Anti-Obesity and Antidiabetic Effects of Fig (Ficus carica L.) Fermented Extract Using Lactobacillus plantarum BT-LP-01
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.1.1. Non-Fermented Fig Preparation
2.1.2. Fermented Fig Preparation
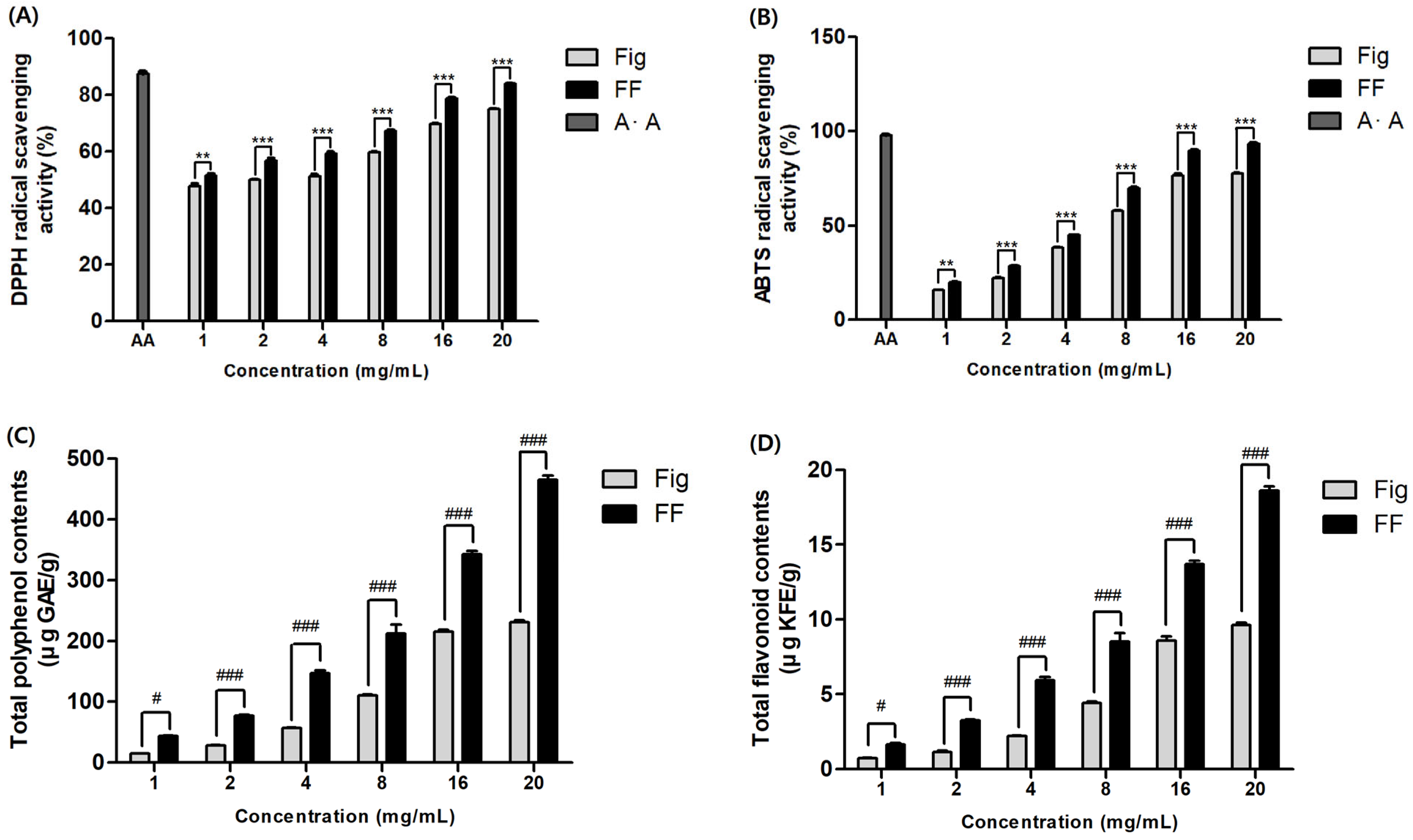
2.2. Determination of Antioxidant Capacity
2.2.1. DPPH Radical Scavenging Assay
2.2.2. ABTS Radical Scavenging Assay
2.3. Total Phenol Content Analysis
2.4. Total Flavonoid Content Analysis
2.5. Animals and Experimental Design
- Normal-Fat Diet group (NFD): Standard diet (10% kcal fat diet, D12450B, research Diets, New Brunswick, NJ, USA) + D·W
- High-Fat Diet group (HFD): High-fat diet (60% kcal fat diet, D12492, research Diets, New Brunswick, NJ, USA) + D·W
- Green tea extract group (GTE): High-fat diet (60% kcal fat diet, D12492, research Diets, New Brunswick, NJ, USA) + green tea extract 50 mg/kg
- Fermented fig 50 mg/kg group (FF 50): High-fat diet (60% kcal fat diet, D12492, research Diets, New Brunswick, NJ, USA) + fermented fig 50 mg/kg
- Fermented fig 125 mg/kg group (FF 125): High-fat diet (60% kcal fat diet, D12492, research Diets, New Brunswick, NJ, USA) + fermented fig 125 mg/kg
- Fermented fig 250 mg/kg group (FF 250): High-fat diet (60% kcal fat diet, D12492, research Diets, New Brunswick, NJ, USA) + fermented fig 250 mg/kg
2.6. Measure of Fasting Blood Glucose and IPGTT
2.7. Blood Biochemical Analysis
2.8. Histopathological
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of FF on Antioxidant
3.2. Effects of FF on Body Weight and Food Intake
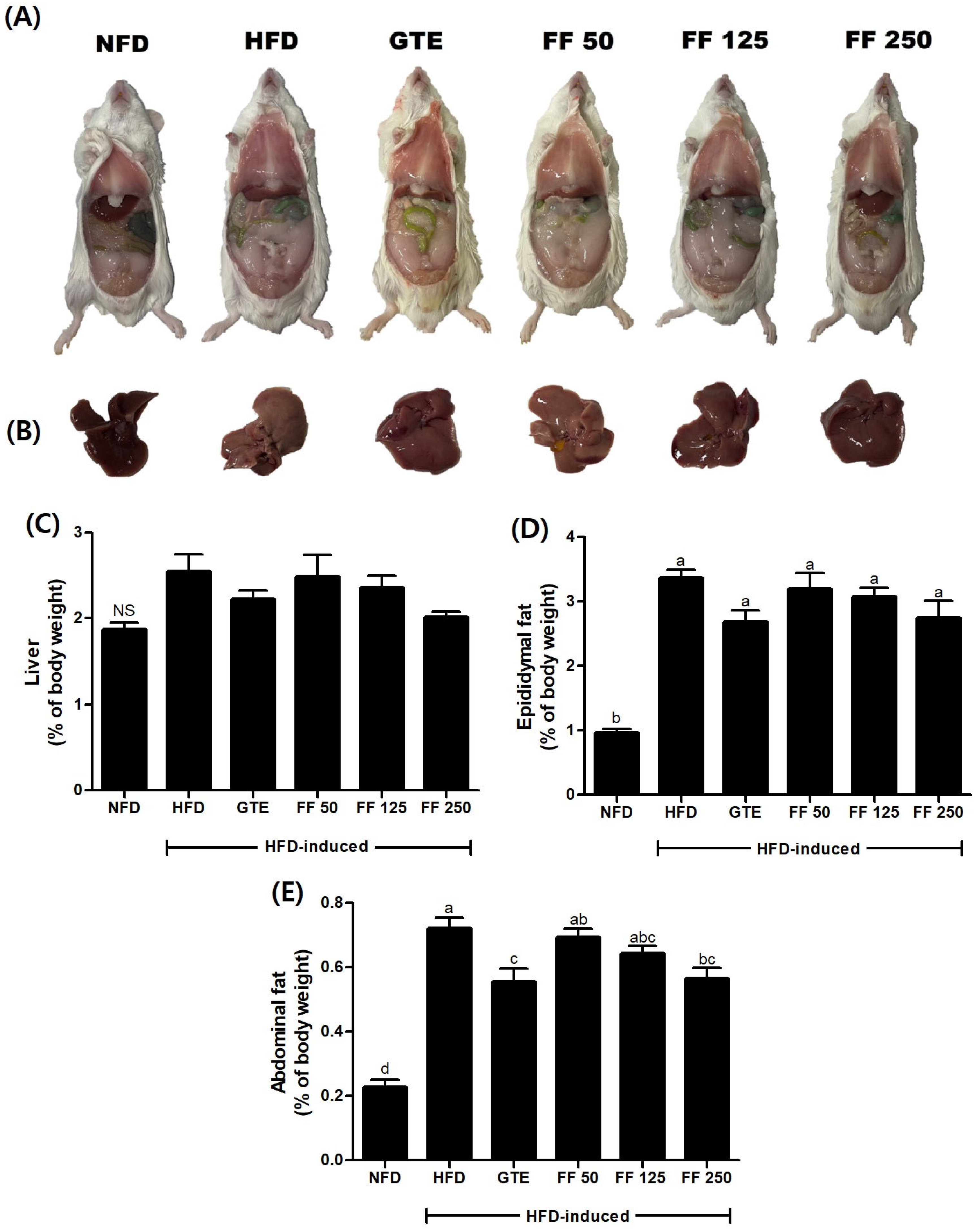
3.3. Effect of FF on Macroscopic Aspects and Organ Weight
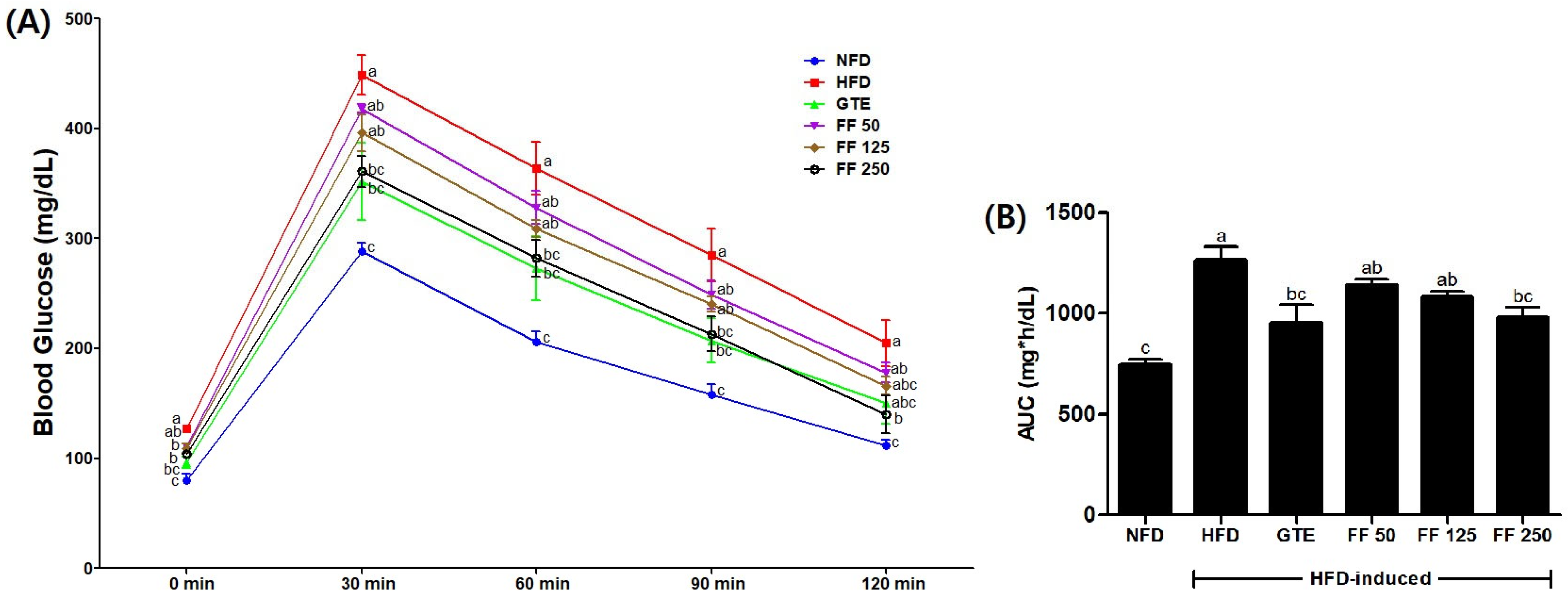
3.4. Effects of FF on Fasting Blood Glucose Levels
3.5. Effects of FF on IPGTT and AUC
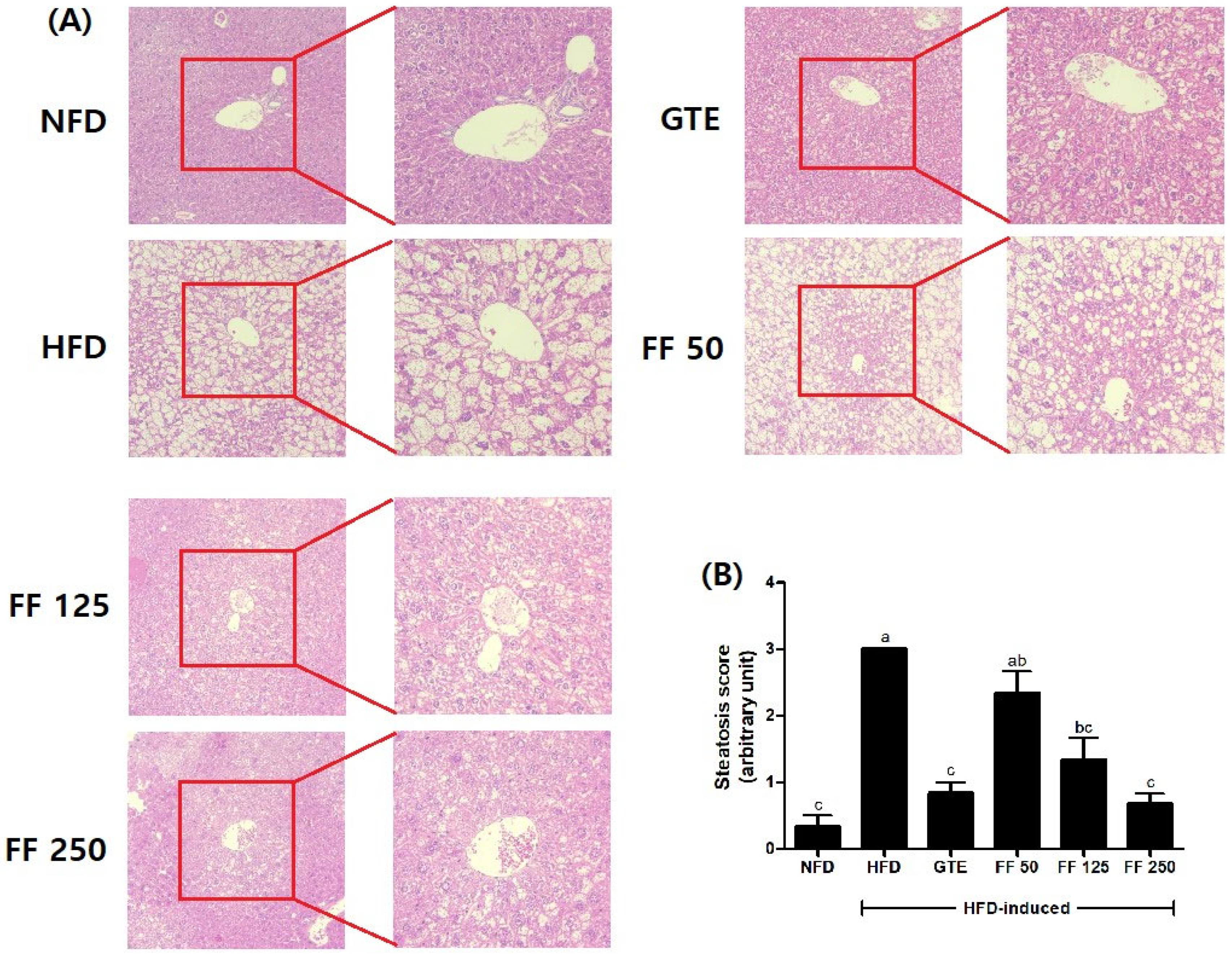
3.6. Effects of FF on Histopathology and Steatosis Score
3.7. Effects of FF on Serum C-P, Insulin, and Glucose Levels
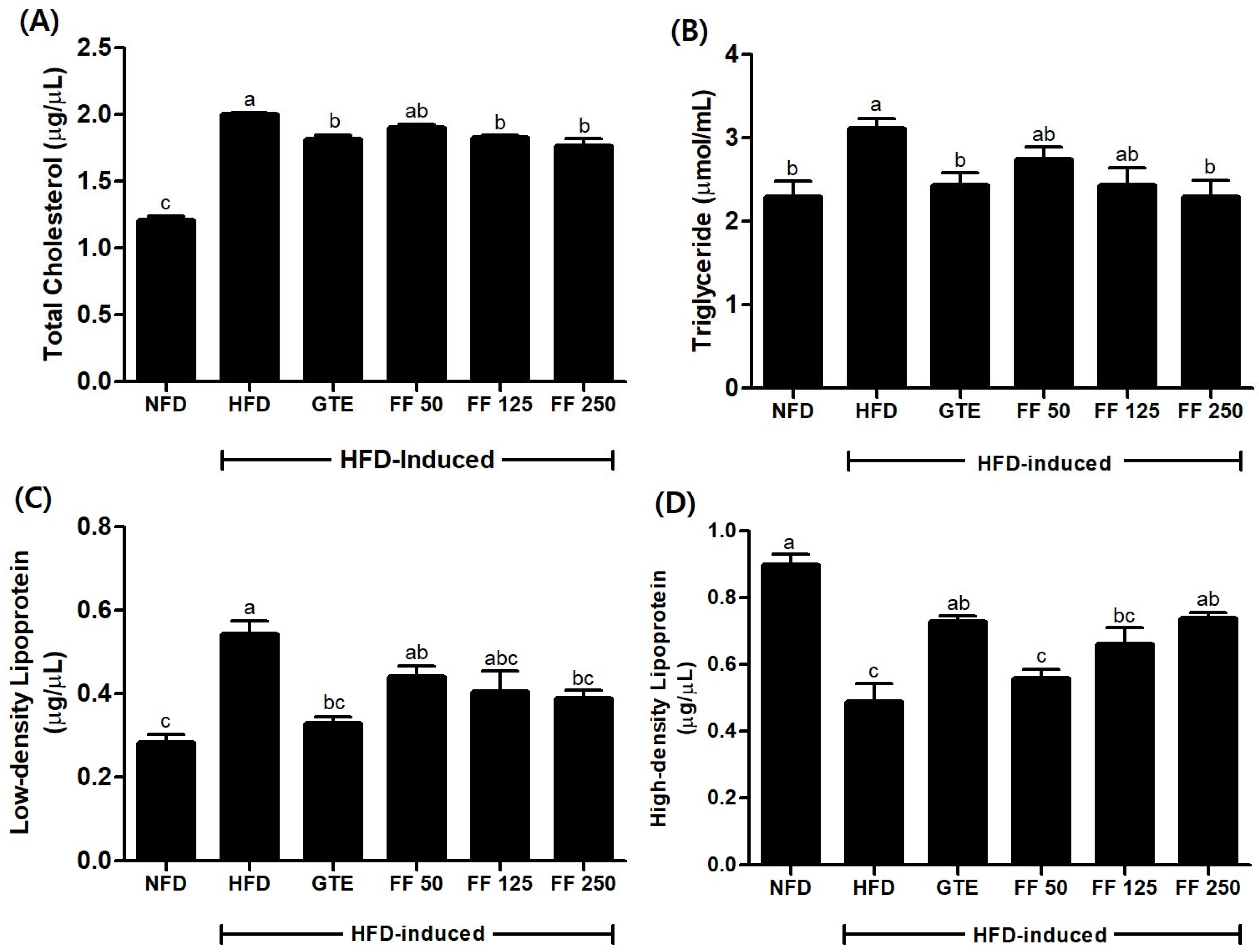
3.8. Effects of FF on Serum Lipid Metabolism
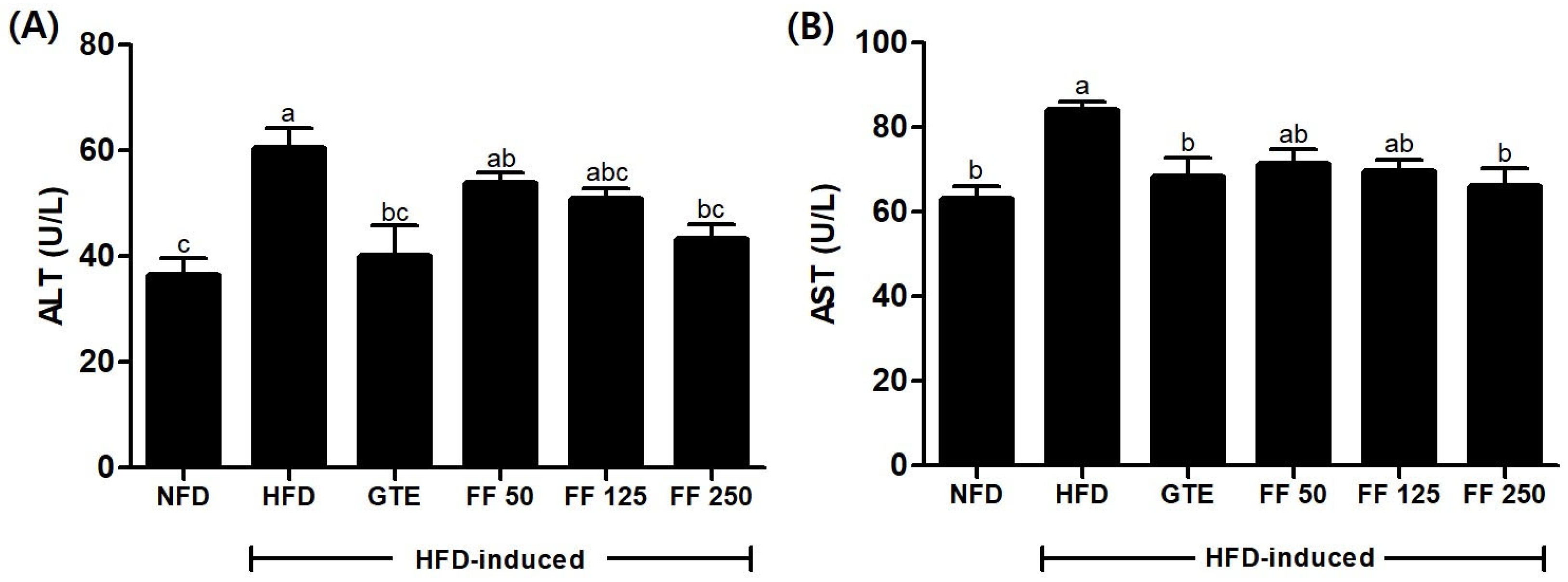
3.9. Effects of FF on Liver Function
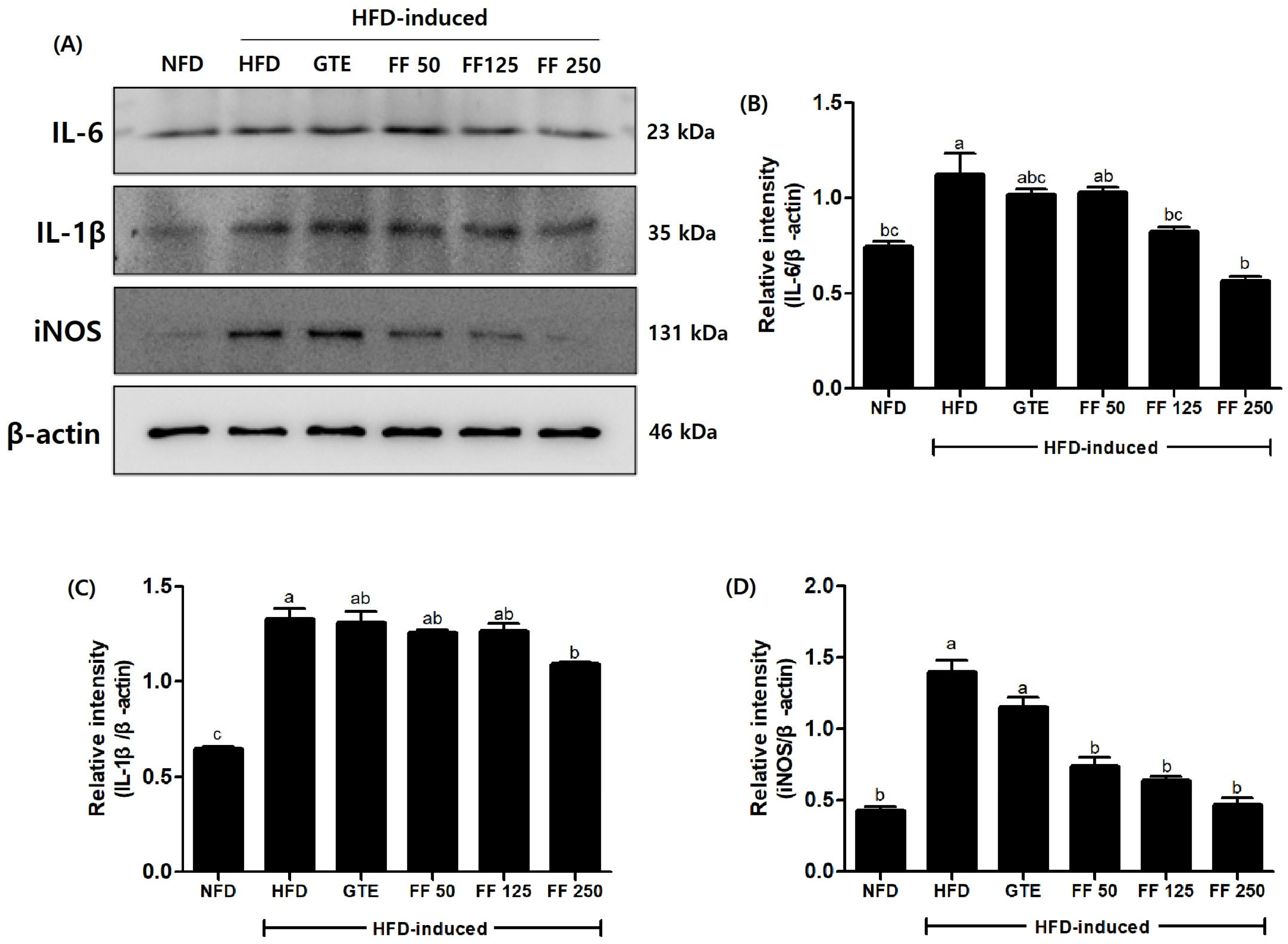
3.10. Effects of FF on Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Chen, H.C.; Farese, R.V., Jr. Inhibition of triglyceride synthesis as a treatment strategy for obesity: Lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 482–486. [Google Scholar] [CrossRef] [PubMed]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2009, 318, 10–14. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- Alessi, M.C.; Lijnen, H.R.; Bastelica, D.; Juhan-Vague, I. Adipose tissue and atherothrombosis. Pathophysiol. Haemost. Thromb. 2003, 33, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Wei, J.; Carns, M.; Hinchcliff, M.; Lee, J.; Whitfield, M.L.; Sodin-Semrl, S.; Varga, J. Levels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: Potential utility as biomarker? Arthritis Res. Ther. 2012, 14, R102. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gstroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Barazzoni, R.; Gortan Cappellari, G.; Ragni, M.; Nisoli, E. Insulin resistance in obesity: An overview of fundamental alterations. Eat. Weight Disord. 2018, 23, 149–157. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Pérez-Pérez, A.; Vilariño-García, T.; Jiménez-Cortegana, C.; Muriana, F.J.G.; Millán-Linares, M.C.; Sánchez-Margalet, V. Nutritional modulation of leptin expression and leptin action in obesity and obesity-associated complications. J. Nutr. Biochem. 2021, 89, 108561. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.L.; Maruthur, N.M.; Singh, S.; Segal, J.B.; Wilson, L.M.; Catterjee, R.; Marinopoulos, S.S.; Puhan, M.A.; Ranasinghe, P.; Block, L.; et al. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann. Intern. Med. 2011, 154, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Song, C.; Li, L.; Wang, T.; Hu, J.; Zhu, L.; Yue, T. Lactobacillus alleviated obesity induced by high-fat diet in mice. J. Food Sci. 2021, 86, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Yibchoc-Anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.S.; Lachance, P.A. Phytosterols and fatty acids in fig (Ficus carica, var. Mission) fruit and tree components. J. Food Sci. 2001, 66, 278–281. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.; Ghoneimy, E.; Abdel-Wahab, A.; Eldeeb, N.; Salem, M.; Ahmed, T. The therapeutic effects of Ficus carica extract as antioxidant and anticancer agent. S. Afr. J. Bot. 2021, 141, 273–277. [Google Scholar] [CrossRef]
- Soltana, H.; Pinon, A.; Limami, Y.; Zaid, Y.; Khalki, L.; Zaid, N.; Salah, D.; Sabitaliyevich, U.Y.; Simon, A.; Liagre, B.; et al. Antitumoral activity of Ficus carica L. on colorectal cancer cell lines. Cell. Mol. Biol. 2019, 65, 6–11. [Google Scholar] [CrossRef]
- Ayuso, M.; Carpena, M.; Taofiq, O.; Albuquerque, T.G.; Simal-Gandara, J.; Oliveira, M.B.P.; Prieto, M.A.; Ferreira, I.C.F.R.; Barros, L. Fig “Ficus carica L.” and its by-products: A decade evidence of their health-promoting benefits towards the development of novel food formulations. Trends Food Sci. Technol. 2022, 127, 1–13. [Google Scholar] [CrossRef]
- Purnamasari, R.; Winarni, D.; Permanasari, A.A.; Agustina, E.; Hayaza, S.; Darmanto, W. Anticancer Activity of Methanol Extract of Ficus carica Leaves and Fruits Against Proliferation, Apoptosis, and Necrosis in Huh7it Cells. Cancer Inf. 2019, 18, 1176935119842576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Song, L.; Xiao, Y.; Lu, S.; Xu, J.; Li, J.; Ren, Z. Lactobacillus plantarum prevents obesity via modulation of gut microbiota and metabolites in high-fat feeding mice. J. Funct. Foods 2020, 73, 104103. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Wang, L.; Zhao, L.; Xia, Y.; Ai, L. Diverse conditions contribute to the cholesterol-lowering ability of different Lactobacillus plantarum strains. Food Funct. 2021, 12, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Borgeraas, H.; Johnson, L.K.; Skattebu, J.; Hertel, J.K.; Hjelmesaeth, J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018, 19, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liong, M.T.; Tsai, Y.C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018, 56, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Yang, L.; Wang, Y.; Jiang, B.; Shen, M.; Ren, D. Reduction of serum cholesterol and its mechanism by Lactobacillus plantarum H6 screened from local fermented food products. Food Funct. 2020, 11, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5986. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Atlasi, R.; Hasani-Ranjbar, S.; Mortazavian, A.M.; Larijani, B. Probiotics supplementation for the obesity management; A systematic review of animal studies and clinical trials. J. Funct. Foods 2019, 52, 228–242. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K.; Chen, R.Y. Effects of fermentation on antioxidant properties and phytochemical composition of soy germ. J. Sci. Food Agric. 2014, 94, 3163–3170. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with Functional Properties: An Approach to Increase Safety and Shelf-Life of Fermented Foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Guo, J.; Jike, X.; Xu, K.; Li, B.; Liu, L.; Fang, Y.; Xu, H.; Lei, H. Phenolic profiles, antioxidant capacities and flavour volatiles in fig (Ficus carica L.) juices from five cultivars fermented by Lactobacillus plantarum and Lactobacillus acidophilus. Int. J. Food Sci. Technol. 2023, 58, 6025–6035. [Google Scholar] [CrossRef]
- Subramaniyan, V.; Sellamuthu, P.S.; Sadiku, E.R. Evaluation of the antifungal activity of cell-free supernatant of Lactobacillus species against the fig (Ficus carica. L) postharvest fungal pathogens through in-vitro and in-silico analysis. Sci. Hortic. 2024, 331, 113182. [Google Scholar] [CrossRef]
- Khezri, S.; Dehgha, P.; Mahmoudi, R.; Jafarlou, M. Fig juice fermented with lactic acid bacteria as a nutraceutical product. Pharm. Sci. 2016, 22, 260–266. [Google Scholar] [CrossRef]
- Long, X.; Zeng, X.; Tan, F.; Yi, R.; Pan, Y.; Zhou, X.; Mu, J.; Zhao, X. Lactobacillus plantarum KFY04 prevents obesity in mice through the PPAR pathway and alleviates oxidative damage and inflammation. Food Funct. 2020, 11, 5460–5472. [Google Scholar] [CrossRef]
- Park, S.Y.; Seong, K.S.; Lim, S.D. Anti-obesity Effect of Yogurt Fermented by Lactobacillus plantarum Q180 in Diet-induced Obese Rats. Korean J. Food Sci. Anim. Resour. 2016, 36, 77–83. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.; Park, Y.S. Anti-diabetic effect of mulberry leaf extract fermented with Lactobacillus plantarum. Korean J. Food Sci. Technol. 2020, 52, 191–199. [Google Scholar]
- Pan, R.; Xu, T.; Bai, J.; Xia, S.; Liu, Q.; Li, J.; Xiao, X.; Dong, Y. Effect of Lactobacillus plantarum fermented barley on plasma glycolipids and insulin sensitivity in subjects with metabolic syndrome. J. Food Biochem. 2020, 44, e13471. [Google Scholar] [CrossRef]
- Bedossa, P.; Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Lee, H.Y.; Lee, H.R.; Yoon, M.C.; Park, S.D.; Lee, Y.T.; Shen, Z.B.; Cui, H.H.; Shin, S.S. Ethyl acetate fraction of GGEx18 modulates feeding efficiency ratio and blood leptin level in high fat diet-fed obese mice. Herb. Formula Sci. 2011, 19, 219–231. [Google Scholar]
- Ezad, S.; Cheema, H.; Collins, N. Statin-induced rhabdomyolysis: A complication of a commonly overlooked drug interaction. Oxf. Med. Case Rep. 2018, 2018, omx104. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Obesity and fat metabolism. J. Korean Soc. Study Obes. 2000, 9, 63–65. [Google Scholar]
- Poret, J.M.; Souza-Smith, F.; Marcell, S.J.; Gaudet, D.A.; Tzeng, T.H.; Braymer, H.D.; Harrison-Bernard, L.M.; Primeaux, S.D. High fat diet consumption differentially affects adipose tissue inflammation and adipocyte size in obesity-prone and obesity-resistant rats. Int. J. Obes. 2018, 42, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir peptides prevent hyperlipidemia and obesity in high-fat-diet-induced obese rats via lipid metabolism modulation. Mol. Nutr. Food Res. 2018, 62, 1700505. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, S.; Qian, L.; Jiang, S.; Tang, Y.; Han, T. DHA-enriched phosphatidylserine ameliorates non-alcoholic fatty liver disease and intestinal dysbacteriosis in mice induced by a high-fat diet. Food Funct. 2021, 12, 4021–4033. [Google Scholar] [CrossRef]
- de Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- van Herpen, N.A.; Schrauwen-Hinderling, V.B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hossain, P.; Kawar, B.; Nahas, M.E. Obesity and diabetes in the developing world—A growing challenge. N. Engl. J. Med. 2007, 356, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, N.H.; Harris, R.A. Pyruvate dehydrogenase kinase-4 deficiency lowers blood glucose and improves glucose tolerance in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E46–E54. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.G.; Greenberg, A.S. Serum TAG analysis differentiates between genetic and obesity-associated NAFLD. Diabetes 2014, 63, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.Z.Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Pomegranate fruit pulp polyphenols reduce diet-induced obesity with modulation of gut microbiota in mice. J. Sci. Food Agric. 2022, 102, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Tomkin, G.H. Atherosclerosis, diabetes and lipoproteins. Expert Rev. Cardiovasc. Ther. 2010, 8, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus paracasei 24 attenuates lipid accumulation in high-fat deit-induced obese mice by regulating the gut microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity 2013, 21, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.O.; Choi, J.S.; Choi, K.H.; Kim, S.; Kim, H.; Chung, D.K. Anti-obesity potential of heat-killed Lactiplantibacillus plantarum K8 in 3T3-L1 cells and high-fat diet mice. Heliyon 2023, 9, e12926. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Olodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: A systematic review and meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef]
- Itskowitz, M.S. Low HDL cholesterol levels. N. Engl. J. Med. 2006, 354, 417–419. [Google Scholar] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and reverse cholesterol transport: Basic mechanisms and their roles in vascular health and disease. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Pratley, R.E.; Weyer, C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia 2001, 44, 929–945. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Liu, C.; Zhou, Y.; Wang, X.; Cheng, B.; Kui, C.; Wang, Y. C-peptide, glycaemic control, and diabetic complications in type 2 diabetes mellitus: A real-world study. Diabetes Metab. Res. Rev. 2022, 38, e3514. [Google Scholar] [CrossRef] [PubMed]
- Leighton, E.; Sainsbury, C.A.; Jones, G.C. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017, 8, 475–487. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-induced TNFα and IL-6 signaling: The missing link between obesity and inflammation—Driven liver and colorectal cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Tang, C.C.; Huang, H.P.; Lee, Y.J.; Tang, Y.H.; Wang, C.J. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem. Toxicol. 2013, 62, 786–796. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; King, Y.; Kim, H.S.; Jeon, Y.J.; Lee, S.H. Inhibition of adipogenesis by diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]


| Diets | Protein | Carbohydrate | Fat | Kcal | |||
|---|---|---|---|---|---|---|---|
| g % | kcal % | g % | kcal % | g % | kcal % | kcal/g | |
| Normal-Fat Diet (NFD, D12450B) | 19.2 | 20 | 67.3 | 70 | 4.2 | 10 | 3.85 |
| High-Fat Diet (HFD, D12492) | 26 | 20 | 26 | 20 | 35 | 60 | 5.24 |
| NFD | HFD | GTE | FF 50 | FF 125 | FF 250 | |
|---|---|---|---|---|---|---|
| Initial body weight (g) | 28.5 ± 0.5 NS | 28.7 ± 0.5 | 28.6 ± 0.4 | 28.6 ± 0.9 | 28.5 ± 0.4 | 28.6 ± 0.4 |
| Final body weight (g) | 42.4 ± 1.3 c | 56.9 ± 1.5 a | 51.3 ± 1.9 abc | 55.2 ± 1.5 ab | 53.7 ± 1.5 abc | 48.8 ± 1.6 bc |
| Body weight gain (g/day) | 1.99 ± 0.15 d | 4.02 ± 0.18 a | 3.25 ± 0.22 ab | 3.81 ± 0.16 ab | 3.60 ± 0.21 abc | 2.89 ± 0.24 c |
| Food intake (g/day) | 3.28 ± 0.30 NS | 3.09 ± 0.25 | 3.41 ± 1.59 | 3.03 ± 0.50 | 3.24 ± 1.24 | 2.93 ± 0.69 |
| FER (%) | 0.61 ± 0.05 d | 1.30 ± 0.06 a | 0.95 ± 0.07 c | 1.26 ± 0.05 ab | 1.11 ± 0.07 abc | 0.99 ± 0.08 bc |
| Fasting Blood Glucose (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| NFD | HFD | GTE | FF 50 | FF 125 | FF 250 | |
| 4 weeks | 75.9 ± 5.5 c | 114.8 ± 4.2 a | 90.2 ± 4.2 bc | 101.0 ± 1.8 ab | 98.2 ± 1.4 b | 96.4 ± 2.0 b |
| 8 weeks | 80.4 ± 5.8 c | 124.4 ± 3.4 a | 96.3 ± 4.8 bc | 111.0 ± 2.9 ab | 114.2 ± 3.9 b | 104.4 ± 3.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Choi, J.; Jang, Y.; Lee, Y.-M.; Kang, M.-H.; Kwon, H.-S.; Kim, S.; Kwon, J. Anti-Obesity and Antidiabetic Effects of Fig (Ficus carica L.) Fermented Extract Using Lactobacillus plantarum BT-LP-01. Appl. Sci. 2024, 14, 6412. https://doi.org/10.3390/app14156412
Choi H, Choi J, Jang Y, Lee Y-M, Kang M-H, Kwon H-S, Kim S, Kwon J. Anti-Obesity and Antidiabetic Effects of Fig (Ficus carica L.) Fermented Extract Using Lactobacillus plantarum BT-LP-01. Applied Sciences. 2024; 14(15):6412. https://doi.org/10.3390/app14156412
Chicago/Turabian StyleChoi, Hwal, Jihye Choi, Yuseong Jang, Young-Min Lee, Myoung-Hak Kang, Hyuck-Se Kwon, Sokho Kim, and Jungkee Kwon. 2024. "Anti-Obesity and Antidiabetic Effects of Fig (Ficus carica L.) Fermented Extract Using Lactobacillus plantarum BT-LP-01" Applied Sciences 14, no. 15: 6412. https://doi.org/10.3390/app14156412






