Application of Biplot Techniques to Evaluate the Potential of Trichoderma spp. as a Biological Control of Moniliasis in Ecuadorian Cacao
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acquisition of Microorganisms
2.2. Culture Media
2.3. Inoculation of Trichoderma spp. In Vitro
2.4. Mycelial Growth Evaluation
- = specific growth rate; it is monotonic and decreasing;
- = microbial population at a given time;
- t = growth time;
- λ = lag phase;
- = initial microbial population.
2.5. In Vitro Antagonism Tests
- RPC= radial mycelial growth of the pathogen (Moniliophthora roreri (MRCP) and Moniliophtora roreri (MMCA)) in control culture on the last day;
- RFP = radial mycelial growth of the pathogen in the presence of the antagonist (Trichoderma spp.) on the final day;
- RIP = radial mycelial growth of the pathogen on the day the confrontation started.
2.6. Field Experiments
- Control treatment: no application of Trichoderma spp. fungal spores;
- Treatment T1: Use of fungal spores of the Trichoderma E22 strain;
- Treatment T2: Use of fungal spores of the strain Trichoderma E25;
- Treatment T3: Use of fungal spores of the Trichoderma E29 strain;
- Treatment T4: Use of fungal spores of the Trichoderma E30 strain;
- Treatment T5: Use of fungal spores of the Trichoderma E39 strain.
- I = Incidence (%);
- DP = Number of diseased fruits;
- TP = Total number of harvested pods.
- E = Efficiency (%);
- FIWoT = Final percentage of the incidence without the application of Trichoderma spp;
- FIWT = Final percentage of incidence with the application of Trichoderma spp.
2.7. Statistical Analysis
2.7.1. PCA Biplot
2.7.2. Descriptive Statistics
3. Results and Discussion
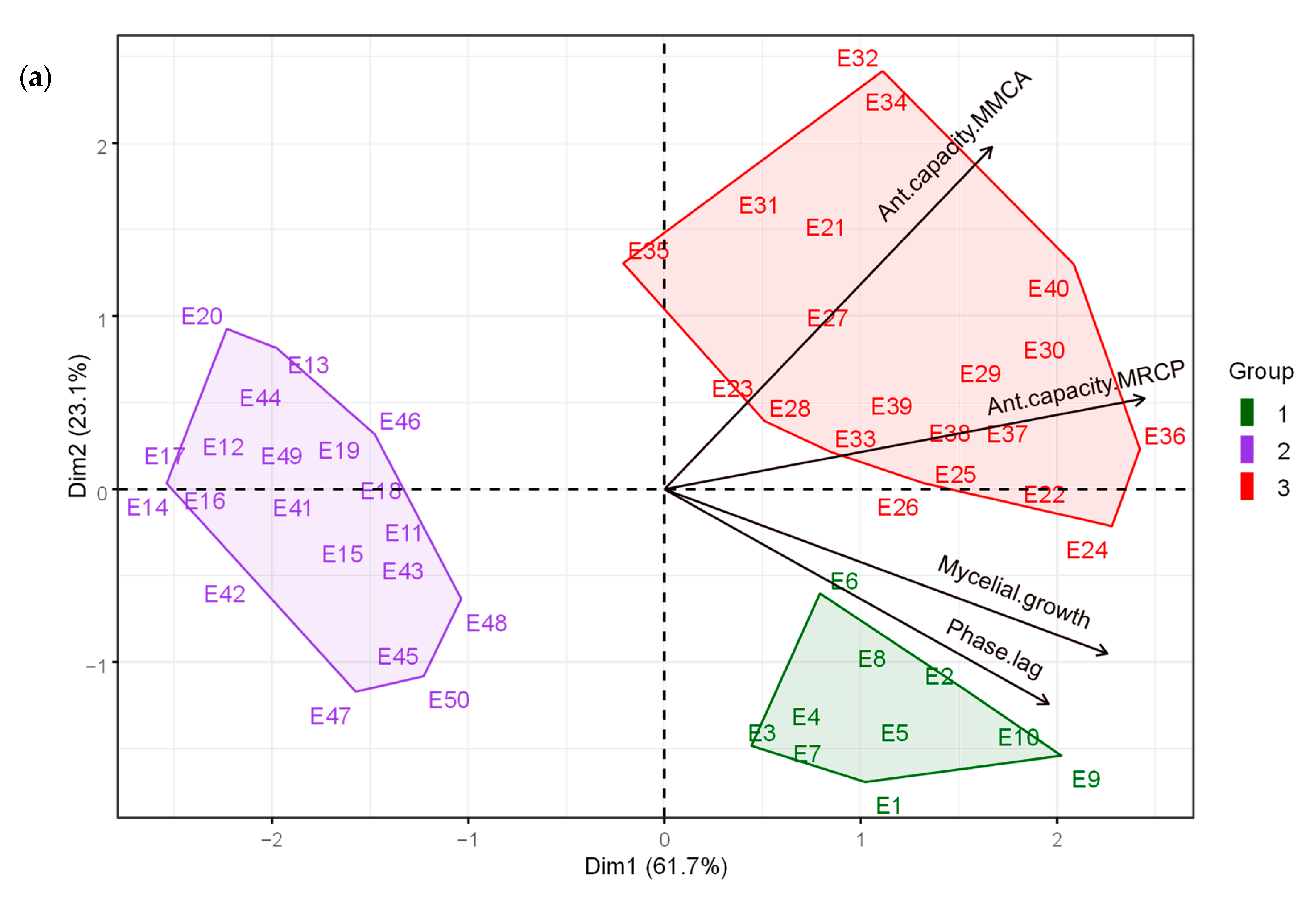
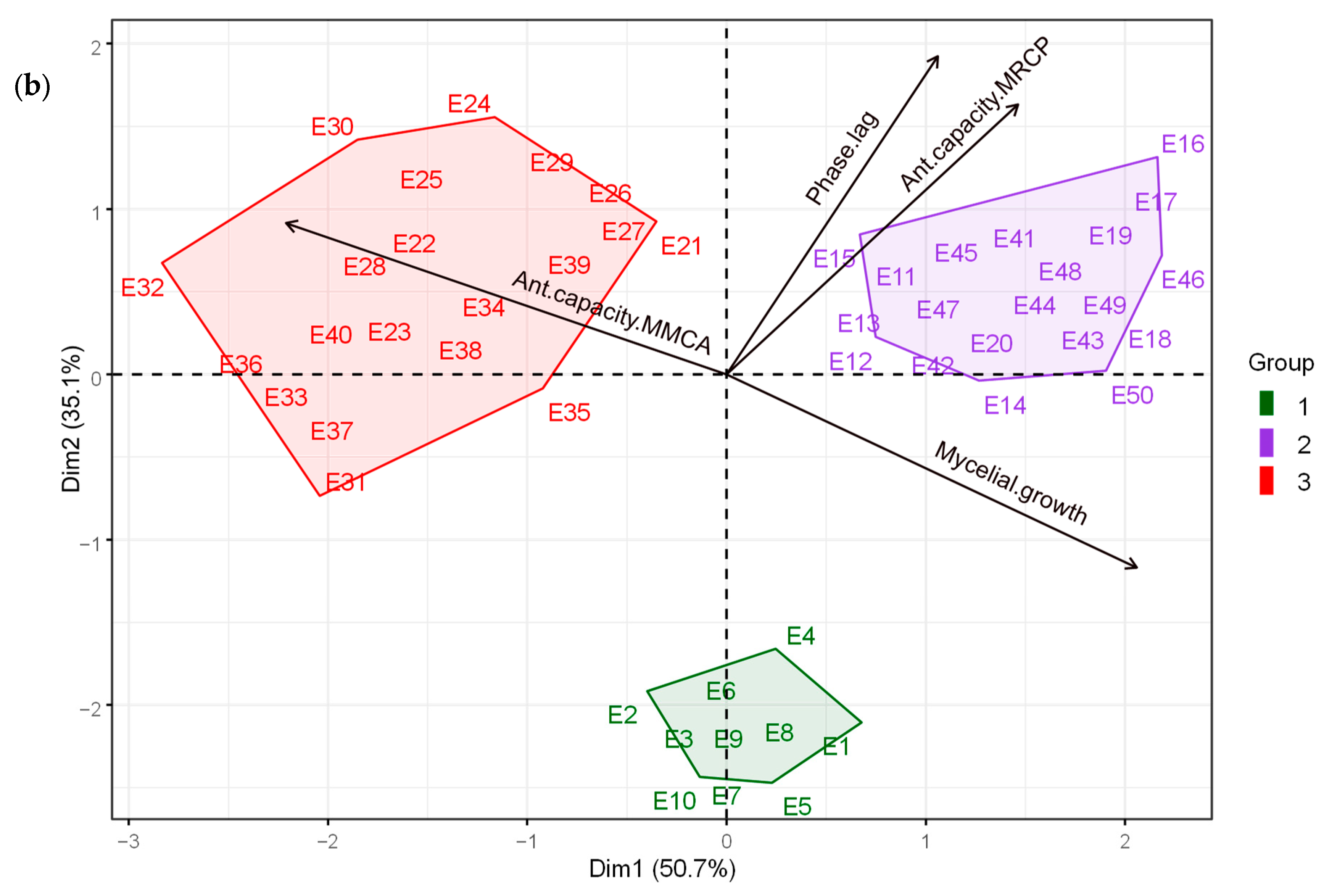
3.1. Statistical Algorithm for Mycelial Characteristics and Antagonistic Capacity of Trichoderma spp. against Moniliopthora roreri
3.2. Results of Biocontrol with Trichoderma spp. in the Field
4. Conclusions
- I.
- The number of Trichoderma strains used in this study was small and may not represent the total diversity that exists in different regions and conditions.
- II.
- The study did not diversify the cultivation conditions of Trichoderma, limiting itself to the use of two different culture media (PDA and MEA).
- III.
- The application of biopreparations in the field was conducted on a single farm; thus, these results do not guarantee replicability on farms in different localities under varying agronomic conditions.
- IV.
- The monitoring period for the application of biopreparations in the field was limited to five months; therefore, the sustainability of Trichoderma usage was not determined.
- I.
- Diversify the Trichoderma strains by testing samples from a wide variety of regions to obtain a more accurate understanding of the potential of specific strains as antagonists.
- II.
- Investigate the effects of different culture media and cultivation conditions (temperature, pH, humidity) on the mycelial growth and antagonism of Trichoderma strains.
- III.
- Conduct trials in multiple localities and under different cultivation conditions to evaluate the efficacy and consistency of biopreparations on a larger scale.
- IV.
- Perform long-term follow-up studies to confirm the persistence of the antagonism and the influence of biopreparations on soil and plant health over time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, H.C.; Bezerra, J.L.; Barreto, R.W. Of Mushrooms and Chocolate Trees: Aetiology and Phylogeny of Witches’ Broom and Frosty Pod Diseases of Cacao. Plant Pathol. 2013, 62, 728–740. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Guevara-Viejó, F.; Vicente-Galindo, P.; Galindo-Villardón, P. Eco-Friendly Biocontrol of Moniliasis in Ecuadorian Cocoa Using Biplot Techniques. Sustainability 2023, 15, 4223. [Google Scholar] [CrossRef]
- CFN. Ficha Sectorial. 2023. Available online: https://www.cfn.fin.ec/wp-content/uploads/downloads/biblioteca/2023/fichas-sectoriales-2-trimestre/Ficha-Sectorial-Cacao.pdf (accessed on 6 May 2024).
- González, L.; Moreira, W.; Dueñas, A. La Cadena de Comercialización Del Cacao Fino de Aroma, Cantón Pichincha, Ecuador. ECA Sinerg. 2022, 13, 86–95. [Google Scholar] [CrossRef]
- Másmela, J. Potential Distribution and Fundamental Niche of Moniliophthora Spp in Cocoa of America and Africa. Agron. Mesoam. 2019, 30, 659–679. [Google Scholar] [CrossRef]
- Sánchez, F.; Garcés, F. Moniliophthora Roreri (Cif y Par) Evans et al. En El Cultivo de Cacao. Sci. Agropecu. 2012, 3, 249–258. [Google Scholar] [CrossRef]
- Díaz, B.; Camargos, P.; Heming, N.; Amorim, L.; De Souza, K.; Peres, K.; Arévalo, E.; Priminho, C.; Guimaraes, E. Characterization of the Microbiota Dynamics Associated with Moniliophthora Roreri, Causal Agent of Cocoa Frosty Pod Rot Disease, Reveals New Viral Species. Front. Microbiol. 2023, 13, 1053562. [Google Scholar] [CrossRef]
- Becker, P.; Esker, P.; Umaña, G. Defense-Related Gene Expression in Response to the Application of Biological Control Agents in Banana—ScienceDirect. Biol. Control 2023, 186, 105317. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Cruz, J. Purificación y Caracterización de Hidrolasas Implicadas En El Micoparasitismo de Trichoderma Harzianum, Universidad de Sevilla. 1994. Available online: https://idus.us.es/bitstream/handle/11441/15805/E_TD_265.pdf?sequence=1&isAllowed=y (accessed on 18 May 2024).
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and Its Role in Biological Control of Plant Fungal and Nematode Disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef]
- Martínez, N. Manejo Integrado de Plagas: Una Solución a La Contaminación Ambiental. Comunidad. Y Salud. 2010, 8, 73–82. [Google Scholar]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Chang, Q.; Hu, J. Retracted: Research and Application of the Data Mining Technology in Economic Intelligence System. Comput. Intell. Neurosci. 2023, 2022, 9813673. [Google Scholar] [CrossRef]
- Mamani, S. MGIDI: Una Metodología Estadística Eficiente Para Análisis Multivariante de Datos Agronómicos. J. Selva Andin. Biosph. 2023, 11, 112–114. [Google Scholar] [CrossRef]
- Guevara-Viejó, F.; Valenzuela-Cobos, J.D.; Vicente-Galindo, P.; Galindo-Villardón, P. Data-Mining Techniques: A New Approach to Identifying the Links among Hybrid Strains of Pleurotus with Culture Media. J. Fungi 2021, 7, 882. [Google Scholar] [CrossRef]
- Valenzuela-Cobos, J.D.; Guevara-Viejó, F.; Cárdenas-Cobo, J.; Lazo-Sulca, R.; Noriega-Verdugo, D.; Garcés-Moncayo, M.F.; Grijalva-Endara, A. Chemical and Productivity Characterization of Parental and Hybrid Strains of Lentinula Edodes Cultivated in Different Agricultural Residues. Emir. J. Food Agric. 2021, 33, 260–265. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A Dynamic Approach to Predicting Bacterial Growth in Food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Florencio-Anastasio, J.G.; Alarcón, A.; García-Ávila, C.d.J.; Ferrera-Cerrato, R.; Quezada-Salinas, A.; Almaraz-Suárez, J.J.; Espinosa-Mendoza, M.; Bocanegra-Flores, D.A.; Hernández-Ramos, L. In vitro Inhibition of Bacteria against Fusarium Oxysporum f. Sp. Cubense Race 2. Rev. Mex. Fitopatol. 2022, 41, 126–142. [Google Scholar] [CrossRef]
- Ramírez-Benítez, J.E.; Arjona Sabido, R.A.; Caamal Velázquez, J.H.; Rodríguez Ávila, N.L.; Solís Pereira, S.E.; Lizama Uc, G. Growth Inhibition and Genetic Modification of Phytophthora Capsici Using Chitosan with Low Degree of Polymerization. Rev. Argent. Microbiol. 2019, 51, 12–17. [Google Scholar] [CrossRef]
- Sánchez, F.L.; Gamboa, E.; Rincón, J. Control Químico y Cultural de La Moniliasis (Moniliophthora Roreri Cif & Par) Del Cacao (Theobroma Cacao L.) En El Estado Barinas. Rev. La Fac. Agron. 2003, 20, 188–194. [Google Scholar]
- Leiva, S.; Oliva, M.; Hernández, E.; Chuquibala, B.; Rubio, K.; García, F.; de la Cruz, M.T. Assessment of the Potential of Trichoderma Spp. Strains Native to Bagua (Amazonas, Peru) in the Biocontrol of Frosty Pod Rot (Moniliophthora Roreri). Agronomy 2020, 10, 1376. [Google Scholar] [CrossRef]
- Gower, J.C.; Le Roux, N.J.; Gardner-Lubbe, S. Biplots: Quantitative Data. Wiley Interdiscip. Rev. Comput. Stat. 2015, 7, 42–62. [Google Scholar] [CrossRef]
- González-Narváez, M.; Ruiz-Barzola, O.; Nieto-Librero, A. Análisis Multivariante: Un Recorrido Por Las Técnicas de Reducción de Dimensiones. “Matemática” ESPOL-FCNM J. 2020, 18, 1–26. [Google Scholar]
- Gabriel, K.R. The Biplot Graphic Display of Matrices with Application to Principal Component Analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Reza, M.; Mohammadi, E.; Razaei, Y. Antagonistic Effects of Trichoderma Species in Biocontrol of Armillaria Mellea in Fruit Trees in Iran. J. Plant Prot. Res. 2008, 48, 213–222. [Google Scholar]
- González, I.; Infante, D.; Peteira, B.; Martínez, B.; Arias, Y.; González, N.; Miranda, I. Caracterización Bioquímica de Aislamientos de Trichoderma Spp. Promisorios Como Agentes de Control Biológico. I. Expresión de Actividad Quitinasa. Rev. Protección Veg. 2010, 25, 58–63. [Google Scholar]
- Limaco, C.C.; Salazar, G.; Silva, J. Evaluation of Enviroment Fungi in Markets from Tacna City-Perú Evaluación de Hongos Ambientales En Mercados de Abastos de La Ciudad de Tacna-Perú. Rev. Ing. Constr. 2019, 34, 5–14. [Google Scholar] [CrossRef]
- Bisset, J. A Revision of the Genus Trichoderma. 11. Infrageneric Classification. Can. J. Bot. 1991, 69, 2357–2372. [Google Scholar] [CrossRef]
- Seng, J.; Herrera, G.; Vaughan, C.S.; Mccoy, M.B. Use of Trichoderma Fungi in Spray Solutions to Reduce Moniliophthora Roreri Infection of Theobroma Cacao Fruits in Northeastern Costa Rica. Rev. Biol. Trop. Int. J. Trop. Biol. 2014, 62, 899–907. [Google Scholar] [CrossRef]
| Strain | Incidence (%) * | Eficience (%) * | Yield (kg/ha) * |
|---|---|---|---|
| Control | 22.23 e ± 1.02 | -- | 630.73 a ± 9.88 |
| T1 | 8.33 b ± 0.20 | 60.56 c ± 0.77 | 831.86 d ± 16.27 |
| T2 | 11.26 c ± 0.40 | 51.26 a ± 1.04 | 677.86 b ± 40.36 |
| T3 | 2.50 a ± 0.40 | 72.46 e ± 2.12 | 976.90 e ± 25.19 |
| T4 | 9.23 b ± 0.75 | 65.13 d ± 1.73 | 761.60 c ± 27.16 |
| T5 | 12.60 d ± 0.78 | 57.36 b ± 1.81 | 673.93 ab ± 20.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guevara-Viejó, F.; Valenzuela-Cobos, J.D.; Noriega-Verdugo, D.; Garcés-Moncayo, M.F.; Basurto Quilligana, R. Application of Biplot Techniques to Evaluate the Potential of Trichoderma spp. as a Biological Control of Moniliasis in Ecuadorian Cacao. Appl. Sci. 2024, 14, 5481. https://doi.org/10.3390/app14135481
Guevara-Viejó F, Valenzuela-Cobos JD, Noriega-Verdugo D, Garcés-Moncayo MF, Basurto Quilligana R. Application of Biplot Techniques to Evaluate the Potential of Trichoderma spp. as a Biological Control of Moniliasis in Ecuadorian Cacao. Applied Sciences. 2024; 14(13):5481. https://doi.org/10.3390/app14135481
Chicago/Turabian StyleGuevara-Viejó, Fabricio, Juan Diego Valenzuela-Cobos, Delia Noriega-Verdugo, María Fernanda Garcés-Moncayo, and Roberto Basurto Quilligana. 2024. "Application of Biplot Techniques to Evaluate the Potential of Trichoderma spp. as a Biological Control of Moniliasis in Ecuadorian Cacao" Applied Sciences 14, no. 13: 5481. https://doi.org/10.3390/app14135481





