Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review
Abstract
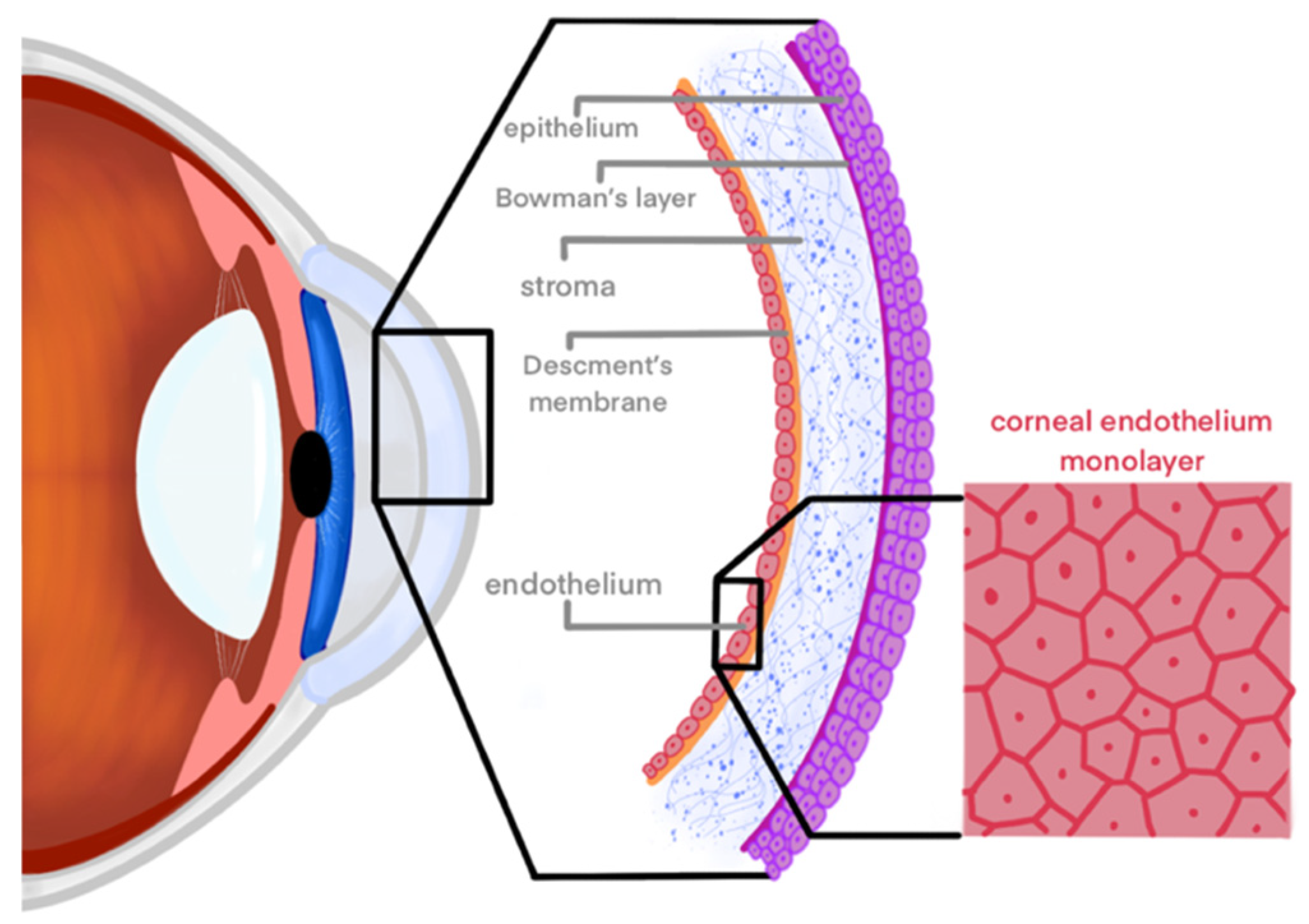
:1. Introduction
1.1. Magnetic Nanotechnology
1.2. Culturing CECs
1.3. Options for Delivery of Cultured CECs
2. Discussion
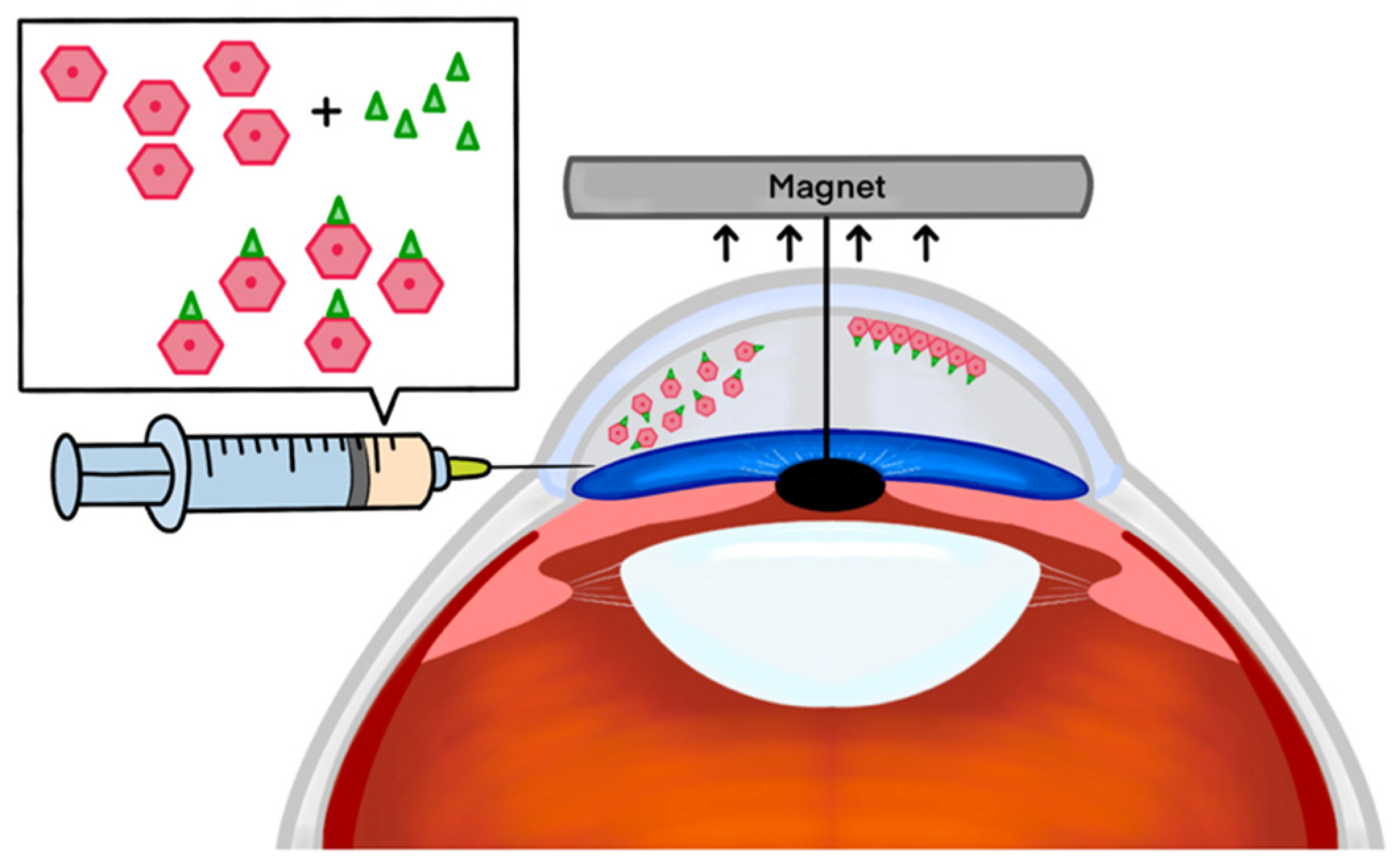
2.1. Effectiveness of Magnetic Nanoparticles in Enhanced Delivery of HCECs
| Moysidis et al. [80] | Xia et al. [81] | Zhao et al. [83] | |
|---|---|---|---|
| Cell type | Cadaveric donor HCECs—50,000 | Cadaveric donor primary HCECs—200,000–600,000 | Donor CECs (origin not specified)—100,000 |
| Experimental Model | In vitro (contact lens model) | Rabbit model (corneal endothelial dysfunction; endothelial cell or Descemet stripping) | Rabbit model (corneal endothelium injury by mechanical destruction) |
| MNPs used | 50 nm diameter superparamagnetic nanoparticles | 50 nm diameter superparamagnetic nanoparticles | Superparamagnetic Fe3O4 nanoparticles in a HA gel matrix (size not specified) |
| Magnet used | Custom made magnet | External neodymium magnet (diameter = 12 mm and height 20 mm) | External neodymium magnet (diameter = 5 mm and height = 20 mm) |
| Control | HCECs without nanoparticles | BSS+ solution | Magnetic PBS solution |
| Results | 2.4-fold increase in cell density compared to gravity | Improved post-operative corneal clarity and reduced corneal thickness | Increased delivery efficiency with HA gel, challenges in uniform distribution |
| Histological findings | Tight junction formation (ZO-1) and functional integration into a monolayer (preserved corneal morphology of transplanted cells) | Tight junction protein expression (ZO-1 and NCAM) and functional integration into a monolayer (preserved corneal morphology of transplanted cells) | Functional integration, irregular cellular distribution |
| Adverse events | N/A as in vitro study | No migration of cells to the iris or trabecular meshwork and no acute fluctuations in IOP | IOP was not investigated as an adverse event |
| Challenges | Saturation effect at higher | Limited follow-up period | Gel degradation impacting cell distribution, impact on IOP not reported |
2.2. Limitations, Safety and Challenges
3. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human Corneal Anatomy Redefined: A Novel Pre-Descemet’s Layer (Dua’s Layer). Ophthalmology 2013, 120, 1778–1785. [Google Scholar] [CrossRef]
- Klyce, S.D. 12. Endothelial pump and barrier function. Exp. Eye Res. 2020, 198, 108068. [Google Scholar] [CrossRef]
- He, Z.; Forest, F.; Gain, P.; Rageade, D.; Bernard, A.; Acquart, S.; Peoc’h, M.; Defoe, D.M.; Thuret, G. 3D map of the human corneal endothelial cell. Sci. Rep. 2016, 6, 29047. [Google Scholar] [CrossRef]
- Okumura, N.; Hirano, H.; Numata, R.; Nakahara, M.; Ueno, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Cell surface markers of functional phenotypic corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7610–7618. [Google Scholar] [CrossRef]
- Parekh, M.; Peh, G.; Mehta, J.S.; Ahmad, S.; Ponzin, D.; Ferrari, S. Effects of corneal preservation conditions on human corneal endothelial cell culture. Exp. Eye Res. 2019, 179, 93–101. [Google Scholar] [CrossRef]
- Polisetti, N.; Joyce, N.C. The culture of limbal stromal cells and corneal endothelial cells. Corneal Regen. Med. Methods Protoc. 2013, 1014, 131–139. [Google Scholar]
- Joyce, N.C.; Meklir, B.; Joyce, S.J.; Zieske, J.D. Cell cycle protein expression and proliferative status in human corneal cells. Investig. Ophthalmol. Vis. Sci. 1996, 37, 645–655. [Google Scholar]
- Chen, K.H.; Harris, D.L.; Joyce, N.C. TGF-beta2 in aqueous humor suppresses S-phase entry in cultured corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2513–2519. [Google Scholar]
- Murphy, C.; Alvarado, J.; Juster, R.; Maglio, M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Investig. Ophthalmol. Vis. Sci. 1984, 25, 312–322. [Google Scholar]
- Age-Related Changes and Diseases of the Ocular Surface and Cornea |IOVS| ARVO Journals. Available online: https://iovs.arvojournals.org/article.aspx?articleid=2127383 (accessed on 5 May 2024).
- Stiemke, M.M.; Edelhauser, H.F.; Geroski, D.H. The developing corneal endothelium: Correlation of morphology, hydration and Na/K ATPase pump site density. Curr. Eye Res. 1991, 10, 145–156. [Google Scholar] [CrossRef]
- Bourne, W.M. Cellular changes in transplanted human corneas. Cornea 2001, 20, 560–569. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative Response of Corneal Endothelial Cells from Young and Older Donors. Investig. Opthalmol. Vis. Sci. 2004, 45, 1743. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Vaiciuliene, R.; Rylskyte, N.; Baguzyte, G.; Jasinskas, V. Risk factors for fluctuations in corneal endothelial cell density (Review). Exp. Ther. Med. 2022, 23, 129. [Google Scholar] [CrossRef]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Krachmer, J.H. Posterior polymorphous corneal dystrophy: A disease characterized by epithelial-like endothelial cells which influence management and prognosis. Trans. Am. Ophthalmol. Soc. 1985, 83, 413–475. [Google Scholar]
- Chan, C.C.; Green, W.R.; Barraquer, J.; Barraquer-Somers, E.; de la Cruz, Z.C. Similarities between Posterior Polymorphous and Congenital Hereditary Endothelial Dystrophies: A Study of 14 Buttons of 11 Cases. Cornea 1982, 1, 155. [Google Scholar] [CrossRef]
- Shields, M.B. Progressive essential iris atrophy, Chandler’s syndrome, and the iris nevus (Cogan-Reese) syndrome: A spectrum of disease. Surv. Ophthalmol. 1979, 24, 3–20. [Google Scholar] [CrossRef]
- Carlson, K.H.; Ilstrup, D.M.; Bourne, W.M.; Dyer, J.A. Effect of silicone elastomer contact lens wear on endothelial cell morphology in aphakic eyes. Cornea 1990, 9, 45–47. [Google Scholar] [CrossRef]
- Bourne, W.M.; Hodge, D.O.; McLaren, J.W. Estimation of corneal endothelial pump function in long-term contact lens wearers. Investig. Ophthalmol. Vis. Sci. 1999, 40, 603–611. [Google Scholar]
- Bourne, W.M.; Nelson, L.R.; Hodge, D.O. Continued endothelial cell loss ten years after lens implantation. Ophthalmology 1994, 101, 1014–1022; discussion 1022–1023. [Google Scholar] [CrossRef]
- Price, M.O.; Feng, M.T.; Price, F.W.J. Endothelial Keratoplasty Update 2020. Cornea 2021, 40, 541. [Google Scholar] [CrossRef]
- Price, M.O.; Price, F.W., Jr. Endothelial keratoplasty—A review. Clin. Exp. Ophthalmol. 2010, 38, 128–140. [Google Scholar] [CrossRef]
- Melles, G.R.; Eggink, F.A.; Lander, F.; Pels, E.; Rietveld, F.J.; Beekhuis, W.H.; Binder, P.S. A surgical technique for posterior lamellar keratoplasty. Cornea 1998, 17, 618–626. [Google Scholar] [CrossRef]
- Melles, G.R.J.; Wijdh, R.H.J.; Nieuwendaal, C.P. A technique to excise the descemet membrane from a recipient cornea (descemetorhexis). Cornea 2004, 23, 286–288. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Stuart, A.J.; Romano, V.; Virgili, G.; Shortt, A.J. Descemet’s membrane endothelial keratoplasty (DMEK) versus Descemet’s stripping automated endothelial keratoplasty (DSAEK) for corneal endothelial failure. Cochrane Database Syst. Rev. 2018, 6, CD012097. [Google Scholar] [CrossRef]
- Grottone, G.T.; Pereira, N.C.; Gomes, J.Á.P. Endothelial keratoplasty: Evolution and horizons. Arq. Bras. Oftalmol. 2012, 75, 439–446. [Google Scholar] [CrossRef]
- Lee, W.B.; Jacobs, D.S.; Musch, D.C.; Kaufman, S.C.; Reinhart, W.J.; Shtein, R.M. Descemet’s stripping endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology 2009, 116, 1818–1830. [Google Scholar] [CrossRef]
- Hurley, D.J.; Murtagh, P.; Guerin, M. Ultrathin Descemet Stripping Automated Endothelial Keratoplasty (UT-DSAEK) versus Descemet Membrane Endothelial Keratoplasty (DMEK)—A systematic review and meta-analysis. Eye 2023, 37, 3026–3032. [Google Scholar] [CrossRef]
- Zafar, S.; Parker, J.S.; de Kort, C.; Melles, G.; Sikder, S. Perceived difficulties and barriers to uptake of Descemet’s membrane endothelial keratoplasty among surgeons. Clin. Ophthalmol. 2019, 13, 1055–1061. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Lim, R.R.; Lakshminarayanan, R.; Mohan, R.R. Nanomedicine Approaches for Corneal Diseases. J. Funct. Biomater. 2015, 6, 277. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic Characterization of Iron Oxide Nanoparticles for Biomedical Applications. Biomed. Nanotechnol. Methods Protoc. 2017, 1570, 47–71. [Google Scholar]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef]
- Misra, R.D.K. Magnetic nanoparticle carrier for targeted drug delivery: Perspective, outlook and design. Mater. Sci. Technol. 2008, 24, 1011–1019. [Google Scholar] [CrossRef]
- Edelman, E.R.; Langer, R. Optimization of release from magnetically controlled polymeric drug release devices. Biomaterials 1993, 14, 621–626. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Vedula, A.; Dubovy, S.R.; Goldberg, J.L. Evaluation of magnetic micro- and nanoparticle toxicity to ocular tissues. PLoS ONE 2011, 6, e17452. [Google Scholar] [CrossRef]
- Giannaccini, M.; Pedicini, L.; De Matienzo, G.; Chiellini, F.; Dente, L.; Raffa, V. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci. Rep. 2017, 7, 43092. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Zhang, S.; Zhong, M.; Fan, H. Ferrite Nanoparticles-Based Reactive Oxygen Species-Mediated Cancer Therapy. Front. Chem. 2021, 9, 651053. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.X.; Gao, Y.J.; Gao, F.P.; Fan, Y.S.; Li, X.J.; Duan, Z.Y.; Wang, H. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 5100–5107. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, K.; Subramanian, S.; Korde, A.; Singh, R.; Sawant, K. Heterogeneous surface architectured pH responsive Metal-Drug Nano-conjugates for mitochondria targeted therapy of Glioblastomas: A multimodal intranasal approach. Chem. Eng. J. 2020, 394, 124419. [Google Scholar] [CrossRef]
- Pankhurst, Q.; Connolly, J.; Jones, S.; Dobson, J. TOPICAL REVIEW: Applications of magnetic nanoparticles in biomedicine. J. Phys. Appl. Phys. 2003, 36. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, F.; Schillinger, U.; Bergemann, C.; Anton, M. Magnetofection: Enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J. Liposome Res. 2003, 13, 29–32. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef]
- Bartakova, A.; Kunzevitzky, N.J.; Goldberg, J.L. Regenerative Cell Therapy for Corneal Endothelium. Curr. Ophthalmol. Rep. 2014, 2, 81–90. [Google Scholar] [CrossRef]
- Baum, J.L.; Niedra, R.; Davis, C.; Yue, B.Y. Mass culture of human corneal endothelial cells. Arch. Ophthalmol. 1979, 97, 1136–1140. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Toh, K.P.; Wu, F.Y.; Tan, D.T.; Mehta, J.S. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS ONE 2011, 6, e28310. [Google Scholar] [CrossRef]
- Frausto, R.F.; Swamy, V.S.; Peh, G.S.L.; Boere, P.M.; Hanser, E.M.; Chung, D.D.; George, B.L.; Morselli, M.; Kao, L.; Azimov, R.; et al. Phenotypic and functional characterization of corneal endothelial cells during in vitro expansion. Sci. Rep. 2020, 10, 7402. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Chng, Z.; Ang, H.P.; Cheng, T.Y.D.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.F.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Bartakova, A.; Kuzmenko, O.; Alvarez-Delfin, K.; Kunzevitzky, N.J.; Goldberg, J.L. A Cell Culture Approach to Optimized Human Corneal Endothelial Cell Function. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1617–1629. [Google Scholar] [CrossRef]
- Takamizawa, S.; Maehata, Y.; Imai, K.; Senoo, H.; Sato, S.; Hata, R.I. Effects of ascorbic acid and ascorbic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells. Cell Biol. Int. 2004, 28, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Schweigerer, L.; Neufeld, G.; Friedman, J.; Abraham, J.A.; Fiddes, J.C.; Gospodarowicz, D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature 1987, 325, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Shima, N.; Kimoto, M.; Yamaguchi, M.; Yamagami, S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8711–8717. [Google Scholar] [CrossRef]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef]
- Ko, M.K.; Kay, E.P. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4495–4503. [Google Scholar] [CrossRef]
- Engelmann, K.; Böhnke, M.; Friedl, P. Isolation and long-term cultivation of human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 1656–1662. [Google Scholar]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Ishizaki, T.; Uehata, M. Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 2000, 325, 273–284. [Google Scholar] [PubMed]
- Ishizaki, T.; Uehata, M.; Tamechika, I.; Keel, J.; Nonomura, K.; Maekawa, M.; Narumiya, S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000, 57, 976–983. [Google Scholar] [PubMed]
- Park, J.H.; Lee, K.; Park, C.Y. Effect of Magnetic Microparticles on Cultivated Human Corneal Endothelial Cells. Transl. Vis. Sci. Technol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wongvisavavit, R.; Parekh, M.; Ahmad, S.; Daniels, J.T. Challenges in corneal endothelial cell culture. Regen. Med. 2021, 16, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.L.; Ong, H.S.; Adnan, K.; Ang, H.P.; Lwin, C.N.; Seah, X.Y.; Lin, S.J.; Mehta, J.S. Functional Evaluation of Two Corneal Endothelial Cell-Based Therapies: Tissue-Engineered Construct and Cell Injection. Sci. Rep. 2019, 9, 6087. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Z.; Qu, J.; Xie, H.; Zhao, J.; Fan, T.; Liu, X.; Zhang, M. Tissue-Engineered Corneal Endothelial Sheets Using Ultrathin Acellular Porcine Corneal Stroma Substrates for Endothelial Keratoplasty. ACS Biomater. Sci. Eng. 2022, 8, 1301–1311. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Park, S.; Leonard, B.C.; Raghunathan, V.K.; Kim, S.; Li, J.Y.; Mannis, M.J.; Murphy, C.J.; Thomasy, S.M. Animal models of corneal endothelial dysfunction to facilitate development of novel therapies. Ann. Transl. Med. 2021, 9, 1271. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK Inhibitor Converts Corneal Endothelial Cells into a Phenotype Capable of Regenerating In Vivo Endothelial Tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.-I.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef] [PubMed]
- Bostan, C.; Thériault, M.; Forget, K.J.; Doyon, C.; Cameron, J.D.; Proulx, S.; Brunette, I. In Vivo Functionality of a Corneal Endothelium Transplanted by Cell-Injection Therapy in a Feline Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1620–1634. [Google Scholar] [CrossRef]
- Mimura, T.; Shimomura, N.; Usui, T.; Noda, Y.; Kaji, Y.; Yamgami, S.; Amano, S.; Miyata, K.; Araie, M. Magnetic attraction of iron-endocytosed corneal endothelial cells to Descemet’s membrane. Exp. Eye Res. 2003, 76, 745–751. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Yanagi, Y.; Usui, T.; Ono, K.; Araie, M.; Amano, S. Sphere Therapy for Corneal Endothelium Deficiency in a Rabbit Model. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3128–3135. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Aurion Biotech. CLARA: A Phase 1/2 Multi-Center, Randomized, Double-Masked, Prospective, Parallel-Arm Study of AURN001 in Subjects with Corneal Edema Secondary to Corneal Endothelial Dysfunction (ABA-1). 2024; Report No.: NCT06041256. Available online: https://clinicaltrials.gov/study/NCT06041256 (accessed on 1 January 2024).
- Worthylake, R.A.; Burridge, K. RhoA and ROCK Promote Migration by Limiting Membrane Protrusions. J. Biol. Chem. 2003, 278, 13578–13584. [Google Scholar] [CrossRef]
- Cornell, L.E.; Wehmeyer, J.L.; Johnson, A.J.; Desilva, M.N.; Zamora, D.O. Magnetic Nanoparticles as a Potential Vehicle for Corneal Endothelium Repair. Mil. Med. 2016, 181 (Suppl. S5), 232–239. [Google Scholar] [CrossRef]
- Moysidis, S.N.; Alvarez-Delfin, K.; Peschansky, V.J.; Salero, E.; Weisman, A.D.; Bartakova, A.; Raffa, G.A.; Merkhofer, R.M.; Kador, K.E.; Kunzevitzky, N.J.; et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 499–509. [Google Scholar] [CrossRef]
- Xia, X.; Atkins, M.; Dalal, R.; Kuzmenko, O.; Chang, K.C.; Sun, C.B.; Benatti, C.A.; Rak, D.J.; Nahmou, M.; Kunzevitzky, N.J.; et al. Magnetic Human Corneal Endothelial Cell Transplant: Delivery, Retention, and Short-Term Efficacy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2438–2448. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Usui, T.; Ishii, Y.; Ono, K.; Yokoo, S.; Funatsu, H.; Araie, M.; Amano, S. Long-term outcome of iron-endocytosing cultured corneal endothelial cell transplantation with magnetic attraction. Exp. Eye Res. 2005, 80, 149–157. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, S.; Li, D.; Li, L.; Ding, X.; Huang, Y.; Li, Y.; Ji, J.; Wang, L.; Fan, Y. Injectable magnetic hyaluronic acid gel for corneal endothelial cells efficient delivery and retention. Appl. Mater. Today 2024, 37, 102090. [Google Scholar] [CrossRef]
- Sun, X.; Song, W.; Teng, L.; Huang, Y.; Liu, J.; Peng, Y.; Lu, X.; Yuan, J.; Zhao, X.; Zhao, Q.; et al. MiRNA 24-3p-rich exosomes functionalized DEGMA-modified hyaluronic acid hydrogels for corneal epithelial healing. Bioact. Mater. 2023, 25, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Jeong, S.H.; Logan, C.M.; Le, P.; Mundy, D.; Chen, F.; Chen, K.M.; Kim, M.; Lee, G.-H.; Na, K.-S.; et al. Supramolecular host-guest hyaluronic acid hydrogels enhance corneal wound healing through dynamic spatiotemporal effects. Ocul. Surf. 2022, 23, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Karvinen, J.; Sorsa, E.; Jönkkäri, I.; Väliaho, J.; Kallio, P.; Ilmarinen, T.; Miettinen, S.; Skottman, H.; Kellomäki, M. Hydrazone crosslinked hyaluronan-based hydrogels for therapeutic delivery of adipose stem cells to treat corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Hong, Y.M.; Chung, W.G.; Park, W.; Lee, J.; Kim, H.K.; Byeon, S.H.; Kim, D.W.; Park, J.-U. Real-time in vivo monitoring of intraocular pressure distribution in the anterior chamber and vitreous chamber for diagnosis of glaucoma. Sci. Adv. 2024, 10, eadk7805. [Google Scholar] [CrossRef] [PubMed]
- Benozzi, J.; Nahum, L.P.; Campanelli, J.L.; Rosenstein, R.E. Effect of hyaluronic acid on intraocular pressure in rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2196–2200. [Google Scholar]
- Emmecell. A Phase 1, Prospective, Multi-Center, Open-Label, Dose-Escalation Study to Assess the Safety, and Tolerability of EO2002 with and without Endothelial Brushing or Descemet Stripping in the Treatment of Corneal Edema (EMME-001). 2024; Report No.: NCT04894110. Available online: https://clinicaltrials.gov/study/NCT04894110 (accessed on 1 January 2024).
- Asociación para Evitar la Ceguera en México. Phase 1, Multiple Dose, Open-Label Study to Assess the Safety and Tolerability of EO2002 Intracameral Injections with or without Topical Ripasudil in the Treatment of Corneal Edema. 2022. Report No.: NCT05636579. Available online: https://clinicaltrials.gov/study/NCT05636579 (accessed on 1 January 2024).
- Markides, H.; Rotherham, M.; Haj, A. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Raju, H.B.; Hu, Y.; Padgett, K.R.; Rodriguez, J.E.; Goldberg, J.L. Investigation of nanoparticles using magnetic resonance imaging after intravitreal injection. Clin. Exp. Ophthalmol. 2012, 40, 100–107. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef]
- Gaharwar, U.S.; Meena, R.; Rajamani, P. Iron oxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in lymphocytes. J. Appl. Toxicol. JAT 2017, 37, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Magaye, R.R.; Yue, X.; Zou, B.; Shi, H.; Yu, H.; Liu, K.; Lin, X.; Xu, J.; Yang, C.; Zhao, J.; et al. Acute toxicity of nickel nanoparticles in rats after intravenous injection. Int. J. Nanomed. 2014, 9, 1393–1402. [Google Scholar]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, S.A.; Rajaei Jafarabadi, M.; Abrishami, M. Temperature Distribution Simulation of the Human Eye Exposed to Laser Radiation. J. Lasers Med. Sci. 2013, 4, 175–181. [Google Scholar] [PubMed]
- Demirci, H.; Slimani, N.; Pawar, M.; Kumon, R.E.; Vaishnava, P.; Besirli, C.G. Magnetic Hyperthermia in Y79 Retinoblastoma and ARPE-19 Retinal Epithelial Cells: Tumor Selective Apoptotic Activity of Iron Oxide Nanoparticle. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of Oxidative Stress in Ocular Diseases: A Balancing Act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and Gold Nanoparticles Exposure to In Vitro Cultured Retina—Studies on Nanoparticle Internalization, Apoptosis, Oxidative Stress, Glial- and Microglial Activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B.K. Nanoceria Prevents Glucose-Induced Protein Glycation in Eye Lens Cells. Nanomaterials 2021, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, B.I.; Cave, G.W.V.; Barnett, Y.; Pierscionek, B. Treatment of Human Lens Epithelium with High Levels of Nanoceria Leads to Reactive Oxygen Species Mediated Apoptosis. Molecules 2020, 25, 441. [Google Scholar] [CrossRef] [PubMed]
| Corneal Endothelial Disease | Current Treatments |
|---|---|
| Fuchs’ Endothelial Corneal Dystrophy | Conservative Management: Hypertonic saline drops Surgical Options: Endothelial keratoplasty (EK), Descemet’s stripping endothelial keratoplasty (DSEK), Descemet’s membrane endothelial keratoplasty (DMEK) |
| Bullous Keratopathy | Conservative Management: Hypertonic saline drops, bandage contact lenses Surgical Options: EK, DSEK, DMEK |
| Endothelial Decompensation due to Contact Lens Wear or Infections | Antimicrobial Therapy: Treatment of underlying infections Surgical Options: EK, DSEK, DMEK |
| Congenital Hereditary Endothelial Dystrophy (CHED) | Medical Management: Symptomatic relief with lubricating drops Surgical Options: EK, DSEK, DMEK |
| Posterior Polymorphous Corneal Dystrophy (PPCD) | Conservative Management: Monitoring for progression Medical Management: Hypertonic saline drops Surgical Options: Glaucoma Drainage Implants, EK, DSEK, DMEK |
| Iridocorneal Endothelial Syndrome (ICE) | Medical Management: Hypertonic saline drops, Topical medications to control intraocular pressure Surgical Options: Trabeculectomy, Glaucoma Drainage Implants, EK, DSEK, DMEK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkelyte, V.; Thompson, P.; Coelho, M.; Woronkowicz, M.; Skopinski, P.; Roberts, H. Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review. Appl. Sci. 2024, 14, 5877. https://doi.org/10.3390/app14135877
Vilkelyte V, Thompson P, Coelho M, Woronkowicz M, Skopinski P, Roberts H. Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review. Applied Sciences. 2024; 14(13):5877. https://doi.org/10.3390/app14135877
Chicago/Turabian StyleVilkelyte, Virginija, Polly Thompson, Maria Coelho, Małgorzata Woronkowicz, Piotr Skopinski, and Harry Roberts. 2024. "Challenges and Advances in Magnetic Nanoparticle-Guided Delivery of Cultured Human Corneal Endothelial Cells—A Review" Applied Sciences 14, no. 13: 5877. https://doi.org/10.3390/app14135877






