Impact of Prolonged Screening and COVID-19 Infection on Acquired Colour Vision Deficiencies Assessed by the Farnsworth–Munsell 100 Hue Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Tools for Testing
2.2.1. Ishihara 32 Plates Edition
2.2.2. Farnsworth–Munsell 100 Hue Test
2.3. Environment Conditions and Testing Procedures
2.4. Data Analysis
2.4.1. Internal Statistical Analysis of the Data Obtained in the Study
- 1
- Correlation Matrix: A correlation matrix has been generated to examine pairwise relationships between variables.
- 2
- A qualitative analysis using ANOVA (Analysis of Variance) was performed to examine the relationships between test results and gender (male or female), as well as between test results and COVID-19 infection status. Fisher’s LSD intervals at the 95% confidence level were calculated and presented to ensure that the analysed factors did not affect the mean values. It is essential to note that the LSD interval is employed exclusively for the comparison of means. A significant difference between the means of the two groups is indicated if their LSD intervals do not overlap.
2.4.2. Analysis through Comparison with Previous Studies
3. Results
3.1. Results Obtained from Statistical Data Analysis
3.1.1. Correlation Matrix
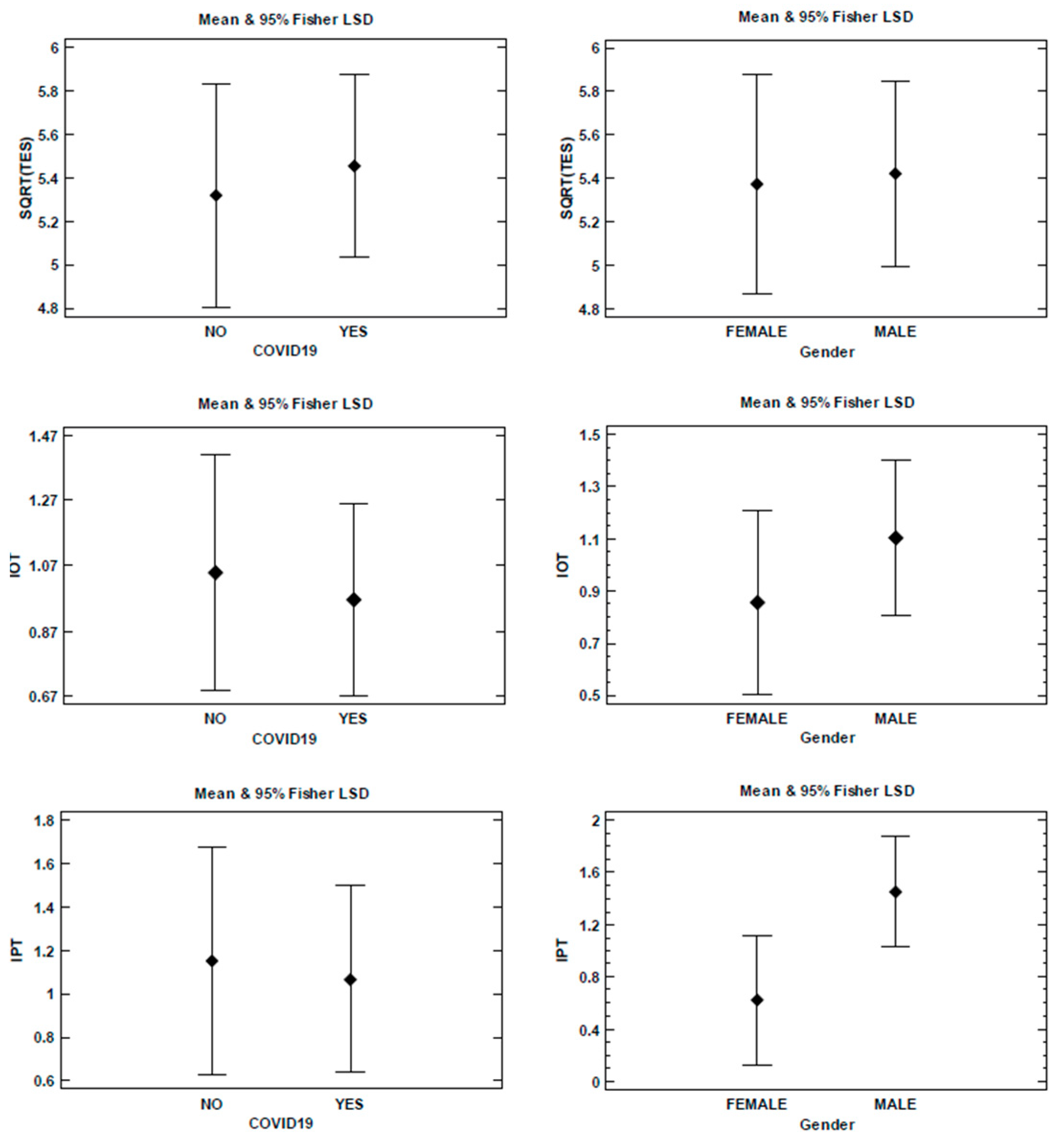
3.1.2. ANOVA Analysis
3.2. Obtained by Analysis through Comparison with Previous Studies
- The average value of and its standard deviation (SD) for the entire set of all 50 subjects;
- The average value of and its SD for the subset of 31 subjects who had previously experienced a COVID-19 infection;
- The average value of and its SD for the subset of 19 subjects who had not experienced a COVID-19 infection.
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serra, G.; Lo Scalzo, L.; Giuffrè, M.; Ferrara, P.; Corsello, G. Smartphone Use and Addiction during the Coronavirus Disease 2019 (COVID-19) Pandemic: Cohort Study on 184 Italian Children and Adolescents. Ital. J. Pediatr. 2021, 47, 150. [Google Scholar] [CrossRef]
- Priya, D.B.; Subramaniyam, M. Fatigue Due to Smartphone Use? Investigating Research Trends and Methods for Analysing Fatigue Caused by Extensive Smartphone Usage: A Review. Work 2022, 72, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, B.; Fennell, C.; Sánchez-Oliver, A.J. Objectively-Assessed Physical Activity, Sedentary Behavior, Smartphone Use, and Sleep Patterns Pre- and during-COVID-19 Quarantine in Young Adults from Spain. Sustainability 2020, 12, 5890. [Google Scholar] [CrossRef]
- Olson, J.A.; Sandra, D.A.; Colucci, É.S.; Al Bikaii, A.; Chmoulevitch, D.; Nahas, J.; Raz, A.; Veissière, S.P.L. Smartphone Addiction Is Increasing across the World: A Meta-Analysis of 24 Countries. Comput. Hum. Behav. 2022, 129, 107138. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhu, D.; Cao, Y. Smartphone Overuse and Visual Impairment in Children and Young Adults: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e21923. [Google Scholar] [CrossRef] [PubMed]
- Chidi-Egboka, N.C.; Jalbert, I.; Golebiowski, B. Smartphone Gaming Induces Dry Eye Symptoms and Reduces Blinking in School-Aged Children. Eye 2023, 37, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Said, O.; Ibrahim, O.; Soliman, A. Visual Sequelae of Computer Vision Syndrome: A Cross-Sectional Case-Control Study. J. Ophthalmol. 2021, 2021, 6630286. [Google Scholar] [CrossRef] [PubMed]
- Golebiowski, B.; Long, J.; Harrison, K.; Lee, A.; Chidi-Egboka, N.; Asper, L. Smartphone Use and Effects on Tear Film, Blinking and Binocular Vision. Curr. Eye Res. 2020, 45, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Mccrann, S.; Loughman, J.; Butler, J.S.; Paudel, N.; Flitcroft, D.I. Smartphone Use as a Possible Risk Factor for Myopia. Clin. Exp. Optom. 2021, 104, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Salim, A.T.; Praveen, A.; Fonseka, D.; Ting, D.S.W.; He, M.G.; Bourne, R.R.A.; Crowston, J.; Wong, T.Y.; Dirani, M. Association between Digital Smart Device Use and Myopia: A Systematic Review and Meta-Analysis. Lancet Digit. Health 2021, 3, e806–e818. [Google Scholar] [CrossRef]
- Mehra, D.; Galor, A. Digital Screen Use and Dry Eye: A Review. Asia-Pac. J. Ophthalmol. 2020, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Sharifi, H.; Bazrafshan, A.; Noori, A.; Karamouzian, M.; Sharifi, A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-Analysis. J. Ophthalmic. Vis. Res. 2021, 16, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Paramei, G.; Griber, Y. Colour Discrimination in Post-COVID-19 Observers Assessed by the Farnsworth-Munsell 100-Hue Test. Res. Sq. 2023. [Google Scholar] [CrossRef]
- DeValois, K.; Webster, M. Color Vision. Scholarpedia 2011, 6, 3073. [Google Scholar] [CrossRef]
- Solomon, S.G.; Lennie, P. The Machinery of Colour Vision. Nat. Rev. Neurosci. 2007, 8, 276–286. [Google Scholar] [CrossRef]
- Gegenfurtner, K.R. Cortical Mechanisms of Colour Vision. Nat. Rev. Neurosci. 2003, 4, 563–572. [Google Scholar] [CrossRef]
- Mollon, J.D. Color Vision: Opsins and Options. Proc. Natl. Acad. Sci. USA 1999, 96, 4743–4745. [Google Scholar] [CrossRef] [PubMed]
- SWANSON, W.; COHEN, J. Color Vision. Ophthalmol. Clin. N. Am. 2003, 16, 179–203. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.P. Acquired Color Vision Deficiency. Surv. Ophthalmol. 2016, 61, 132–155. [Google Scholar] [CrossRef]
- Bowmaker, J.K.; Dartnall, H.J.; Mollon, J.D. Microspectrophotometric Demonstration of Four Classes of Photoreceptor in an Old World Primate, Macaca Fascicularis. J. Physiol. 1980, 298, 131–143. [Google Scholar] [CrossRef]
- Ahnelt, P.K.; Kolb, H.; Pflug, R. Identification of a Subtype of Cone Photoreceptor, Likely to Be Blue Sensitive, in the Human Retina. J. Comp. Neurol. 1987, 255, 18–34. [Google Scholar] [CrossRef]
- Hofer, H.; Carroll, J.; Neitz, J.; Neitz, M.; Williams, D.R. Organization of the Human Trichromatic Cone Mosaic. J. Neurosci. 2005, 25, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Nathans, J.; Merbs, S.L.; Sung, C.-H.; Weitz, C.J.; Wang, Y. Molecular genetics of human visual pigments. Annu. Rev. Genet. 1992, 26, 403–424. [Google Scholar] [CrossRef]
- Hart, W.M. Acquired Dyschromatopsias. Surv. Ophthalmol. 1987, 32, 10–31. [Google Scholar] [CrossRef]
- Simunovic, M.P. Colour Vision Deficiency. Eye 2010, 24, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Modarres, M.; Mirsamadi, M.; Peyman, G.A. Prevalence of Congenital Color Deficiencies in Secondary-School Students in Tehran. Int. Ophthalmol. 1996, 20, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Verriest, G. Further Studies on Acquired Deficiency of Color Discrimination. J. Opt. Soc. Am. 1963, 53, 185–195. [Google Scholar] [CrossRef]
- Oh, Y.-S.; Kim, J.-S.; Chung, S.-W.; Song, I.-U.; Kim, Y.-D.; Kim, Y.-I.; Lee, K.-S. Color Vision in Parkinson’s Disease and Essential Tremor. Eur. J. Neurol. 2011, 18, 577–583. [Google Scholar] [CrossRef]
- Pieri, V.; Diederich, N.J.; Raman, R.; Goetz, C.G. Decreased Color Discrimination and Contrast Sensitivity in Parkinson’s Disease. J. Neurol. Sci. 2000, 172, 7–11. [Google Scholar] [CrossRef]
- Müller, T.; Kuhn, W.; Büttner, T.; Przuntek, H. Distorted Colour Discrimination in Parkinson’s Disease Is Related to Severity of the Disease. Acta Neurol. Scand. 1997, 96, 293–296. [Google Scholar] [CrossRef]
- Kim, H.J.; Ryou, J.H.; Choi, K.T.; Kim, S.M.; Kim, J.T.; Han, D.H. Deficits in Color Detection in Patients with Alzheimer Disease. PLoS ONE 2022, 17, e0262226. [Google Scholar] [CrossRef] [PubMed]
- Barton, F.B.; Fong, D.S.; Knatterud, G.L. Classification of Farnsworth-Munsell 100-Hue Test Results in the Early Treatment Diabetic Retinopathy Study. Am. J. Ophthalmol. 2004, 138, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Quiceno, J.I.; Capparelli, E.; Sadun, A.A.; Munguia, D.; Grant, I.; Listhaus, A.; Crapotta, J.; Lambert, B.; Freeman, W.R. Visual Dysfunction Without Retinitis in Patients with Acquired Immunodeficiency Syndrome. Am. J. Ophthalmol. 1992, 113, 8–13. [Google Scholar] [CrossRef]
- Cruysberg, J.R.M.; Pinckers, A. Acquired Color Vision Defects in Compressive Optic Neuropathy. Neuro-Ophthalmology 1982, 2, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Iregren, A.; Andersson, M.; Nylén, P. Color Vision and Occupational Chemical Exposures: I. An Overview of Tests and Effects. Neurotoxicology 2002, 23, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Willmann, G.; Ivanov, I.V.; Fischer, M.D.; Lahiri, S.; Pokharel, R.K.; Werner, A.; Khurana, T.S. Effects on Colour Discrimination during Long Term Exposure to High Altitudes on Mt Everest. Br. J. Ophthalmol. 2010, 94, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Almustanyir, A.; Hovis, J.K.; Alduhayan, R.; Alhassan, M.; Bokhary, K.; Alabdulkader, B. Evaluation of the Third Edition of the City University Colour Vision Test. Ophthalmic Physiol. Opt. 2022, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Birch, J. Worldwide Prevalence of Red-Green Color Deficiency. J. Opt. Soc. Am. A 2012, 29, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Hardy, L.H.; Rand, G.; Rittler, M.C. Tests for the Detection and Analysis of Color-Blindness I The Ishihara Test: An Evaluation. J. Opt. Soc. Am. 1945, 35, 268–275. [Google Scholar] [CrossRef]
- Farnsworth, D. The Farnsworth-Munsell 100-Hue Test for the Examination of Color Discrimination; Manual; Munsell Color, Macbeth, Division of Kollmorgen Instruments Corp.: New Windsor, NY, USA, 1957. [Google Scholar]
- Kinnear, P.R. Proposals for Scoring and Assessing the 100-HUE Test. Vis. Res. 1970, 10, 423–433. [Google Scholar] [CrossRef]
- Knoblauch, K.; Saunders, F.; Kusuda, M.; Hynes, R.; Higgins, K.E.; de Monasterio, F.M. Quantitative Comparison of Farnsworth and Kinnear Plots of the Farnsworth-Munsell 100-Hue Test. In Colour Vision Deficiencies IX; Springer: Dordrecht, The Netherlands, 1989; pp. 413–416. [Google Scholar]
- Mäntyjärvi, M. Normal Test Scores in the Farnsworth–Munsell 100 Hue Test. Doc. Ophthalmol. 2001, 102, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Gurnani, B. Revisiting Color Vision Standards and Testing Methods in Various Occupational Groups. Indian J. Ophthalmol. 2022, 70, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Racheva, K.; Totev, T.; Natchev, E.; Bocheva, N.; Beirne, R.; Zlatkova, M. Color Discrimination Assessment in Patients with Hypothyroidism Using the Farnsworth–Munsell 100 Hue Test. J. Opt. Soc. Am. A 2020, 37, A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Kasputytė, R.; Slatkevičienė, G.; Liutkevičienė, R.; Glebauskienė, B.; Bernotas, G.; Tamašauskas, A. Changes of Visual Functions in Patients with Pituitary Adenoma. Medicina 2013, 49, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Susilo, T. Normal Colour Perception in Developmental Prosopagnosia. Sci. Rep. 2021, 11, 13741. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.S.; Podgor, M.J.; Collier, B.; Gunkel, R.D. Color Vision and Age in a Normal North American Population. Graefe’s Arch. Clin. Exp. Ophthalmol. 1991, 229, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, P.R. New Farnsworth-Munsell 100 Hue Test Norms of Normal Observers for Each Year of Age 5-22 and for Age Decades 30–70. Br. J. Ophthalmol. 2002, 86, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Verriest, G.; Van Laethem, J.; Uvijls, A. A New Assessment of the Normal Ranges of the Farnsworth-Munsell 100-Hue Test Scores. Am. J. Ophthalmol. 1982, 93, 635–642. [Google Scholar] [CrossRef]
- Blindor, C. Ishihara 38 Plates CVD Test. Available online: https://www.color-blindness.com/ishihara-38-plates-cvd-test/ (accessed on 20 April 2023).
- Almustanyir, A.; Alduhayan, R.; Alhassan, M.; Bokhary, K.; Alabdulkader, B. Evaluation of the Waggoner Computerized D15 Color Vision Test Using an IPad Device. J. Opt. Soc. Am. A 2021, 38, 1647–1655. [Google Scholar] [CrossRef]
- ISO-CIE ISO/CIE DIS 23603.2; Standard Method of Assessing the Spectral Quality of Daylight Simulators for Visual Appraisal and Measurement of Colour. ICS: 17.180.20—Colours and Measurement of Light 2018. ISO: Geneva, Switzerland, 2018.
| SMD | PCMD | OMD | SHT | TES | THD | IPT | IOT | OLD | |
|---|---|---|---|---|---|---|---|---|---|
| SMD a | 1.00 | −0.27 | 0.11 | 0.00 | 0.26 | 0.49 | −0.06 | −0.21 | −0.22 |
| PCMD b | −0.27 | 1.00 | −0.18 | −0.27 | 0.09 | 0.54 | 0.46 | 0.22 | 0.34 |
| OMD c | 0.11 | −0.18 | 1.00 | 0.06 | 0.40 | 0.45 | −0.24 | 0.26 | 0.02 |
| SHT d | 0.00 | −0.27 | 0.06 | 1.00 | −0.22 | −0.19 | 0.00 | −0.12 | −0.23 |
| TES e | 0.26 | 0.09 | 0.40 | −0.22 | 1.00 | 0.46 | 0.05 | 0.27 | 0.12 |
| THD f | 0.49 | 0.54 | 0.45 | −0.19 | 0.46 | 1.00 | 0.21 | 0.18 | 0.14 |
| IPT g | −0.06 | 0.46 | −0.24 | 0.00 | 0.05 | 0.21 | 1.00 | 0.44 | 0.20 |
| IOT h | −0.21 | 0.22 | 0.26 | −0.12 | 0.27 | 0.18 | 0.44 | 1.00 | 0.14 |
| OLD i | −0.22 | 0.34 | 0.02 | −0.23 | 0.12 | 0.14 | 0.20 | 0.14 | 1.00 |
| All Subjects | Subjects with COVID-19 | Subjects No COVID-19 |
|---|---|---|
| 5.40 ± 1.58 | 5.46 ± 1.62 | 5.32 ± 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncho Santonja, M.; Jordán, J.; Micó, B.; López, D.; Tortajada Montañana, I.; Peris-Fajarnés, G. Impact of Prolonged Screening and COVID-19 Infection on Acquired Colour Vision Deficiencies Assessed by the Farnsworth–Munsell 100 Hue Test. Appl. Sci. 2024, 14, 5563. https://doi.org/10.3390/app14135563
Moncho Santonja M, Jordán J, Micó B, López D, Tortajada Montañana I, Peris-Fajarnés G. Impact of Prolonged Screening and COVID-19 Infection on Acquired Colour Vision Deficiencies Assessed by the Farnsworth–Munsell 100 Hue Test. Applied Sciences. 2024; 14(13):5563. https://doi.org/10.3390/app14135563
Chicago/Turabian StyleMoncho Santonja, María, Jorge Jordán, Bárbara Micó, Daniel López, Ignacio Tortajada Montañana, and Guillermo Peris-Fajarnés. 2024. "Impact of Prolonged Screening and COVID-19 Infection on Acquired Colour Vision Deficiencies Assessed by the Farnsworth–Munsell 100 Hue Test" Applied Sciences 14, no. 13: 5563. https://doi.org/10.3390/app14135563






