Lawsonia inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid
Abstract
1. Introduction
2. Material Preparation
2.1. Preparation of Materials
2.1.1. Preparation of Mild Steel Specimens
2.1.2. Plant Extract Preparation
2.2. Weight-Loss Measurements
2.3. Electrochemical Measurements
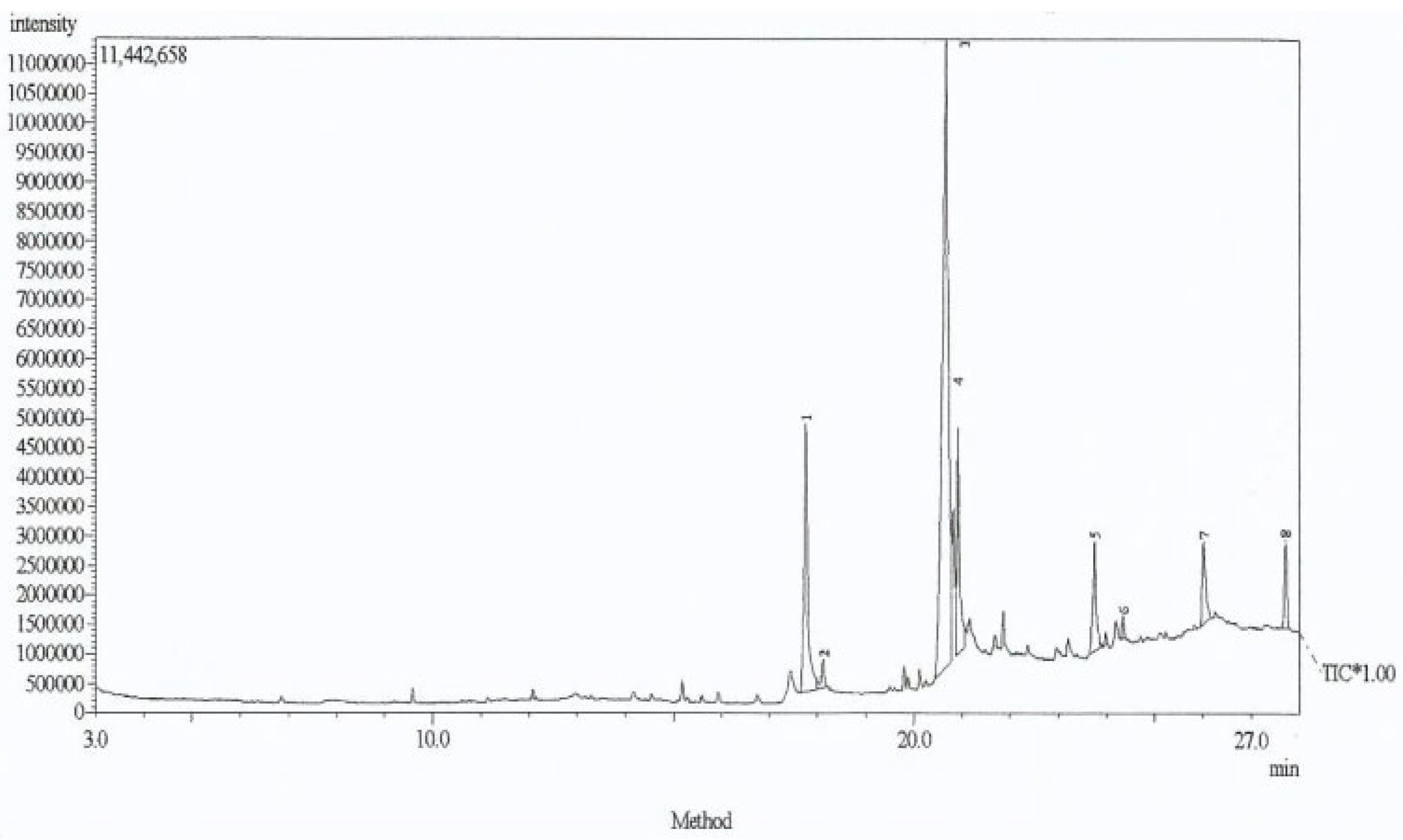
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Measurements
2.5. SEM (Scanning Electron Microscopy)
3. Results and Discussion
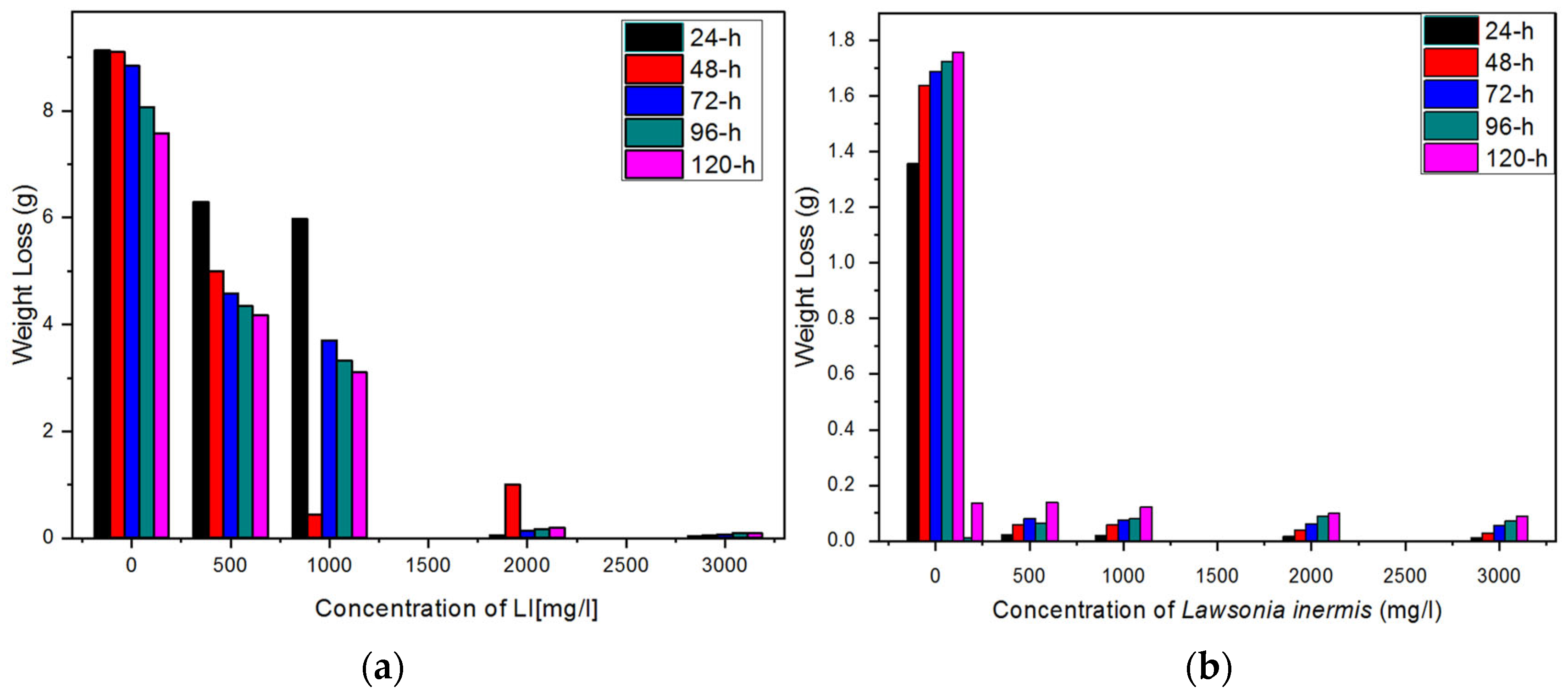
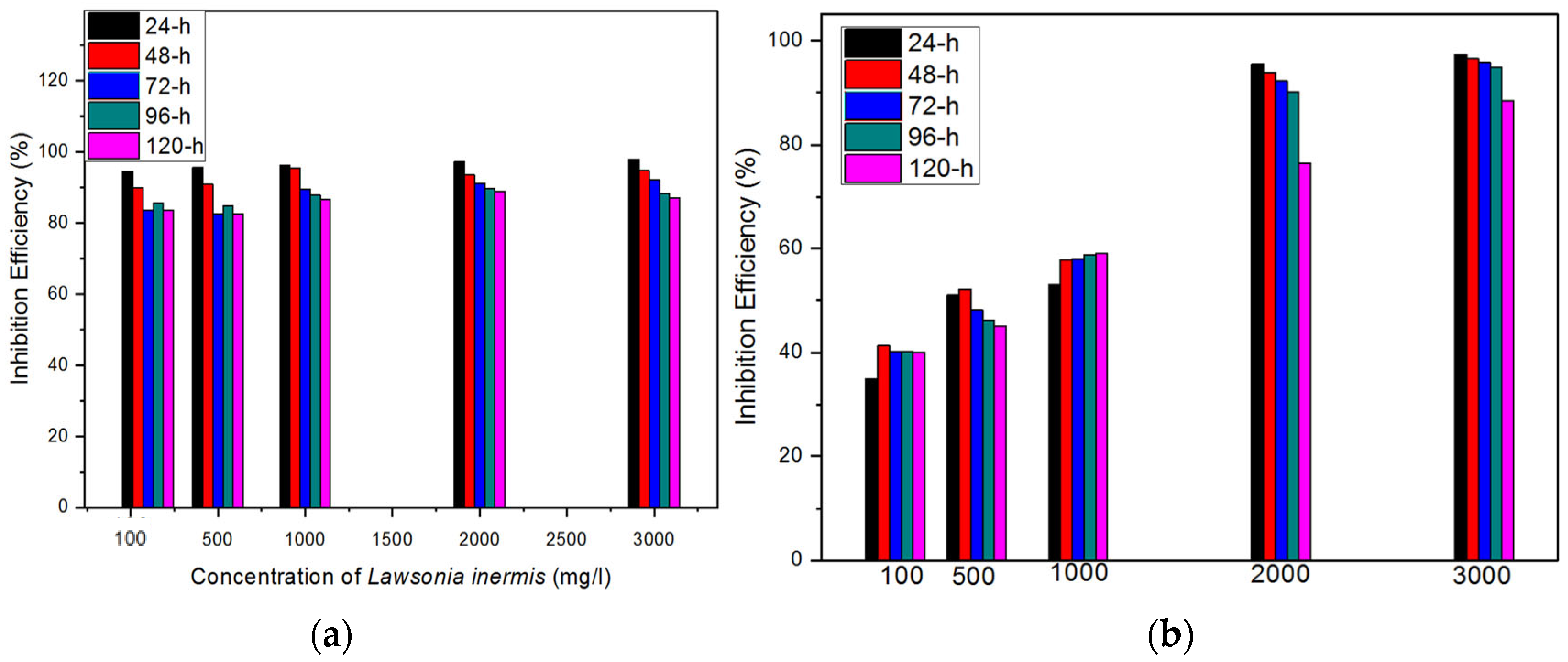
3.1. Gravimetric Results
3.2. Electrochemical Measurements
Potentiodynamic Polarisation (Pdp) Results for Mild Steel
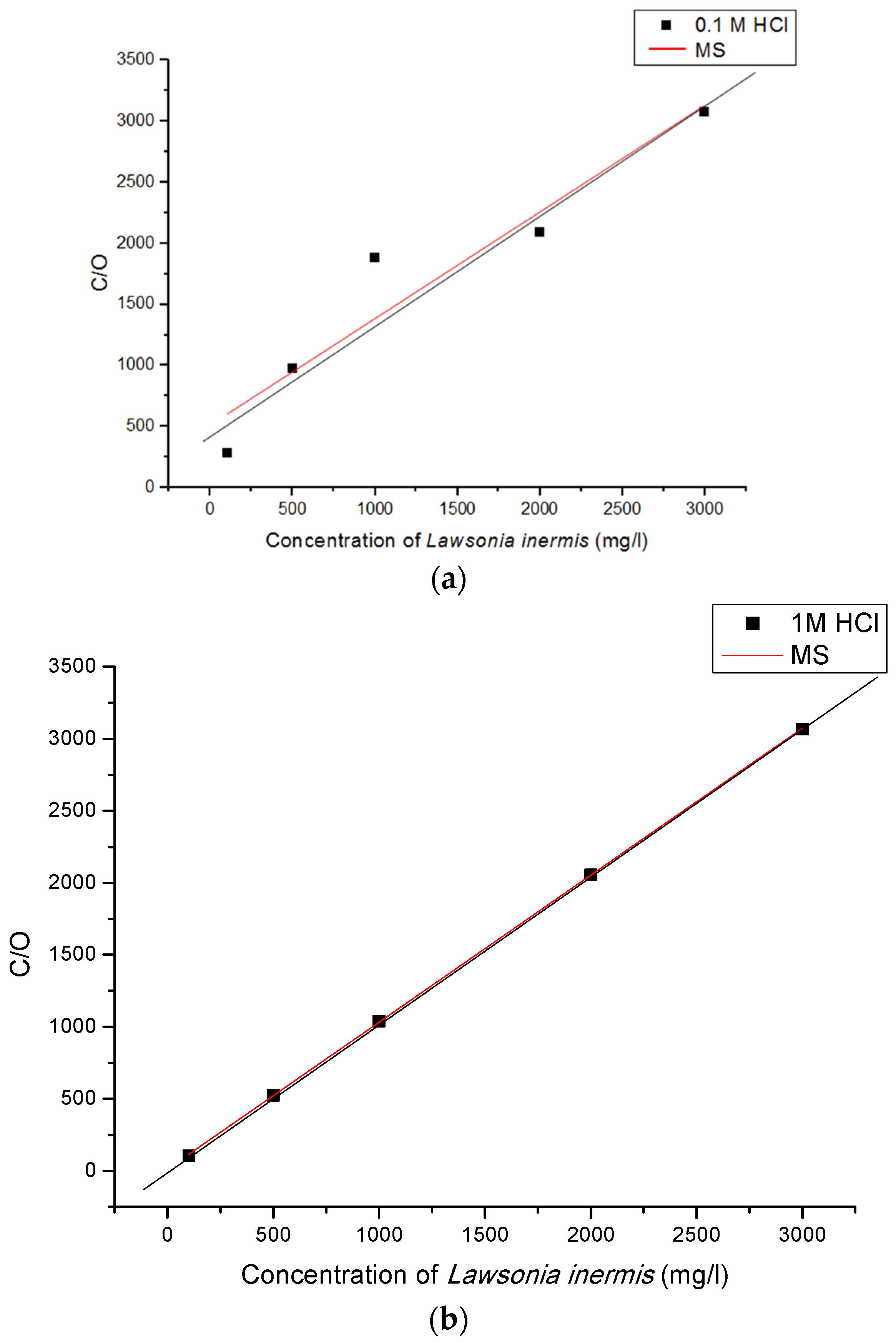
3.3. Adsorption Consideration of Lawsonia inermis Plant Extract
Linear Regression Analysis
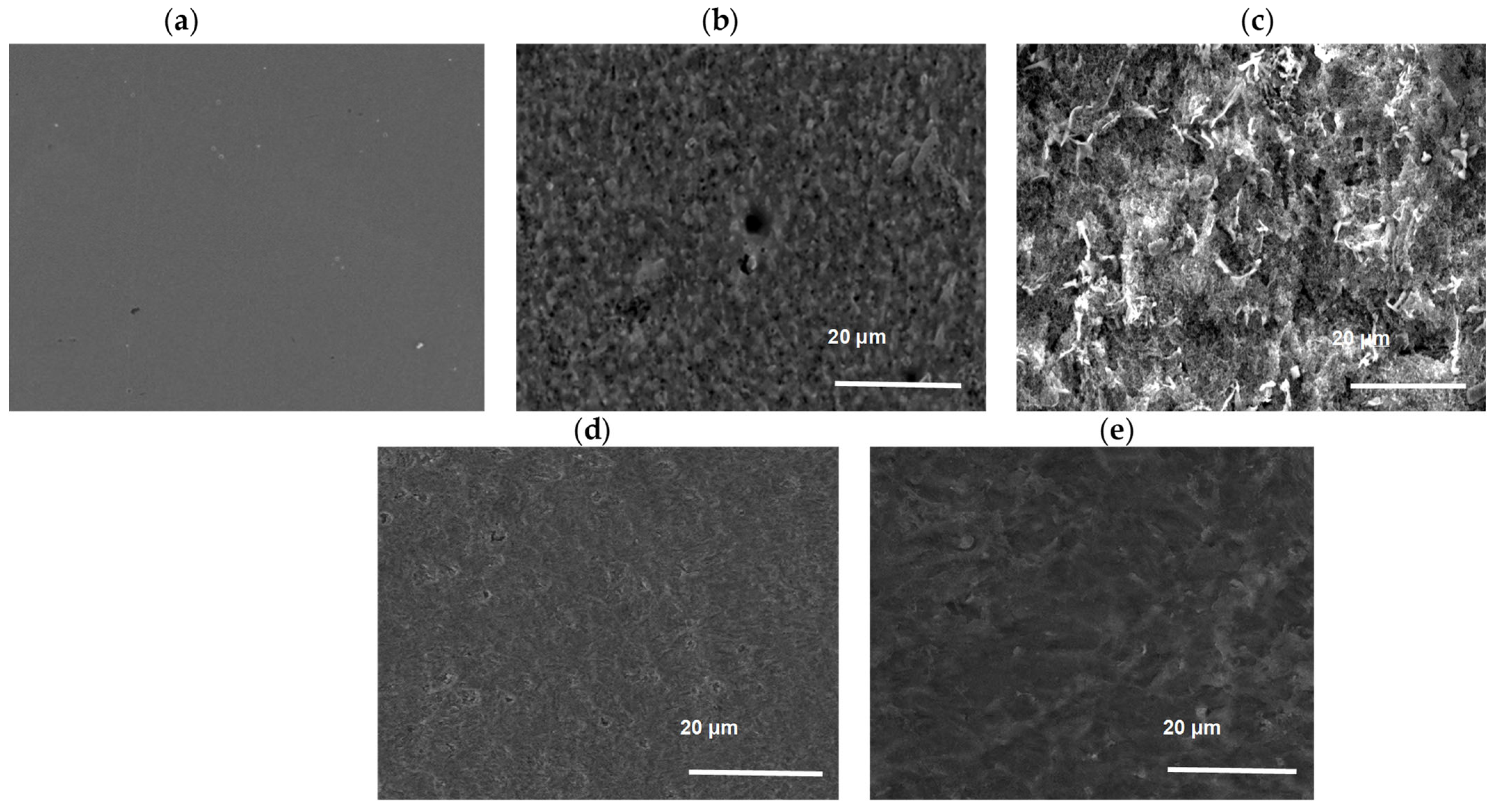
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. GC-MS Analysis Results for Lawsonia inermis
4. Conclusions
- The extract of the Lawsonia inermis noticeably inhibited the corrosion of the mild steel in the HCl acidic environment, the effect of which increased with the increase in the concentration of the plant extract, thus presenting Lawsonia inermis as an effective corrosion inhibitor;
- The Langmuir adsorption isothermal analysis reveals that the adsorption of Lawsonia inermis’s phytochemicals on the mild steel’s surface follows a combination of physisorption and weak chemisorption mechanisms. This indicates that the inhibitor molecules form a protective layer on the metal’s surface, thereby reducing the corrosion rate;
- The potentiodynamic polarisation measurements demonstrate that the Lawsonia inermis extract alters both the cathodic and anodic reactions and shifts the corrosion potential to more positive values, leading to improved corrosion resistance of the mild steel;
- This study highlights the importance for optimising the concentration of the Lawsonia inermis extract for the maximum corrosion inhibition efficiency. Concentrations ranging from 2000 mg/L to 3000 mg/L demonstrate excellent inhibition efficiencies, suggesting the effectiveness of the extract at higher concentrations;
- In addition, the organic compounds identified in Lawsonia inermis played a role by forming cross-links to create a thin film layer over the steel’s surface, which inhibited the oxidation of the metal and reduced corrosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghali, E.; Elboujdaini, M.; Omanovic, S. Corrosion Basics: An Introduction; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Behpour, M.; Ghoreishi, S.M.; Khayatkashani, M.; Soltani, N.; Salavati-Niasari, M.; Hamadanian, M. Electrochemical and Theoretical Investigation on the Corrosion Inhibition of Mild Steel by Thiosalicylaldehyde Derivatives in Hydrochloric Acid Solution. Corros. Sci. 2008, 50, 2172–2181. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Ebrahimi, M.; Aliofkhazraei, M. Corrosion Inhibition of Mild Steel in 1M HCl Solution by Henna Extract: A Comparative Study of the Inhibition Performance with Chemical Inhibitor. Arab. J. Sci. Eng. 2014, 39, 2927–2933. [Google Scholar]
- Prakash, C.V.; Udayabhanu, R.N.; Kamath, S. A Review on Lawsonia inermis Linn. (Henna). Int. J. Green Pharm. 2018, 12, S208–S215. [Google Scholar]
- Devi, N.; Karthiga, N.; Keerthana, R.; Umasankareswari, T.; Krishnaveni, A.; Singh, G.; Rajendran, S. Extracts of leaves as corrosion inhibitors—An overview and corrosion inhibition by an aqueous extract of henna leaves (Lawsonia inermis). Int. J. Corros. Scale Inhib. 2020, 9, 1169–1193. [Google Scholar] [CrossRef]
- Zulkifli, F.; Ali, N.; Yusof, M.S.M.; Khairul, W.M.; Rahamathullah, R.; Isa, M.I.N.; Nik, W.B.W. The Effect of Concentration of Lawsonia inermis as a Corrosion Inhibitor for Aluminum Alloy in Seawater. Adv. Phys. Chem. 2017, 2017, 8521623. [Google Scholar] [CrossRef]
- Selvi, T.M.; Nancy, K.; Florence, J.F. A study on the effect of pharmacologically active Lawsonia inermis linn. leaf extract on zinc electrodeposition on mild steel. Int. J. Res. Dev. Pharm. Life Sci. 2015, 2, 505–516. [Google Scholar]
- Singh, A.; Quraishi, M.A. Green corrosion inhibitors: An overview. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2018; pp. 1–38. [Google Scholar]
- Rahman, H.; Seleim, S.; Hafez, A.; Helmy, A. Study of electropolishing inhibition of steel using natural products as a green inhibitor in ortho-phosphoric acid. Green Chem. Lett. Rev. 2015, 8, 88–94. [Google Scholar] [CrossRef]
- El-Shamy, A.; El-Hadek, M.; Nassef, A.; El-Bindary, R. Optimization of the Influencing Variables on the Corrosion Property of Steel Alloy 4130 in 3.5 wt.% NaCl Solution. J. Chem. J. Chem. 2020, 2020, 9212491. [Google Scholar] [CrossRef]
- El-Bindary, R.; El-Shamy, A.; El-Hadek, M.; Nassef, A. Statistical Analysis of the Inhibition of Carbon Steel Corrosion in 3.5 wt.% NaCl Solution Using Lawsonia Extract. Port-Said Eng. Res. J. 2020, 25, 101–113. [Google Scholar] [CrossRef]
- Brixi, N.; Cherif, R.; Bezzar, A.; Sail, L.; Aït-Mokhtar, A. Effectiveness of henna leaves extract and its derivatives as green corrosion inhibitors of reinforcement steel exposed to chlorides. Eur. J. Environ. Civ. Eng. 2021, 26, 5912–5930. [Google Scholar] [CrossRef]
- Rani, O. Pengaruh Inhibitor Ekstrak Daun Inai (Lawsonia inermis L) Terhadap Laju Korosi Baja St37 dalam Larutan HCl 3%. J. Energy Mater. Instrum. Technol. 2021, 2, 104–109. [Google Scholar] [CrossRef]
- Njoku, V.O.; Oguzie, E.E.; Obi, C.; Ayuk, A.A. Baphianitidaleaves extract as a green corrosion inhibitor for the corrosion of mild steel in acidic media. Adv. Chem. 2014, 2014, 808456. [Google Scholar] [CrossRef]
- Oguzie, E.E. Corrosion inhibitive effect and adsorption properties of Hibiscus sabdariffa extract on mild steel in acidic media. Port. Electrochim. Acta 2008, 26, 303–314. [Google Scholar] [CrossRef]
- Ihebrodike, M.M.; Michael, C.N.; Kelechukwu, B.O.; Lebe, A.N.; Chidiebere, M.A.; Francis, C.E.; Oguzie, E.E. Experimental and theoriticalassessment of the inhibiting action of Aspiliaafricana extract on corrosion of aluminum alloy AA3003 in HCl. J. Mater. Sci. 2012, 47, 2559–2572. [Google Scholar]
- Akawu, I.; Ajibola, V.O.; Gimba, C.E.; Paul, E.D.; Adamu, I.K.; Gulumbe, N. Corrosion inhibition of Caesalpinia coriaria (DIVI-DIVI) pods extract on mild steel in 1m solutions of HCL AND H2SO4. J. Chem. Soc. Niger. 2017, 41. [Google Scholar]
- Dananjaya, S.H.; Edussariva, M.; Dissanayake, A.S. Inhibition action of Lawsone on the corrosion of mild steel in Acidic Media. Online J. Sci. Technol. 2012, 2, 32–36. [Google Scholar]
- Nagiub, A.M.; Mahross, M.H.; Khalil HF, Y.; Mahran BN, A.; Yehia, M.M.; El-Sabbah MM, B. Azo dye compounds as corrosion inhibitors for dissolution of mild steel in HCl acid. Port Electrochim. Acta 2013, 31, 119–139. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Chidiebere, M.A.; Oguzie, K.L.; Adindu, C.B.; Momoh-yahaya, H. Biomass extracts for materials protection: Corrosion inhibition of mild steel in acidic media by Terminalia chebula. Chem. Eng. Commun. 2014, 201, 790–803. [Google Scholar] [CrossRef]
- Jones, A.; Brown, B. Corrosion Inhibition by Natural Extracts. J. Corros. Sci. 2020, 15, 150–165. [Google Scholar]
- Enyoh, C.E.; Wang, Q. Adsorption and toxicity characteristics of ciprofloxacin on differently prepared polyethylene terephthalate microplastics from both experimental and theoretical perspectives. J. Water Pro. Eng. 2023, 53, 103909. [Google Scholar] [CrossRef]
- Pamela, I.O.; Emmanuel, C.N.; Ichou, H.; Berdimurodov, E.; Dagdag, O.; Kenneth, O.A.; Blessing, C.A.; Aliev, N.; Berisha, A.; AlObaid, A. Chrysophyllum albidum extract as a new and green protective agent for metal. Can. Metall. Q. 2024, 1–16. [Google Scholar] [CrossRef]
- Chile, N.E.; Haldhar, R.; Godffrey, U.K.; Chijioke, O.C.; Umezuruike, E.A.; Ifeoma, O.P.; Oke, M.O.; Ichou, H.; Arrousse, N.; Kim, S.-C.; et al. Theoretical Study and Adsorption Behavior of Urea on MildSteel in Automotive Gas Oil (AGO) Medium. Lubricants 2022, 10, 157. [Google Scholar] [CrossRef]
- Okeke, P.I.; Emeghara, K.C.; Ibe Geogeline, C.; Oseghale, T.I.; Maduka, O.G.; Emereonye, R.U. Inhibitive Action and Adsorption Character of Water Extract of Solanum melongena for the Corrosion of Mild Steel in 0.5 M H2SO4. IOSR J. Appl. Chem. 2016, 9, 20–25, e-ISSN 2278-5736. [Google Scholar] [CrossRef]
- Okeke, P.I.; Maduka, O.G.; Emereonye, R.U.; Akalezi, C.O.; Mughele, K.; Achinihu, I.O.; Azubuike, N.E. Corrosion Inhibition Efficacy of Cninodosculus chayamansa Extract on Aluminum Metal in Acidic and Alkaline Media. Int. J. Sci. Technoledge 2015, 3, 227–234, ISSN 2321-919x. [Google Scholar]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Wu, J. Super-hydrophobic metal-complex film fabricated electrochemically on copper as a barrier to corrosive medium. Corros. Sci. 2014, 83, 317–326. [Google Scholar] [CrossRef]
- Lundgren, S.M.; Persson, K.; Mueller, G.; Kronberg, B.; Clarke, J.; Chtaib, M.; Claesson, P.M. Unsaturated Fatty Acids in Alkane Solution: Adsorption to Steel Surfaces. Langmuir 2007, 23, 10598–10602. [Google Scholar] [CrossRef] [PubMed]
- Miralrio, A.; Espinoza Vázquez, A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Akalezi, C.O.; Maduabuchi, A.C.; Enenebeaku, C.K.; Oguzie, E.E. Experimental and DFT evaluation of adsorption and inhibitive properties of Moringa oliefera extract on mild steel corrosion in acidic media. Arab. J. Chem. 2020, 13, 9270–9282. [Google Scholar] [CrossRef]


| System | Ecorr | Icorr | Ѳ | IE (%) |
|---|---|---|---|---|
| 1 M HCl | −500.4 | 183 | ||
| 100 mg/dm3 LI | −468.3 | 113.5 | 0.381 | 38.1 |
| 1000 mg/dm3 LI | −461.9 | 26.6 | 0.855 | 85.5 |
| 0.1 M HCl | −496.2 | 145.7 | ||
| 100 mg/dm3 LI | −490.3 | 85.6 | 0.412 | 41.2 |
| 1000 mg/dm3 LI | −497.9 | 15.8 | 0.892 | 89.2 |
| Isotherm | Intercept | Slope | Kads | Ln Kads | R2 | |
|---|---|---|---|---|---|---|
| Langmuir Mild steel (0.1 M) | 513.3307 | 0.8728 | 0.002 | 6.267 | 0.8899 | −15.787 |
| Langmuir Mild steel (1 M HCl) | 12.2050 | 1.0203 | 0.0819 | 2.503 | 0.9999 | −6.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okore, G.; Ejiogu, B.; Okeke, P.; Amanze, K.; Okore, S.; Oguzie, E.; Enyoh, C.E. Lawsonia inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Appl. Sci. 2024, 14, 6392. https://doi.org/10.3390/app14156392
Okore G, Ejiogu B, Okeke P, Amanze K, Okore S, Oguzie E, Enyoh CE. Lawsonia inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Applied Sciences. 2024; 14(15):6392. https://doi.org/10.3390/app14156392
Chicago/Turabian StyleOkore, Glory, Blessing Ejiogu, Pamela Okeke, Kenneth Amanze, Sunday Okore, Emeka Oguzie, and Christian Ebere Enyoh. 2024. "Lawsonia inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid" Applied Sciences 14, no. 15: 6392. https://doi.org/10.3390/app14156392
APA StyleOkore, G., Ejiogu, B., Okeke, P., Amanze, K., Okore, S., Oguzie, E., & Enyoh, C. E. (2024). Lawsonia inermis as an Active Corrosion Inhibitor for Mild Steel in Hydrochloric Acid. Applied Sciences, 14(15), 6392. https://doi.org/10.3390/app14156392








