils isolated from the blood of UC patients was enhanced compared to healthy individuals, while no such correlation was noted in the CD group. The presented results indicate the primary role of neutrophils in UC compared to CD. Therefore, the observed correlation between P-selectin and IL-1α may be related to the interactions of P-selectin with neutrophils and their subsequent activation inducing the local oxidative stress, leading to the release of IL-1α.
The observed moderate correlation between E-selectin and IL-12p70 serum levels may also be explained by the interaction of adhesive molecules with immune cells. E-selectin binds to ligands located on the surface of neutrophils, monocytes, and T cells [10]. The binding of monocytes to E-selectin was related in the study by Davis et al. [26] with the phosphorylation of protein kinase B (also known as AKT) in human monocytes. The activation of AKT is suggested to fulfill an antiapoptotic role; therefore, the observed upregulation of AKT upon binding to E-selectin may prolong the survival of monocytes after migration to inflamed tissue. However, these results were not observed in the case of P-selectin, indicating that AKT activation in monocytes is limited to the binding of these immune cells to E-selectin. Regarding the fact that IL-12p70 is released from, among others, macrophages, the observed relationship may be related to the prolonged survival of macrophages releasing IL-12p70 upon ligation with E-selectin.
The inflammatory mediator that correlated most often with other analyzed biomarkers in this study was TNF-α, which is a pleiotropic cytokine with a well-established role in the development of ulcerative colitis. TNF-α is engaged in the activation of macrophages, the amplification of the T cell response, and the increase in the expression of adhesive molecules. In this research, we noted a significant moderate correlation between the level of TNF-α and E-selectin in the serum of patients with UC. The influence of TNF-α on the expression of adhesive molecules can be confirmed by Rios-Navarro et al.’s [27] study, in which TNF-α increased the expression of E-selectin in human umbilical vein endothelial cells (HUVECs). This effect of TNF-α on E-selectin expression was not only intermittent, but also reversed by the exposition of the HUVEC cell culture to anti-TNF-α antibodies. These findings confirm the relationship between TNF-α and E-selectin observed in our study. Another significant and moderate relationship was noted in the case of the TNF-α and CCL-2 serum profile in the UC group. The observed correlation may be confirmed with the above-mentioned study of Magnusson et al. [23], in which 14 weeks of anti-TNF-α treatment with infliximab reduced the expression of CCL2 in the serum, as well as in intestinal tissue collected from UC patients responding to the treatment; however, no such difference was noted in the case of the non-responding group. The role of TNF-α in the upregulation of CCL2 level during UC may be further confirmed by the study of Aomatsu et al. [28], in which, after 24 h of exposition to TNF-α, the expression of CCL2 increased significantly in human colonic myofibroblasts. In Aomatsu et al.’s study, the increase in CCL2 expression was related to the TNF-α-induced activation of the NF-κB pathway, which plays a crucial role in the development of ulcerative colitis [28,29]. The strongest correlation in our study was noted between the serum profile of IL-1α and TNF-α in patients with UC. The observed relationship may be related to the stimulating role of IL-1α in TNF-α expression. This hypothesis can be confirmed by Turner et al.’s [30] study, in which researchers analyzed the potential of IL-1α in the induction of different proinflammatory cytokines in human cardiac myofibroblasts. The researchers noted that IL-1α stimulated the expression of TNF-α in a dose-dependent manner. Moreover, Turner et al. noted that the upregulation of TNF-α was mediated by the NF-κB and MAPK pathways, which also play an important role in the development of inflammatory process in ulcerative colitis [29,31]. Therefore, during UC, IL-1α may also induce the expression of TNF-α through the NF-κB and MAPK pathways, contributing to the development of the inflammatory process across the intestines.
5. Conclusions
The aim of this study was to identify new biological markers, which could be useful in the differential diagnosis of IBD to Crohn’s disease or ulcerative colitis. The conducted analyses revealed a significant increase in the serum profile of E-selectin, P-selectin, IL-1α, and IL-12p70 in patients with CD compared to UC, indicating a potential use of these proinflammatory mediators as biomarkers supporting the differential diagnosis between CD and UC. Moreover, a crucial relationship has been noted between the serum concentration of CCL2 and TNF-α and the disease activity in the group of patients with ulcerative colitis. This observed correlation may therefore highlight the possible role of CCL2 and TNF-α in monitoring the disease activity, as well as in evaluating the effectiveness of the implemented treatment. Considering the complexity of interactions between proinflammatory mediators during the development of CD and UC, in this study, we also analyzed the correlations between the analyzed chemokines, cytokines, and adhesion molecules. Statistical analyses revealed a significant relationship between the concentration of E-selectin and IL-12p70; P-selectin and IL-1α; and TNF-α with E-selectin, CCL2, and IL-1α in the serum of patients with UC. The obtained results present crucial relations between proinflammatory mediators, which can provide some new information about the initiation and development of ulcerative colitis.
Author Contributions
A.G.—investigation, biochemical analysis, writing—original draft preparation; G.W.—investigation, biochemical analysis; Y.K.-K.—data interpretation, writing—review and editing; D.I.—data interpretation, writing—review and editing; P.O.—data interpretation, writing—review and editing; K.K.-V.—conceptualization, investigation, writing—original draft preparation, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia in Katowice, Poland, grant No. PCN-2-076/K/1/I.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Medical University of Silesia in Katowice (protocol code KNW/0022/KB/309/15).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Ananthakrishnan, A.N.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative Colitis and Crohn’s Disease Have Similar Burden and Goals for Treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Feakins, R.M. Ulcerative colitis or Crohn’s disease? Pitfalls and problems. Histopathology 2014, 64, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Faubion, W.A.; Papadakis, K.A. Targeting Specific Immunologic Pathways in Crohn’s Disease. Gastroenterol. Clin. N. Am. 2017, 46, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Bris, R.; Saez, A.; Herrero-Fernandez, B.; Rius, C.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Sato, N.; Mizuno, N.; Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun Rev. 2022, 21, 103017. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Tutdibi, E.; Goedicke-Fritz, S.; Schöpe, J.; Zemlin, M.; Nourkami-Tutdibi, N. Serum cytokines MCP-1 and GCS-F as potential biomarkers in pediatric inflammatory bowel disease. PLoS ONE 2023, 18, e0288147. [Google Scholar] [CrossRef]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Pooley, N.; Ghosh, L.; Sharon, P. Up-regulation of E-selectin and intercellular adhesion molecule-1 differs between Crohn’s disease and ulcerative colitis. Dig. Dis. Sci. 1995, 40, 219–225. [Google Scholar] [CrossRef]
- Magro, F.; Araujo, F.; Pereira, P.; Meireles, E.; Diniz-Ribeiro, M.; Velosom, F.T. Soluble selectins, sICAM, sVCAM, and angiogenic proteins in different activity groups of patients with inflammatory bowel disease. Dig. Dis. Sci. 2004, 49, 1265–1274. [Google Scholar] [CrossRef]
- Polek, A.; Sobiczewski, W.; Matowicka-Karna, J. P-selektyna i jej rola w niektórych chorobach. PHMD 2009, 63, 465–470. [Google Scholar] [PubMed]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1α: A comprehensive review on the role of IL-1α in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Schunk, S.J.; Triem, S.; Schmit, D.; Zewinger, S.; Sarakpi, T.; Becker, E.; Hütter, G.; Wrublewsky, S.; Küting, F.; Hohl, M.; et al. Interleukin-1α Is a Central Regulator of Leukocyte-Endothelial Adhesion in Myocardial Infarction and in Chronic Kidney Disease. Circulation 2021, 144, 893–908. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Kałużna, K.; Jura-Półtorak, A.; Derkacz, A.; Olczyk, K. Circulating Profile of ECM-Related Proteins as Diagnostic Markers in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 5618. [Google Scholar] [CrossRef]
- Lahiff, C.; Safaie, P.; Awais, A.; Akbari, M.; Gashin, L.; Sheth, S.; Lembo, A.; Leffler, D.; Moss, A.C.; Cheifetz, A.S. The Crohn’s disease activity index (CDAI) is similarly elevated in patients with Crohn’s disease and in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2013, 37, 786–794. [Google Scholar] [CrossRef]
- Ikeya, K.; Hanai, H.; Sugimoto, K.; Osawa, S.; Kawasaki, S.; Iida, T.; Maruyama, Y.; Watanabe, F. The Ulcerative Colitis Endoscopic Index of Severity More Accurately Reflects Clinical Outcomes and Long-term Prognosis than the Mayo Endoscopic Score. J. Crohn’s Colitis 2016, 10, 286–295. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Biancone, L.; Marasco, R.; Morrone, G.; Marasco, O.; Luzza, F.; Pallone, F. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 1997, 112, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, L.D.; Pistiki, A.; Giamarellos-Bourboulis, E.J.; Georgitsi, M.; Damoraki, G.; Polymeros, D.; Dimitriadis, G.D.; Triantafyllou, K. Activation of NLRP3 Inflammasome in Inflammatory Bowel Disease: Differences Between Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2017, 62, 2348–2356. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Voudoukis, E.; Karmiris, K.; Koutroubakis, I.E. Multipotent role of platelets in inflammatory bowel diseases: A clinical approach. World J. Gastroenterol. 2014, 20, 3180–3190. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Strid, H.; Isaksson, S.; Bajor, A.; Lasson, A.; Ung, K.A.; Öhman, L. Response to infliximab therapy in ulcerative colitis is associated with decreased monocyte activation, reduced CCL2 expression and downregulation of Tenascin C. J. Crohn’s Colitis. 2015, 9, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Kaplan, G.G. The role of TNFα in ulcerative colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Davies, J.M.; Radford, K.J.; Begun, J.; Levesque, J.P.; Winkler, I.G. Adhesion to E-selectin primes macrophages for activation through AKT and mTOR. Immunol. Cell Biol. 2021, 99, 622–639. [Google Scholar] [CrossRef]
- Ríos-Navarro, C.; de Pablo, C.; Collado-Diaz, V.; Orden, S.; Blas-Garcia, A.; Martínez-Cuesta, M.Á.; Esplugues, J.V.; Alvarez, A. Differential effects of anti-TNF-α and anti-IL-12/23 agents on human leukocyte-endothelial cell interactions. Eur. J. Pharmacol. 2015, 765, 355–365. [Google Scholar] [CrossRef]
- Aomatsu, T.; Imaeda, H.; Takahashi, K.; Fujimoto, T.; Kasumi, E.; Yoden, A.; Tamai, H.; Fujiyama, Y.; Andoh, A. Tacrolimus (FK506) suppresses TNF-α-induced CCL2 (MCP-1) and CXCL10 (IP-10) expression via the inhibition of p38 MAP kinase activation in human colonic myofibroblasts. Int. J. Mol. Med. 2012, 30, 1152–1158. [Google Scholar] [CrossRef]
- McDaniel, D.K.; Eden, K.; Ringel, V.M.; Allen, I.C. Emerging Roles for Noncanonical NF-κB Signaling in the Modulation of Inflammatory Bowel Disease Pathobiology. Inflamm. Bowel. Dis. 2016, 22, 2265–2279. [Google Scholar] [CrossRef]
- Turner, N.A.; Das, A.; Warburton, P.; O’Regan, D.J.; Ball, S.G.; Porter, K.E. Interleukin-1α stimulates proinflammatory cytokine expression in human cardiac myofibroblasts. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1117-27. [Google Scholar] [CrossRef]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP kinases in inflammatory bowel disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).