Development of Dehydrogenation System for Liquid Organic Hydrogen Carrier with Enhanced Reaction Rate
Abstract
:1. Introduction
2. Preliminary Experiments
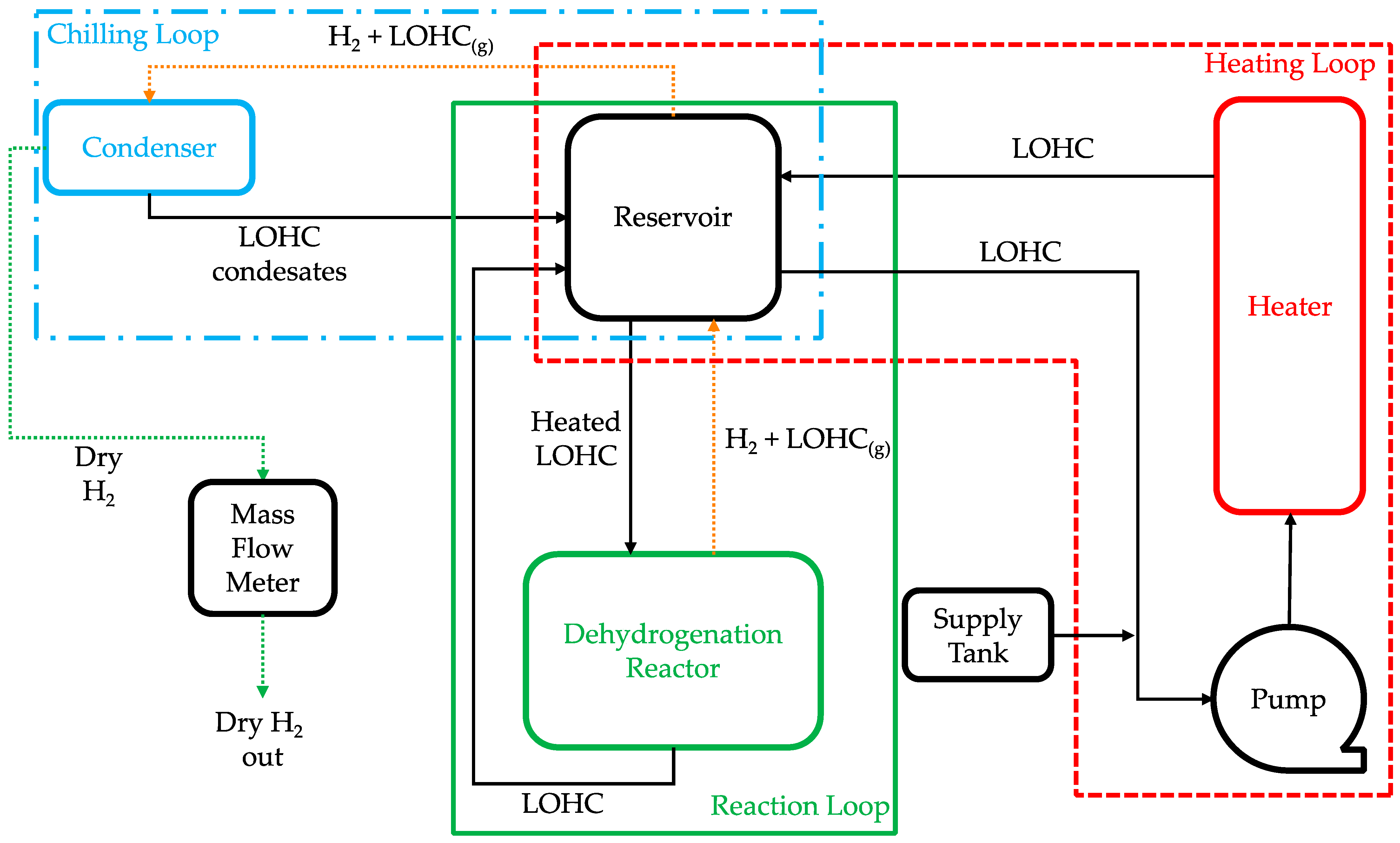
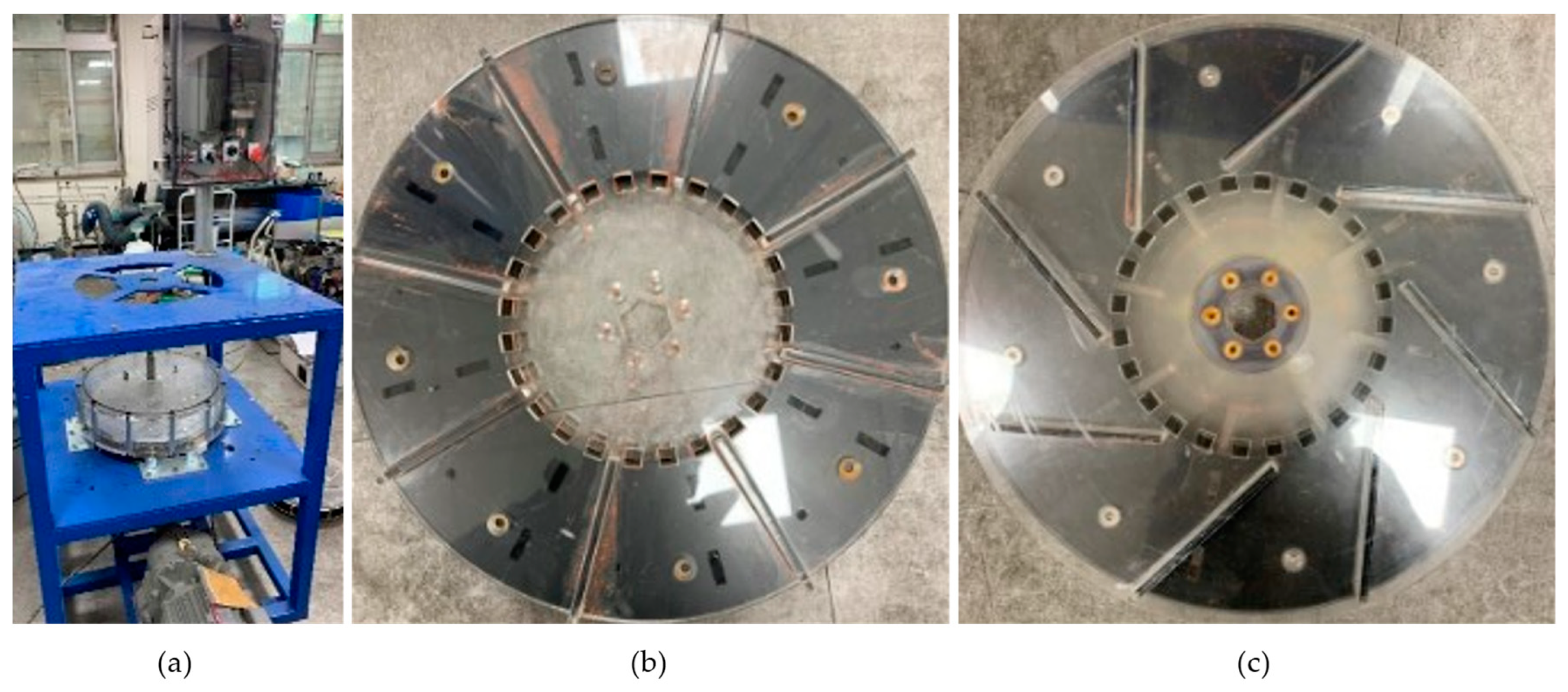
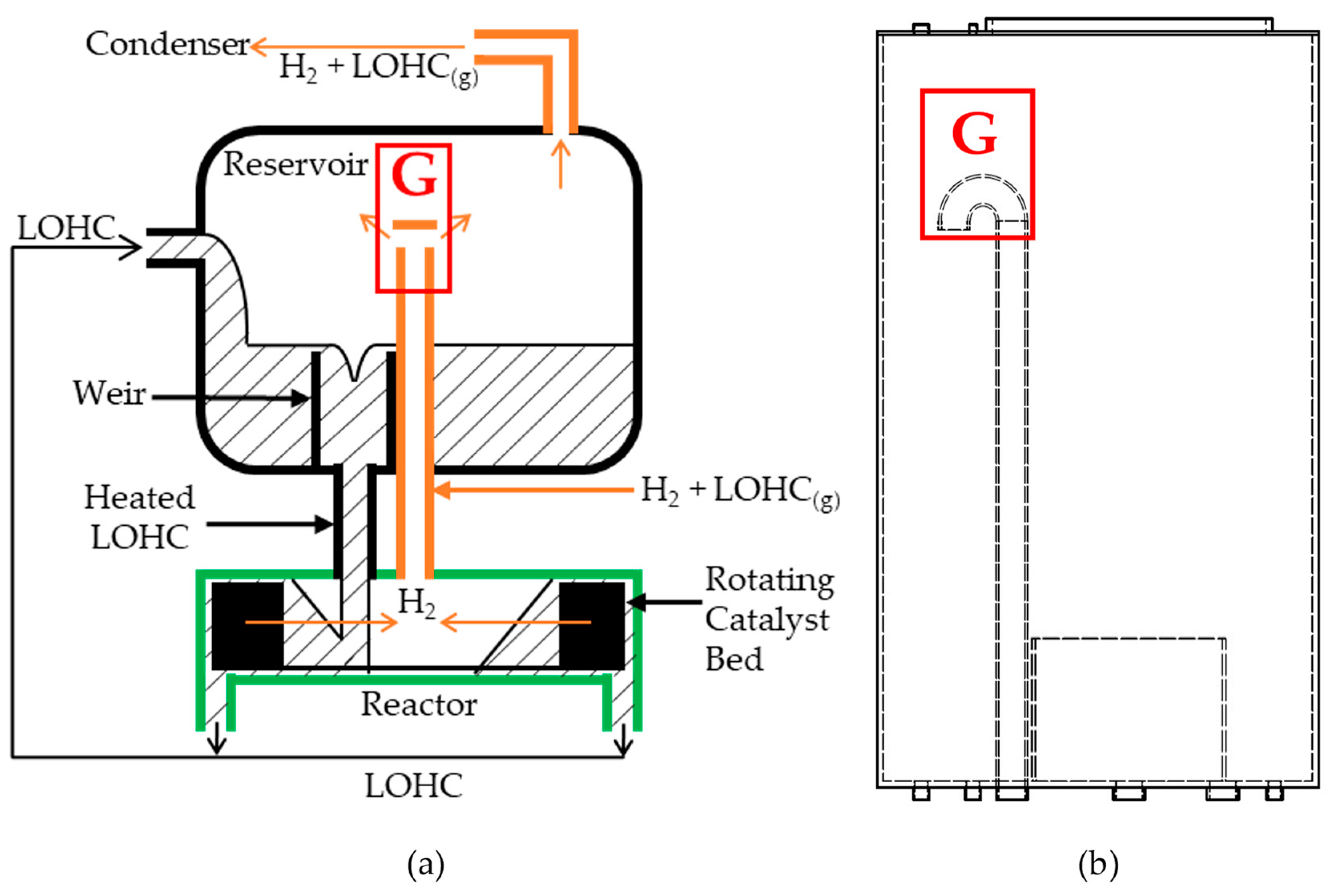
2.1. Reactor System Design Concepts
2.2. LOHC Material and Catalyst
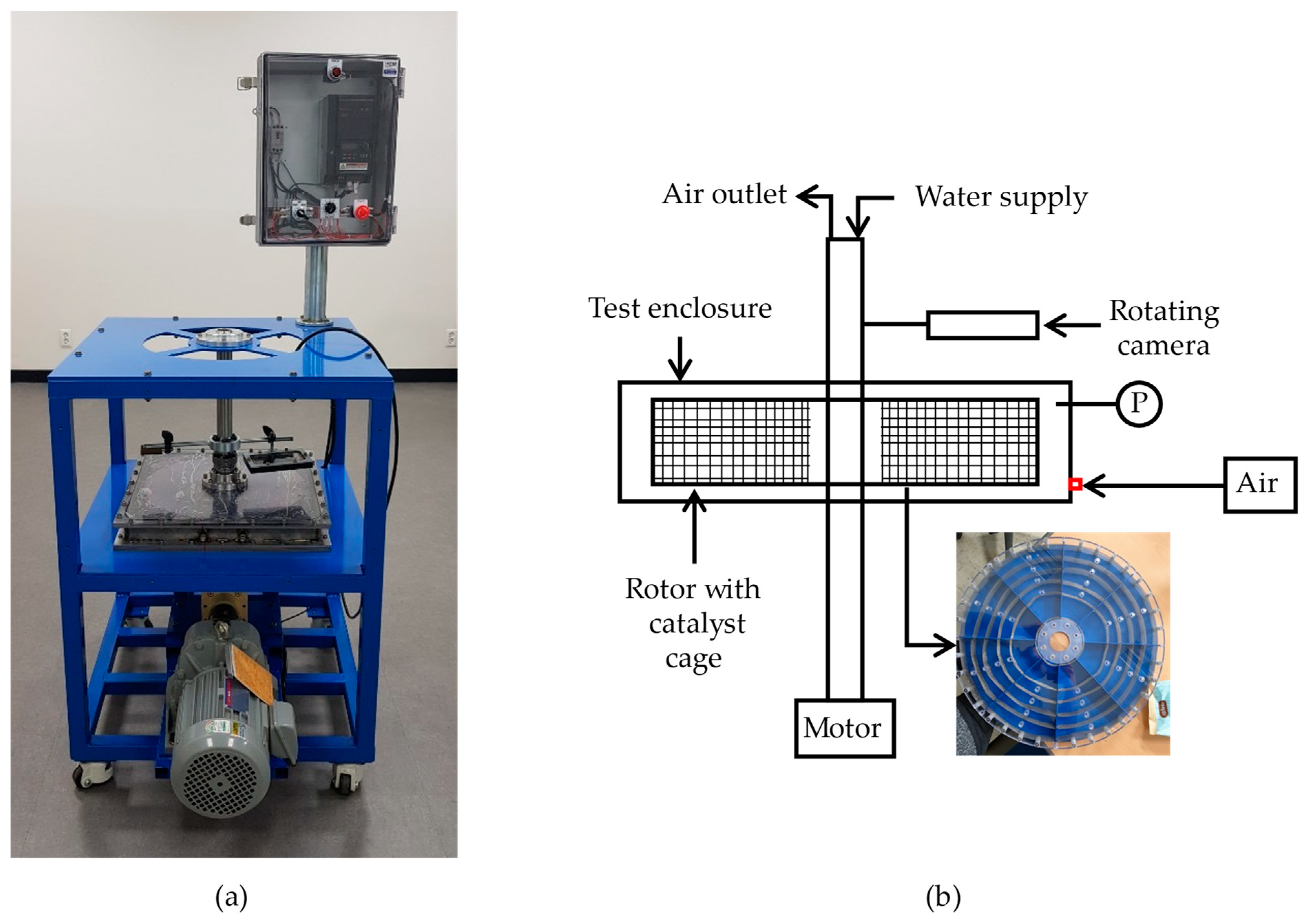
2.3. Gas Infiltration through Catalyst Bed
2.4. Flow Visualization Experiment
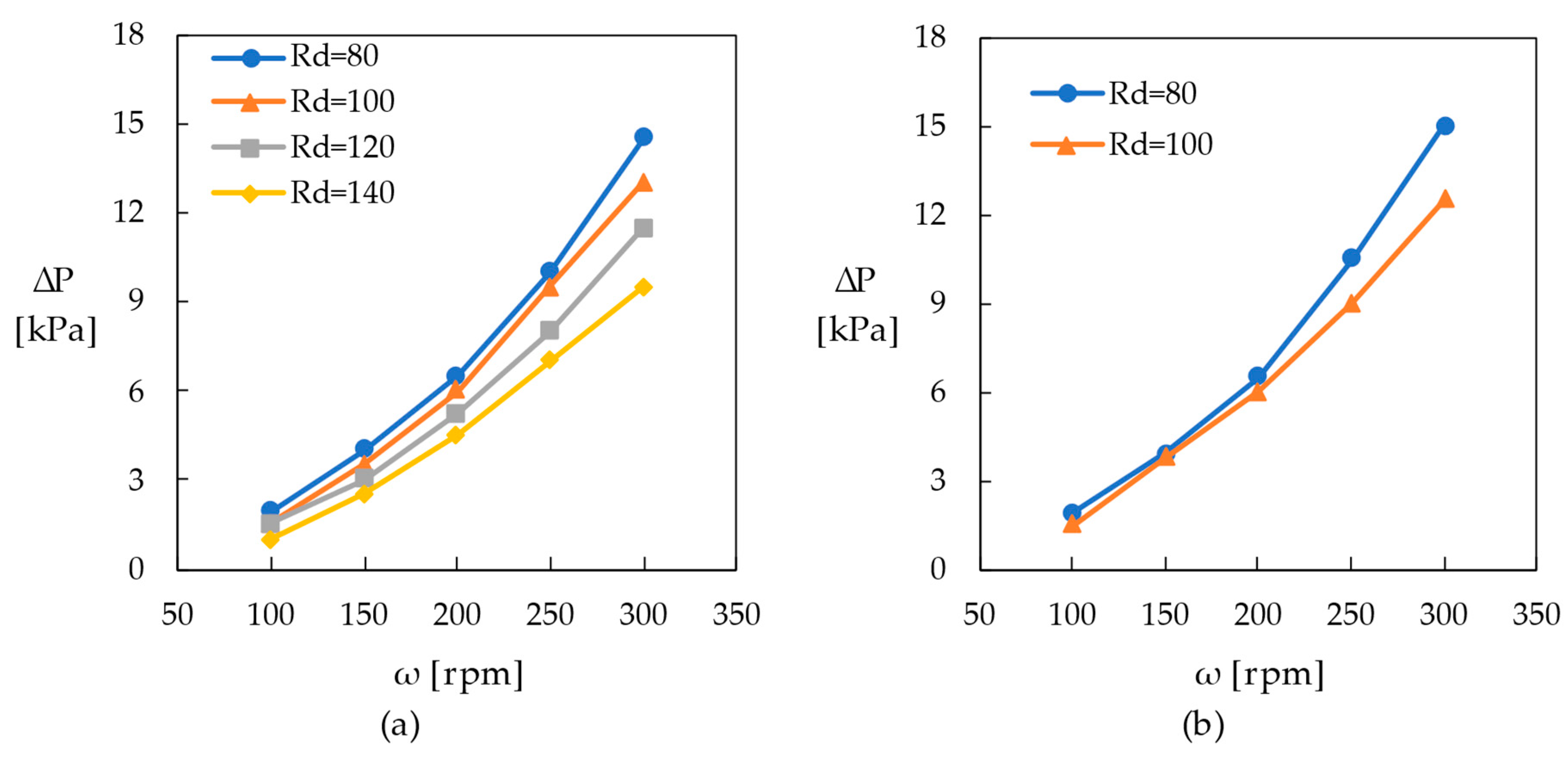
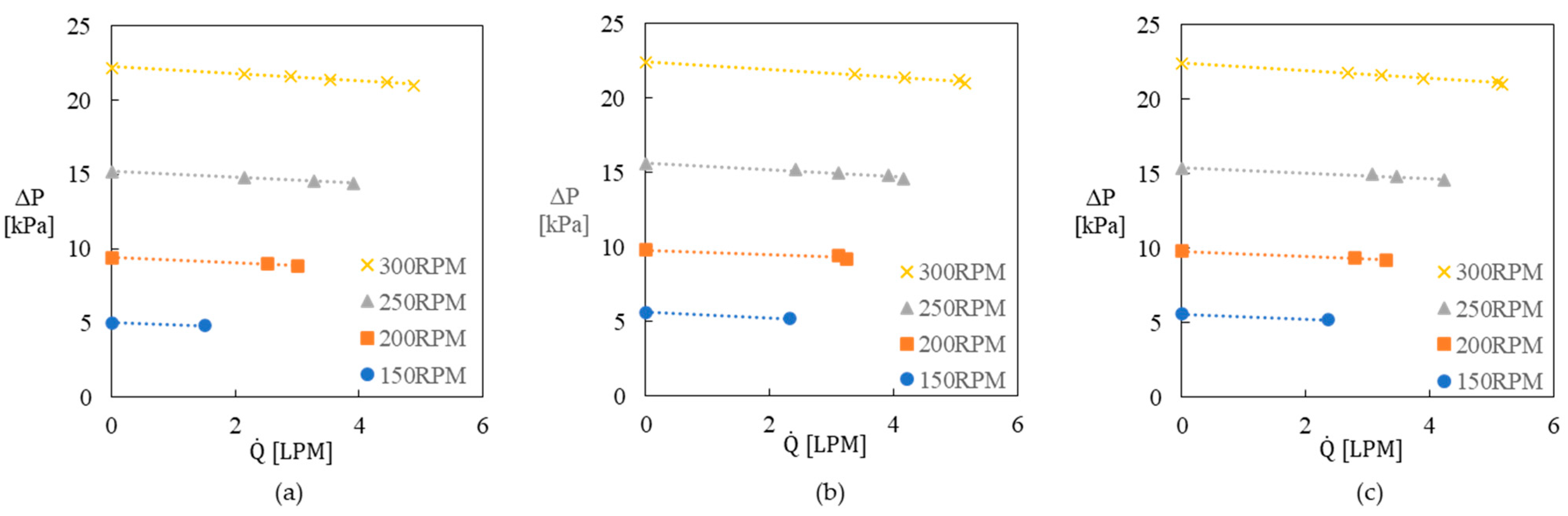
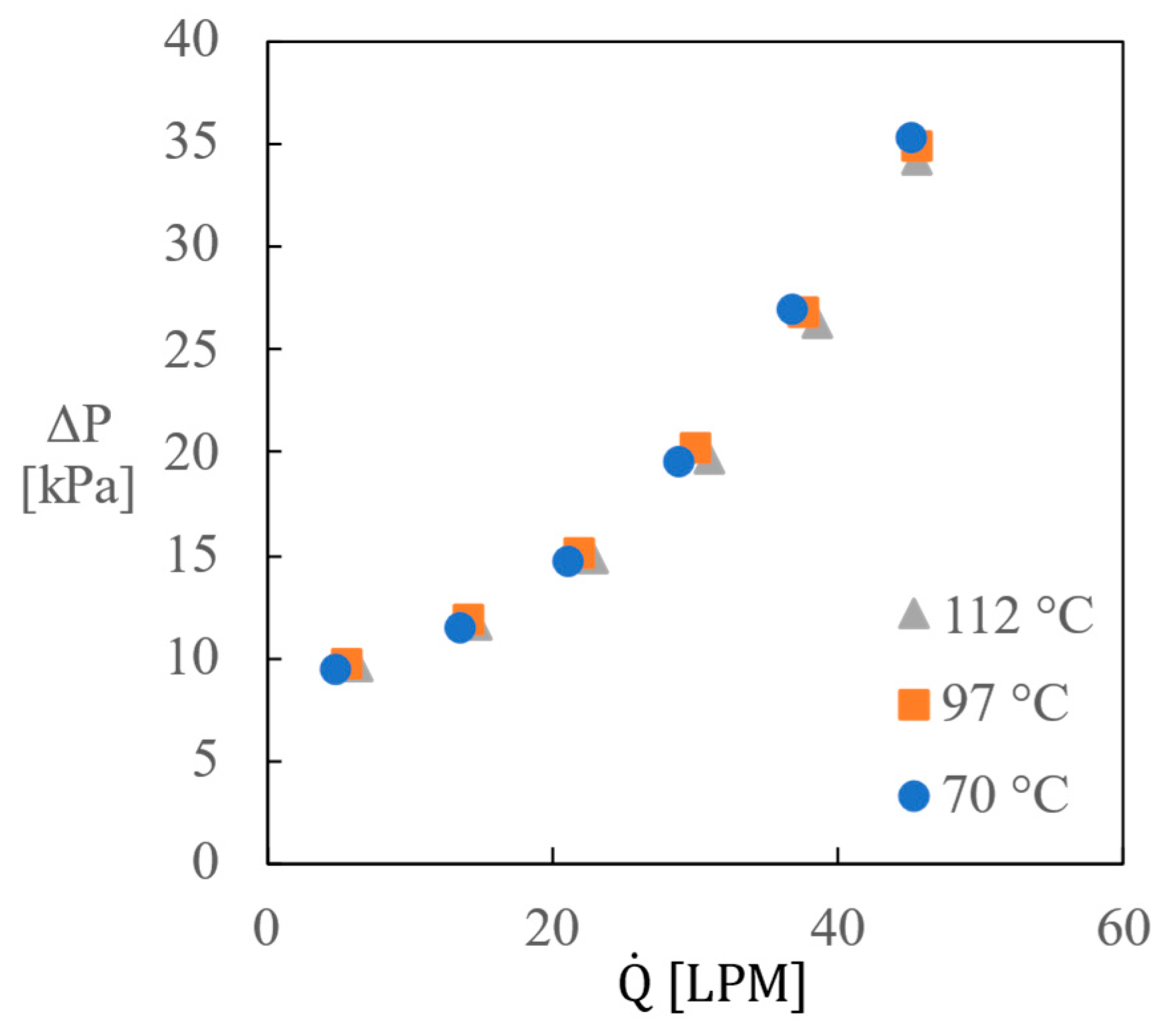
2.5. Reactor Pumping Performance and Heater Flow Resistance
3. Dehydrogenation Experiment
4. Results and Discussion
| mol/L | Molar concentration of H18-DBT | |
| mol/s | Inlet molar flow of H18-DBT | |
| mol/kg s | Reaction rate | |
| kg | Weight of catalyst | |
| Fractional conversion of H18-DBT. |
5. Conclusions
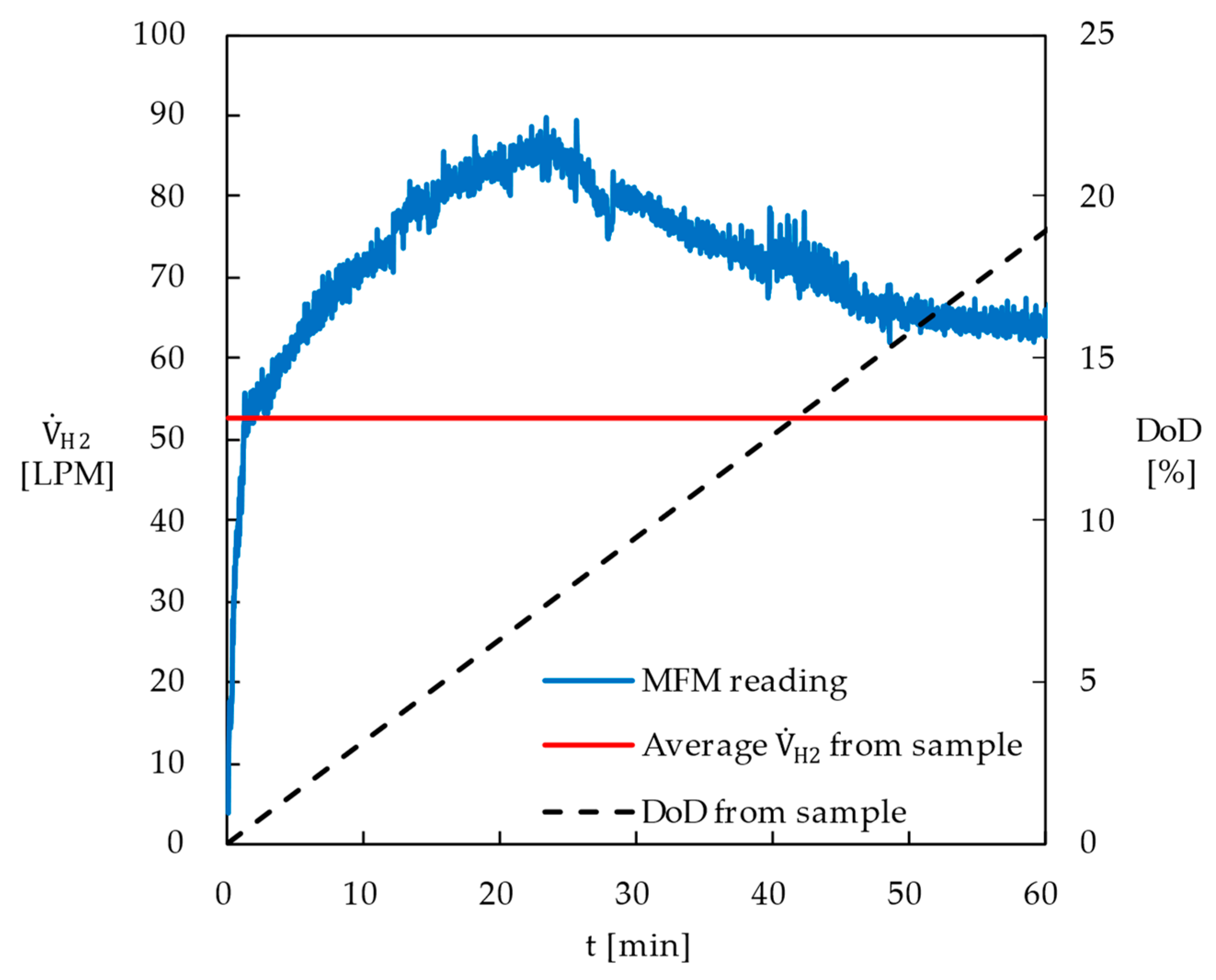
- The dehydrogenation reactor developed in this study demonstrated superior performance compared to conventional fixed-bed or batch reactors, primarily due to the efficient separation of hydrogen bubbles from the catalyst surface. Using 1.5 kg of commercially available 0.5 wt% Pt catalyst and 26.3 L of perhydro-DBT, the degree of dehydrogenation reached 54.2% in 295 min.
- The average hydrogen production rate during the first hour was measured at 52.6 LPM, which was approximately 148% of the predicted value based on the reaction kinetics of the 5.0 wt% Pt catalyst from Sigma-Aldrich®.
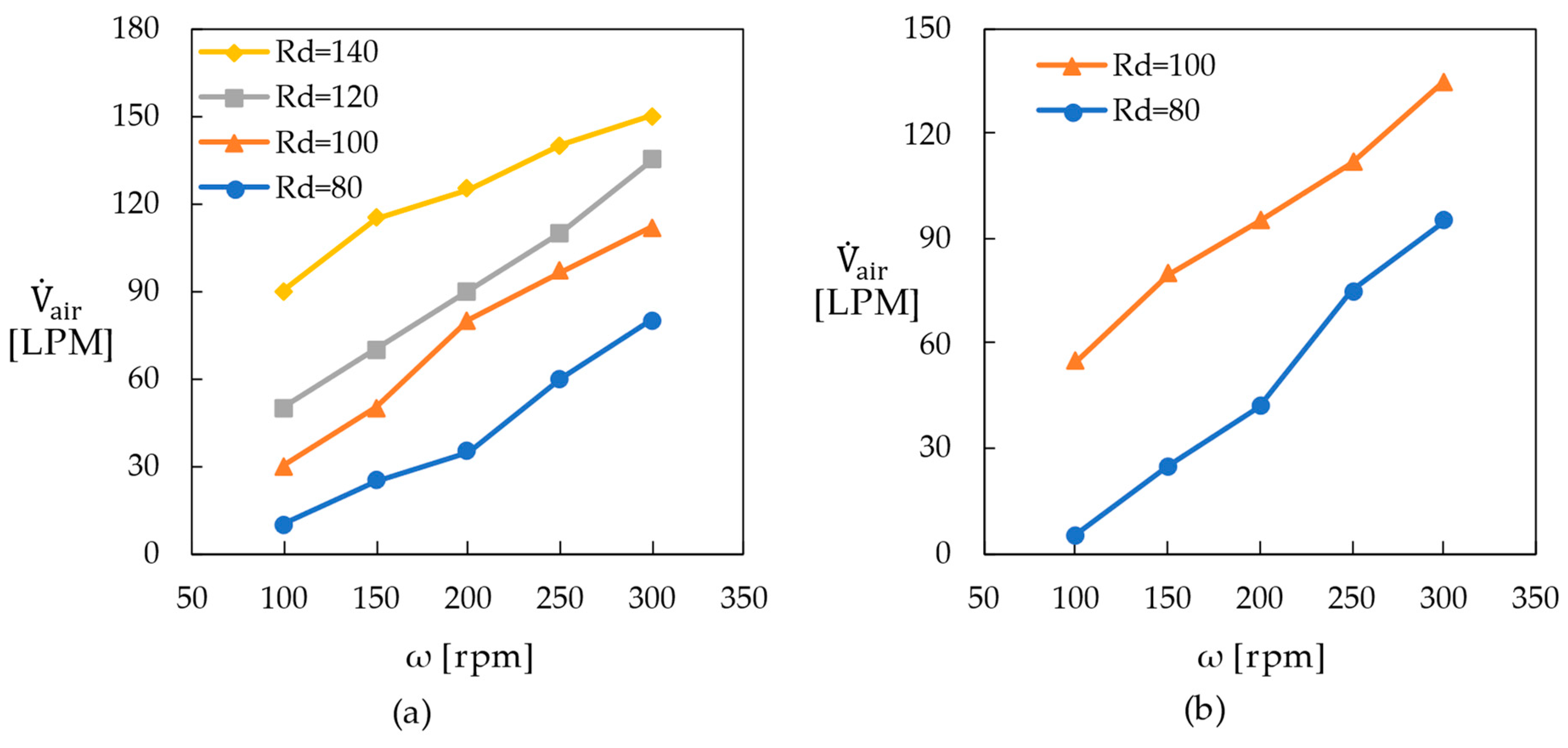
- The preliminary gas infiltration experiments indicated that the proposed reactor isolated 42–95 LPM of hydrogen production at a rotation speed of 200 rpm when the catalyst volume was 2.8 L. The amount of liquid within the reactor was automatically adjusted according to changes in the hydrogen production rate.
- The bounding surfaces of the rotating catalyst bed neighboring the stationary walls of the reactor needed to be highly permeable to utilize the secondary flow. Moreover, the catalyst pellets needed to be located within a small effective distance from this permeable wall. Finally, the bottom wall of the rotor must be an impermeable plate that extends to the center of rotation and be installed with the narrowest gap from the reactor’s bottom wall.
- The flow through the reaction loop could be driven by the pumping capacity of the reactor for rotation speeds > 200 rpm. However, the amount of convective heat transfer along the heating surface sufficient to avoid thermal degradation must be accomplished by employing a separate heating loop with an adequately sized pump.
- If LOHC vapor was not adequately removed from the gas mixture produced by the reactor, the MFM measurement results could not be trusted.
- The degradation characteristics of the LOHC material need to be studied to provide the criteria required in the design of the heating mechanism.
- A condenser that effectively removes LOHC vapor from the produced hydrogen without any accompanying significant flow resistance needs to be developed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of Environmental and Economic Aspects of Various Hydrogen Production Methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen Storage Methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-Free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A Future Energy Supply Based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment Based on Chemical and Economic Properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Liquid Organic and Inorganic Chemical Hydrides for High-Capacity Hydrogen Storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Naseem, M.; Usman, M.; Lee, S. A Parametric Study of Dehydrogenation of Various Liquid Organic Hydrogen Carrier (LOHC) Materials and Its Application to Methanation Process. Int. J. Hydrogen Energy 2021, 46, 4100–4115. [Google Scholar] [CrossRef]
- Bollmann, J.; Schmidt, N.; Beck, D.; Preuster, P.; Zigan, L.; Wasserscheid, P.; Will, S. A Path to a Dynamic Hydrogen Storage System Using a Liquid Organic Hydrogen Carrier (LOHC): Burner-Based Direct Heating of the Dehydrogenation Unit. Int. J. Hydrogen Energy 2023, 48, 1011–1023. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Higo, T. Recent Trends on the Dehydrogenation Catalysis of Liquid Organic Hydrogen Carrier (LOHC): A Review. Top. Catal. 2021, 64, 470–480. [Google Scholar] [CrossRef]
- Amende, M.; Gleichweit, C.; Schernich, S.; Höfert, O.; Lorenz, M.P.A.; Zhao, W.; Koch, M.; Obesser, K.; Papp, C.; Wasserscheid, P.; et al. Size and Structure Effects Controlling the Stability of the Liquid Organic Hydrogen Carrier Dodecahydro-N-Ethylcarbazole during Dehydrogenation over Pt Model Catalysts. J. Phys. Chem. Lett. 2014, 5, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, E.; Taillades-Jacquin, M.; Rozière, J.; Jones, D.J. High-Purity Hydrogen Generation via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Bösmann, A.; Brückner, N.; Thöming, J.; Wasserscheid, P.; Stolte, S. Environmental and Health Impact Assessment of Liquid Organic Hydrogen Carrier (LOHC) Systems—Challenges and Preliminary Results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- Energy Efficiency & Renewable Energy. Multiyear Research, Development, and Demonstration Plan; Department of Energy, United States: Washington, DC, USA, 2012; p. 1219578. [CrossRef]
- Park, S.; Naseem, M.; Lee, S. Experimental Assessment of Perhydro-Dibenzyltoluene Dehydrogenation Reaction Kinetics in a Continuous Flow System for Stable Hydrogen Supply. Materials 2021, 14, 7613. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, M.; Ihonen, J. Techno-Economic Feasibility of Road Transport of Hydrogen Using Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2020, 45, 32098–32112. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Łukasik, N.; Wysocka, I.; Rogala, A.; Gębicki, J. Recent Progress on Hydrogen Storage and Production Using Chemical Hydrogen Carriers. Energies 2022, 15, 4964. [Google Scholar] [CrossRef]
- Nielsen, M.; Alberico, E.; Baumann, W.; Drexler, H.-J.; Junge, H.; Gladiali, S.; Beller, M. Low-Temperature Aqueous-Phase Methanol Dehydrogenation to Hydrogen and Carbon Dioxide. Nature 2013, 495, 85–89. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emel’yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-Toluene Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Aslam, R.; Müller, K.; Müller, M.; Koch, M.; Wasserscheid, P.; Arlt, W. Measurement of Hydrogen Solubility in Potential Liquid Organic Hydrogen Carriers. J. Chem. Eng. Data 2016, 61, 643–649. [Google Scholar] [CrossRef]
- Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Benzyltoluene/Dibenzyltoluene-Based Mixtures as Suitable Liquid Organic Hydrogen Carrier Systems for Low Temperature Applications. Int. J. Hydrogen Energy 2020, 45, 14897–14906. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhang, C.; Ding, Z.; Jin, X. Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects. Energies 2024, 17, 1940. [Google Scholar] [CrossRef]
- Kwak, Y.; Moon, S.; Ahn, C.; Kim, A.-R.; Park, Y.; Kim, Y.; Sohn, H.; Jeong, H.; Nam, S.W.; Yoon, C.W.; et al. Effect of the Support Properties in Dehydrogenation of Biphenyl-Based Eutectic Mixture as Liquid Organic Hydrogen Carrier (LOHC) over Pt/Al2O3 Catalysts. Fuel 2021, 284, 119285. [Google Scholar] [CrossRef]
- Müller, K.; Aslam, R.; Fischer, A.; Stark, K.; Wasserscheid, P.; Arlt, W. Experimental Assessment of the Degree of Hydrogen Loading for the Dibenzyl Toluene Based LOHC System. Int. J. Hydrogen Energy 2016, 41, 22097–22103. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen Storage Using a Hot Pressure Swing Reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef]




| Experiment Temperature (°C) | H18-DBT | H0-DBT | ||||
|---|---|---|---|---|---|---|
| Density (kg/m3) | Viscosity (mPa·s) | Vapor Pressure (Pa) | Density (kg/m3) | Viscosity (mPa·s) | Vapor Pressure (Pa) | |
| 20 | 913.4 | 252.3 | 4.22 × 10−41 | 1044 | 44.18 | 9.91 × 10−5 |
| 70 | 881.5 | 15.96 | 9.91 × 10−17 | 1008 | 6.457 | 5.08 × 10−2 |
| 97 | 864.2 | 6.426 | 6.61 × 10−10 | 989.1 | 3.272 | 4.95 × 10−1 |
| 112 | 854.7 | 4.403 | 3.99 × 10−7 | 978.3 | 2.428 | 1.61 × 100 |
| 300 | 734.6 | 2.399 | 1.89 × 104 | 843.9 | 0.7911 | 9.56 × 103 |
| Study | Reactor Type | Catalyst | H18-DBT Capacity | Result |
|---|---|---|---|---|
| Jorschick et al. [27] | Batch | 0.3 wt% Pt | 1.1 mol | DoD = 40% in 192 min |
| Brückner et al. [11] | Batch | 0.5 wt% Pt | 0.1 mol | DoD = 40% in 120 min 1 DoD = 51% in 210 min 2 |
| Present study | Batch | 0.5 wt% Pt | 82.8 mol | DoD = 54% in 295 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Usman, M.; Park, S.; Lee, S.; Song, M.H. Development of Dehydrogenation System for Liquid Organic Hydrogen Carrier with Enhanced Reaction Rate. Appl. Sci. 2024, 14, 5803. https://doi.org/10.3390/app14135803
Lee J, Usman M, Park S, Lee S, Song MH. Development of Dehydrogenation System for Liquid Organic Hydrogen Carrier with Enhanced Reaction Rate. Applied Sciences. 2024; 14(13):5803. https://doi.org/10.3390/app14135803
Chicago/Turabian StyleLee, Juhan, Muhammad Usman, Sanghyoun Park, Sangyong Lee, and Myung Ho Song. 2024. "Development of Dehydrogenation System for Liquid Organic Hydrogen Carrier with Enhanced Reaction Rate" Applied Sciences 14, no. 13: 5803. https://doi.org/10.3390/app14135803







